Abstract
Kale (Brassica oleracea L. Acephala Group) is the rich source of medicinal value sulphur compounds, glucosinolates (GLSs). The aim of this study was to investigate the effect of different proportion of sulphur (S) supplementation levels on the accumulation of GLSs in the leaves of the kale cultivar ('TBC'). High performance liquid chromatography (HPLC) separation method guided to identify and quantify six GSLs including three aliphatic (progoitrin, sinigrin and gluconapin) and three indolyl (glucobrassicin, 4-methoxyglucobrassicin and neoglucobrasscin) respectively. Analysis of these distinct levels of S supplementation revealed that the accumulation of individual and total GLSs was directly proportional to the S concentration. The maximum levels of total GLSs (26.8 µmol/g DW) and glucobrassicin (9.98 µmol/g DW) were found in lower and upper parts of the leaves supplemented with 1 mM and 2 mM S, respectively. Interestingly, aliphatic GSLs were noted predominant in all the parts (50.1, 59.3 and 56% of total GSLs). Among the aliphatic and indolyl GSLs, sinigrin and glucobrassicin account 35.3 and 30.88% of the total GSLs. From this study, it is concluded that supply of S enhance the GSLs accumulation in kale.
Keywords: Brassica oleracea acephala, Sulphur, Glucosinolates, HPLC
1. Introduction
Consuming fresh leafy vegetables provide health benefits to humans beyond the advantage of having the processed foods. Vegetables of the Brassica group are mainly consumed as fresh leaves and juice can be conveniently used to meet the recommendations of daily fruits and vegetables (Chun et al., 2013, Chun et al., 2018, Al-Dhabi et al., 2015, Kim et al., 2015). Fresh leaf and juice of kale (Brassica oleracea var. acephala) commonly consumed in Korea are rich in nutrients such as amino acids, vitamins, minerals, dietary fiber and minerals, as well as of a high variety of phytochemicals, namely carotenoids, glucosinolates (GLs) and phenols (Chung et al., 1993, Perez-Balibrea et al., 2011, Chun et al., 2017, Park et al., 2016). Among the phytochemicals, glucosinolates (GLSs) have many advantages as it has the ability to influence the human health as pharmaceutical, nutraceutical, or as food product, especially in curing colon cancer and lung cancer (Clarke, 2010, Melchini et al., 2013, Seo et al., 2014, Park et al., 2014a, Park et al., 2014b, Park et al., 2014c, Lee et al., 2014, Lee et al., 2015, Lee et al., 2016, Fu et al., 2016, Pandey et al., 2017). GLSs are a diverse group of sulphur-rich anionic secondary metabolites containing the precursor amino acids, methionine, tryptophan and phenylalanine in the structure. The degradation products of GLSs such as isothiocyanates, thiocyanates, nitriles, epithionitriles or oxazolidine-2-thionesknown to have many biological function, particularly, bladder, colon and lung cancer treatment (Cartea and Velasco, 2008, Park et al., 2013, Seo et al., 2014, Thavarajah et al., 2016, Armesto et al., 2017, Biegańska-Marecik et al., 2017).
Kale is a biannual crop, and is an important vegetable belonging to the Brassica family, which has been distributed commonly in the Europe and the United states, whereas, in the recent years, it has been cultivated in Korea, China and Japan. The bolting stem part of the kale is commonly consumed, because the textures of bolting stem are tender and crisp and the flavor is attractive. GLSs contain both nitrogen (N) and sulphur (S) and therefore their concentrations in vegetables are influenced by the addition of N and S fertilizer (Falk et al., 2007, Groenbaek et al., 2016). The content of GSL is affected by several environmental factors such as temperature, light, soil type and fertilizer applications (Cartea and Velasco, 2008). Increasing nitrogen (N) tends to decrease the total GSL content in different Brassica crops (Chen et al., 2006, Li et al., 2007, Groenbaek et al., 2016), however the influence of N and S on GSL content in Brassicaceae family has been widely studied (Rangkadilok et al., 2004, Falk et al., 2007). Therefore, the objective of this study was to examine the effect of S on GSL concentration in kale to enhance their nutritional and health-promoting properties.
