Abstract
Resveratrol, a polyphenol found in various plants, including grapes, plums and peanuts has shown various medIRInal properties, including antioxidant, protection of cardiovascular disease and cancer risk. However, the effects of resveratrol on spinal cord reperfusion injury have not been investigated. Hence, the present study was designed to evaluate the effect of resveratrol on nitric oxide synthase (iNOS)/p38MAPK signaling pathway and to elucidate its regulating effect on the protection of spinal cord injury. Spinal cord ischemia–reperfusion injury (IRI) was performed by the infrarenal abdominal aorta with mini aneurysm clip model. The expressions of iNOS and p38MAPK and the levels of biochemical parameters, including nitrite/nitrate, malondialdehyde (MDA), advanced oxidation products (AOPP), reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) were measured in control and experimental groups. IRI-induced rats treated with 10 mg/kg resveratrol protected spinal cord from ischemia injury as supported by improved biological parameters measured in spinal cord tissue homogenates. The resveratrol treatment significantly decreased the levels of plasma nitrite/nitrate, iNOS mRNA and protein expressions and phosphorylation of p38MAPK in IRI-induced rats. Further, IRI-produced free radicals were reduced by resveratrol treatment by increasing enzymatic and non-enzymatic antioxidant levels such as GSH, SOD and CAT. Taken together, administration of resveratrol protects the damage caused by spinal cord ischemia with potential mechanism of suppressing the activation of iNOS/p38MAPK pathway and subsequent reduction of oxidative stress due to IRI.
Keywords: Spinal cord, Resveratrol, Antioxidant, p38MAPk, iNOS
1. Introduction
Ischemic reperfusion injury (IRI) of spinal cord generally occurs in an acute situation after operations performed on the thoracic and thoraco-abdominal aorta and interrupts the blood circulation (Kanellopoulos et al., 1997). This may result in paraplegia associated with a variety of extended neurophysiological alterations (Wang et al., 2008). Neurologic injury caused by ischemia–reperfusion injury of the spinal cord has been a frequency between 3% and 24% (Cambria et al., 1997). The pathogenesis of neurologic injury after IRI is mainly due to oxidative stress, excitotoxIRIty and energy failure (Dawson et al., 1992, Choi, 1988). Among these factors, oxidative stress with massive production of reactive oxygen species (ROS), including free radicals and lipid peroxides is also involved in the neurological vascular injuries (Xing et al., 2008). Numerous studies reported that increased oxidative stress due to IRI is associated with decreased enzymatic and non-enzymatic antioxidant defense potential, such as glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) activities (Naik et al., 2006, Morsy et al., 2010).
In addition, oxidative stress induced by ischemia is leading to alteration of various genes and signaling pathways. It has been found that nitric oxide synthase (iNOS), which is the key enzyme that catalyzes the synthesis of nitric oxide (NO) from l-arginine, is increased in neurotoxicity or neuroprotection following IRI neural tissues (Lipton, 1999, Matsumoto et al., 1999). In addition, various signaling pathways have been activated in blocking certain signaling pathways for the treatment of secondary damage following IRI (Park et al., 2008, Song et al., 2013a, Song et al., 2013b). For instance, the cellular responses to a diverse array of stimuli are directed by mitogen-activated protein kinase (MAPK) signal transduction pathway. Among MAPK superfamily, the p38MAPK pathway is responsible for stress signals from a variety of cells (Lawrence et al., 2008, Raman et al., 2007).
Over the past few decades, research has been focused on numerous dietary and botanical natural compounds, which have antioxidant properties that can reduce or prevent spinal cord from IRI. In this regard, resveratrol (3,4′,5-trihydroxystilbene), a polyphenol is found in various plants, including grapes, plums and peanuts. It is also present in wines, especially red wines and to a much lesser extent in white wines (Shigematsu et al., 2003, Ramadevi et al., 2016). In addition to anti-cancer, anti-inflammatory, cardioprotective properties, one of the biological activities of resveratrol has been attributed to involve its antioxidant potential (Fremont, 2000, Kalaiselvi et al., 2016, Neelamkavil and Thoppil, 2016, Valsan and Raphael, 2016). Resveratrol has been shown to inhibit membrane lipid peroxidation, scavenge free radicals by increasing antioxidant levels and inhibit platelet aggregation and protect several organs from I/R injury (de la Lastra and Villegas, 2007, Noorudheen and Chandrasekharan, 2016). Though several earlier studies focused on resveratrol as antioxidant inducer in different diseases, the role of resveratrol on the protection of spinal cord reperfusion injury remains unexplored.
Therefore, the aim of this study is to evaluate whether the administration of resveratrol can protect the spinal cord reperfusion injury in rats against high oxidative stress induced by ischemia. Further, we investigate the role of resveratrol in the inhibition of iNOS expression and p38MAPK signaling pathway.
2. Materials and methods
2.1. Animals
Male Sprague–Dawley rats of 10–11 weeks of age were obtained from the Shanghai SLAC Laboratory Animal Co. Ltd and weighed between 200 and 250 g. All rats were maintained in accordance with Institutional Animal care and use Committee guidelines. The rates were housed at 22 °C with a relative humidity of 40–60% and a 12 h light/dark cycle and provided ad libitum standard chow with free access of water. The ethical approval for the experiment was obtained from the Institutional Ethics Committee and the rats were divided into five groups (Table 1). The resveratrol doses were selected based on the previous studies (Sinha et al., 2002, Kiziltepe et al., 2004).
