Abstract
Prevention of food spoilage and food poisoning pathogens is usually achieved by use of chemical preservatives which have negative impacts including: human health hazards of the chemical applications, chemical residues in food & feed chains and acquisition of microbial resistance to the used chemicals. Because of such concerns, the necessity to find a potentially effective, healthy safer and natural alternative preservatives is increased. Within these texts, Plant extracts have been used to control food poisoning diseases and preserve foodstuff. Antimicrobial activity of five plant extracts were investigated against Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi using agar disc diffusion technique. Ethanolic extracts of Punica granatum, Syzygium aromaticum, Zingiber officinales and Thymus vulgaris were potentially effective with variable efficiency against the tested bacterial strains at concentration of 10 mg/ml while extract of Cuminum cyminum was only effective against S. aureus respectively. P. granatum and S. aromaticum ethanolic extracts were the most effective plant extracts and showed bacteriostatic and bactericidal activities against the highly susceptible strains of food borne pathogenic bacteria (S. aureus and P. aeruginosa) with MIC's ranged from 2.5 to 5.0 mg/ml and MBC of 5.0 and 10 mg/ml except P. aeruginosa which was less sensitive and its MBC reached to 12.5 mg/ml of S. aromaticum respectively. These plant extracts which proved to be potentially effective can be used as natural alternative preventives to control food poisoning diseases and preserve food stuff avoiding healthy hazards of chemically antimicrobial agent applications.
Keywords: Food spoilage, Herbal plants, Antibacterial activity, Natural preservatives, MIC
1. Introduction
Food poisoning is considered as one of the most common cause of illness and death in developing countries (Doughari et al., 2007, Pirbalouti et al., 2009, Sapkota et al., 2012). Most of food poisoning reports are associated with bacterial contamination especially members of Gram negative bacteria like Salmonella typhi, Escherichia coli and Pseudomonas aeruginosa (Solomakos et al., 2008, Pandey and Singh, 2011). Other Gram positive bacteria including Staphylococcus aureus and Bacillus cereus have been also identified as the causal agents of food borne diseases or food spoilage (Braga et al., 2005). Prevention of food spoilage and their etiological agent is traditionally achieved by the use of chemical preservatives (Yamamura et al., 2000, Shan et al., 2007). Despite of the proven efficiency of these chemical preservative in prevention and outbreak control of food poisoning diseases, their repeated applications has resulted in the accumulation of chemical residues in food and feed chain, acquisition of microbial resistance to the applied chemicals and unpleasant side effects of these chemicals on human health (Akinyemi et al., 2006, Bialonska et al., 2010). Because of such concern, efforts have been focused on developing a potentially effective, healthy safer and natural food preservatives. Within these contexts is the utilization of plant extracts as antimicrobial agents for food preservation (Nasar-Abbas and Kadir, 2004, Hara_Kudo et al., 2004, Mathabe et al., 2005). These plant extracts considered as natural sources of antimicrobial agents, regarded as nutritionally safe and easily degradable (Cowan, 1999, Duffy and Power, 2001, Berahou et al., 2007, Chika et al., 2007). The antimicrobial activity exhibited by plant extracts against food poisoning bacteria has been demonstrated by several researchers (Delgado et al., 2004, Alzoreky and Nakahara, 2003, Verma et al., 2012, Akinpelu et al., 2015). Gupta et al. (2010) investigated antibacterial activity of five ethanolic and aqueous plant extracts against S. aureus, Pseudomonas aeruginosa and Bacillus subtilis and their results showed that the ethanolic extracts of four plants (Achyranthes aspera, Cynodon dacynodon dactylon, Lantana camara and Tagetes patula) were effective against all tested microorganisms with MIC's ranged from 25 to 125 mg/ml. Sapkota et al. (2012) studied antibacterial effect of guava leaves, garlic and ginger against some human microbial pathogens and they ascertained that ginger was only effective against S. aureus while guava and garlic were effective against all tested microorganisms. Akinpelu et al. (2015) investigated antibacterial potential of crude and butanolic extracts of Persea americana against Bacillus cereus implicated in food poisoning. The extracts exhibited antibacterial activity at concentrations of 25 and 10 mg/ml with MBC of both extracts ranged between 3.12 and 12.5 mg/ml respectively. Moreover, antimicrobial activity of different natural substances such as medicinal plant extract have been investigated against food borne bacteria. For example; Ahmad and Beg, 2001, Kokoska et al., 2002, Ateb and Erdo_Urul, 2003, Rios and Recio, 2005 tested the suppression of food borne bacteria and their diseases by medical plant extracts. The extract of three