Abstract
The itaconic acid (IA) world market is expected to exceed 216 million of dollars by 2020 as a result of an increasing demand for bio-based chemicals. The potential of this organic acid produced by fermentation mainly with filamentous fungi relies on the vast industrial applications of polymers derived from it. The applications may be as a superabsorbent polymer for personal care or agriculture, unsaturated polyester resin for the transportation industry, poly(methyl methacrylate) for electronic devices, among many others. However, the existence of other substitutes and the high production cost limit the current IA market. IA manufacturing is done mainly in China and other Asia–Pacific countries. Higher economic feasibility and production worldwide may be achieved with the use of low-cost feedstock of local origin and with the development of applications targeted to specific local markets. Moreover, research on the biological pathway for IA synthesis and the effect of medium composition are important for amplifying the knowledge about the production of that biochemical with great market potential.
Keywords: Bio-based chemicals, Bio-based polymers, Fungal fermentative processes, Itaconic acid, Itaconic acid polymers, Itaconic acid trading market, Sustainable materials
Introduction
The world demand for eco-friendly products is constantly growing. Many bioprocesses are under development to align the environmental and economic aspects of manufacturing renewable products, which are not all feasible yet (Bailey 2016). The continuing effort and research, associated with government policies that promote sustainable programs, effectively nurtures the growth of bio-based product market (Report Linker 2017).
Bio-based organic acids are part of the portfolio of profitable and renewable chemicals. The combination of those two important factors results in an increasing demand of those acids. Moreover, the stringent restrictions imposed by governmental regulators in many countries have been encouraging companies to seek alternative renewable products and biotechnological processes (Report Linker 2017). The challenge is not only to obtain eco-friendly products, but to have equivalency in quality and quantity for competing with the products already available on the corresponding market (Bailey 2016).
Itaconic acid (IA) is a bio-based chemical with great potential for the chemical market and attractive end use applications (Weastra 2012). Even though the chemical properties of the organic acid enable a vast possibility of applications, IA is currently considered a niche market (Transparency Market Research 2015). The expected expansion of IA on the appropriate market depends on the development of the technologies for producing IA and its derivatives. Innovation, price competitiveness, and global expansion are key components for any product to achieve success in the renewable market. Improvements in production medium, as well as the application of the most appropriate fermentation conditions for achieving high IA yield, are some of the investigations regarding the develop of technologies for IA production (Krull et al. 2017).
IA origin and definition
IA can be synthesized either chemically or biochemically. The former has never been produced commercially due to the numerous stages for IA synthesis and the low efficiency of the process, while the latter is obtained by fermentation mainly using filamentous fungi with a significant production yield (Pfeifer et al. 1952; Kautola et al. 1989). A more detailed description about IA production is presented in later sections.
IA is presented in the form of white crystals and it is chemically defined as an unsaturated dicarboxylic acid with one of the carboxylic groups conjugated to a methyl group. Some of IA characteristics are listed on Table 1.
Table 1.
| IUPAC name | 2-Methylidenebutanedioic acid |
| Synonyms | Itaconic acid; 2-methylenesuccinic acid; methylelesuccinic acid; propylenedicarboxylic acid; methylenebutanedioic acid |
| Abbreviation | IA |
| CAS Number | 97-65-4 |
| Molecular formula | C5H6O4 |
| Molar mass (g/mol) | 130.09874 |
| Melting point | 165–168 °C |
| Appearance (Color) | White |
| Appearance (Form) | Powder or crystals |
| Density (g/cm3 at 25 °C) | 1.573 |
| Solubility in water (g/100 mL, 20 °C) | 8.31 |
| Acidity (pKa) | pKa1 = 3.84 pKa2 = 5.55 |
A brief history about IA
IA was discovered in 1837, described by Baup as a product obtainable from the pyrolysis of citric acid and it was named citric acid (Turner and Liebig 1841; Kane et al. 1945; Tate 1967). In 1840, Crassus described it as the product of the third stage of thermal decomposition of citric acid and proposed the name itaconic acid (Turner and Liebig 1841). At that time, the chemical route was the only one known.
The IA chemical synthesis is as follows:
distillation of citric acid;
oxidation of isopropene or from mesityl oxide to citraconic acid and subsequent isomerization;
carboxylation of acetylene derivatives, for example, propargyl chloride or butynoates;
condensation of succinate or succinic anhydride with formaldehyde to generate citraconic acid with subsequent isomerization.
This sequence of chemical reactions, however, is not economically feasible. The requirement for several stages resulted in an unsatisfactory yield and used components that were not readily available (Merger and Liebe 1991).
IA was first polymerized by Swarts as a form of ethyl ester in 1873 (Tate 1967). Dialkyl ester polymers were developed with properties close to glass at a process that lasted about 3 days (Hope 1927). Despite the interesting properties, it was only possible to obtain IA at a low scale due to the already mentioned low efficiency of the chemical route, which was a potential limitation for obtaining the end-product on a large scale.
In 1931, Kinoshita first reported the production of IA by the microbial route. In his study, a filamentous fungus isolated from salted prune juice was cultivated under surface fermentation conditions in the presence of concentrated solutions of sugars and high concentrations of chlorides, reaching yield of up to 0.24 (g IA/g substrate). Because the used microorganism was an IA producer, the filamentous fungus was named Aspergillus itaconicus (Kinoshita 1931). The production condition of that study, however, was never developed commercially (Kane et al. 1945).
Calam et al. (1939) presented that some A. terreus strains produced IA in Czapek-Dox medium containing 50 g/L of glucose. That was the first study which demonstrated that A. terreus was able to produce IA (0.12 g IA/g substrate after 25 days). The authors also showed that not all strains—5 out of the 6 strains tested—produced this organic acid to the extracellular medium at the conditions used (Calam et al. 1939).
The homopolymerization of IA was described in 1958, which was done with hydrochloric acid and potassium persulfate (Marvel and Shepherd 1959). Because of the interesting properties of IA polymers, further investigation was done to reach higher final concentrations of IA from different strains.
According to Miall (1978), in 1945, Moyer and Coghill evaluated 30 A. terreus cultures from the traditional microbial cell bank Northern Regional Research Laboratory (currently, Agricultural Research Service—ARS), identifying the strain NRRL 265 as the only IA producer. In the same year, Lockwood and Reeves analyzed 308 strains isolated from soil samples and A. terreus NRRL 1960 was chosen for pilot scale processes (Willke and Vorlop 2001). Since then, A. terreus strain NRRL 1960 has been the most studied among researchers to obtain IA. A. terreus NRRL 1960 is stored in different international cell banks with the following codes: ATCC 10020, ATCC 20589, DSM 826, CBS 11646, among others (NRRL 2017).
The production of IA by microbial route was first patented in 1945 (Kane et al. 1945). Pfizer Company accomplished 28% of the theoretical yield for producing IA with sucrose after 14 days of fermentation (Kane et al. 1945). IA was included in the company’s product portfolio in 1945 (Okabe et al. 2009).
Other bioprocess conditions allowed Lockwood and Ward (1945) to obtain 30 g of IA from 100 g of glucose (42% of the theoretical yield). In the following years, the strain Aspergillus terreus NRRL 1960 was used for larger scale production, with different nitrogen sources in a 20 L bioreactor (Nelson et al. 1952). At that stage, studies were done on a large scale (between 1130 and 2270 L), and in semi-continuous fermentation (Pfeifer et al. 1952). Other microorganisms have been reported as IA producers, such as Ustilago zeae (Miall 1978), and by a number of Candida sp. strains (Horitsu et al. 1983).
In 2004, IA was listed as one of the 12 most promising chemicals available from biomass according to the United States Department of Energy report (Werpy and Petersen 2004). The document selected IA and 11 others from an initial list of more than 300 bio-based building blocks regarding the potential markets of the chemicals and their derivatives, and the technical complexity in producing those chemicals. Since that report, which included succinic, fumaric and malic acid among other chemicals, IA gained a significant interest in the scientific community and it stimulated vast research about the improvement in IA production and its applications (Kuenz et al. 2012; Klement and Büchs 2013).
The homopolymerization process of IA on a large scale was a challenge in the early 21th century (Werpy and Petersen 2004). The improvement of the economic feasibility for obtaining IA depended on the development of the polymerization techniques that would decrease production cost to produce the homopolymer. That barrier was overcome by researchers from the University of New Hampshire, and the technology was licensed to Itaconix®, which proceeded to develop IA products from the poly(itaconic acid) (PIA) (Durant 2011).