Table 1.
Rats were divided into five groups with seven rats in each group, as follows.
| Groups | Treatment |
|---|---|
| Group I (Con) | Control rats received 1 ml of vehicle (Saline) by intraperitoneal injection without surgery |
| Group II (Resv) | Rats received 1 ml of resveratrol (10 mg/kg body weight) by intraperitoneal injection without surgery |
| Group III (Sham) | Rats subjected to laparotomy and infrarenal abdominal aorta dissection without clamping of the aorta |
| Group IV (IRI) | Rats received 1 ml of vehicle (saline) by intraperitoneal injection following a 45-min spinal cord ischemia–reperfusion (IRI) |
| Group V (IRI + Resv) | Rats received 1 ml of resveratrol (10 mg/kg body weight) by intraperitoneal injection following a 45-min spinal cord ischemia–reperfusion |
2.2. Induction of spinal cord ischemia–reperfusion injury
Spinal cord ischemia was induced according to previously published article (Lafci et al., 2013, Lemmon et al., 2014). Rats were initially anesthetized with intraperitoneal injection of ketamine (50 mg/kg) and xylazine (5 mg/kg), followed by a half dose of ketamine if required during the procedure. The rat body temperature was monitored by a rectal probe inserted into the rectum and was adjusted between 37 and 38 °C using a heating pad. All animals were prepared in a sterile fashion. After rats had been stabilized, the retroperitoneum was opened and the abdominal aorta was reached through laparotomy with a standard midline incision. Vascular clamps were placed between the left renal arteries and proximal to the aortic bifurcation with a mini aneurysm clip. The animals were subjected to ischemia using cross clamping for 45 min. After completion of procedures, the wounds were closed with proline sutures. All animals were fed on a standard diet and water ad libitum after surgery. All animals were anesthetized and sacrificed after 48 h of spinal cord ischemia–reperfusion procedure. Blood samples were collected in tubes containing potassium oxalate and sodium fluoride. The 3, 4, 5 lumbar segments of the spinal cord were harvested immediately via posterior approach.
2.3. Determination of Nitrite/Nitrate (NOx) levels
The nitrite/nitrate (NOx) levels in plasma were estimated based on the Griess reaction. Briefly, the enzymatic conversion of nitrate to nitrite by nitrate reductase is measured at 540 nm by spectrophotometry.
2.4. Western blot analysis
Total protein was extracted from spinal cord tissues of control and treated groups using a lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% (v/v) NP-40, 1 mM PMSF with protease inhibitor cocktail. The protein concentrations of the supernatant were determined using BCA and stored at -80C. The equal amount proteins (50 μg/well) were separated in 10% SDS–PAGE, transferred to a nitrocellulose membrane. The membranes were incubated overnight at 4 °C with primary antibodies specific for iNOS, p-p38, total p38 and β-actin. The corresponding secondary IgG antibodies with alkaline phosphatase markers were used and incubated for 2 h at room temperature. The membranes were developed using ECL reagent. Protein signals were quantified by ChemiDoc and after being normalized to cognate b-actin signals.
2.5. Quantitative RT-PCR analysis
Total RNA was extracted from tissues using a Trizol kit. cDNA was synthesized from 2 μg of total RNA using Oligo(dT)12–18 primer and Superscript II reverse transcriptase. SYBR green master mix was used to measure iNOS and GAPDH mRNA expression with following primers: iNOS primers were 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and 5′-GGGTGTCAGAGTCTTGTGCCTTTGG-3′; GAPDH primers were 5′-TGGTGAAGGTCGGTGTGAAC-3′ and 5′-TTCCCATTCTCAGCCTTGAC-3′. The PCR reactions were carried with 20 μl mixture containing 100 ng of cDNA, 10 μl of SYBR green master mix and 1 μl of each specific primer. The PCR reactions were run on the Bio-Rad iCycler. The iNOS mRNA expression levels were normalized with endogenous control (GAPDH) and expressed as relative to control.
2.6. Estimation of malondialdehyde (MDA) and advanced oxidation products (AOPP) levels
MDA is the secondary product of lipid peroxidation, which was measured by the method of Ohkawa et al. (1979). Briefly, trichloroacetic acid (TCA) and thiobarbituric acid (TBA) were added to the tissue homogenates and their absorbance was measured at 532 nm. The AOPP were measured in tissue homogenates by addition of MgCl2 and phosphotungstate. AOPP were immediately measured in the supernatant and absorbance was read at 340 nm under acidic conditions. The level of MDA and AOPP was expressed as nM of MDA or AOPP reactants/100 g of wet tissue.
2.7. Estimation of reduced glutathione (GSH)
The levels of reduced glutathione were measured as described by Ellman (1959). The tissue homogenates were mixed with 5% TCA. The contents were mixed well and 1.0 ml of Ellman’s reagent and 0.3 M disodium hydrogen phosphate were added. The absorbance was read at 420 nm against a blank containing TCA. The amount of glutathione is expressed as mg/g of protein.
2.8. Estimation superoxide dismutase (SOD) activity
The activity of SOD was measured as described by Misra and Fridovich (1972). Briefly, ethanol and chloroform were added to tissue homogenates followed by adding 0.6 mM EDTA solution and 0.1 M carbonate-bicarbonate buffer. 1.8 mM epinephrine was added to initiate the reaction and absorbance was read at 480 nm. The enzyme activity is expressed as 50% inhibition of epinephrine in one minute/mg protein.