2. Materials and methods
2.1. Chemicals
HPLC grade-acetonitrile (CH3CN) and methanol (CH3OH) were obtained from J.T. Baker Chemical Co. (Phillipsburg, NJ, USA). DEAE-Sephadex A-25, sinigrin (2-propenyl GSL) and aryl sulfatase (type H-1, EC 3.1.6.1) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant materials and culture conditions
Kale seeds 'TBC' was purchased from the Asia seed Company (Seoul, Republic of Korea). The seeds were sown in 72 hole-plug tray with bed soil by spraying water intermittently on February 20, 2014. The plantlets were transplanted to a port (23 × 23 × 18 cm) containing vermiculite (ca. 500 g) after 5 weeks (33 days after sowing, DAS). The plants were grown in a greenhouse at Chungnam National University. The average temperature, quantity of light, relative humidity is each 17.0 °C, 344.4 µmol m−2 s−1 and 66.0% respectively. After one week (34 DAS), each kale was initially treated with different 1/8 N-P-K (10–1–5) millimolar (mM) concentrations. From 40 DAS, every two days interval, kale was treated with nutrient solution consisted of 0.0, 0.5, 1.0 and 2.0 mM sulphur (S) till 68 DAS (Table 1). After 68 DAS (April 29), the leaves were harvested and divided into three groups according to upper, middle and lower portions from the ground. And then, they were measured the length and fresh weight. The leaves were freeze-dried at −70 °C in a freeze dried for three days, measured the dry weight, powdered using mortar and pestle and stored at desiccator until chemical analysis.
Table 1.
Composition of nutrient solutions for different S molar concentrations (mM).
| Nutrients | S treatment (mM) |
|||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 2.0 | |
| KCl | 5.0 | 5.0 | 5.0 | 5.0 |
| Ca(NO3)2·4H2O | 2.0 | 2.0 | 2.0 | 2.0 |
| NH4NO3 | 2.5 | 2.5 | 2.5 | 2.5 |
| NH4H2PO4 | 1.0 | 1.0 | 1.0 | 1.0 |
| MgSO4·7H2O | – | 0.5 | 1.0 | 1.0 |
| MgCl2 | 1.0 | 0.5 | – | – |
| Na2SO4 | – | – | – | 1.0 |
| Micronutrients | Concentrations | |||
| ppm | g/L | |||
| MnCl2 | 0.50 | 1.80 | ||
| H3BO3 | 0.50 | 2.86 | ||
| ZnSO4 | 0.05 | 0.22 | ||
| (NH4)6Mo7O24·4H2O | 0.01 | 0.09 | ||
| CuSO4·5H2O | 0.02 | 0.08 | ||
| Fe-EDTA | 3.00 | 22.62 | ||
2.3. Extraction of crude glucosinolates (GSLs) and their desulfation
Desulfo (DS) - GSLs were extracted according to the procedure of Kim et al. (2007) and ISO 9167-1 (1992). Briefly, crude GSLs from freeze-dried materials (100 mg) were extracted with 1.5 ml of 70% (v/v) boiling methanol in water bath at 70 °C for 5 min. After centrifugation (12,000g, 4 °C, 10 min), the supernatant was immediately transferred to a clean test tube, and the residue was further re-extracted twice to complete extraction of GSLs. The combined supernatant was considered as the crude of GSLs. Separately 0.5 mg of sinigrin was dissolved in 5 ml ultra-pure water which used as an external standard (0.001792 µmol). Desulfation of the crude extracts were performed on a Sephadex A-25 DEAE (ca. 40 mg as dry matrix) column previously activated as [H]+ form with 0.5 M sodium acetate. Desulfation of sinigrin (external standard) was also carried out separately as the same process. The crude GSL extracts were loaded onto a pre-equilibrated column, and the column was then washed with 1 ml (×3 times) of ultra-pure water to remove neutral and positive ions. After loading of aryl sulfatase (E.C.3.1.6.1) (75 µl), the desulfation reaction was performed overnight (16–18 h) at room temperature. The desulfated GSLs were eluted with 0.5 ml (×3 times) of ultra-pure water. The eluates were filtered through 0.45 µm Teflon PTFE syringe filter and analyzed immediately by HPLC or stored at −20 °C until further chemical analysis.