medicinal plants used in Nigerian folk medicine showed a highly antibacterial activity against some food borne pathogens. All extracts exhibited a strong antimicrobial activity against Salmonella enteritidis, E. coli and S. aureus but in variable degree and with different MIC's depending upon the plant extract and pathogenic organism. (Ahmad et al., 1998, Akinyemi et al., 2006). In addition, Sher, 2009, Venkatesan and Karrunakaran, 2010, Pirbalouti et al., 2010 investigated antimicrobial activity of eight medicinal plants against E. coli, Bacillus cereus and Listeria monocytogenes. The most effective extracts were those obtained from Myrtus communis and Thymus daenensis with MIC values ranged between 0.039 and 10 mg/ml. Antimicrobial activity of Punica granatum against food poisoning bacteria was proved by several investigators (Prashanth et al., 2001, Negi and Jayaprakasha, 2003, Voravuthikunchai et al., 2005, Naz et al., 2007, Nuamsetti et al., 2012). Antibacterial activity of Punica, Citrus and Allium extracts against food borne spoilage bacteria was investigated by Verma et al. (2012). All plant extracts was potentially effective against S. typhi, E. coli, B. cereus and S. aureus implicated in food spoilage but the extract of Punica granatum was the most effective extract with concentration of 500 mg/ml. Ethanolic P. granatum peels extracts was found to be potentially effective against Micrococcus luteus, S. aureus, Bacillus megaterium and Gram negative bacteria like E. coli and P. aeruginos in concentration ranged between 30 and 50 µg/ml. (Duman et al., 2009, Sadeghian et al., 2011, Dey et al., 2012). Antimicrobial activity of ethanolic Punica granatum extract and its fractions showed a highly antibacterial activity against Gram positive (S. aureus and B. cereus) and Gram negative bacteria (E. coli and S. typhi) causing food poisoning and these extracts can be used for prevention of food borne diseases or as preservative in food industry (Alzoreky, 2009, Mahboubi et al., 2015). Spices extracts used as food additives were potentially effective against some food poisoning bacteria and their antibacterial activity was investigated by several researchers (Ozcan and Erkmen, 2001, Nevas et al., 2004, Parekh and Sumitra, 2007, Abdulrahman et al., 2010). Cinnamon extract was found to be the most effective spice against all tested strain while the weakest antimicrobial activity was displayed by cumin, ginger and clove respectively. Antimicrobial activity of clove (S. aromaticum) against Gram negative bacteria and food borne pathogens was investigated by Sakagami et al., 2000, Mahfuzul_Hoque et al., 2007, Saeed and Tariq, 2008, Pandey and Singh, 2011. Some researchers reported that ethanolic clove extract was potentially active against S. aureus, Vibrio parahaemolyticus and P. aeruginosa while it was inactive against E. coli and Salmonella enteritidis (Mahfuzul_Hoque et al., 2007). Other researchers ascertained activity of clove oil against all tested pathogenic bacteria while Vibrio cholera, S. typhi and Klebsiella pneumonia were found to be resistant to aqueous clove extract (Saeed and Tariq, 2008, Saeed et al., 2013). Moreover, the methanolic clove extract was reported to be potentially effective against S. aureus, P. aeruginosa and E. coli with MIC ranged from 0.1 to 2.31 mg/ml (Pandey and Singh, 2011). Antimicrobial activity of cumin seeds (Cuminum cyminum) extract was reported to be potentially effective against several strains of Gram positive and Gram negative bacteria implicated in food poisoning with variable MIC's (Arora and Kaur, 1999, Shan et al., 2007, Chaudry and Tariq, 2008). MIC's of cumin extract effectiveagainstE.coli, P. aeruginosa, S. aureus and B. pumilus were ranged between 6.25 and 25 mg/ml (Dua et al., 2013) while a higher concentration ranged between 20 and 60 mg/ml were previously reported by Sheikh et al. (2010). Seven ethanolic and aqueous plant extracts were investigated against some clinically pathogenic bacteria. Ethanolic Punica granatum extract was effective against all tested bacterial pathogens with MIC of 0.2 mg/ml. Zingiber officinales extract was also effective against P. aeruginosa and K. pneumonia while Thymus kotschyana was potentially effective against S. aureus and E. coli (Qader et al., 2013). Rasooli et al. (2006) investigated antimicrobial activity of some thyme essential oils against food borne pathogens (Listeria monocytogenes). Most of food poisoning diseases have been associated with bacterial contaminations particulary members of Gram negative bacteria like E. coli, S. typhi & P. aeruginosa and Gram positive like S. aureus & B. cereus. Research concerning the efficiency of Syzygium aromaticum, Thymus vulgaris, Punica granatum, Zingiber officinales and Cuminum cyminum against the previous etiological food spoilage bacteria are scanty in Arabian area. Therefore, the present study aimed to evaluate antibacterial activity of these plant extract against food poisoning diseases caused by S. aureus, B. cereus, E. coli, S. typhi and P. aeruginosa in vitro.