2.9. Estimation of catalase (CAT) activity
The CAT activity was measured as described by Takahara et al. (1960). Briefly, 50 mM phosphate buffer was added to the tissue homogenate and the reaction was started by the addition of 30 mM H2O2 solution. The absorbance was read at 240 nm. The enzyme activity is expressed as μmoles of H2O2 decomposed/min/mg protein.
2.10. Statistical analysis
Data were expressed as mean ± standard deviation (SD). Significant differences were assessed using Student’s t test and p value of less than 0.05 was considered as statistically significant.
3. Results
3.1. Effect of resveratrol on plasma nitrite/nitrate levels
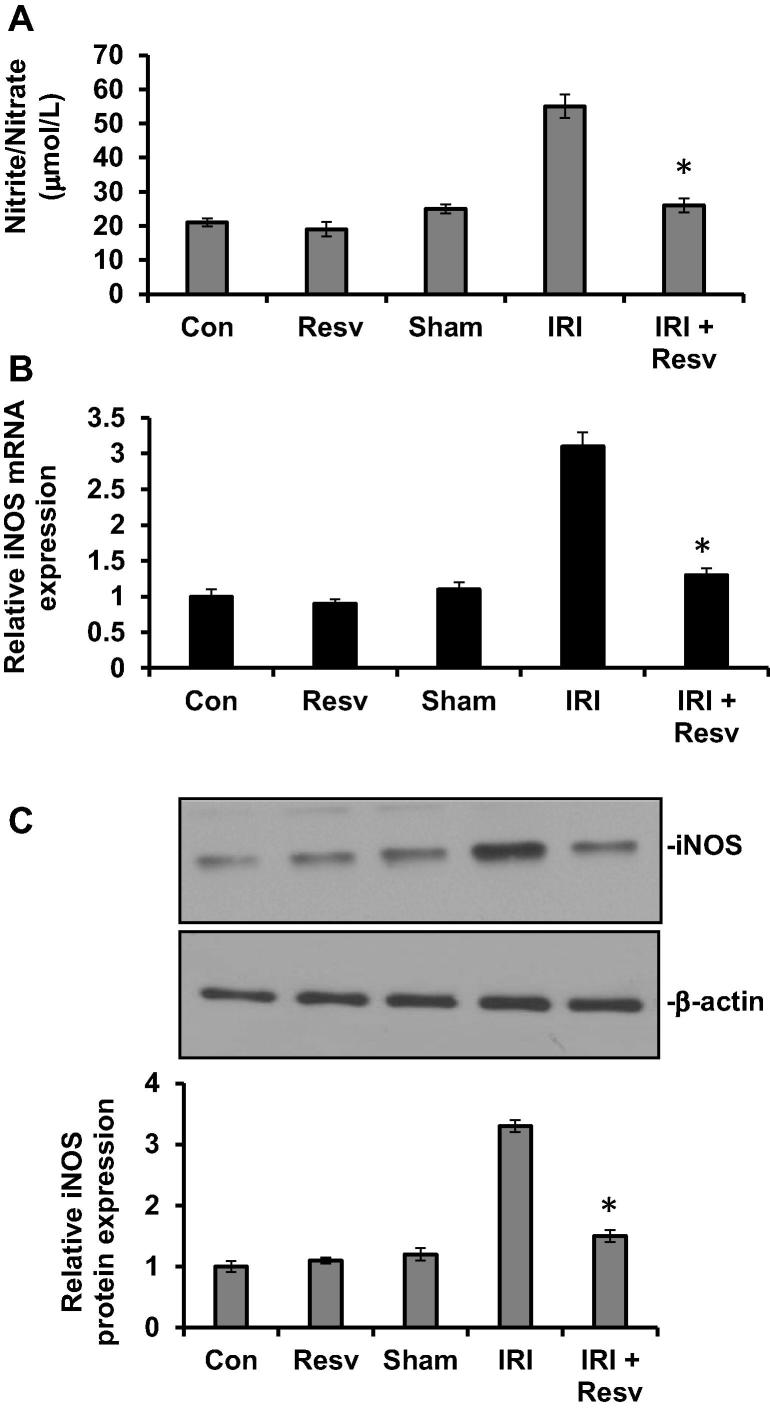
To investigate whether emic reperfusion injury (IRI)-induced nitrite/nitrate level is altered by resveratrol, we measured circulating nitrite/nitrate levels in plasma of experimental groups. The rats treated with resveratrol alone (Resv) and sham surgery (Sham) groups did not show change in plasma as compared to vehicle treated control group. However, the plasma nitrite/nitrate level was significantly increased in IRI group compared with control group. On the other hand, administration of resveratrol to IRI rats (IRI + RESV) resulted in a significant decrease in plasma nitrite/nitrate level (Fig.1A).
Figure 1.
Effect of resveratrol on plasma nitrite/nitrate and iNOS mRNA and protein expressions. (A) The plasma levels of nitrite/nitrate were measured in rats as described in materials and methods. (B) RNA was extracted from spinal cord tissue homogenates and iNOS mRNA levels measured using primers as indicated in materials and methods. The iNOS mRNA expression levels were normalized with endogenous control (GAPDH) and expressed as relative to control. (C) The iNOS protein levels were determined in spinal cord tissue homogenates by western blot as described in materials and methods. β-actin used as a loading control. Protein signals were quantified by ChemiDoc and normalized to β-actin signals, are presented as relative expression. Con: Control group; Resv: resveratrol control group; Sham: Sham group; IRI: ischemia–reperfusion injury group; IRI + Resv: resveratrol treated ischemia–reperfusion injury group. Results are expressed as mean ± SD (n = 7). ⁎p < 0.001 compared with IRI group.