2.4. Separation and identification of glucosinolates
DS-GSLs were analyzed by 1200 series HPLC system (Agilent Technologies, CA, USA) equipped with an Inertsil ODS-3 (C18) column 150 × 3.0 mm i.d., particle size 3 µm (GL Science, Tokyo, Japan). The HPLC analysis was carried out with a flow rate of 1.0 ml/min at a column oven temperature of 40 °C and a wavelength of 227 nm. The solvent system employed was (A) ultra-pure water (PURELAB Option-Q, ELGA) and (B) 100% acetonitrile. The solvent program was used as follows: 0 min to 2 min solvent B 0%, 7 min solvent B 10%, then kept constant at solvent B 31% for 16–19 min, and then kept constant at solvent B 0% for 10 min (total 40 min). The individual GSLs were quantified with the external standard sinigrin with their HPLC area and response factors (ISO 9167-1, 1992). For the identification of the individual GSLs, the MS analysis was carried out with an ESI interface operated in the positive ion mode. The MS operating conditions were as follows: ion spray voltage, 5.5 kV; curtain gas (20 Pa), nebulizing gas (50 Pa) and heating gas (50 Pa), high purity nitrogen (N2); heating gas temperature, 550 °C; spectra range, m/z 100–800 (scan time 4.8 s). In this study, all the samples were designated as GSLs even though DS-GSLs were determined.
3. Results and discussion
3.1. Effect of sulphur supplementation on plant growth
From the data presented in Table 2, it is clear that the fresh and dry weight of kale leaves was comparatively increased as a result of the supplementation of different levels of S treatment. The fresh weight of an upper and middle leaf was increased by 84% and 157% in 2.0 mM S and by 66% and 150% in dry weight by the application in 2.0 mM S, respectively. These results are slightly similar to Zaki et al. (2009) and Bimova and Pokluda (2009), who obtained the Brassica plant (broccoli and cabbage) growth by the supplementation of N and S.
Table 2.
Growth of kale “TBC” leaves under sulphur treatment.
| S treatments (mM) | Leaf length (cm) | Fresh weight (g) | Dry weight (g) | Water content (%) | |
|---|---|---|---|---|---|
| 0.0 | Upper | 15.8 ± 1.4 | 19.7 ± 4.7 | 2.14 ± 0.42 | 89.0 ± 0.3 |
| Middle | 13.5 ± 1.3 | 21.0 ± 5.6 | 2.03 ± 0.53 | 90.3 ± 0.6 | |
| Lower | 6.8 ± 0.9 | 15.1 ± 5.5 | 1.26 ± 0.43 | 91.6 ± 0.4 | |
| 0.5 | Upper | 23.2 ± 1.7 | 35.4 ± 4.2 | 3.78 ± 0.35 | 89.3 ± 0.7 |
| Middle | 19.7 ± 1.1 | 45.6 ± 7.9 | 4.36 ± 0.33 | 90.4 ± 0.9 | |
| Lower | 6.0 ± 0.5 | 32.2 ± 4.1 | 2.85 ± 0.59 | 91.2 ± 0.9 | |
| 1.0 | Upper | 21.5 ± 2.5 | 34.2 ± 6.2 | 3.47 ± 0.62 | 89.9 ± 0.5 |
| Middle | 17.4 ± 2.5 | 48.3 ± 10.4 | 4.38 ± 1.09 | 91.0 ± 0.4 | |
| Lower | 6.1 ± 0.7 | 37.2 ± 3.9 | 3.24 ± 0.40 | 91.3 ± 0.2 | |
| 2.0 | Upper | 23.4 ± 1.2 | 36.2 ± 7.4 | 3.56 ± 0.11 | 90.0 ± 1.7 |
| Middle | 20.1 ± 0.3 | 54.0 ± 6.1 | 5.03 ± 0.49 | 90.7 ± 0.4 | |
| Lower | 7.3 ± 0.9 | 36.0 ± 7.9 | 3.15 ± 0.57 | 91.2 ± 0.6 | |
3.2. Identification of glucosinolates
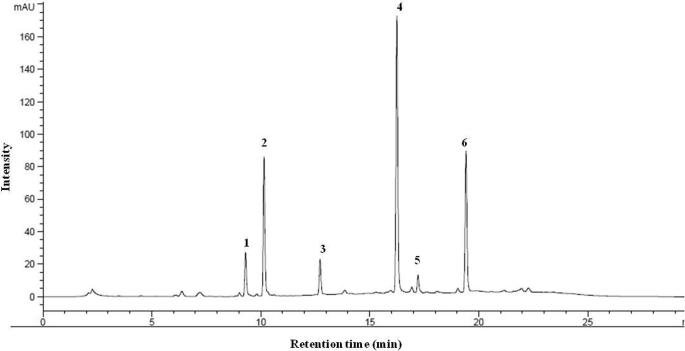