More recently, IA was identified to be secreted by mammalian immune cells, such as macrophages, responsible for the antimicrobial activity by those cells in situations of inflammatory conditions (Sugimoto et al. 2011). IA was previously detected in the lungs from mice infected with tuberculosis, but it had been assumed that the metabolite was produced by the contaminating bacteria (Shin et al. 2011; Cordes et al. 2015). Michelucci et al. (2013) identified that, in mammalian cells, IA is produced by the immunoresponsive gene 1(Irgl), a highly expressed gene by macrophages in inflammation. That IA characteristic was explored by Bajpai et al. (2016) as a component of antimicrobial biofilm with potential application in the biomedical field.
IA producer microorganisms
Some microorganisms are able to synthesize IA, but with different productions capacities. The main IA producers are from the species A. terreus, which are used for producing the acid on a commercial scale (Saha 2017). The requirement for systems that result in higher IA productivity and higher yields (product/consumed substrate) encourages many researchers to find different IA producers (Voll et al. 2012). Table 2 lists some of the producing microorganisms, as well as the characteristic of the process.
Table 2.
Itaconic acid producers and their fermentative processes
| Microorganism | Feedstock | Working volume and fermentation type | IA (g/L) | Yield (Y IA/substrate) | Time |
|---|---|---|---|---|---|
| A. terreus NRRL 265a (Lockwood and Moyer 1945) | Sucrose | 50 mL, Submerged fermentation | 48.7 | n.d. | 11 d |
| Mutant of A. terreus NRRL 265 and 1960b (Nubel et al. 1962) | Beet and sugarcane molasses | 2L, submerged fermentation | 50 | n.d. | n.d. |
| A. terreus NRRL 1960a (Kautola et al. 1985) | Xylose and glucose | 14 L, immobilized microorganism in submerged fermentation | 30 | 0.54 g IA/g S | 4 d |
| A. terreus DSM 23081a (Kuenz et al. 2012) | Glucose | 100 mL, Submerged fermentation | 90 | n.d. | 13 d |
| A. terreus NRRL 1960a (Kocabas et al. 2014) | Corn cob, cotton twig and sunflower stalk with glucose | 100 mL, simultaneous enzymatic hydrolysis and fermentation, in submerged medium | 18 | n.d. | 12 d |
| A. terreus TN484-M1a (Dwiarti et al. 2007) | Corn sorghum | 3L, submerged fermentation | 48 | 0.34 g IA/g S | 6 d |
|
A. terreus CECT 20365a (Vassilev et al. 2013) |
Sugar beet press-mud and dry olive residues and glycerol | 10 g, solid-state fermentation | n.d. | 44 g IA/kg | 5 d |
| Mutant of A. terreus ATCC 10020a (Tsai et al. 2001) | Glucose, sucrose, fructose, mannose, starch hydrolysate and molasse | 45 mL, semi-solid-state fermentation | n.d. | 0.55 g IA/g | 120 h |
| Mutant of A. niger ATCC 1015a (Blumhoff et al. 2013) | Glucose | 100 mL, submerged fermentation | 1.2 | n.d. | 10–13 d |
| Candida sp.b (Tabuchi et al. 1981) | Glucose | 25 mL, submerged fermentation | 35 | n.d. | 5 d |
| Mutant of E. colid (Okamoto et al. 2014) | Glucose | 1.5 L, submerged fermentation | 4.3 | 0.13 g IA/g S | 5 d |
| H. mompa TANAKAa (Araki et al. 1957) | Sweet potato | 14 mm of the feedstock, Solid-state fermentation | 0.5 | n.d. | n.d. |
| Pseudozyma antarctica Y-7808c (Levinson et al. 2006) | Glucose | 1L, submerged fermentation | 30 | 0.38 g IA/g S | 6 d |
| Ustilago maydis MB215c (Maassen et al. 2014) | Glucose | 2 or 6 L, submerged fermentation | 44.5 | 0.24 g IA/g S | 6 d |
| Ustilago maydis MB215c (Carstensen et al. 2013) | Glucose | 2L, submerged fermentation | 4 | n.d. | n.d. |
| Mutant of Yarrowia lipolytica PO1fb (Blazeck et al. 2015) | Glucose | 1.5 L, submerged fermentation | 1.2 | 0.058 g IA/g S | 7 d |
The microorganism classifications are different kingdom and types: afilamentous fungus, byeast, cbasidiomycetes or dbacteria
It is noticed that the filamentous fungus strain Aspergillus terreus produces the highest IA concentrations in glucose medium, but Ustilago maydis is also a promising microorganism for IA synthesis. The technical difficulties regarding the use of filamentous fungus compared to bacteria or yeast encourage the research for different IA producers. The bioprocess with filamentous fungi is usually sensitive to hydro-mechanical stress in submerged fermentation (Voll et al. 2012) and its filamentous growth characteristics can be operationally more complicated than other microorganisms mentioned.
Ustilago maydis is a basidiomycete, which is a non-pathogenic microorganism when presented as a free-living yeast-like cell and plant pathogenic as the filamentous form (Levinson et al. 2006; Rafi et al. 2014). Despite the advantages of using basidiomycete, the highest production obtained from that microorganism is about 0.2 g IA/g glucose (Maassen et al. 2014), which is still much lower than the highest concentrations produced by A. terreus (0.48 IA/g glucose) in batch fermentation in laboratory scale (Kuenz et al. 2012).
Although research tends to focus on Aspergillus and Ustilago strains, different studies have demonstrated IA production capacity by other microorganism species. Helicobasidium mompa produces IA at low values − 0.25 to 0.5 g/L IA (Araki et al. 1957). Candida sp. (Tabuchi et al. 1981) was genetically modified and it produced about 0.35 g IA/g glucose. Despite the potential results, to the best of the authors knowledge, no further studies were presented regarding IA production by that Candida sp. Pseudozyma antarctica was also reported as IA producer, which synthesized 0.1 g IA/g substrate, in medium containing either glucose or fructose (Levinson et al. 2006). Genetically modified E. coli was also less efficient than A. terreus, with final production of about 0.14 g IA/g glucose by the fourth day of fermentation (Okamoto et al. 2014). Despite the different strategies applied for IA production with other microorganisms, A. terreus is still the current, dominant choice for IA production on a commercial scale.
The urge of increasing IA production also drives research on metabolic manipulation of microorganisms (Kuenz et al. 2012). Genetic modification has been done on Aspergillus niger, which is expected to produce higher IA concentrations than the bioprocess done with A. terreus, since the latter produces over 200 g/L of citric acid (also an organic acid) and has a very similar metabolic system to its parental strain (Blumhoff et al. 2013). The efforts done to promote IA production capacity on A. niger have been an important tool for comprehension of the IA pathway in A. terreus (Steiger et al.2016).
Metabolic system of IA production by Aspergillus terreus
Different studies regarding the metabolic pathway of IA synthesis have been done mainly with A. terreus (Tevz et al. 2010; Huang et al. 2014a, b). Currently, it is highly accepted that cis-aconitate decarboxylase (CAD) is responsible for the final transformation of cis-aconitate to itaconate (Hossain et al. 2016; Jiménez-Quero et al. 2016).
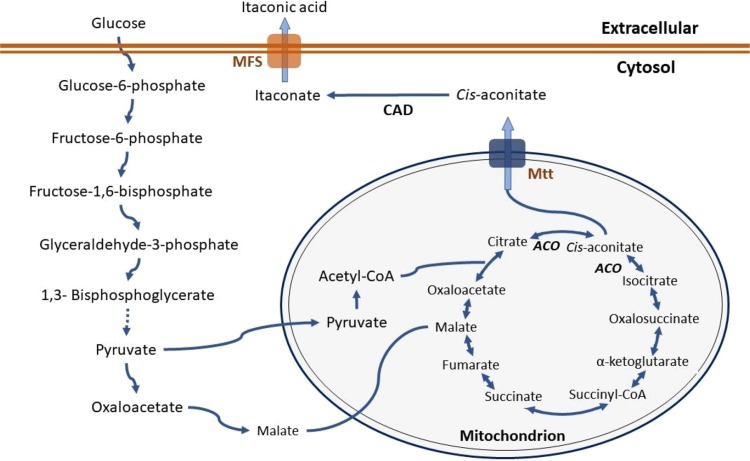
In IA synthesis from glucose, the substrate enters the cell and it is degraded mainly via the glycolysis route. Both malate and pyruvate produced in the cytosol enters the mitochondria to the TCA cycle, where cis-aconitate is produced. Cis-aconitate is transported to the cytosol through a mitochondrial tricarboxylate transporter (Mtt), where CAD synthesizes the itaconate production (Li et al. 2013). Finally, itaconate is externalized through major facilitator superfamily proteins (MFS). The kinetic profile of IA production shows that a slow or null cell growth rate prevails during IA production (Kuenz et al. 2012). This is explained by the deviation of cis-aconitate from the mitochondria to the cytosol, i.e., the TCA cycle is incomplete and cell growth is limited or null. Figure 1 illustrates the metabolic pathway from glucose.