3.2. Effect of resveratrol on iNOS mRNA and protein expressions
To further determine the effect of resveratrol on IRI-induced iNOS expressions, iNOS mRNA and protein expressions were measured by qRT-PCR and western blot, respectively. The iNOS mRNA (Fig.1B) and protein (Fig.1C) expressions were not altered in control, resveratrol alone and sham surgery groups. The levels of iNOS mRNA and protein expressions were significantly increased in IRI group, whereas it was reversed back to normal level, when IRI-induced rats were administrated with resveratrol (IRI + RESV), suggesting that resveratrol reduced the nitrite/nitrate production and iNOS expression.
3.3. Effect of resveratrol on p38MAPK pathway
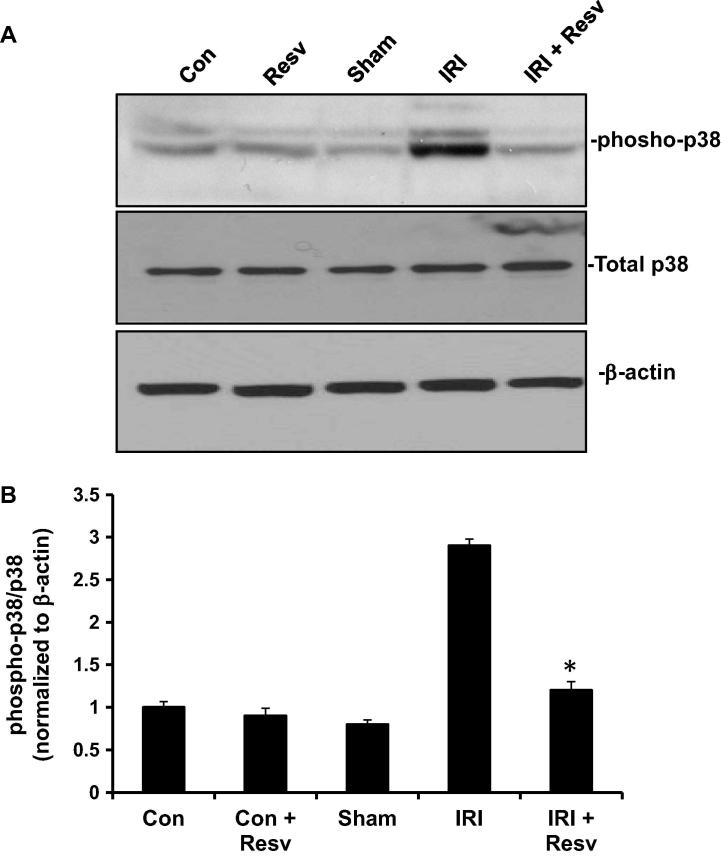
To examine whether IRI activates stress-induced p38 MAPK pathway and it can be altered by resveratrol, we measured the phosphorylation of p38 MAPK, as an indicator for activation of p38 MAPK pathway. As shown in Fig.2A and B, the phosphorylation of p38 MAPK did not alter in control, resveratrol and sham groups. However, p38 MAPK was markedly phosphorylated in IRI group. On the other hand, the IRI-induced rats treated with resveratrol (IRI + RESV) had a significantly decreased phosphorylation of p38 as compared to IRI group. The total p38 MAPK expression did not change in any of the groups.
Figure 2.
Effect of resveratrol on p38MPAK pathway. (A) The protein expressions of phospho-p38 MPAK, total MAPK and β-actin were determined in spinal cord tissue homogenates by western blot as described in materials and methods. β-actin used as a loading control. (B) Protein signals were quantified by ChemiDoc and normalized to β-actin signals, are presented as relative expression. Con: Control group; Resv: resveratrol control group; Sham: Sham group; IRI: ischemia–reperfusion injury group; IRI + Resv: resveratrol treated ischemia–reperfusion injury group. Results are expressed as mean ± SD (n = 7). ⁎p < 0.001 compared with IRI group.