High performance liquid chromatography (HPLC) analyses of kale are shown in Fig. 1. Six GSLs including three aliphatic GSLs (progoitrin, sinigrin and gluconapin) and three indolyl GSLs (glucobrassicin, 4-methoxyglucobrassicin and neoglucobrasscin) were eluted and separated individually in HPLC chromatogram based on the retention time (Table 2). Each peaks and retention time were coincided with the previous report (Lee et al., 2014). With respect to the sinigrin response factors, the identified GSLs response factors were varied 5.8 fold.
Fig. 1.
HPLC chromatogram of glucosinolates (GSLs) isolated from kale. Peak numbers refer to the GSLs listed in Table 2. Peak No. 1, progoitrin; 2, sinigrin; 3, gluconapin; 4, glucobrassicin; 5, 4-methoxyglucobrassicin; 6, neoglucobrasscin.
The total GSL content ranged from 1.04 to 26.8 µmol/g DW (mean 14.27) (Table 3). The results revealed that the addition of various concentrations of S solution exhibited comparative differences in the GSL contents occurred among the upper, middle and lower portions of kale. The aliphatic GSLs were predominant, representing average 63% and 67.5% of the total GSL content, in the upper and middle portion of kale supplemented with 5 mM S, whereas, at 2 mM concentration aliphatic GSLs were noted as 50.1, 59.3 and 56% of upper, middle and lower portions, respectively. Among the aliphatic GSL, sinigrin detected as the predominant (mean 3.12 µmol/g DW) and contributed 35.35% of total GSLs and 67% of the total aliphatic GSLs. At 0.5 mM concentration of S, individual and total GSLs contents were comparatively less, clearly suggested that 0.5 mM concentration did not enhance the accumulation of GSLs. Closer observation of the results revealed that the accumulation of total GSLs were directly proportional to the concentration of S addition. The total GSLs in upper portion were 10.59, 12.9 and 26.8 µmol/g DW, in middle portion were 4.02, 6.58 and 12.63 µmol/g DW and in the lower portion were 1.02, 2.62 and 3.53 µmol/g DW, respectively at 0.5, 1.0 and 2 mM S addition. Schonhof et al. (2007) and Vallejo et al. (2003) reported that the addition of S ranging from 0.075 to 0.6 gram/plant increase the total and individual GSLs in Brassica plants. The present investigation showed that 1 mM and 2 mM S were optimum for the synthesis of GSLs in kale. Interestingly indolyl GSLs detected in the range of 1.27–13.35 µmol/g DW and shared <30% of the total GSLs and glucobrassicin documented the highest (0.45–9.9 µmol/g DW), whereas 4-methoxyglucobrassicin and neoglucobrasscin contributed a mean less than 30% of the total indolyl GSLs content, similarly, Kim et al. (2002) that S application relatively increased the indolyl GSLs in turnip and Rangkadilok et al. (2004) reported the increased concentration gypsum enhanced the total and indolyl GSLs in broccoli. In general, glucobrassicin was higher in Brassica vegetables (Zhang and Talalay, 1994) (see Table 4).