Fig. 1.
Itaconic acid biosynthesis pathway in Aspergillus terreus. Mtt: Mitochondrial tricarboxylate transporter; MFS: major facilitator superfamily proteins; CAD: cis-aconitate decarboxylase; ACO: aconitase. [Adapted from Klement and Büchs (2013) and Huang et al (2014b)]
Dwiarti et al. (2002) were able to characterize the enzyme CAD for the first time and Kanamasa et al. (2008) identified the gene responsible for its coding. Those findings confirmed the CAD enzyme function in synthesizing IA and identified the region where the gene is located (Lai et al. 2007). Bentley and Thiessen (1957) identified that the enzyme is inhibited by heavy metals. Despite these conclusions, up to now, the regulation of IA synthesis is not yet completely known (Klement and Büchs 2013).
A deep understanding of the effect of nutrients on cultivation and production is of paramount importance given that the regulation of CAD is possibly related to the limitation of the essential element other than carbon (Welter 2000). Further knowledge about A. terreus metabolic regulation, as well as the effect of medium components and operation conditions, is of great importance for increasing IA production.
IA: a bio-based chemical with potential market growth and a wide range of applications
The technical barriers of IA synthesis over 10 years ago were the formation of other co-products during the fermentation and the need to increase the fermentation’s yield and productivity (Werpy and Petersen 2004). Despite the technological advances, there are still limitation concerning the production process, and the decrease of industrial costs is still desirable (Weastra 2012).
The IA market is characterized as a niche among other chemicals mainly due to limited assimilation of IA products in the market and the large availability of substitutes for those. The decrease of production costs would increase IA economic feasibility and expand the commercial interest for IA (Transparency Market Research 2015). Because of its market potential in a world scenario with increasing demand for bio-based chemicals, the IA market is expected to exceed 216 million of dollars by 2020 (Global Industry Analysis 2016).
The advantages of IA products over other chemicals from fossil source include its biodegradability (as homopolymer), non-toxicity, and a variety of possible derivatives as polymers. Some IA applications are under development, some of them still need further research and development (R&D) to be economically feasible, while some others are closer to reaching the commercial market. Some of the vast number of end-products available in the literature are presented on Table 3.
Table 3.
Characteristics and application of polymers produced with IA
| Polymer | End-product properties | Applications |
|---|---|---|
| Poly(itaconic acid) (PIA) (Sadeghi and Hosseinzadeh 2008) | This homopolymer produces superabsorbent polymer (SAP) with hydrophobic properties due to functional crosslinks. SAP can absorb hundreds or thousands of times of its mass, although it is soluble in water | Disposable diapers and feminine absorbents, retention agents in agriculture, concrete additives, soil correctives, controlled drug release system. It is a potent candidate for replacing SAP from acrylic acid |
| Poly(acrylamide-co-itaconic acid) P(AM-co-IA) (Shi et al. 2014) | It forms superabsorbent hydrogel microspheres with high absorption capacity, similar to poly(itaconic acid) | Soil amendments, water shutoff agents, and drug delivery carriers |
| Poly(acrylonitrile-co-itaconic acid) (PAI) (Nguyen-Thai and Hong 2014) | (PAI) has high electronic conductivity | Carbon-based soft electronic devices |
| Polystyrene-core(PSt)/ poly(butyl acrylate) (PBA) co-itaconic acid)-shell and PBA-core/ (PSt-co-IA)-shell polymers (Aguiar et al. 1999; Rabelero et al. 2013) | It may be presented as shell-core polymer produced with polystyrene. It changes from elastic to rigid by increasing the amount of IA incorporated into the shell, with properties close to those of a rigid plastic | Coating, adherent material, or high strength plastics |
| Poly(itaconic acid–co–bisacrylamide) (Bednarz et al. 2014) | Hydrogels formulated with deep eutectic solvents (DES) composed of high-density crosslinks with the ability to absorb metal ions | Absorbents of metal cations (Cu2+, Co2+, Ni2+) |
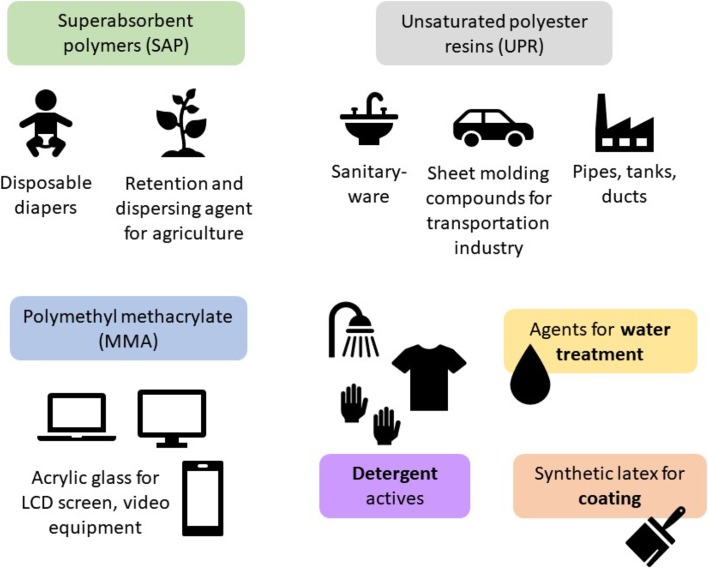
Currently, the most promising applications of IA are as synthetic latex, methyl methacrylate (MMA), unsaturated polyester resins (UPR), and superabsorbent polymer (SAP) (Global Market Insights 2016), illustrated on Fig. 2.
Fig. 2.
Some of the most potential itaconic acid market applications
Synthetic latex represents over 50% of the global market share of IA products, mostly used for polymer stabilization—SBR latex (styrene–butadiene rubber). As this niche market is already the most demanding of IA, it is expected to have less prominent growth over the next years (Global Market Insights 2016).
The expansion of the MMA market, currently produced from acetone cyanohydrin (Weastra 2012), is expected to further fuel IA demand. MMA requirements in liquid–crystal display (LCD) screens, smartphones screen, and video equipment are some of the mostly likely applications of IA (Global Market Insights 2016).
The use of IA as UPR, which can also be produced with maleic anhydride (Weastra 2012), is directed to marine, construction, and transportation industries. Because of its similar structure to maleic anhydride, IA is a potent bio-based chemical for substituting non-renewable chemicals (Global Market Insights 2016).
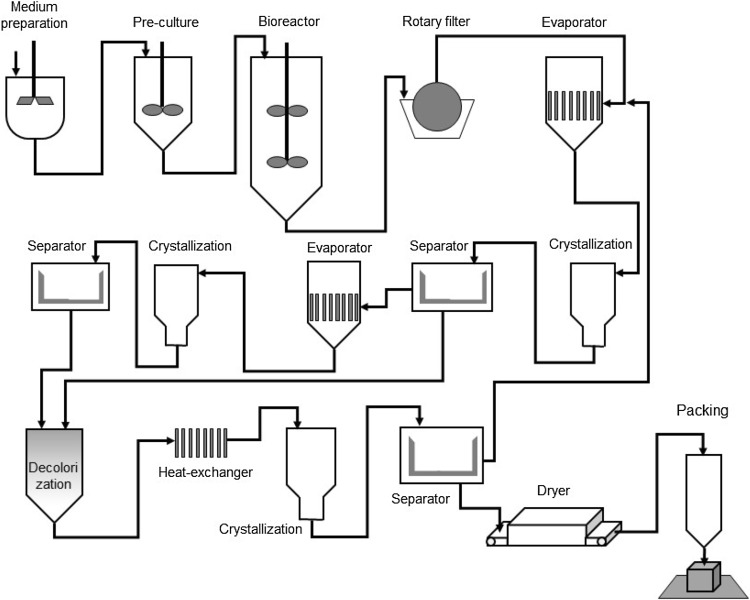
IA large-scale production
The process of IA production was well described by Okabe et al. (2009). According to the authors, the industrial production of IA is a five-step process. The fermentation step concerns in IA synthesis from microorganism, whose cells are removed together with other solid particles at the end of the process by filtration process. The solid-free broth goes to the concentration step where a liquid of over 350 g/L of IA is obtained. The concentrated liquid passes through two series of crystallization processes, at 15 °C. The crystals formed are decolorized by treatment with activated charcoal at 80 °C. In the case of a large-scale industrial process, the decolorization process can be optimized. The decolorized broth is evaporated and recrystallized before going to the drying and packing steps. If the production requires a high degree of purity, the product goes through a purification process, such as solvent extraction, ion exchange, and a new decolorization. Each stage has high efficiency for recovering IA: 95% in the filtration step, 98% of the concentration process, and 95% in the crystallization and drying. The total recovery of the process is approximately 80%. The production steps are illustrated in Fig. 3.