3.4. Effect of resveratrol on lipid peroxidation (MDA) and oxidation (AOPP) products
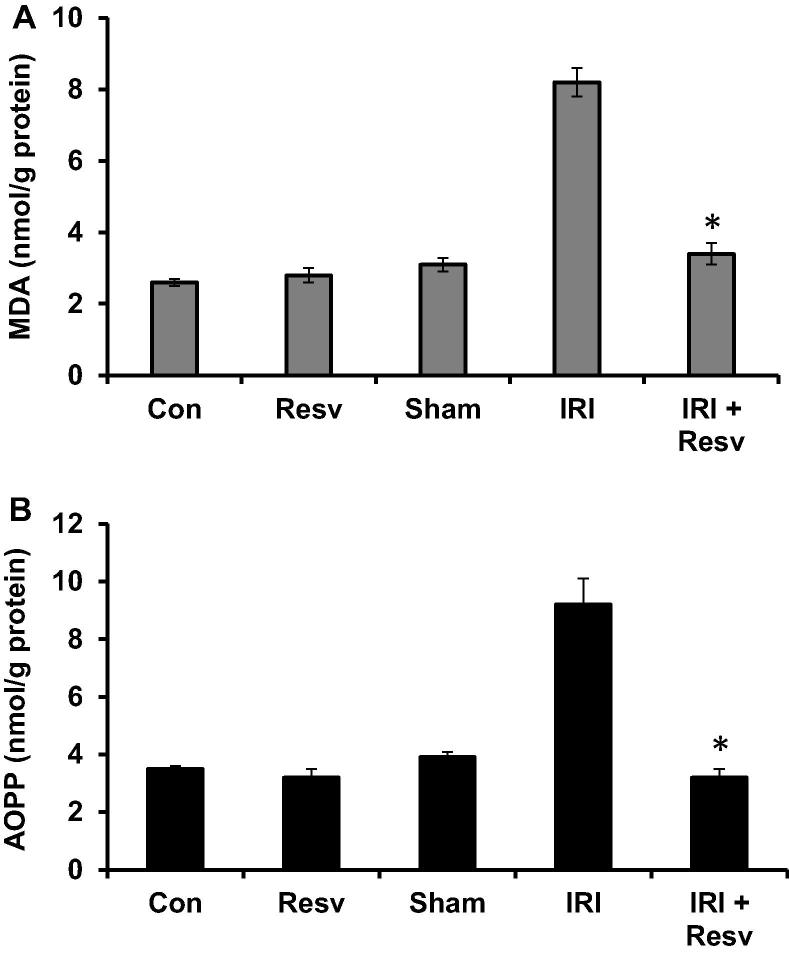
We further investigate the effect of resveratrol on IRI-produced reactive oxygen species, lipid peroxidation (MDA) and oxidation (AOPP) product levels were measured. The levels of MDA (Fig.3A) and AOPP (Fig.3B) in spinal cord homogenates were significantly increased in IRI group as compared with control, resveratrol alone and sham –surgery groups. While administration of resveratrol to IRI-induced rats (IRI + RESV) produced a significant reduction of MDA and AOPP levels compared to IRI group.
Figure 3.
Effect of resveratrol on lipid peroxidation (MDA) and oxidation (AOPP) products. (A) Malondialdehyde (MDA) and (B) advanced oxidation products (AOPP) were measured in spinal cord tissue homogenates and expressed as nmol/g protein. Con: Control group; Resv: resveratrol control group; Sham: Sham group; IRI: ischemia–reperfusion injury group; IRI + Resv: resveratrol treated ischemia–reperfusion injury group. Results are expressed as mean ± SD (n = 7). ⁎p < 0.001 compared with IRI group.
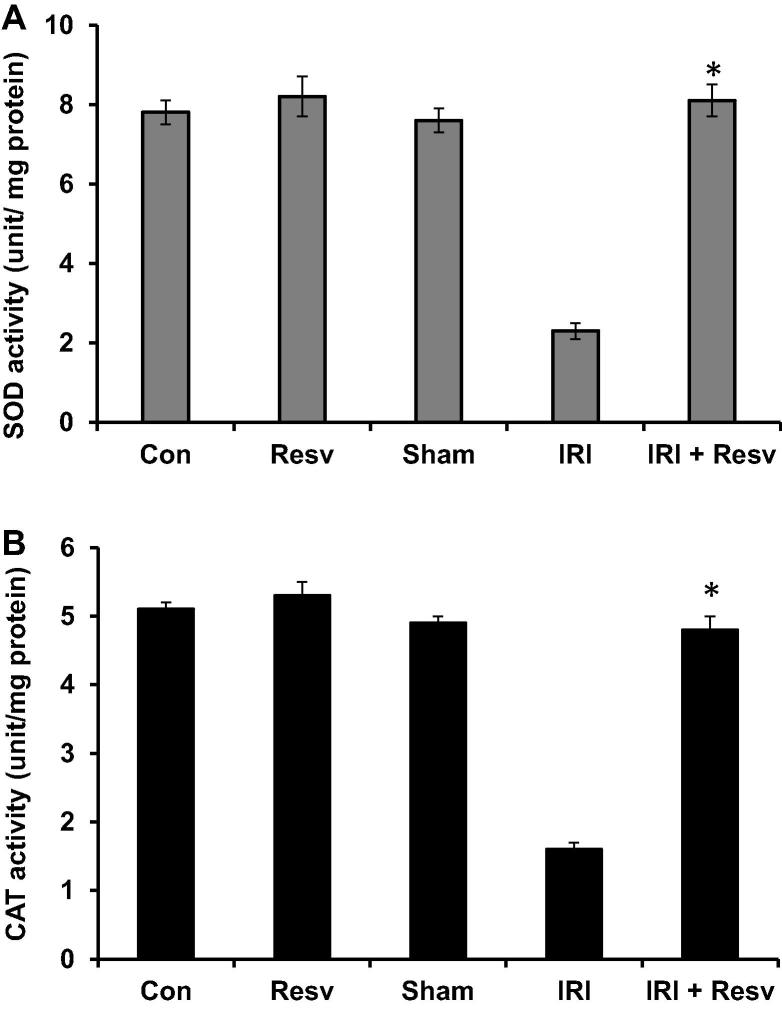
3.5. Effect of resveratrol on GSH, SOD and CAT antioxidant activities
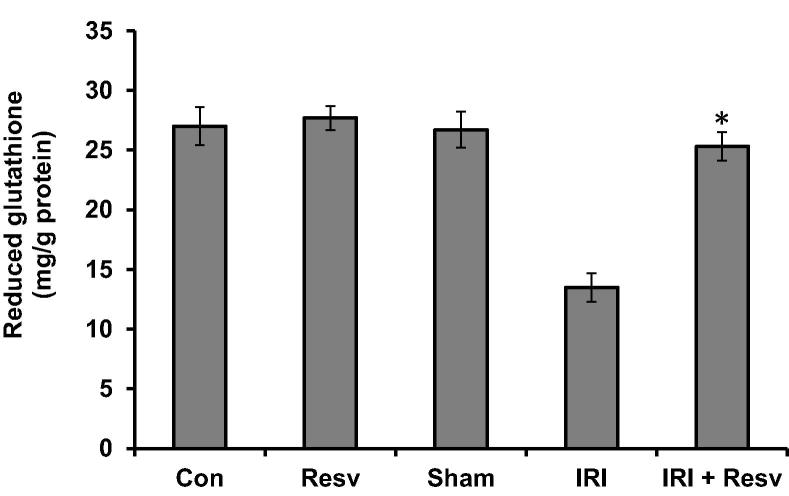
To examine the enzymatic and non-enzymatic antioxidant activities in experimental groups, we measured GSH (non-enzymatic), SOD and CAT (enzymatic) activities in spinal cord homogenates. The level of GSH (Fig. 4) and activities of SOD (Fig.5A) and CAT (Fig.5B) did not alter in control, resveratrol and sham-surgery groups. Induction of IRI in rats resulted in significant reduction of GSH, SOD and CAT activities as compared with control group. However, the IRI-induced rats treated with resveratrol significantly increased aforementioned enzymatic and non-enzymatic levels as compared to IRI group.