Table 3.
Glucosinolates identified in kale.
| No.a | RTb (min) | Trivial names | Semisystematic names of R-groups | Compound groups | [M+H]+ (m/z) | Response factorc |
|---|---|---|---|---|---|---|
| 1 | 9.26 | Progoitrin | (2R)-2-Hydroxy-3-buteny | Aliphatic | 310 | 1.09 |
| 2 | 10.11 | Sinigrin | 2-Propenyl | Aliphatic | 280 | 1 |
| 3 | 12.68 | Gluconapin | 3-Butenyl | Aliphatic | 294 | 1.11 |
| 4 | 16.20 | Glucobrassicin | 3-Indolymethyl | Indolyl | 369 | 0.29 |
| 5 | 17.18 | 4-Methoxyglucobrassicin | 4-Methoxy-3-indolylmethyl | Indolyl | 399 | 0.25 |
| 6 | 19.40 | Neoglucobrasscin | N-Methoxy-3-indolylmethyl | Indolyl | 399 | 0.2 |
No., the elution order of glucosinolates from HPLC chromatogram (Fig. 1).
RT (retention time).
The international organization for standardization (ISO 9167-1, 1992).
Table 4.
Glucosinolate contents (μmol/g dry wt.) in kale ‘TBC’ by sulphur solutions (mM) (n = 3).
| No.a | Trivial name | S 0.0* | S 0.5 |
S 1.0 |
S 2.0 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper, Middle, Lower | Upper | Middle | Lower | Upper | Middle | Lower | Upper | Middle | Lower | ||
| 1 | Progoitrin | NDb | 1.02 ± 0.19 | 0.38 ± 0.09 | ND | 1.74 ± 0.84 | 0.77 ± 0.24 | 0.22 ± 0.19 | 3.60 ± 0.31 | 1.38 ± 0.45 | 0.35 ± 0.05 |
| 2 | Sinigrin | ND | 4.33 ± 1.42 | 1.57 ± 0.59 | 0.29 ± 0.25 | 4.95 ± 0.85 | 2.81 ± 0.49 | 0.90 ± 0.36 | 7.92 ± 0.15 | 4.23 ± 1.23 | 1.14 ± 0.25 |
| 3 | Gluconapin | ND | 0.86 ± 0.12 | 0.29 ± 0.25 | ND | 1.35 ± 0.65 | 0.86 ± 0.25 | 0.23 ± 0.22 | 1.93 ± 0.11 | 1.89 ± 0.66 | 0.49 ± 0.11 |
| 4 | Glucobrassicin | ND | 2.74 ± 0.98 | 1.13 ± 0.39 | 0.45 ± 0.33 | 3.31 ± 0.80 | 1.59 ± 0.54 | 0.83 ± 0.51 | 9.99 ± 0.39 | 4.16 ± 2.24 | 1.02 ± 0.63 |
| 5 | 4-Methoxyglucobrassicin | ND | 0.28 ± 0.03 | 0.17 ± 0.03 | 0.20 ± 0.05 | 0.28 ± 0.05 | 0.19 ± 0.03 | 0.27 ± 0.13 | 0.34 ± 0.00 | 0.24 ± 0.07 | 0.32 ± 0.11 |
| 6 | Neoglucobrasscin | ND | 1.36 ± 0.70 | 0.48 ± 0.03 | 0.11 ± 0.04 | 1.26 ± 0.46 | 0.36 ± 0.07 | 0.18 ± 0.02 | 3.03 ± 0.41 | 0.74 ± 0.48 | 0.20 ± 0.17 |
| Total | 10.59 ± 2.56 | 4.02 ± 1.29 | 1.04 ± 0.51 | 12.90 ± 3.56 | 6.58 ± 1.51 | 2.62 ± 1.34 | 26.80 ± 1.34 | 12.63 ± 4.89 | 3.53 ± 1.07 | ||
GSLs in the non-treatment (S 0.0 mM) were not detected.