2. Materials and methods
2.1. Plants extraction preparation
Plant materials of five plant species included in this study (Table 1) were collected from local market of Riyadh, Saudi Arabia. The collected plants were watery washed, disinfected, rinsed with distilled water and finally dried in shade. The dried plant material of each plant species was grounded into fine powder to pass 100 mm sieve. 50 g of the fine powder was soaked in 200 ml of ethanol with stirring for 48 h., filtered through double layers of muslin, centrifuged at 9000 rpm for 10 min and finally filtered again through Whatman filter paper No. (41) to attain a clear filtrate. The filtrates were evaporated and dried at 40 °C under reduced pressure using rotatory vacuum evaporator. The extract yields were weighted, stored in a small bottles in fridge at 5 °C and their yield percentages were calculated using the following formula: Extract yield% = R/S × 100 (where R; weight of extracted plants residues and S; weight of plant raw sample).
Table 1.
The ethnobotanical data of employed plant species and their extract yield percentage.
| Plant species | Family | Local name | Common name | Plant part used | Extract pH | Extract yield (%) |
|---|---|---|---|---|---|---|
| Cuminum cyminum | Apiaceae | Kammun | Cumin | Seeds | 6.2 | 3.12 |
| Punica granatum | Lythraceae | Romman | Pomegranate | Peels | 4.7 | 9.74 |
| Syzygium aromaticum | Myrtaceae | Koronfil | Clove | Flowers | 5.3 | 4.38 |
| Thymus vulgaris | Lamiaceae | Za'ater | Thyme | Leaves | 6.8 | 6.54 |
| Zingiber officinale | Zingiberaceae | Zanjabil | Ginger | Rhizome | 7.1 | 5.26 |
2.2. Antibacterial activity of the plant extracts
2.2.1. Bacterial strains
The antibacterial potency of each plant extract was evaluated using five bacterial strains causing food poisoning diseases. Two strains of Gram positive (Staphylococcus aureus and Bacillus cereus) and three strains of Gram negative (Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa) bacteria. The bacterial strains were provided from the culture collection of Botany and Microbiology Dept. King Saud University, Riyadh, K.S.A.
2.2.2. Inoculums preparation
Each bacterial strain was subcultured overnight at 35 °C in Mueller-Hilton agar slants. The bacterial growth was harvested using 5 ml of sterile saline water, its absorbance was adjusted at 580 um and diluted to attain viable cell count of 107 CFU/ml using spectrophotometer.
2.2.3. Antibacterial activity of plants extract
The disk diffusion method is used to evaluate antimicrobial activity of the each plant extract. The plant extract residues (50 mg) were re- dissolved in 2.5 ml of ethanol, sterilized through Millipore filter (0.22 µm) then loaded over sterile filter paper discs (8 mm in diameter) to obtain final concentration of 10 mg/disc. Ten ml of Mueller-Hilton agar medium was poured into sterile Petri dishes (as a basal layer) followed with 15 ml of seeded medium previously inoculated with bacterial suspension (100 ml of medium/1 ml of 107 CFU) to attain 105 CFU/ml of medium. Sterile filter paper discs loaded with plant extract concentration of (10 mg/ml) were placed on the top of Mueller-Hilton agar plates. Filter paper discs loaded with 5 µg of Gentamycin was used as positive control. The plates were kept in the fridge at 5 °C for 2 h. to permit plant extracts diffusion then incubated at 35 °C for 24 h. The presence of inhibition zones were measured by Vernier caliper, recorded and considered as indication for antibacterial activity.
2.2.4. Determination of minimum inhibitory concentrations (MIC's) of the effective plants extract
MIC is defined as the lowest concentration of the antimicrobial agent that inhibits the microbial growth after 24 h. of incubation. The most effective plant extracts which exhibiting a strong antibacterial activity at 10 mg/ml was manipulated to determine their MIC using disk diffusion method and evaluate their efficiency in controlling bacterial strains causing food poisoning diseases. Different concentrations of the effective plant extract (1.25, 2.5, 5.0, 10.0, 12.5 and 15.0 mg/ml) were prepared separately by dissolving 50 mg in 2.5 ml of ethanol, sterilized through Millipore filter and loaded their requisite amount over sterilized filter paper discs (8 mm in diameter). Mueller-Hilton agar was poured into sterile Petri dishes and seeded with bacterial suspensions of the pathogenic strains. The loaded filter paper discs with different concentrations of the effective plant extract were placed on the top of the Mueller-Hilton agar plates. The plates were kept in the fridge at 5 °C for 2 h. then incubated at 35 °C for 24 h. The inhibition zones were measured by Vernier caliper and recorded against the concentrations of the effective plant extracts.