Figure 4.
Effect of resveratrol on the level of reduced glutathione (GSH). Reduced glutathione levels were measured in spinal cord tissue homogenates and expressed as mg/g protein. Con: Control group; Resv: resveratrol control group; Sham: Sham group; IRI: ischemia–reperfusion injury group; IRI + Resv: resveratrol treated ischemia–reperfusion injury group. Results are expressed as mean ± SD (n = 7). ⁎p < 0.001 compared with IRI group.
Figure 5.
Effect of resveratrol on the activities of SOD and CAT. SOD and CAT enzyme activities were measured in spinal cord tissue homogenates and expressed as unit/mg protein. Con: Control group; Resv: resveratrol control group; Sham: Sham group; IRI: ischemia–reperfusion injury group; IRI + Resv: resveratrol treated ischemia–reperfusion injury group. Results are expressed as mean ± SD (n = 7). ⁎p < 0.001 compared with IRI group.
4. Discussion
Over production of ROS and free radicals are the most important components to modulate the pathogenesis of neurologic dysfunction with IRI (Genestra, 2007). The energy-depleted cells by reoxygenation causes increased production of free radicals (Santhosh et al., 2016). The loss of membrane lipid functions by peroxidation results in cell death and production of cytokines and activation of neutrophils, which contribute to generation of free radicals (Ilhan et al., 1999, Kuroda and Siesjo, 1997). The other possible mechanism of neuronal death is excitotoxicity with release of glutamate and activation of N-methyl D-aspartate receptors that initiate excessive Ca 2_ influx and activate a chain of reactions that lead to neuronal death.
Surgical methods to the spinal cord on the thoracic and thoraco-abdominal aorta may cause temporary or permanent ischemia of the spinal cord resulting in a variety of extended neurophysiological alterations. Several strategies have been used to maintain spinal cord blood flow to increase spinal cord tolerance for ischemia, including distal aortic perfusion, intrathecal vasodilators, re-attachment of intercostal and lumbar vessels and decreasing cerebrospinal fluid pressure. These approaches also help to reduce reperfusion injury such as free radical scavengers and immune system modulation (Aslan et al., 2009, Mauney et al., 1995). Further, numerous studies reported that naturally occurring compounds such as a-tocopherol, quercetin (Song et al., 2013a, Song et al., 2013b) and syringic acid (Tokmak et al., 2015) has been shown to prevent IRI-injury in rats by increasing antioxidant status. Although resveratrol are known to have a variety of biological activities, including chemopreventive, membrane lipid peroxidation, and scavenge free radicals by increasing antioxidant levels, their neuroprotective roles have not been investigated.
In the present study, we demonstrated that the IRI-induced rats treated with 10 mg/kg resveratrol protected spinal cord from ischemia injury as supported by improved biological parameters measured in spinal cord tissue homogenates. The resveratrol treatment significantly decreased the levels of plasma nitrite/nitrate, iNOS mRNA and protein expressions and phosphorylation of p38MAPK in IRI-induced rats. Further, IRI-produced free radicals were reduced by resveratrol treatment by increasing enzymatic and non-enzymatic antioxidant levels.
Over production of NO can bind to superoxide anions, which results in generation of strong oxidant ONOO− and resulting in severe oxidative stress in injured spinal cord tissue. In addition, iNOS also plays an important role to produce secondary oxidative stress for the pathogenesis of neurologic dysfunction with IRI. In addition, several studies have been focused on the cross-talk between iNOS/p38MAPK signaling pathway (Conti et al., 2007, Maggio et al., 2012). For example, iNOS expression was upregulated by LPS-induced p38MAPK in astrocytes and macrophages and the iNOS expression was inhibited by SB203580, a specific p38MAPK inhibitor (Bhat et al., 1998). Therefore, we sought to examine if resveratrol can inhibit iNOS/p38MAPK pathway through suppression of oxidative stress following IRI. As consistent with previous studies, our data also showed that IRI-induced rats significantly increased nitrite/nitrate, iNOS mRNA and protein expressions. Further, the phosphorylation of p38MAPK is increased to activate p38MAPK in IRI-induced rats. Administration of resveratrol to IRI rats significantly decreases iNOS expressions and consecutively decreases phosphorylation of p38MAPK expression. These data suggest that IRI injury activates p38MAPK/iNOS signaling pathway and that can be inhibited by resveratrol treatment (Serasanambati and Chilakapati, 2016).
