Abstract
This study aims to present an integrated process that can be used to produce biomedical and biological active components from the fruit shell of Camellia oleifera Abel. Through the Foss method, Aldehyde, acid compounds, acyl and alcohol compounds account for 22.7, 15.93, 0.24 and 61.13% of the extractives which were extracted from Camellia oleifera fruit shell by methanol solvents. Furfural, Pyrazole-4-carboxaldehyde, 1-methyl- and 5-Hydroxymethylfurfural account for 4.74, 1.22 and 58.78% of the extractives which were extracted from the fruit shell of Camellia oleifera Abel by ethanol solvents. Aldehyde, acid and amine compounds account for 5.01, 56.18 and 7.20% of the extractives which were extracted from the fruit shell of Camellia oleifera Abel by ethyl acetate solvents. The extractives of fresh flesh of bayberry were rich in rare drug, biomedical and biological activities.
Keywords: Camellia oleifera, Extractives, Biomolecules, GC–MS
1. Introduction
Camellia oleifera is from the family of Thecae and widely planted in southeast China (Zhu et al., 2013, Zhang et al., 2011). China has affluent resources of Camellia oleifera with an annual output of more than 560 million tons (Jin, 2012). People call its seed oil as tea oil which contains abundant unsaturated fatty acids such as oleic acid and linoleic acid (Ong et al., 2017). Compared with olive oil, it is a kind of high quality edible oil, which is better than olive oil to some extent (Sahari et al., 2004, Zhang et al., 2008). The fruit shell of Camellia oleifera is an important byproduct in woody edible oil production. Approximately 0.54 tons of the shell is produced from 1 ton of Camellia oleifera nuts (Jin, 2012). The main components of the fruit shell of Camellia oleifera are cellulose, hemicellulose, and lignin. The fruit shell of Camellia oleifera can be used to produce saponin, tannin, xylose, xylitol, furfural, potassic salt, and medium (Wang et al., 2008, Chen et al., 2010) as well as activated carbon, efficient deodorant, protein feed and culture medium. Therefore, the deep processing of oil tea shell can significantly improve the additional value of the fruit shell of Camellia oleifera.
Some researches indicate that the extractives of the fruit shell of Camellia oleifera could scavenge free radical, inhibit lipid peroxidation, decrease serum glucose level of alloxan-induced diabetic mice and inhibit the growth of transplanted ascites tumor (Zhu et al., 2007a, Zhu et al., 2007b). Recent studies indicate that the extractives of the fruit shell of Camellia oleifera have DPPH radical-scavenging ability (Zhang et al., 2010, Gao et al., 2017, Daya and Pant, 2017). In addition, Shen found that the extractives of the fruit shell of Camellia oleifera could reduce body weight of high-fat diet induced mice (Shen et al., 2008). However, the fruit shell of Camellia oleifera is usually regarded as agricultural waste and people often discard or burn it instead of effectively using it because of its undeveloped economic benefit (Zhu et al., 2013, Zhang et al., 2011). Moreover, the degradation of the fruit shell of Camellia oleifera may cause odor gas pollution and liquid and solid pollution. Therefore, the development of comprehensive and high value-added use of the fruit shell of Camellia oleifera could promote the rapid and sustainable development of the economy and improve its efficiency. In this study, we use a variety of extractants to extract the functional components of the fruit shell of Camellia oleifera, and the extractives are analyzed by GC–MS to further utilize Camellia oleifera resources.
2. Materials and methods
2.1. Materials
The fruit shell of Camellia oleifera was collected from the Key Laboratory of Forestry Biotechnology, Hunan Province, China and was powdered and kept at −3 °C in vacuum. Methanol, ethanol and ethyl acetate were purchased from Hunan Huihong Reagent CO., Ltd, Hunnan Province, China, which were prepared for the subsequent experiments and were all chromatographic grade. Cotton bag and cotton thread were extracted in methanol, ethanol and ethyl acetate solutions for 12 h, respectively. The rotary evaporator was purchased from Gongyi Yuhua Instrument CO., Ltd, Henan Province, China. The anhydrous sodium sulfate was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd, Tianjin, China. GC–MS was purchased from Agilent Technologies, Inc., America.
2.2. Experiment methods
Extraction: Weighed three copies of the fruit shell of Camellia oleifera, each about 40 g (0.1 mg accuracy), and then parceled each into a cotton bag and tied by cotton thread, and marked. Extraction was carried out in 300 ml methanol, ethanol and ethyl acetate solvents by the Foss method for 6 h at a temperature of 60, 70 and 70 °C, respectively. After extraction, the methanol, ethanol and ethyl acetate were removed by the rotary evaporator, respectively. After dried with anhydrous sodium sulfate, the resulting extractives were stored at -3 °C. We named the three kinds of extractives as K1, K2 and K3 samples which were extracted by methanol, ethanol and ethyl acetate, respectively.