No., the elution order of glucosinolates.
ND, not detected.
In conclusion, different ratio of S supply enhanced the aliphatic GSL and total GSL contents in kale. Increasing levels of S proportional to the synthesis of GSLs concluded the role of S fertilizers and GSL profiles in young plant foods (kale). Sulphur fertilizers are applied to kale to enhance the anticancer phytochemicals yield when soil nutrients are limiting.
Conflict of interest statement
The corresponding author and the contributing authors declare that they have no conflicts of interest in this research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Dhabi N.A., Arasu M.V., Kim S.J., Uddin Md.R., Park W.T., Lee S.Y., Park S.U. Methyl jasmonate- and light-induced glucosinolate and anthocyanin biosynthesis in radish seedlings. Nat. Prod. Commun. 2015;10(7):1211–1214. [PubMed] [Google Scholar]
- Armesto J., Gómez-Limia L., Carballo J., Martínez S. Impact of vacuum cooking and boiling, and refrigerated storage on the quality of galega kale (Brassica oleracea var. acephala cv. Galega) LWT - Food Sci. Technol. 2017;79:267–277. [Google Scholar]
- Biegańska-Marecik R., Radziejewska-Kubzdela E., Marecik R. Characterization of phenolics, glucosinolates and antioxidant activity of beverages based on apple juice with addition of frozen and freeze-dried curly kale leaves (Brassica oleracea L. var. acephala L.) Food Chem. 2017;230:271–280. doi: 10.1016/j.foodchem.2017.03.047. [DOI] [PubMed] [Google Scholar]
- Bimova P., Pokluda R. Impact of fertilizers on total antioxidants capacity in head cabbage. Hort. Sci. 2009;26:21–25. [Google Scholar]
- Cartea M.E., Velasco P. Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochem. Rev. 2008;7:213–229. [Google Scholar]
- Chen X.J., Zhu Z.J., Ni X.L., Qian Q.Q. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. chinensis. Agric. Sci. China. 2006;5(8):603–608. [Google Scholar]
- Chun J.-H., Arasu M.V., Lim Y.-P., Kim S.-J. Variation of major glucosinolates in different varieties and lines of rocket salad. Hort. Environ. Biotechnol. 2013;54(3):206–213. [Google Scholar]
- Chun J.-H., Kim N.-H., Seo M.-S., Jin M., Park S.U., Arasu M.V., Kim S.-J., Al-Dhabi N.A. Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis) Saudi J. Biol. 2018;25:71–82. doi: 10.1016/j.sjbs.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J.-H., Kim S., Arasu M.V., Al-Dhabi N.A., Chung D.Y., Kim S.-J. Combined effect of Nitrogen, Phosphorus and Potassium fertilizers on the contents of glucosinolates in rocket salad (Eruca sativa Mill.) Saudi J. Biol. Sci. 2017;24:436–443. doi: 10.1016/j.sjbs.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.Y., Kim H.W., Yoon S. Analysis of antioxidant nutrients in green yellow vegetable juice. Korean J. Food Sci. Technol. 1993;31:880–886. [Google Scholar]
- Clarke D.B. Glucosinolates, structures and analysis in food. Anal. Methods. 2010;2:310–325. [Google Scholar]
- Falk K.L., Tokuhisa J.G., Gershenzon J. The effect of sulfur nutrition on plant glucosinolate content: Physiology and molecular mechanisms. Plant Biol. 2007;9:573–581. doi: 10.1055/s-2007-965431. [DOI] [PubMed] [Google Scholar]