Fig. 3.
Diagram of itaconic acid industrial production by A. terreus
IA global production
IA was first commercially produced by Pfizer Company, in 1945. Since then, other companies such as Iwata Chemical (started at 1970, in Japan), Rhodia (started at 1995, in France), and Cargill (started at 1996, in the USA) have been great producers (Okabe et al. 2009). The production interruption by Cargill, Pfizer, and Rhodia made China the current largest IA producer (El-Imam and Du 2014).
China has been receiving robust investments from companies and from the Chinese government, including in bioprocesses industries. The increasing research background, human resources, and financial support has provided the biotechnology industry growth over recent years in that country (Huang et al. 2010).
Among many Chinese companies that produces IA, the Qingdao Kehai Biochemistry Company is responsible for about 50% of the total capacity of IA production in China, or 18% worldwide, with 10,000 Mt/year (Huang et al. 2010). That company is part of the Qingdao Langyatai Group and exports IA (not final products nor derivates) mainly to North and South America, and Western and Eastern Europe (China 2017).
The last reports show that only three countries are currently responsible for the world production: China, India, and USA (Global Industry Analysis 2016). The main current players are Alpha Chemika (India), Chenggdu Jindai Biology Engineering Co., Ltd. (China), Jinan Huaming Biochemistry Co., Ltd. (China), Qingdao Kehai Biochemistry Co., Ltd. (China), Shandong Kaison Biochemistry Co., Ltd. (China), Zhejiang Guoguang Biochemistry Co., Ltd. (China), and Itaconix Co. (USA).
In fact, the Asia–Pacific region market should serve as an example for developing the IA market in other countries. The existence of many domestic manufactures among IA players in that region represents the moderately fragmented IA market. The development of technologies and applications addressed to those niche and local markets may be the key strategy to expand IA production in other countries (Global Market Insights 2016).
One example of targeting local opportunities is the possibility of growth in Europe because of product control by the government. Currently, European Union regulations to stop the manufacture of detergents produced from sodium tri(poly)phosphate (STPP) may be substituted with IA derivatives. In Germany, the IA market benefits from environmental government practices and its size reached 2.8 million in 2015. South Africa, Saudi Arabia, and the United Arab Emirates may also be targets for IA applications as a result of rising preferences for bio-based products in those countries (Global Market Insights 2016).
Medium requirement for a high yield IA production
Multiple parameters influence metabolites production, such as medium composition, pH, temperature, the presence or absence of trace elements, and many others (Vrabl et al. 2012). Among them, the carbon source used is very important for producing economic feasible IA. The requirement of high initial concentration of sugars to obtain high yields reflects on high cost with feedstock if pure substrates, such as glucose or sucrose, are used.
The knowledge about the sufficient concentration to be used for high IA production without the use of excessive substrate affects the production final cost and depends on the strain. The highest IA yields (> 0.8 mol IA mol glucose) are achieved with over 100 g/L of glucose by A. terreus NRRL 1960, without a significant increase neither decrease in the final yield with substrate up to 200 g/L (Karaffa et al. 2015). Different results were obtained with A. terreus NRRL 1963, which presented an inhibition effect with concentrations higher than 160 g/L of glucose (Welter 2000). Kuenz et al. (2012), however, showed that similar concentrations are obtained by A. terreus NRRL 1993, A. terreus NRRL 1960, and A. terreus DSM 23081 (0.7 mol IA/mol glucose).
Considering the kinetics properties of CAD, an essential enzyme for IA production, its KM value for its main substrate, cis-aconitic acid, is 2.45 mM at pH 6.2 and 37 °C (Dwiarti et al. 2002). The low affinity to its substrate in IA synthesis indicated by the high KM value demonstrates the need for high substrate concentration for achieving high production yields (Cordes et al. 2015).
Different nitrogen sources, such as yeast extract or corn steep liquor, were used in the early studies (Pfeifer et al. 1952), but the complexity and varied composition of those reactants are undesirable factors for developing a stable production platform. IA fermentation with urea or ammonium nitrate resulted in low fermentation rates according to Nelson et al. (1952) and Pfeifer et al. (1952). However, ammonium nitrate (NH4NO3) has been used as nitrogen source in many other studies with high IA yield (Kautola et al. 1991; Kuenz et al. 2012).
Regarding the nitrogen source concentration, Vassilev et al. (1992) showed that, for immobilized cells, the rate of IA production in the absence of nitrogen is higher than with an initial concentration of 4 g/L of NH4NO3. Those results indicated that the nitrogen consumption is related to cell production rather than IA synthesis (Vassilev et al. 1992). Welter (2000) evaluated the combination of NH4NO3 with KH2PO4, and it was observed that, for initial 94 g/L of glucose, minimal cell growth and high IA production are obtained with 0.08 g/L KH2PO4 and 2 g/L of NH4NO3. Kuenz et al. (2012) chose to use 3 g/L NH4NO3 rather than 1.5 g/L to avoid insufficient nitrogen source supply, even though both the initial concentrations of NH4NO3 resulted in similar results.
Other medium components can influence IA production such as Fe, Mn—below 5 µg/L (Karaffa et al. 2015), Mg, Cu, Zn, P, N, and carbon source concentration (Batti and Schweiger 1963; Kautola et al. 1991; Willke and Vorlop 2001; Li et al. 2012; Karaffa et al. 2015).
IA production by low-cost feedstock
Studies have shown that some residues are suitable as carbon source for IA production, with some examples presented on Table 2. The limitations of IA production in some media are related to A. terreus sensitivity to medium impurities, which are not yet well defined (Hiller et al. 2014). However, the literature does not detail which components and at which concentration they impair IA production. Despite that sensitivity, some studies show the capacity of A. terreus to produce IA from waste material. The importance in evaluating IA production from residues relies on the possibility of IA production in different countries depending on the abundance of the specific residue. Using low-cost feedstock from local source, IA production economic feasibility may promote further application to the market.
Reddy and Singh (2002) showed that 20 and 30 g/L IA were produced, respectively, from market refuse fruits and hydrolyzed corn starch with A. terreus mutant. Petruccioli et al. (1999) obtained 18 g IA/L from corn starch feedstock, while Dwiarti et al. (2007) obtained about 50 g IA/L using hydrolysate sago starch. The use of molasse medium requires a previous treatment for removing the impurities for a high IA yield process (Maassen et al. 2014).
Corn cob, a lignocellulosic residue, was used in a two-step process: first, xylanase was produced by A. terreus, which was further used on the second step of the process concerning the hydrolysis of the lignocellulosic feedstock (with addition of commercial xylanase) for obtaining fermentable sugars for IA production, also by A. terreus (about 8 g/L IA) (Kocabas et al. 2014). A different lignocellulosic material, beech wood hydrolysate, was used for IA production and about 13 g/L IA was produced by A. terreus in solid-state reactor after the removal of phenolic components with anion and cation exchangers (Sieker et al. 2012).
Sieker et al. (2012) showed that IA production was only achieved when beech wood hydrolysate was detoxified by a mixture of anion and cation exchangers (among other pretreatment analyzed), achieving maximum concentration of almost 4.5 g IA/L for a submerged culture (glucose and xylose from hydrolyzed wood). The treatment used almost completely removed the phenolic compounds and organic acids and decreased the salt ions, whereas rice husks hydrolysate pretreated with CaO(s) produced 1.9 g/L IA (Pedroso et al. 2017).
IA production from residues should consider the cost for feedstock treatment to evaluate a real feasibility of the material. A wider knowledge of the potential inhibitors is important for using less expensive carbon sources with minimal pretreatment (Klement and Büchs 2013).
Aspergillus terreus oxygen strict requirement for IA production
Different studies described the direct relation between aeration and IA production, and the requirement for continuous oxygen supply throughout the bioprocess is of significant important. Pfeifer et al. (1952) and Nelson et al. (1952) were probably the first to report the need for continuous aeration to reach high IA yields. Nelson et al. (1952) described that a 20 min interruption in the air flow after 54 h of fermentation was enough to drastically decrease IA production rates (the values were not detailed). Pfeifer et al. (1952) described that it was only possible to reverse the damage of no IA production (related to 15–60 min interruptions in air flow) if extranutrients were added to the medium. Despite the occurrence of further IA production, the final IA concentration was lower compared to the assay which was continuously aerated.