GC/MS determination: GC condition: quartz capillary column was 30 mm × 0.25 mm × 0.25 um; the temperature of column was 60 °C; program warming was 6 °C/min; the temperature of the inlet was 250 °C; column flow was 1.0 ml/min; pre-column pressure was 80 kPa; split ratio was 40:1; and carrier gas was high helium.
MS condition: ionization mode was EI; the electron energy was 70 Ev; the temperature of transmission line was 250 °C; the temperature of ion source was 230–250 °C; the temperature of quadrupole was 150–200 °C; quality range was 30–500 M/Z; the wiley7 n.1 standard spectrum and computer were used for qualitative search.
3. Results
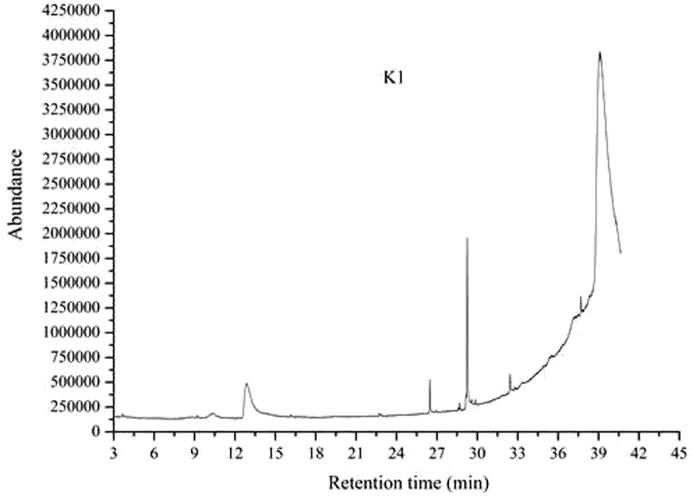
Fig. 1 shows the total ion chromatograms of the three kinds of extractives which are analyzed by GC–MS.
Fig. 1.
Total ion chromatograms of the fruit shell of Camellia oleifera which were extracted by methanol, ethanol and ethyl acetate, respectively.
The spectrum of each peak is retrieved by using the computer and wiley7 n.1 standard spectrum, according to the laws of the mass spectrum cracking to checking, and peak area normalization method is used to calculate the content of each component. The specific results are shown in Table 1, Table 2, Table 3.
Table 1.
GC–MS Analysis of K1 sample.
| No. | Retention time (min) | Peak area (%) | Component |
|---|---|---|---|
| 1 | 12.87 | 22.07 | 5-Hydroxymethylfurfural |
| 2 | 26.471 | 2.27 | n-Hexadecanoic acid |
| 3 | 28.691 | 0.42 | 9,12-Octadecadienoyl chloride (Z,Z)- |
| 4 | 29.158 | 0.95 | 9,12-Octadecadienoic acid (Z,Z)- |
| 5 | 29.25 | 12.71 | cis-Vaccenic acid |
| 6 | 32.435 | 1.50 | 1-Heptatriacotanol |
| 7 | 37.692 | 1.47 | .gamma.-Sitosterol |
| 8 | 39.088 | 43.32 | .gamma.-Sitosterol |
| 9 | 39.131 | 1.46 | .gamma.-Sitosterol |
| 10 | 39.156 | 13.84 | .gamma.-Sitosterol |
Table 2.
GC–MS Analysis of K2 sample.
| No. | Retention time (min) | Peak area (%) | Component |
|---|---|---|---|
| 1 | 3.696 | 4.74 | Furfural |
| 2 | 5.172 | 0.65 | – |
| 3 | 6.143 | 1.22 | Pyrazole-4-carboxaldehyde, 1-methyl- |
| 4 | 12.446 | 58.78 | 5-Hydroxymethylfurfural |
| 5 | 28.697 | 0.46 | – |
| 6 | 29.225 | 1.16 | – |
| 7 | 38.116 | 21.47 | – |
| 8 | 38.621 | 5.44 | – |
| 9 | 39.961 | 1.54 | – |
| 10 | 40.527 | 4.54 | – |
Table 3.
GC–MS analysis of K3 sample.
| No. | Retention time (min) | Peak area (%) | Component |
|---|---|---|---|
| 1 | 22.708 | 2.68 | Coniferyl aldehyde |
| 2 | 26.489 | 10.98 | n-Hexadecanoic acid |
| 3 | 26.901 | 2.33 | 3,5-Dimethoxy-4-hydroxycinnamaldehyde |
| 4 | 29.275 | 45.20 | cis-Vaccenic acid |
| 5 | 29.877 | 1.38 | 9-Octadecenamide, (Z)- |
| 6 | 32.435 | 5.82 | 9-Octadecenamide, (Z)- |
| 7 | 34.907 | 2.68 | – |
| 8 | 37.016 | 9.32 | – |
| 9 | 37.692 | 7.07 | – |
| 10 | 39.439 | 12.54 | – |
4. Discussion
Table 1 shows that K1 samples are identified four categories of components totally, including one subcategory of aldehyde compounds, three subcategories of acid compounds, one subcategory of acyl compounds and two subcategories of alcohol compounds which account for 22.7, 15.93, 0.24 and 61.13% of the K1 samples. Obviously, the representative compound is γ-Sitosterol which accounts for 59.63%.