- Fu R., Zhang Y., Guo Y., Peng T., Chen F. Hepatoprotection using Brassica rapa var. rapa L. seeds and its bioactive compound, sinapine thiocyanate, for CCl4-induced liver injury. J. Funct. Foods. 2016;22:73–81. [Google Scholar]
- Groenbaek M., Jensen S., Neugart S., Schreiner M., Kidmose U., Kristensen H.L. Nitrogen split dose fertilization, plant age and frost effects on phytochemical content and sensory properties of curly kale (Brassica oleracea L. var. sabellica) Food Chem. 2016;197:530–538. doi: 10.1016/j.foodchem.2015.10.108. [DOI] [PubMed] [Google Scholar]
- International Standards Organisation (ISO), (1992). Rapeseed: Determination of glucosinolates content - Part 1: Method using High performance liquid chromatography, ISO 9167-1:1992 (E). Geneva, Switzerland, pp. 1–9.
- Kim H.H., Bong S.J., Al-Dhabi N.A., Arasu M.V., Park S.U. Variation of aminoacid contents of pale green purple Kohlrabis (Brassica oleracea var. gongylodes) Asian J. Chem. 2015;27(7):2675–2677. [Google Scholar]
- Kim S.-J., Kawaharada C., Jin S., Hashimoto M., Ishii G., Yamauchi H. Structural elucidation of 4-(cystein-S-yl)butyl glucosinolate from the leaves of Eruca sativa. Biosci. Biotechnol. Biochem. 2007;71:114–121. doi: 10.1271/bbb.60400. [DOI] [PubMed] [Google Scholar]
- Kim S.-J., Matsuo T., Watanabe M., Watanabe Y. Effect of nitrogen and sulphur application on the glucosinolate content in vegetable turnip rape (Brassica rapa L.) Soil Sci. Plant Nut. 2002;48:43–49. [Google Scholar]
- Lee D.S., Jeon D.S., Park S.G., Arasu M.V., Al-Dhabi N.A., Kim S.C., Kim S.J. Effect of cold storage on the contents of glucosinolates in Chinese cabbage (Brassica rapa L. ssp. pekinensis) South Ind. J. Biol. Sci. 2015;1(1):38–42. [Google Scholar]
- Lee M.K., Arasu M.V., Park S., Byeon D.H., Chung S.-O., Park S.U., Lim Y.-P., Kim S.-J. LED lights enhance metabolites and antioxidants in Chinese cabbage and kale. Braz. Arch. Biol. Technol. 2016;259:e16150546. [Google Scholar]
- Lee M.-K., Chun J.-H., Byeon D.H., Chung S.-O., Park S.U., Park S., Arasu M.V., Al-Dhabi N.A., Lim Y-P., Kim S-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT - Food Sci. Technol. 2014;58:93–101. [Google Scholar]
- Li S., Schonhof I., Krumbein A., Li L., Stutzel H., Schreiner M. Glucosinolate concentration in turnip (Brassica rapa ssp. rapifera L.) roots as affected by nitrogen and sulfur supply. J. Agric. Food Chem. 2007;55:8452–8457. doi: 10.1021/jf070816k. [DOI] [PubMed] [Google Scholar]
- Melchini A., 1, Traka MH., Catania S., Micel N., Taviano MF., Maimone P., Francisco M., Mithen RF., Costa C. Antiproliferative activity of the dietary isothiocyanate erucin, a bioactive compound from cruciferous vegetables, on human prostate cancer cells. Nutr. Cancer. 2013;65(1):132–138. doi: 10.1080/01635581.2013.741747. [DOI] [PubMed] [Google Scholar]
- Pandey C., Augustine R., Panthri M., Zia I., Bisht N.C., Gupta M. Arsenic affects the production of glucosinolate, thiol and phytochemical compounds: a comparison of two Brassica cultivars. Plant Phys. Biochem. 2017;111:144–154. doi: 10.1016/j.plaphy.2016.11.026. [DOI] [PubMed] [Google Scholar]