The aeration requirement for maintaining the cell’s capacity of producing IA is so important that Larsen and Eimhjellen (1955) conducted the separation of IA-producing A. terreus cells—non-proliferating mycelia—from the fermentation broth with constant aeration. The authors described that if the aeration process was not maintained throughout the separation process, the endogenous IA was not expelled to the extracellular medium (acidified tap water).
Riscaldati et al. (2000) showed that during the cell growth phase, there is a higher demand for oxygen, as well as for phosphorous and nitrogen consumption. When cell concentration reached a slow cell growth rate, the dissolved oxygen (DO) slowly increased from under 20% DO to almost 40 or 80% DO, depending on the initial pH or aeration rate. Kuenz et al. (2012) also described the occurrence of a drastic decrease of dissolved oxygen to 20% DO in the beginning of the fermentation (the first day of a 10 days’ fermentation). However, by the 8th day until the end of the fermentation, the value was not higher than 40% DO. The continuous need for oxygen supply even when cell growth is at low rates indicates that oxygen requirement is higher for IA production than cell maintenance.
Gyamerah (1995) showed that A. terreus cultivated in glucose medium had different behavior in IA production after 1, 3, 5, or 10 min of interruption of oxygen supply after 100 h of fermentation. By the third day, the reestablishment of aeration—after stopping air supply for 10 min—resulted in, at most, only 52% of the IA produced on the assay with continuous oxygen supply by the third day. That behavior was similar to the observations by Lin et al. (2004). The shorter interruption periods (3 and 5 min) resulted in less severe decrease of IA production (respectively, about 77% and 66% less IA compared with the assay without interruption) (Gyamerah 1995). This indicates that the capacity of IA production after the pause in oxygen supply is also related to the duration of the interruption period.
The reason for the significantly lower IA production when oxygen supply is completely interrupted has not yet been clarified. Based on the evaluation of different studies, this study states the following hypothesis: the system responsible for the drastic interruption of IA production, which is related to the period of interruption in oxygen supply, might be related to an inhibition effect of cis-aconitate inside the mitochondria or the cytosol.
A requirement of a readily transportation system of cis-aconitate from the mitochondria to the cytosol has been evidenced (Huang et al. 2014a, b). Cis-aconitic acid is unstable in environments with a pH under 7 (Ambler and Roberts 1948), and it is spontaneously converted to trans-aconitic acid, the thermodynamically more stable form of the substance (Steiger et al. 2016). Trans-aconitate has been described as an inhibitor of at least two mitochondrial enzymes—aconitase (Laube et al. 1994) and fumarase (Rebholz and Northrop 1994). At sufficient oxygen concentration, A. terreus promptly transports cis-aconitate to the cytosol by Mtt transporter (from the mitochondria to the cytosol), which is further converted to itaconate by CAD (Huang et al. 2014a, b).
In the occasion of aeration interruption, the energy applied for transporting H+ and IA could be impaired. The Mtt transporters would have a lower activity in the absence of oxygen, and cis-aconitate would accumulate inside the mitochondria. In the occasional malfunctioning of H+ transportation due to the lack of oxygen, the pH inside the cell would decrease and promote the formation of trans-aconitate, and thus, the inhibition of important enzymes from the TCA cycle. Such inhibition effect would prevent further IA production and substrate consumption, as the enzymatic system would be damaged. The longer the period of interruption in oxygen supply, the greater the conversion of cis to trans-aconitate might be.
The hypothesis also suggests that the negative effect of cis-aconitate conversion to trans-aconitate during the lack of oxygen supply is more effective to the mitochondrial enzymatic system. The supposition may be supported by the observation of Gyamerah (1995) studies, who showed that the inhibition of mitochondria membrane transporters results in higher decrease of IA production (> 90%) than the inhibition of cell membrane transporters (< 9%).
IA production and medium pH
In IA production systems, environments in which the pH is not regulated during the fermentation, the microorganism tends to acidify the medium to a very low pH (< 2). IA synthesis is strongly related to the initial pH, as the entire or part of the enzymatic system responsible for IA production may function in an acid environment (Larsen and Eimhjellen 1955). Different metabolites were produced depending on the pH value considered for regulating the entire fermentation process. In pH 2.1, the main products by A. terreus were IA, carbonic gas, and cells, while the fermentation in pH 6 produced L-malic, succinic, fumaric acids, carbon dioxide, and cells (Larsen and Eimhjellen 1955).
Among the existing hypotheses for the transport of organic acids to the extracellular medium by microorganisms, three of them are described below (Vrabl et al. 2012).
Hypothesis of overflow metabolism The expulsion of organic acids out into the extracellular medium is considered one of the mechanisms employed by the cell to release energy in a situation in which growth is limited by a non-carbon nutrient and a carbon source is in excess. The hypothesis is subdivided in relation to the location of the bottleneck causing this release, which may be glycolysis, TCA cycle, or respiratory chain. In several studies, the phenomenon of overflow metabolism is associated with the increase of glycolytic flow;
Hypothesis of charge balance It is considered that when the H+/substrate transport system is prevented, the transport of the organic acid anions is the main form of compensation of the ion flow for the excretion of H+ by the enzyme H+-ATPase. This operation prevents the plasma membrane from being hyperpolarized in a way detrimental to the cell. In an environment where pH is low, most of the excreted protons return to the interior of the cell via the protons of nutrients. In an environment with high pH, especially in cultures with NaOH addition as a control of the excreted protons, the entrance of the proton into the cell is impaired, requiring a new charge flow. The release in the medium of organic acids can balance the proton flow almost stoichiometrically.
Hypothesis of aggressive acidification The hypothesis, developed for A. niger strain, describes that the filamentous fungus releases the acid in the extracellular environment, and the acid environment results in a medium with less probability of contamination from other microorganisms. Assuming that the organic acids transported through the membrane are completely protonated (uncharged), these compounds would be the major source of acidification of the medium.
Krull et al. (2017) demonstrated that the need for an acid environment, with fermentation broth pH under 2, is only essential in the beginning of the fermentative process. Their findings with a genetically modified A. terreus strain showed that, after the initial drop to 1.6, which is necessary for IA production, the rise and maintenance of the pH at 3–3.4 increases the final IA concentration (around 150 g/L IA). The optimized condition that allowed such concentration involved not only pH adjustment, but also a fed-batch operating system. The final yield of 0.58 g IA/g glucose is, thus, not higher than other studies without pH regulation.
Advances in IA research and intellectual properties
The analysis of research trends provides an indication of the state-of-the-art technology applied to IA. Scopus® [one of the largest abstract and citation database of peer-reviewed literature (Scopus 2017)] and Derwent World Patent Index™ (DWPI) [one of the most comprehensive collection of global patent data in English (Clarivate 2017)] were used to compile, respectively, scientific articles and patent documents to evaluate the scientific and technological advances in IA production.
The set of articles was selected using the following criteria: English scientific articles containing the words “itaconic acid” on the title, published from 1910 (earliest year available on the database) to 2016. The set of patents was selected with similar standard: patents containing the words “itaconic acid” on the title published from 1910 (earliest year available on the database) until 2016. The databases provided 640 articles and 1033 patents, from which important information such as title, abstract, publication year, and priority country of the patents—first country where the invention is filled (OECD 2017)—were used to evaluate the advances of IA technologies.
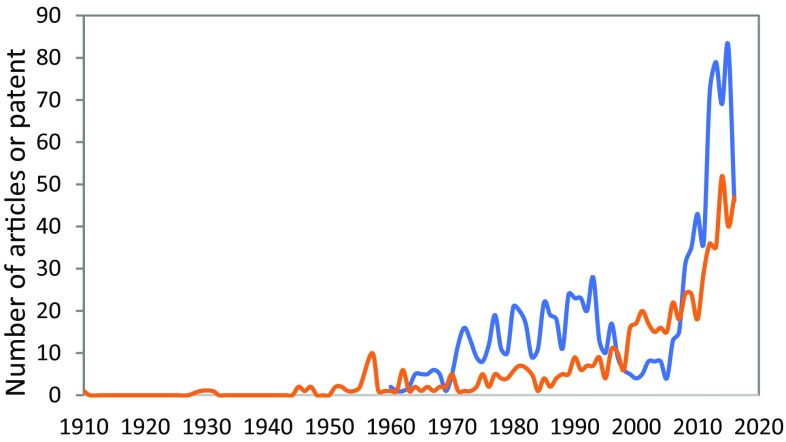
The number of scientific articles and patent increased significantly during the period analyzed (Fig. 4), which confirms the high interest in developing technologies regarding IA production and its derivatives. The steep slope observed for the profile of number of patents, which initiated in 2007, corresponds mostly to the numerous Chinese patents about IA published on that period—76% of the 509 patents published from 2007 to 2016. The frequent world crises that result in drastic fluctuations of oil prices highly motivated the development of alternative technologies to partially substitute products from non-renewable sources (Macrotrends 2017), including the development of IA technologies. In China, the investments from government and companies increased the country’s participation in the advances of the world’s research (Huang et al. 2010).