2.2.5. Determination of minimum bactericidal concentrations (MBC's) of the effective plants extract
Streaks were taken from the two lowest concentrations of the plant extract plates exhibiting invisible growth (from inhibition zone of MIC plates) and subcultures onto sterile Tryptone soya agar (TSA) plates. The plates were incubated at 35 °C for 24 h. then examined for bacterial growth in corresponding to plant extract concentration. MBC was taken as the concentration of plant extract that did not exhibiting any bacterial growth on the freshly inoculated agar plates.
3. Results and discussion
3.1. Plants extraction yield
The ethanobotanical data of the employed plants and their extract percentage yield are illustrated in Table 1. The extract of 50 g of dried plant materials with ethanol yielded plant extract residues ranged from 1.56 to 4.87 g. The highest yield of plant extract was obtained from Punica granatum (4.87 g) followed by Thymus vulgaris (3.27 g) while Cuminum cyminum give the lowest extract yield respectively.
3.2. Antibacterial activity of plants extract
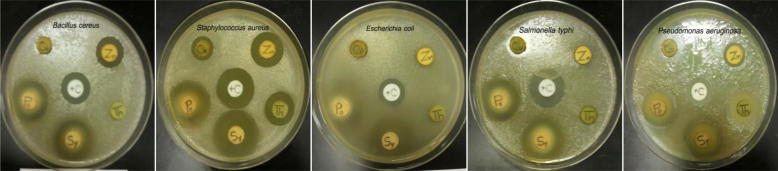
Five plant species were investigated to evaluate their antibacterial activity against food poisoning bacteria including two strains of Gram positive bacteria (B. cereus & S. aureus) and three strains of Gram negative bacteria (E. coli, S. typhi & P. aeruginosa) using disc diffusion method. Evaluation of antibacterial activity of these plant extracts was recorded in Table 2 and illustrated in Fig. 1. The results revealed that all plant extracts were potentially effective in suppressing microbial growth of food poisoning bacteria with variable potency. P. granatum was the most effective extract retarding microbial growth of all tested pathogenic bacteria at concentration of 10 mg/ml while extract of C. cyminum was effective only against S. aureus. Other plant extracts showed variable antimicrobial activity against food poisoning bacterial strains. S. aromaticum exhibited inhibitory effect against four of the pathogenic strains (B. cereus, S. aureus, E. coli & P. aeruginosa) whereas Z. officinales was effective against three of them (B. cereus, S. aureus & P. aeruginosa) and T. vulgaris was effective against S. aureus and P. aeruginosa.
Table 2.
Antimicrobial screening test of ethanolic plants extract (10 mg/ml) against some bacterial strains of food poisoning diseases.
| Plant species | Inhibition zones (mm) |
||||
|---|---|---|---|---|---|
| Gram (+ve) pathogenic bacteria |
Gram (−ve) pathogenic bacteria |
||||
| B. cereus | S. aureus | E. coli | S. typhi | P. aeruginosa | |
| Cuminum cyminum | 0.0 ± 0.0 | 9.5 ± 0.74 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Punica granatum | 16.3 ± 0.57 | 18.5 ± 0.13 | 14.2 ± 0.61 | 9.7 ± 0.22 | 16.1 ± 0.46 |
| Syzygium aromaticum | 14.6 ± 0.37 | 15.8 ± 0.41 | 11.9 ± 0.34 | 0.0 ± 0.0 | 13.4 ± 0.11 |
| Thymus vulgaris | 0.0 ± 0.0 | 17.6 ± 0.31 | 0.0 ± 0.0 | 0.0 ± 0.0 | 14.7 ± 0.25 |
| Zingiber officinales | 8.3 ± 0.46 | 15.4 ± 0.23 | 0.0 ± 0.0 | 0.0 ± 0.0 | 11.2 ± 0.17 |
| Gentamycin (5 µg) | 16.8 ± 0.37 | 20.5 ± 0.24 | 15.6 ± 0.53 | 18.7 ± 0.61 | 13.1 ± 0.35 |
Data are means of three replicates (n = 3) ± standard error.
Fig. 1.
Growth inhibition of some food poisoning bacterial strains caused by plant extracts. Cu, Cumin; Po, pomegranate; Sy, Clove; Th, Thyme; Zn, Ginger and +C, positive control.
Results of antimicrobial activity of the five plant extracts can suggested that S. typhi was the most resistant strain to plant extracts followed by E. coli while S. aureus and P. aeruginosa were the most susceptible strains to the extracted plants respectively. Moreover, P. granatum and S. aromaticum extracts were the most effective extracts and showed a strong antibacterial activity against food poisoning bacteria. Hence, experiments were conducted to determine their minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) against the most susceptible bacterial strains (S. aureus and P. aeruginosa).