In the present study, MDA and AOPP levels, as a marker lipid peroxidation and protein oxidation, respectively, were significantly increased in the IRI group as compared to control group. In consistent with our data, other studies also have been reported that chronic constriction injury induced spinal cord insults in the sciatic nerve in rats. The over production of free radicals is well correlated with production of oxidative protein product. Moreover, the AOPP as mediator of oxidative stress and the levels of AOPPare also correlated with MDA and cytokines levels (Pekarkova et al., 2001). The enzymatic and non-enzymatic antioxidants such as glutathione, SOD and CAT are the primary defense against reactive oxygen species. These antioxidants have been shown to form an important adaptive response to peroxidative stress (Sgaravatti et al., 2009). In the current study, the levels of GSH, SOD and CAT significantly decreased in IRI rats, whereas administration of resveratrol increased these antioxidants levels, suggesting that the resveratrol treated rats were being protected from ROS.
5. Conclusion
In conclusion, administration of resveratrol protects the damage caused by spinal cord ischemia in rats. In addition, we confirmed from the present study that resveratrol scavenge free radicals by exerting its antioxidant effects and blocking iNOS/p38MAPK signaling pathway. However, further detail studies are warranted to determine whether resveratrol can be implemented as a neuroprotective agent for the treatment of spinal cord ischemia–reperfusion injury in human.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aslan A., Cemek M., Buyukokuroglu M.E., Altunbas K., Bas O., Yurumez Y., Cosar M. Dantrolene can reduce secondary damage after spinal cord injury. Eur. Spine. J. 2009;18(10):1442–1451. doi: 10.1007/s00586-009-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N.R., Zhang P., Lee J.C., Hogan E.L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci. 1998;18(5):1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambria R.P., Davison J.K., Zannetti S., L’Italien G., Brewster D.C., Gertler J.P., Moncure A.C., LaMuraglia G.M., Abbott W.M. Clinical experience with epidural cooling for spinal cord protection during thoracic and thoracoabdominal aneurysm repair. J. Vasc. Surg. 1997;25(2):234–241. doi: 10.1016/s0741-5214(97)70365-3. [DOI] [PubMed] [Google Scholar]
- Choi D.W. Glutamate neurotoxIRIty and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Conti A., Miscusi M., Cardali S., Germanò A., Suzuki H., Cuzzocrea S., Tomasello F. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res. Rev. 2007;54(1):205–218. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Dawson T.M., Dawson V.L., Snyder S.H. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann. Neurol. 1992;32(3):297–311. doi: 10.1002/ana.410320302. [DOI] [PubMed] [Google Scholar]
- de la Lastra C.A., Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem. Soc. Trans. 2007;35(5):1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66(8):663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19(9):1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Ilhan A., Koltuksuz U., Ozen S., Uz E., Ciralik H., Akyol O. The effects of caffeic acid phenethyl ester (CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Eur. J. Cardiothorac. Surg. 1999;16(4):458–463. doi: 10.1016/s1010-7940(99)00246-8. [DOI] [PubMed] [Google Scholar]
- Kalaiselvi V., Binu T.V., Radha S.R. Preliminary phytochemical analysis of the various leaf extracts of Mimusops elengi L. South Indian J. Biol. Sci. 2016;2(1):24–29. [Google Scholar]
- Kanellopoulos G.K., Kato H., Hsu C.Y., Kouchoukos N.T. Spinal cord ischemic injury. Development of a new model in the rat. Stroke. 1997;28(12):2532–2538. doi: 10.1161/01.str.28.12.2532. [DOI] [PubMed] [Google Scholar]
- Kiziltepe U., Turan N.N., Han U., Ulus A.T., Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia–reperfusion injury. J. Vasc. Surg. 2004;40(1):138–145. doi: 10.1016/j.jvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Kuroda S., Siesjo B.K. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin. Neurosci. 1997;4(4):199–212. [PubMed] [Google Scholar]
- Lafci G., Email H.S.G., Korkmaz K., Erdem H., Cicek O.F., Nacar O.A., Yildirim L., Kaya E., Ankarali H. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J. Cardiothorac. Surg. 2013;8:64. doi: 10.1186/1749-8090-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M.C., Jivan A., Shao C., Duan L., Goad D., Zaganjor E., Osborne J., McGlynn K., Stippec S., Earnest S., Chen W., Cobb M.H. The roles of MAPKs in disease. Cell Res. 2008;18(4):436–442. doi: 10.1038/cr.2008.37. [DOI] [PubMed] [Google Scholar]
- Lemmon V.P., Ferguson A.R., Popovich P.G., Xu X.M., Snow D.M., Igarashi M., Beattie C.E., Bixby J.L. MIASCI Consortium. Minimum information about a spinal cord injury experiment: a proposed reporting standard for spinal cord injury experiments. J. Neurotrauma. 2014;31(15):1354–1361. doi: 10.1089/neu.2014.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S.A. Neuronal protection and destruction by NO. Cell Death Differ. 1999;6(10):943–951. doi: 10.1038/sj.cdd.4400580. [DOI] [PubMed] [Google Scholar]