Fig. 4.
Number of scientific articles and patent documents selected from the databases Scopus® and Derwent World Patent Index™, respectively. The documents selection was done considering articles and patents which contained the words “itaconic acid” on the title, published from the initial years available on the databases until 2016. For the article selection, the documents were restricted to the English language. Scientific articles (orange line) and patent documents (blue line)
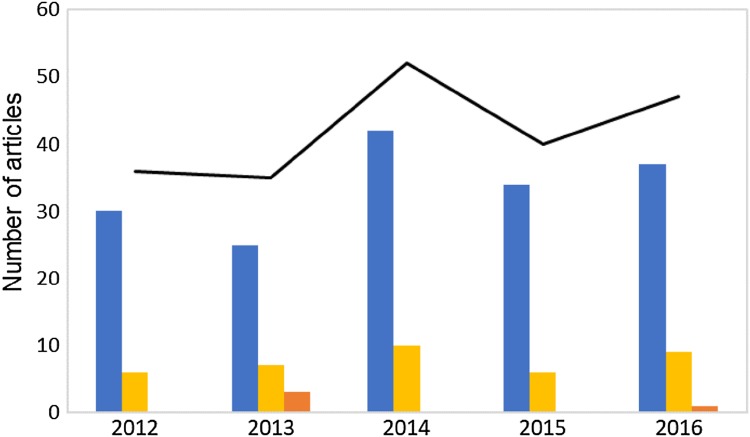
Figure 5 represents a classification of the most recent articles, published from 2012 to 2016, which were separated by the main subject addressed by each document. The studies related to the development of IA derivatives represent 80% of the total articles in that period, and the second most frequent subject was the development of IA fermentative processes, with 18% of the studies analyzed. It was not observed a significant fluctuation of the number of articles that concerns those classifications throughout the period analyzed. The scientific articles about the metabolic pathway of IA production, including the improvement of different strains for higher yield production, were less frequent, with only 2% of the articles from the analyzed period. The evaluation indicates that the recent research concerning IA is substantially more directed for developing IA end-products the rather than the advances in fermentative processes and microorganism modification for improving the final IA yield.
Fig. 5.
Number of scientific articles from the database Scopus containing on the title the words “itaconic acid”, published from 2012 to 2016. Each of the 210 articles was characterized by the main subject related to itaconic acid (IA) synthesis or application: polymer development or application (blue bars), fermentative process development (yellow bars), study of metabolic pathway (orange bars), and the total number of articles in each year (black line)
The most recent patents analyzed, published from 2012 to 2016 (348 patent documents), were separated by the priority country or its region. The priority country of a patent is frequently where the requesting institution is located. The analysis indicated that 86% of the inventions were first patented in China, which is a further indicator of that country’s high interest in developing IA technology. The second region where IA patents were mostly deposited is Asia (5%), which Japan is the priority country with more than 60% of those patents. The USA and Europe occupies the third and fourth positions (respectively, 4 and 3.4% of the total patents from 2012 to 2016). South America was the priority country for only 0.86% of the patents from the analyzed period. This reflects the robust Chinese investment in IA technologies and the Chinese position on the current global IA market (section IA global market).
The analysis of the advances in IA innovations, whether by the published articles or patents, indicates that the IA technology development is currently more directed to the improvement of IA products and their applications. It is important to note that the high number of patent documents evidences the significant interest of the organic acid application, as many patents are deposited by companies or institutions with the intention to apply the inventions on the market. This is a high indication of the expansion of IA on the available market for renewable sources. Moreover, it shows that improvements for IA processes have been done, and that the interest in IA products concern different counties, but mainly China, which is also the current greater IA world producer (Global Industry Analysis 2016).
Conclusion
Itaconic acid (IA) is a promising bio-based chemical with vast application in chemical industry. The increasing demand of bio-based products is a gateway for the development of IA derivatives. The current knowledge about IA metabolic pathway mainly by Aspergillus terreus allows a good understanding of its synthesis process, but further comprehension such as inhibitory components is necessary to achieve high yields with residue feedstocks. IA current niche market may increase with innovation and specific market targeting, in addition to the use of low-cost feedstock.
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the financial support of this work.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Aguiar A, Gonzalez-Villegas S, Rabelero M, Mendizabal E, Puig JE, Domínguez JM, Katime I. Core-shell polymers with improved mechanical properties prepared by microemulsion polymerization. Macromolecules. 1999;32:6767–6771. doi: 10.1021/ma981703s. [DOI] [Google Scholar]
- Ambler JA, Roberts EJ. The effect of pH on the stability of cis-aconitic acid in dilute solution. J Org Chem. 1948;13:399–402. doi: 10.1021/jo01161a013. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamazaki Y, Suziki N. Production of itaconic acid by Helicobasidium mompa TANAKA. Jpn J Phytopathol. 1957;22:83–87. doi: 10.3186/jjphytopath.22.83. [DOI] [Google Scholar]
- Bailey A (2016) Recent activity on bioproducts that enable biofuels in the bioenergy technologies office. In: https://energy.gov/sites/prod/files/2016/11/f34/bailey_bioenergy_2016.pdf. Accessed 23 Oct 2017
- Bajpai SK, Jyotishi P, Bajpai M. Synthesis of nanosilver loaded chitosan/poly(acrylamide-co-itaconic acid) based inter-polyelectrolyte complex films for antimicrobial applications. Carbohydr Polym. 2016;154:223–230. doi: 10.1016/j.carbpol.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Batti M, Schweiger LB (1963) Process for the production of itaconic acid. Patent US 3 078 217
- Bednarz S, Fluder M, Galica M, Bogdal D, Maciejaszek I. Synthesis of hydrogels by polymerization of itaconic acid-choline chloride deep eutectic solvent. J Appl Polym Sci. 2014;131:1–8. doi: 10.1002/app.40608. [DOI] [Google Scholar]
- Bentley R, Thiessen CP. Biosynthesis of itaconic acid in Aspergillus terreus III. The properties and reaction mechanism of cis-aconitic acid decarboxylase. J Biol Chem. 1957;226:703–720. [PubMed] [Google Scholar]
- Blazeck J, Hill A, Jamoussi M, Pan A, Miller J, Alper HS. Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab Eng. 2015;32:66–73. doi: 10.1016/j.ymben.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Blumhoff ML, Steiger MG, Mattanovich D, Sauer M. Targeting enzymes to the right compartment: metabolic engineering for itaconic acid production by Aspergillus niger. Metab Eng. 2013;19:26–32. doi: 10.1016/j.ymben.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Calam CT, Oxford AE, Rainstrick H. Studies in the biochemistry of micro-organisms: itaconic acid, a metabolic product of a strain of Aspergillus terreus Thom. Biochem J. 1939;33:1488–1495. doi: 10.1042/bj0331488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen F, Klement T, Büchs J, Melin T, Wessling M. Continuous production and recovery of itaconic acid in a membrane bioreactor. Bioresour Technol. 2013;137:179–187. doi: 10.1016/j.biortech.2013.03.044. [DOI] [PubMed] [Google Scholar]
- China (2017) Qingdao Kehai Biochemistry CO. Ltd. http://itaconic.en.china.cn/op/CorpInfo/index.htm. Accessed 1 Aug 2017
- Clarivate (2017) Derwent world patents index. In: https://clarivate.com/wp-content/uploads/2017/10/IP_DWPI_Sell_Sheet_0617.pdf. Accessed 31 Oct 2017
- Cordes T, Michelucci A, Hiller K. Itaconic acid: the surprising role of an industrial compound as a Mammalian antimicrobial metabolite. Annu Rev Nutr. 2015;35:451–473. doi: 10.1146/annurev-nutr-071714-034243. [DOI] [PubMed] [Google Scholar]