3.3. Minimum inhibitory concentrations (MIC's) of the effective plants extract
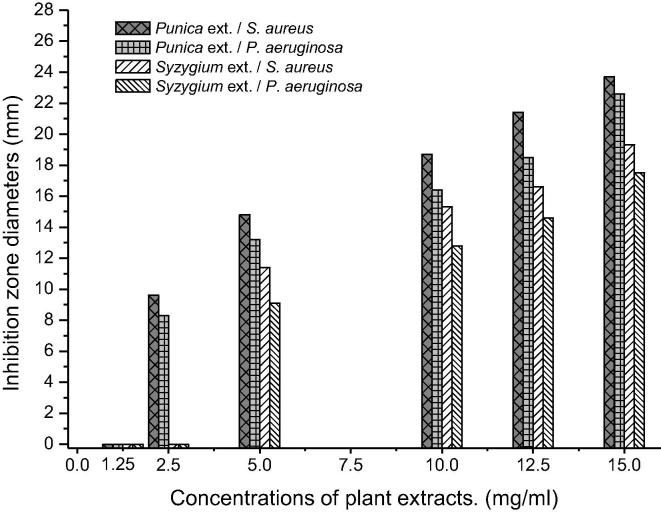
The MIC and MBC of the most effective plant extracts (P. granatum and S. aromaticum) were employed by disc diffusion method to evaluate their bacteriostatic and bactericidal properties. The concentration effect of the effective plant extracts were reported in Table 3 and illustrated in Fig. 2. The inhibitory effect of P. granatum extract started at 2.5 mg/ml with inhibition zones of 9.6 and 8.3 mm against S. aureus and P. aeruginosa while extract of S. aromaticum suppressed bacterial growth of these strains at concentration of 5 mg/ml with inhibition zones of 11.4 and 9.2 mm respectively.
Table 3.
MIC's of the most effective plant extract against S. aureus and P. aeruginosa.
| Plant ext. | Conc. mg/ml | Inhibition zones (mm) |
|
|---|---|---|---|
| Gram (+ve) pathogenic bacteria | Gram (−ve) pathogenic bacteria | ||
| S. aureus | P. aeruginosa | ||
| P. granatum | 1.25 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2.50 | 9.6 ± 0.65 | 8.3 ± 0.95 | |
| 05.0 | 14.8 ± 0.83 | 13.2 ± 1.1 | |
| 10.0 | 18.7 ± 0.75 | 16.4 ± 0.56 | |
| 12.5 | 21.4 ± 0.46 | 18.5 ± 0.36 | |
| 15.0 | 23.7 ± 0.35 | 22.6 ± 0.74 | |
| S. aromaticum | 1.25 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2.50 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| 05.0 | 11.4 ± 0.37 | 9.1 ± 0.80 | |
| 10.0 | 15.3 ± 0.85 | 12.8 ± 0.45 | |
| 12.5 | 16.6 ± 0.13 | 14.7 ± 0.52 | |
| 15.0 | 19.3 ± 0.65 | 17.5 ± 0.35 | |
Data are means of three replicates (n = 3) ± standard error.
Fig. 2.
MIC's of the effective plant extracts against S. aureus and P. aeruginosa.
3.4. Minimum bactericidal concentrations (MBC's) of the effective plants extract
The MBC was confirmed by absence of bacterial growth of the tested strains streaked form inhibition zone corresponding to their lowest MIC's. P. granatum extract showed potentially bactericidal activity against the tested pathogenic bacteria (S. aureus and P. aeruginosa) with MBC of 5 mg/ml while MBC of S. aromaticum extract reached to 10 mg/ml except P. aeruginosa which was less sensitive and its minimal bactericidal concentration reached to 12.5 mg/ml. The results of MIC and MBC of the effective plant extracts suggested that P. granatum and S. aromaticum can be used to control and prevent food borne bacteria and food poisoning diseases. Bacterial strains included in this study were chosen for their importance in food spoilage and food poisoning. S. aureus considered as the one of the most common source of food borne disease while B. cereus, E. coli, S. typhi and P. aeruginosa produce toxins and other metabolites that induce human gastroenteritis diseases. P. granatum extract suppressing microbial growth of all tested bacterial strains followed by extract of S. aromaticum which appear to be potentially effective against four bacterial strains and less effective against S. typhi.