- Park C.H., Baskar T.B., Park S.-Y., Kim S.-J., Arasu M.V., Al-Dhabi N.A., Kim J.K., Park S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus) Molecules. 2016;21:157. doi: 10.3390/molecules21020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.-H., Arasu M.V., Park N.-Y., Choi Y.-J., Lee S.-W., Al-Dhabi N.A., Kim J.B., Kim S.-J. Variation of glucoraphanin and glucobrassicin: anticancer components in Brassica during processing. Food Sci. Technol. (Campinas) 2013;33:624–631. [Google Scholar]
- Park S., Arasu M.V., Lee M.-K., Chun J.-H., Seo J.M., Al-Dhabi N.A., Kim S.-J. Analysis and metabolite profiling of glucosinolates, anthocyanins and free amino acids in inbred lines of green and red cabbage (Brassica oleracea L.) LWT - Food Sci. Technol. 2014;58:58. doi: 10.1016/j.foodchem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Park S., Arasu M.V., Lee M.-K., Chun J.-H., Seo J.M., Lee S.W., Al-Dhabi N.A., Kim S.-J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.) Food Chem. 2014;145:77–85. doi: 10.1016/j.foodchem.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Park S., Arasu M.V., Nan J., Choi S.-H., Lim Y.P., Park J.-T., Al-Dhabi N.A., Kim S.-J. Metabolite profiling of phenolics, anthocyanins and flavonols in cabbage (Brassica oleracea var. capitata) Ind. Crops Prod. 2014;60:8–14. [Google Scholar]
- Perez-Balibrea S., Moreno D.A., Garcia-Viguera C. Genotypic effects on the phytochemical quality of seeds and sprouts from commercial broccoli cultivars. Food Chem. 2011;125:348–354. [Google Scholar]
- Rangkadilok N., Nicolas M.E., Bennett R.N., Eagling D.R., Premier R.R., Taylor P.W.J. The effect of sulfur fertilizer on glucoraphanin levels in broccoli (B. oleracea L. var italica) at different growth stages. J. Agric. Food Chem. 2004;52(9):2632–2639. doi: 10.1021/jf030655u. [DOI] [PubMed] [Google Scholar]
- Schonhof I., Blankenburg D., Muller S., Krumbein A. Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 2007;170:65–72. [Google Scholar]
- Seo J.-M., Arasu M.V., Kim Y.-B., Park S.U., Kim S.-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2014;177(15):204–213. doi: 10.1016/j.foodchem.2014.12.094. [DOI] [PubMed] [Google Scholar]
- Thavarajah D., Thavarajah P., Abare A., Basnagala S., Lacher C., Smith P., Combs G.F., Jr. Mineral micronutrient and prebiotic carbohydrate profiles of USA-grown kale (Brassica oleracea L. var. acephala) J. Food Comp. Anal. 2016;52:9–15. [Google Scholar]
- Vallejo F., Garcia-Viguera C., Tomas-Barberan F.A. Changes in broccoli (Brassica oleracea L. var. italica) health promoting compounds with inflorescence development. J. Agric. Food Chem. 2003;51:3776–3782. doi: 10.1021/jf0212338. [DOI] [PubMed] [Google Scholar]
- Zaki M.F., Abdelhafez A.A.M., El-dewiny Y. Camilia. Influence of biofertilization and nitrogen sources on growth, yield and quality of broccoli (Brassica oleracea L. var. italica) Egypt. J Appl. Sci. 2009;24:86–111. [Google Scholar]
- Zhang Y., Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54(7 Suppl.):1976s–1981s. [PubMed] [Google Scholar]