- Durant YG (2011) Development of integrated production of polyitaconic acid from Northeast hardwood biomass. https://reeis.usda.gov/web/crisprojectpages/0220209-development-of-integrated-production-of-polyitaconic-acid-from-northeast-hardwood-biomass.html. Accessed 2 Aug 2017
- Dwiarti L, Yamane K, Yamatani H, Prihardi K, Okabe M. Purification and characterization of cis-aconitic acid decarboxylase from Aspergillus terreus TN484-M1. J Biosci Bioeng. 2002;94:29–33. doi: 10.1016/S1389-1723(02)80112-8. [DOI] [PubMed] [Google Scholar]
- Dwiarti L, Otsuka M, Miura S, Yaguchi M, Okabe M. Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour Technol. 2007;98:3329–3337. doi: 10.1016/j.biortech.2006.03.016. [DOI] [PubMed] [Google Scholar]
- El-Imam AA, Du C. Fermentative itaconic acid production. J. Biodiversity, Bioprospect Dev. 2014;1:1–8. [Google Scholar]
- Global Industry Analysis (2016) The global itaconic acid market. http://www.strategyr.com/MarketResearch/Itaconic_Acid_IA_Market_Trends.asp. Accessed 4 May 2017
- Global Market Insights (2016) Itaconic acid market. https://www.gminsights.com/industry-analysis/itaconic-acid-market. Accessed 4 Jun 2017
- Gyamerah MH. Oxygen requirement and energy relations of itaconic acid fermentation by Aspergillus terreus NRRL 1960. Appl Microbiol Biotechnol. 1995;44:20–26. doi: 10.1007/BF00164475. [DOI] [Google Scholar]
- Hiller K, Cordes T, Michelucci A (2014) Biotechnological production of itaconic acid. WO2014161988A1
- Hope E (1927) Manufacture of glass or glass-like objects. Patent US 1 644 131
- Horitsu H, Takahashi Y, Tsuda J, Kawai K, Kawano Y. Production of itaconic acid by Aspergillus terreus immobilized in polyacrylamide gels. Eur J Appl Microbiol Biotechnol. 1983;18:358–360. doi: 10.1007/BF00504745. [DOI] [Google Scholar]
- Hossain AH, Li A, Brickwedde A, Wilms L, Caspers M, Overkamp K, Punt PJ. Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb Cell Fact. 2016;15:130. doi: 10.1186/s12934-016-0527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Huang L, Lin J, Xu Z, Cen P. Organic chemicals from bioprocesses in China. In: Tsao GT, Ouyand P, Chen J, editors. Advances in biochemical engineering/biotechnology. Heidelberg: Springer; 2010. pp. 43–71. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen M, Lu X, Li Y, Li X, Li J-J. Direct production of itaconic acid from liquefied corn starch by genetically engineered Aspergillus terreus. Microb Cell Fact. 2014;13:108. doi: 10.1186/s12934-014-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Lu X, Li Y, Li X, Li J-J. Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb Cell Fact. 2014;13:119. doi: 10.1186/s12934-014-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Quero A, Pollet E, Zhao M, Marchioni E, Avérous L, Phalip V. Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus strains. J Microbiol Biotechnol. 2016;26:1557–1565. doi: 10.4014/jmb.1603.03073. [DOI] [PubMed] [Google Scholar]
- Kanamasa S, Dwiarti L, Okabe M, Park EY. Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl Microbiol Biotechnol. 2008;80:223–229. doi: 10.1007/s00253-008-1523-1. [DOI] [PubMed] [Google Scholar]
- Kane JH, Finlay AC, Amann PF (1945) Production of itaconic acid. Patent US 2 385 283
- Karaffa L, Díaz R, Papp B, Fekete E, Sándor E, Kubicek CP. A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Appl Microbiol Biotechnol. 2015;99:7937–7944. doi: 10.1007/s00253-015-6735-6. [DOI] [PubMed] [Google Scholar]
- Kautola H, Vahvaselka M, Linko Y-Y, Linko P. Itaconic acid production by immobilized Aspergillus terreus from xylose and glucose. Biotechnol Lett. 1985;7:167–172. doi: 10.1007/BF01027812. [DOI] [Google Scholar]
- Kautola H, Vassilev N, Linko Y-Y. Itaconic acid production by immobilized Aspergillus terreus on sucrose medium. Biotechnol Lett. 1989;11:313–318. doi: 10.1007/BF01024510. [DOI] [Google Scholar]
- Kautola H, Rymowicz W, Linko Y, Linko P (1991) Itaconic acid production by immobilized Asperyillus terreus with varied metal additions. Appl Microbiol Biotechnol 35:154–158
- Kinoshita K. Über eine neue Aspergillus-Art, Asp. itaconicus nov. spec. Bot Mag Tokyo. 1931;45:45–60. doi: 10.15281/jplantres1887.45.45. [DOI] [Google Scholar]
- Klement T, Büchs J. Itaconic acid: a biotechnological process in change. Bioresour Technol. 2013;135:422–431. doi: 10.1016/j.biortech.2012.11.141. [DOI] [PubMed] [Google Scholar]
- Kocabas A, Ogel ZB, Bakir U. Xylanase and itaconic acid production by Aspergillus terreus NRRL 1960 within a biorefinery concept. Ann Microbiol. 2014;64:75–84. doi: 10.1007/s13213-013-0634-9. [DOI] [Google Scholar]
- Krull S, Hevekerl A, Kuenz A, Prüße U. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl Microbiol Biotechnol. 2017;101:4063–4072. doi: 10.1007/s00253-017-8192-x. [DOI] [PubMed] [Google Scholar]
- Kuenz A, Gallenmüller Y, Willke T, Vorlop K-D. Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biotechnol. 2012;96:1209–1216. doi: 10.1007/s00253-012-4221-y. [DOI] [PubMed] [Google Scholar]
- Lai L-ST, Hung C-S, Lo C-C. Effects of lactose and glucose on production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J Biosci Bioeng. 2007;104:9–13. doi: 10.1263/jbb.104.9. [DOI] [PubMed] [Google Scholar]
- Larsen H, Eimhjellen KE. The mechanism of itaconic acid formation by Aspergillus terreus 1. The effect of acidity. Biochem J. 1955;60:135–139. doi: 10.1042/bj0600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube H, Kennedy MC, Beinert H. Crystal structures of aconitate with trans-aconitate and nitrocitrate bound. J Mol Biol. 1994;237:437–451. doi: 10.1006/jmbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- Levinson WE, Kurtzman CP, Kuo TM. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzyme Microb Technol. 2006;39:824–827. doi: 10.1016/j.enzmictec.2006.01.005. [DOI] [Google Scholar]
- Li A, Pfelzer N, Zuijderwijk R, Punt P. Enhanced itaconic acid production in Aspergillus niger using genetic modification and medium optimization. BMC Biotechnol. 2012;12:57. doi: 10.1186/1472-6750-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Pfelzer N, Zuijderwijk R, Brickwedde A, Cora Z, Punt P. Reduced by-product formation and modified oxygen availability improve itaconic acid production in Aspergillus niger. Appl Microbiol Biotechnol. 2013;97:3901–3911. doi: 10.1007/s00253-012-4684-x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Li Y, Huang M, Tsai Y (2004) Intracellular expression of Vitreoscilla hemoglobin in Aspergillus terreus to alleviate the effect of a short break in aeration during culture. Biotechnol Lett 26:1067–1072 [DOI] [PubMed]
- Lockwood LB, Moyer AJ (1945) Method for the production of itaconic acid. Patent US 2 462 981
- Lockwood LB, Ward GE. Fermentation process for itaconic acid. Ind Eng Chem. 1945;37:405–406. doi: 10.1021/ie50424a029. [DOI] [Google Scholar]
- Maassen N, Panakova M, Wierckx N, Geiser E, Zimmermann M, Bölker M, Klinner U, Blank LM. Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng Life Sci. 2014;14:129–134. doi: 10.1002/elsc.201300043. [DOI] [Google Scholar]
- Macrotrends (2017) Crude oil prices—70 year historical chart. In: http://www.macrotrends.net/1369/crude-oil-price-history-chart. Accessed 31 Oct 2017
- Marvel CS, Shepherd TH. Polymerization reactions of itaconic acid and some of its derivaties. J Org Chem. 1959;24:599–605. doi: 10.1021/jo01087a006. [DOI] [Google Scholar]
- Merger F, Liebe J. Preparation of 1,1-disubstituted ethylene componds. Patent US. 1991;4(997):955. [Google Scholar]
- Miall LM (1978) Organic acids. In: Rose AH (ed) Economic microbiology: primary products of metabolism. Academic, pp 47–119
- Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci USA. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GEN, Traufler DH, Kelley S, Lockwood LB. Production of itaconic acid by Aspergillus terreus in 20-liter fermentors. Ind Eng Chem. 1952;44:1166–1168. doi: 10.1021/ie50509a062. [DOI] [Google Scholar]