These results are in accordance with those of Verma et al., 2012, Qader et al., 2013, Mahboubi et al., 2015. A great variation in MIC of P. granatum extract demonstrated in several investigation may be due to considerable variation in their method of extraction, constituents as well as bacterial strains used. Also, variation in MIC of different plant extracts may arise from variation in their chemical constituents and volatile nature of their constituents. On the other hand, S. aromaticum extract was found to be effective with concentration of (10 mg/ml) against B. cereus, S. aureus, E. coli and P. aeruginosa suppressing their growth with inhibition zones of 14.6, 15.8, 11.9 and 13.4 mm respectively. These results are in accordance with that of Mahfuzul_Hoque et al., 2007, Pandey and Singh, 2011. Cumin was found to be ineffective in controlling the other bacterial strains and these results were contrasted with that of Dua et al. (2013) who reported cumin with potentially effective with MIC ranged from 6.25 to 12.5 mg/ml .On the other hand, a higher concentration of cumin extract reached to 60 mg/ml may be required to be effective against food spoilage bacteria and these results were coincident with that previously reported by Sheikh et al. (2010). Several researchers investigated the efficiency of plant extracts and their effective compounds as antimicrobial agents to control growth of food borne and spoilage bacteria. Some researchers have suggested that antimicrobial components of the plant extracts (terpenoid, alkaloid and phenolic compounds) interact with enzymes and proteins of the microbial cell membrane causing its disruption to disperse a flux of protons towards cell exterior which induces cell death or may inhibit enzymes necessary for aminoacids biosynthesis (Burt, 2004, Gill and Holley, 2006). Other researchers attributed the inhibitory effect of these plant extracts to hydrophobicity characters of these plants extracts which enable them to react with protein of microbial cell membrane and mitochondria disturbing their structures and changing their permeability (Friedman et al., 2004, Tiwari et al., 2009). The present study suggested that plant extracts which proved to be potentially effective can be used as natural preservatives to control food poisoning diseases and preserve food avoiding application of healthy hazards of chemical preservatives.
4. Conclusion
Food spoilage is often caused by the growth of many pathogenic bacterial strains. Prevention of food spoilage in food industry and food stuff is mainly based on the application of chemical preservatives. The adverse effects of these chemical preservatives on human health increases the demand to search for potentially effective, healthy safer and natural food preservative. The plant extracts which proved to be potentially effective as (P. granatum and S. aromaticum) can be used as natural alternative preventives to control food poisoning diseases and preserve food stuff avoiding healthy hazards of chemically antimicrobial agent applications.
Declaration of conflict of interest
None.
Acknowledgments
The Authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RG-1438-090.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdulrahman M.S., Thangaraj S., Salique S.M., Khan K.F., Natheer S.E. Antimicrobial and biochemical analysis of some spices extracts against food spoilage pathogens. Int. J. Food Safety. 2010;12:71–75. [Google Scholar]
- Ahmad I., Beg A.Z. Antimicrobial and photochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmocol. 2001;74:113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Mehmood Z., Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998;62:183–193. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- Akinpelu D.A., Aiyegoro O.A., Akinpelu O.F., Okah A.I. Stem bark extract and fraction of Persea americana (Mill) exhibits bactericidal activities against strains of Bacillus cereus associated with food poisoning. Molecules. 2015;20:416–429. doi: 10.3390/molecules20010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi K.O., Oluwa O.K., Omomigbehin E.O. Antimicrobial activity of crude extracts of three medicinal plants used in South-West Nigerian folk medicine on some food borne bacterial pathogens. Afr. J. Trad. Compl. Altern. Med. 2006;3(4):13–22. [Google Scholar]
- Alzoreky N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009;134:244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Alzoreky N.S., Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003;80:223–230. doi: 10.1016/s0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- Arora D.S., Kaur J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents. 1999;12:257–262. doi: 10.1016/s0924-8579(99)00074-6. [DOI] [PubMed] [Google Scholar]