- Maggio D.M., Chatzipanteli K., Masters N., Patel S.P., Dietrich W.D., Pearse D.D. Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J. Neurotrauma. 2012;29(12):2244–2249. doi: 10.1089/neu.2012.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Iida Y., Wakamatsu H., Ohtake K., Nakakimura K., Xiong L., Sakabe T. The effects of N(G)-nitro-l-arginine-methyl ester on neurologic and histopathologic outcome after transient spinal cord ischemia in rabbits. Anesthesia Analgesia. 1999;89(3):696–702. doi: 10.1097/00000539-199909000-00031. [DOI] [PubMed] [Google Scholar]
- Mauney M.C., Blackbourne L.H., Langenburg S.E., Buchanan S.A., Kron I.L., Tribble C.G. Prevention of spinal cord injury after repair of the thoracic or thoracoabdominal aorta. Ann. Thorac. Surg. 1995;59(1):245–252. doi: 10.1016/0003-4975(94)00815-O. [DOI] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- Morsy M.D., Mostafa O.A., Hassan W.N. A potential protective effect of alpha-tocopherol on vascular complication in spinal cord reperfusion injury in rats. J. Biomed. Sci. 2010;17:55. doi: 10.1186/1423-0127-17-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A.K., Tandan S.K., Dudhgaonkar S.P., Jadhav S.H., Kataria M., Prakash V.R., Kumar D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-l-cysteine in rats. Eur. J. Pain. 2006;10(7):573–579. doi: 10.1016/j.ejpain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Indian J. Biol. Sci. 2016;2(1):41–45. [Google Scholar]
- Noorudheen N., Chandrasekharan D.K. Effect of ethanolic extract of Phyllanthus emblica on captan induced oxidative stress in vivo. South Indian J. Biol. Sci. 2016;2(1):95–102. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarkova I., Parara S., Holecek V., Stopka P., Trefil L., Racek J., Rokyta R. Does exogenous melatonin influence the free radicals metabolism and pain sensation in rat? Physiol. Res. 2001;50(6):595–602. [PubMed] [Google Scholar]
- Ramadevi S., Kaleeswaran B., Natarajan P. Phytochemicals analysis and antimicrobial activity of Ruellia patula L. against pathogenic microorganisms. South Indian J. Biol. Sci. 2016;2(2):306–313. [Google Scholar]
- Raman M., Chen W., Cobb M.H. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Santhosh S.K., Venugopal A., Radhakrishnan M.C. Study on the phytochemical, antibacterial and antioxidant activities of Simarouba glauca. South Indian J. Biol. Sci. 2016;2(1):119–124. [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of nuclear factor kappa B (NF-kB) in human diseases – a review. South Indian J. Biol. Sci. 2016;2(4):368–387. [Google Scholar]
- Sgaravatti A.M., Magnusson A.S., Oliveira A.S., Mescka C.P., Zanin F., Sgarbi M.B., Pederzolli C.D., Wyse A.T., Wannmacher C.M., Wajner M., Dutra-Filho C.S. Effects of 1,4-butanediol administration on oxidative stress in rat brain: study of the neurotoxIRIty of gamma-hydroxybutyric acid in vivo. Metab. Brain Dis. 2009;24(2):271–282. doi: 10.1007/s11011-009-9136-7. [DOI] [PubMed] [Google Scholar]
- Shigematsu S., Shida S., Hara M., Takahashi N., Yoshimatsu H., Sakata T., Korthuis R.J. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic. Biol. Med. 2003;34(7):810–817. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- Sinha K., Chaudhary G., Gupta Y.K. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71(6):655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- Song Y., Liu J., Zhang F., Zhang J., Shi T., Zeng Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci. 2013;92(24–26):1215–1221. doi: 10.1016/j.lfs.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Song Y., Zeng Z., Jin C., Zhang J., Ding B., Zhang F. Protective effect of ginkgolide B against acute spinal cord injury in rats and its correlation with the Jak/STAT signaling pathway. Neurochem. Res. 2013;38(3):610–619. doi: 10.1007/s11064-012-0959-y. [DOI] [PubMed] [Google Scholar]
- Takahara S., Hamilton H.B., Neel J.v., Kobara T.Y., Ogura Y., Nishimura E.T. Hypocatalasemia: a new genetic carrier state. J. Clin. Invest. 1960;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokmak M., Yuksel Y., Sehitoglu M.H., Guven M., Akman T., Aras A.B., Cosar M., Abbed K.M. The neuroprotective effect of syringic acid on spinal cord ischemia/reperfusion injury in rats. Inflammation. 2015;38(5):1969–1978. doi: 10.1007/s10753-015-0177-2. [DOI] [PubMed] [Google Scholar]
- Valsan A., Raphael K.R. Pharmacognostic profile of Averrhoa bilimbi Linn. Leaves. South Indian J. Biol. Sci. 2016;2(1):75–80. [Google Scholar]
- Wang J.Y., Shen J., Gao Q., Ye Z.G., Yang S.Y., Liang H.W., Bruce I.C., Luo B.Y., Xia Q. Ischemic post conditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39(3):983–990. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- Xing B., Chen H., Zhang M., Zhao D., Jiang R., Liu X., Zhang S. Ischemic post-conditioning protects brain and reduces inflammation in a rat model of focal cerebral ischemia/reperfusion. J. Neurochem. 2008;105(5):1737–1745. doi: 10.1111/j.1471-4159.2008.05276.x. [DOI] [PubMed] [Google Scholar]