- Nguyen-Thai NU, Hong SC. Controlled architectures of poly(acrylonitrile-co-itaconic acid) for efficient structural transformation into carbon materials. Carbon N Y. 2014;69:571–581. doi: 10.1016/j.carbon.2013.12.068. [DOI] [Google Scholar]
- NRRL (2017) ARS Culture Collection Database, mold, Aspergillus terreus NRRL 1960. https://nrrl.ncaur.usda.gov/cgi-bin/usda/mold/repo. Accessed 7 Aug 2017
- Nubel RC, Ratajak W, Ratajak EJ (1962) Process for producing itaconic acid. Patent US 3 044 941
- OECD (2017) Economic analysis and statistics division directorate for science, technology and industry. In: https://www.oecd.org/sti/sci-tech/37569498.pdf. Accessed 29 Oct 2017
- Okabe M, Lies D, Kanamasa S, Park EY. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol. 2009;84:597–606. doi: 10.1007/s00253-009-2132-3. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Chin T, Hiratsuka K, Aso Y, Tanaka Y, Takahashi T, Ohara H. Production of itaconic acid using metabolically engineered Escherichia coli. J Gen Appl Microbiol. 2014;60:191–197. doi: 10.2323/jgam.60.191. [DOI] [PubMed] [Google Scholar]
- Pedroso GB, Montipó S, Mario DAN, Alves SH, Martins AF. Building block itaconic acid from left-over biomass. Biomass Convers Biorefinery. 2017;7:23–35. doi: 10.1007/s13399-016-0210-1. [DOI] [Google Scholar]
- Petruccioli M, Pulci V, Federici F. Itaconic acid production by Aspergillus terreus on raw starchy materials. Lett Appl Microbiol. 1999;28:309–312. doi: 10.1046/j.1365-2672.1999.00528.x. [DOI] [Google Scholar]
- Pfeifer VF, Vojnovich C, Heger EN. Itaconic acid by fermentation with Aspergillus terreus. Ind Eng Chem. 1952;44:2975–2980. doi: 10.1021/ie50516a055. [DOI] [Google Scholar]
- PubChem (2017) Itaconic acid. In: https://pubchem.ncbi.nlm.nih.gov/compound/811. Accessed 11 Aug 2017
- Rabelero M, Trujillo A, Ceja I, Caché G, Mendizábal E, Esquena J, Solans C, Puig JE. Effects of the functionalizing agent, itaconic acid, on the mechanical properties of microemulsion-made core/shell polymers. Polym Eng Sci. 2013;53:1529–1535. doi: 10.1002/pen.23404. [DOI] [Google Scholar]
- Rafi M, Hanumanthu MG, Rao DM, Venkateswarlu K. Production of itaconic acid by Ustilago maydis from agro wastes in solid state fermentation. J BioScience Biotech. 2014;3:163–168. [Google Scholar]
- Rebholz K, Northrop D. Kinetics of enzymes with iso-mechanisms: dead-end inhibition of fumarase and carbonic anhydrase II. Arch. Biochem Biophys. 1994;312:227–233. doi: 10.1006/abbi.1994.1303. [DOI] [PubMed] [Google Scholar]
- Reddy CS, Singh R. Enhanced production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour Technol. 2002;85:69–71. doi: 10.1016/S0960-8524(02)00075-5. [DOI] [PubMed] [Google Scholar]
- Report Linker (2017) Global bio-based chemicals market forecast 2017–2025. In: https://www.reportlinker.com/p05001382/Global-Bio-Based-Chemicals-Market-Forecast.html. Accessed 24 Oct 2017
- Riscaldati E, Moresi M, Federici F, Petruccioli M. Effect of pH and stirring rate on itaconate production by Aspergillus terreus. J Biotechnol. 2000;83:219–230. doi: 10.1016/S0168-1656(00)00322-9. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Hosseinzadeh H. Synthesis of starch-poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel with salt and pH-responsiveness properties as a drug delivery system. J Bioact Compat Polym. 2008;23:381–404. doi: 10.1177/0883911508093504. [DOI] [Google Scholar]
- Saha BC. Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. J Ind Microbiol Biotechnol. 2017;44:303–315. doi: 10.1007/s10295-016-1878-8. [DOI] [PubMed] [Google Scholar]
- Scopus (2017) Scopus. In: https://www.elsevier.com/solutions/scopus. Accessed 28 Oct 2017
- Shi D, Gao Y, Sun L, Chen M. Superabsorbent poly(acrylamide-co-itaconic acid) hydrogel microspheres: preparation, characterization and absorbency. Polym Sci. 2014;56:275–282. [Google Scholar]
- Shin JH, Yang JY, Jeon BY, Yoon YJ, Cho SN, Kang YH, Ryu DH, Hwang GS. 1H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J Proteome Res. 2011;10:2238–2247. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- Sieker T, Poth S, Tippkötter N, Ulber R (2012) Itaconic acid production from beech wood hydrolysates. https://www.mv.uni-kl.de/biovt/forschung/poster/LCB2_02. Accessed 2 Jun 2017
- Steiger MG, Punt PJ, Ram AFJ, Mattanovich D, Sauer M. Characterizing MttA as a mitochondrial cis-aconitic acid transporter by metabolic engineering. Metab Eng. 2016;35:95–104. doi: 10.1016/j.ymben.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Sakagami H, Yokote Y, Onuma H, Kaneko M, Mori M, Sakaguchi Y, Soga T. Non-targeted metabolite profiling in activated macrophage secretion. Metabolomics. 2011;8:624–633. doi: 10.1007/s11306-011-0353-9. [DOI] [Google Scholar]
- Tabuchi T, Sugisawa T, Ishidori T, Nakahara T, Sugiyama J. Itaconic acid fermentation by a yeast belonging to the genus Candida. Agric Biol Chem. 1981;45:475–479. [Google Scholar]
- Tate BE. Polymerization of itaconic acid derivatives. Adv Polym Sci. 1967;5:214–232. doi: 10.1007/BFb0051282. [DOI] [Google Scholar]
- Transparency Market Research (2015) Itaconic acid market for synthetic latex, unsaturated polyester resins, detergents, superabsorbent polymers (SAP), and other applications—global industry analysis, size, share, growth, trends and forecast 2015—2023. http://www.transparencymarketresearch.com/itaconic-acid-market.html. Accessed 4 May 2017
- Tsai Y-C, Huang M-C, Lin S-F, Su Y-C (2001) Method for the production of itaconic acid using Aspergillus terreus solid state fermentation. Patent US 6 171 831 B1
- Turner E, Liebig J. Elements of chemistry. 6. London: Taylor and Walton; 1841. [Google Scholar]
- Vassilev N, Kautola H, Linko Y-Y. Immobilized Aspergillus terreus in itaconic acid production from glucose. Biotechnol Lett. 1992;14:201–206. doi: 10.1007/BF01023359. [DOI] [Google Scholar]
- Vassilev N, Medina A, Mendes G, Galvez A, Martos V, Vassileva M. Solubilization of animal bonechar by a filamentous fungus employed in solid state fermentation. Ecol Eng. 2013;58:165–169. doi: 10.1016/j.ecoleng.2013.06.029. [DOI] [Google Scholar]
- Voll A, Klement T, Gerhards G, Büchs J, Marquardt W. Metabolic modelling of itaconic acid fermentation with Ustilago maydis. Chem Eng Trans. 2012;27:367–372. [Google Scholar]
- Vrabl P, Fuchs V, Pichler B, Schinagl CW, Burgstaller W. Organic acid excretion in Penicillium ochrochloron increases with ambient pH. Front Microbiol. 2012;3:1–10. doi: 10.3389/fmicb.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weastra SRO (2012) Determination of market potential for selected platform chemicals—itaconic acid, succinic adis, 2,5-furandicarboxylic acid. http://www.bioconsept.eu/wp-content/uploads/BioConSepT_Market-potential-for-selected-platform-chemicals_report1.pdf. Accessed 6 Sep 2016
- Welter K (2000) Biotechnische Produktion von Itaconsäure aus nachwachsenden Rohstoffen mit immobilisierten Zellen. Dissertation. Technische Universität Braunschweig
- Werpy T, Petersen G (2004) Top value added chemicals from biomass volume I—results of screening for potential candidates from sugars and synthesis gas. In: https://www.nrel.gov/docs/fy04osti/35523.pdf. Accessed 15 Aug 2016
- Willke T, Vorlop KD. Biotechnological production of itaconic acid. Appl Microbiol Biotechnol. 2001;56:289–295. doi: 10.1007/s002530100685. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang B, Yang S. Production of citric, itaconic, fumaric and malic acids in filamentous fungal fermentations. In: Yang S-T, El-Enshasy HA, Thongchul N, editors. Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Hoboken: Wiley; 2013. p. 24. [Google Scholar]