- Ateb D.A., Erdo_Urul O.T. Antimicrobial activities of various medicinal and commercial plant extracts. Turk. J. Biol. 2003;27:157–162. [Google Scholar]
- Berahou A., Auhmani A., Fdil N., Benharref A., Jana M., Gadhi C.A. Antibacterial activity of Quercus ilex bark’s extracts. J. Ethnopharmacol. 2007;112:426–429. doi: 10.1016/j.jep.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Bialonska D., Ramnani P., Kasimsetty S.G., Muntha K.R., Gibson G.R., Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010;140:175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Braga L.C., Shupp J.W., Cummings C., Jett M., Takahashi J.A., Carmo L.S. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J. Ethnopharmacol. 2005;96:335–339. doi: 10.1016/j.jep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential application in foods: a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chaudry N.M.A., Tariq P. In vitro antibacterial activities of Kalonji, Cumin and Poppy seeds. Pak. J. Bot. 2008;40(1):461–467. [Google Scholar]
- Chika C.O., Jude N.O., Ifeanyi C.O., Anyanwu N.B. Antibacterial activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta. J. Am. Sci. 2007;3(3):11–16. [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado B., Palop A., Fernandez P.S., Periago P.M. Combined effect of thymol and cymene to control the growth of Bacillus cereus vegetative cells. Eur. Food Res. Technol. 2004;218:188–193. [Google Scholar]
- Dey D., Debnath S., Hazra S., Ghosh S., Ray R., Hazra B. Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing Gram-negative bacilli. Food Chem. Toxicol. 2012;50:4302–4309. doi: 10.1016/j.fct.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Doughari J.H., Pukuma M.S., De N. Antibacterial effects of Balanites aegyptiaca L. Drel. and Moringa oleifera Lam. on Salmonella typhi. Afr. J. Biotechnol. 2007;6(19):2212–2215. [Google Scholar]
- Dua A., Gaurav G., Balkar S., Mahajan R. Antimicrobial properties of methanolic extract of cumin (Cuminum cyminum) seeds. Int. J. Res. Ayurveda Pharm. 2013;4(1):104–107. [Google Scholar]
- Duffy C.F., Power R.F. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int. J. Antimicrob. Agents. 2001;17:527–529. doi: 10.1016/s0924-8579(01)00326-0. [DOI] [PubMed] [Google Scholar]
- Duman A.D., Ozgen S.K.M., Dayisoylu K.S., Eribl N., Durgac C. Antimicrobial activity of six Pomegranate (Punica granatum L.) varieties and their Relation to some of their pomological and phytonutrient characteristics. Molecules. 2009;14:1808–1817. doi: 10.3390/molecules14051808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M., Henika P.R., Levin C.E., Mandrell R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agri. Food Chem. 2004;52:6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- Gill A.O., Holley R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006;108:1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Gupta R.N., Kartik V., Manoj P., Singh P.S., Alka G. Antibacterial activities of ethanolic extracts of plants used in flok medicine. Int. J. Res. Ayurveda Pharm. 2010;1(2):529–535. [Google Scholar]
- Hara_Kudo Y., Kobayashi A., Sugita-Konishi Y., Kondo k. Antibacterial activity of plants used in cooking for aroma and taste. J. Food Prot. 2004;67:2820–2824. doi: 10.4315/0362-028x-67.12.2820. [DOI] [PubMed] [Google Scholar]
- Kokoska L., Polesny Z., Rada V., Nepovim A., Vanek T. Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol. 2002;82:51–53. doi: 10.1016/s0378-8741(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Mahboubi A., Asgarpanah J., Sadaghiqani P.N., Faizi M. Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. Var. pleniflora flower (Golnar) against bacterial strains causing food borne diseases. BMC Complem. Altern. Med. 2015;15:366–373. doi: 10.1186/s12906-015-0887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfuzul_Hoque M., Bari M.L., Juneja V.K., Kawamoto S. Antimicrobial activity of cloves and cinnamon extracts against food borne pathogens and spoilage bacteria and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. J. Food Sci. Technol. 2007;72:9–21. [Google Scholar]
- Mathabe M.C., Nikolova R.V., Lall N., Nyazema N.Z. Antibacterial activities of medicinal plants used for the treatment of diarrhea in Limpopo Province, South Africa. J. Ethnopharmocol. 2005;105:286–293. doi: 10.1016/j.jep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Nasar-Abbas S.M., Kadir Halkman A. Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int. J. Food Microbiol. 2004;97:63–69. doi: 10.1016/j.ijfoodmicro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Naz S., Siddiqi R., Ahmad S., Rasool S.A., Sayeed S.A. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 2007;72:341–345. doi: 10.1111/j.1750-3841.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Negi P.S., Jayaprakasha G.K. Antioxidant and antibacterial activities of Punica granatum peel extracts. J. Food Sci. 2003;68:1473–1477. [Google Scholar]
- Nevas M., Korhonen A.R., Lindtrom M., Turkki P., Korkeala H. Antibacterial efficiency of Finnish spices essential oils against pathogenic and spoilage bacteria. J. Food Prot. 2004;67:199–202. doi: 10.4315/0362-028x-67.1.199. [DOI] [PubMed] [Google Scholar]
- Nuamsetti T., Dechayuenyong P., Tantipailbulvut S. Antibacterial activity of pomegranate fruit peels and arils. Sci. Asia. 2012;38:319–322. [Google Scholar]
- Ozcan M., Erkmen O. Antimicrobial activity of the essential oils of Turkish plant spices. Eur. Food. Res. Technol. 2001;212:658–660. [Google Scholar]
- Pandey A., Singh P. Antibacterial activity of Syzygium aromaticum (Clove) with metal ion effect against food borne pathogens. Asian J. Plant Sci. Res. 2011;1(2):69–80. [Google Scholar]
- Parekh J., Sumitra C. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr. J. Biomed. Res. 2007;10:175–181. [Google Scholar]
- Pirbalouti A.G., Chaleshtori A.R., Tajbakhsh E., Momtaz H., Rahimi E., Shahin F. Bioactivity of medicinal plants extracts against Listeria monocytogenes isolated from food. J. Food. Agric. Environ. 2009;7:132–135. [Google Scholar]
- Pirbalouti A.G., Jahanbazi P., Enteshari S., Malekpoor F., Hamedi B. Antimicrobial activity of some Iranian medicinal plants. Arch. Biol. Sci. Belgrade. 2010;62(3):633–642. [Google Scholar]
- Prashanth D.J., Asha M.K., Amit A. Antibacterial activity of Punica granatum. Fitoterapia. 2001;72:171–173. doi: 10.1016/s0367-326x(00)00270-7. [DOI] [PubMed] [Google Scholar]
- Qader M.K., Khalid N.S., Abdullah A.M. Antibacterial activity of some plant extracts against clinical pathogens. Int. J. Microbiol. Immunol. Res. 2013;1(5):53–56. [Google Scholar]
- Rasooli I., Rezaei M.B., Allameh A. Ultrastructural studies on antimicrobial efficacy of thyme essential oils on Listeria monocytogenes. Int. J. Infect. Dis. 2006;10:236–241. doi: 10.1016/j.ijid.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Rios J.L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;4:80–100. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Sadeghian A., Ghorbani A., Mohamadi_Nejad A., Rakhshandeh H. Antimicrobial activity of aqueous and methanolic extracts of pomegranate fruit skin. Avicenna J. Phytomed. 2011;1(2):67–73. [Google Scholar]
- Saeed S., Tariq P. In vitro Antibacterial activity of clove against Gram negative bacteria. Pak. J. Bot. 2008;40(5):2157–2160. [Google Scholar]
- Saeed M., Nadeem M., Khan M.R., Shabbir M.A., Shehzad A., Amir R.M. Antimcrobial activity os Syzygium aromaticum extracts against food spoilage bacteria. Afr. J. Microbiol. Res. 2013;7(41):4848–4856. [Google Scholar]
- Sakagami Y., Kaikoh S., Kajimura K., Yokoyama H. Inhibitory Effect of Clove Extracts on Verotoxin Production by Enterohemorrhagic Escherichia coli O157: H7. Biocontrol Sci. 2000;5:47–49. [Google Scholar]
- Sapkota R., Dasgupta R., Nancy, Rawat D.S. Antibacterial effects of plants extracts on human microbial pathogens & microbial limit tests. Int. J. Res Pharm. Chem. 2012;2(4):926–936. [Google Scholar]
- Shan B., Cai Y., Brooks J.D., Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sheikh M.I., Islam S., Rahman Atikur, Rahman Mostafizur, Rahman Mushiur, Rahman Muzanur, Rahmanm Abdur, Alam F. Control of some human pathogenic bacteria by seed extracts of cumin (cuminum cyminum L.) Agric. Conspec. Sci. 2010;75(1):39–44. [Google Scholar]
- Sher A. Antimicrobial activity of natural products from medicinal plants. Gomal. J. Med. Sci. 2009;7(1):72–78. [Google Scholar]
- Solomakos N., Govaris A., Koidis P., Botsoglou N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008;80:159–166. doi: 10.1016/j.meatsci.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Tiwari B.K., Valdramidi V.P., O'Donnell C.P., Muthukumarappan K., Bourke P., Cullen P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009;57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- Venkatesan D., Karrunakaran C.M. Antimicrobial activity of selected Indian medicinal plants. J. Phytol. 2010;2(2):44–48. [Google Scholar]
- Verma V., Singh R., Tiwari R.K., Srivastava N., Verma S. Antibacterial activity of extracts of Citrus, Allium and Punica against food borne spoilage. Asian J. Plant Sci. Res. 2012;2(4):503–509. [Google Scholar]
- Voravuthikunchai S.P., Sririrak T., Limsuwan S., Supawita T., Iida T., Hond T. Inhibitory effect of active compounds from Punica granatum pericarp on verocytotoxin production by Enterohemorrhagic Escherichia coli O157: H7. J. Health Sci. 2005;51:590–596. [Google Scholar]
- Yamamura A., Murai A., Takamatsu H., Watabe K. Antimicrobial effect of chemical preservatives on enterohemorrhagic Escherichia coli O157: H7. J. Health Sci. 2000;46:204–208. [Google Scholar]