As can be seen from Table 2, the K2 samples are identified one category of components totally, including furfural, Pyrazole-4-carboxaldehyde, 1-methyl- and 5-Hydroxymethylfurfural which account for 4.74, 1.22 and 58.78% of the K2 samples. And the representative compound is 5-Hydroxymethylfurfural.
According to Table 3, K3 samples are identified three categories of components totally, including two subcategories of aldehyde compounds, one subcategory of acid compounds and one subcategory of amine compounds which account for 5.01, 56.18 and 7.20% of the K3 samples (Sharma and Yadav, 2017, Franco et al., 2017; Kumuruzzaman and Sarker, 2017). And cis-Vaccenic acid is the representative compound which accounts for 45.20%.
Consequently, three different extraction methods show that γ-Sitosterol, 5-Hydroxymethylfurfural and cis-Vaccenic acid are representative compounds in the fruit shell of Camellia oleifera, and there are most of the species of acid compounds in the fruit shell of Camellia oleifera. Especially, γ-Sitosterol is pure natural, non-toxic and has no side effects thus suitable for pharmaceutical, food and cosmetics additives. 5-Hydroxymethylfurfural and furfural with good application value, could be used as raw materials for organic synthesis and be used in synthetic resins, varnishes, pesticides, coatings and other fields (Rosatella et al., 2011, Chheda et al., 2007). Some acid compounds of the fruit shell of Camellia oleifera such as cis-Vaccenic acid could be used to produce a variety of chlorate, and n-Hexadecanoic has Anti-cancer and anti-tumor functions (Zhang et al., 2010, Shen et al., 2008). 9-Octadecenamide, (Z)- are often used as chemical additives and modifier (Yoshida et al., 1994, Wang et al., 2008, Chen et al., 2010).
In addition, the methanol could extract γ-Sitosterol easier from the fruit shell of Camellia oleifera; the ethanol could extract 5-Hydroxymethylfurfural easier from the fruit shell of Camellia oleifera; the ethyl acetate could extract cis-Vaccenic acid easier from the fruit shell of Camellia oleifera. Thus, there are many potential values of the extraction of the fruit shell of Camellia oleifera and extraction methods are also critical.
5. Conclusion
In this study, the fruit shell of Camellia oleifera is extracted by methanol, ethanol and ethyl acetate and there are twelve kinds of compounds. The methanol could extract γ-Sitosterol easier from the fruit shell of Camellia oleifera, aldehyde, acid compounds, acyl and alcohol compounds accounting for 22.7, 15.93, 0.24 and 61.13% of the extractives. The ethanol could extract 5-Hydroxymethylfurfural easier from the fruit shell of Camellia oleifera, furfural, Pyrazole-4-carboxaldehyde, 1-methyl- and 5-Hydroxymethylfurfural accounting for 4.74, 1.22 and 58.78% of the extractives. The ethyl acetate could extract cis-Vaccenic acid easier from the fruit shell of Camellia oleifera, Aldehyde, acid and amine compounds accounting for 5.01, 56.18 and 7.20% of the extractives. According to the relative contents, the extractives of the fruit shell of Camellia oleifera are rich in rare drug and biomedical activities.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yizhang Xie, Email: 2908889550@QQ.com.
Wanxi Peng, Email: pengwanxi@163.com.
References
- Chen J.H., Wu H.Y., Liau B.C., Chang C.M.J., Jong T.T., Wu L.C. Identification and evaluation of antioxidants defatted camellia oleifera, seeds by isopropanol salting-out pretreatment. Food Chem. 2010;121(4):1246–1254. [Google Scholar]
- Chheda J.N., Román-Leshkov Y., Dumesic J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono-and poly-saccharides. Green Chem. 2007;9(4):342–350. [Google Scholar]
- Daya B., Pant K. Biomonitoring of Wetland Using Macrophytes and Macroinvertebrates. Malay. J. Sustain. Agri. 2017;1(1):11–14. [Google Scholar]
- Franco H., Quinchuela T.G., Macancela N.A., Mero P.C. Participative analysis of socio-ecological dynamics and interactions. A case study of the manglaralto coastal aquifer, santa elena-ecuador. Malaysian. J. Sustain. Agri. 2017;1(1):19–22. [Google Scholar]
- Gao W., Wang Y., Wang W., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharma. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X. Bioactivities of water-soluble polysaccharides from fruit shell of Camellia oleifera Abel: Antitumor and antioxidant activities. Carbohydr. Polym. 2012;87(3):2198–2201. [Google Scholar]
- Ong S.Q., Lee B.B., Tan G.P., Maniam S. Capacity of black soldier fly and house fly larvae in treating the wasted rice in Malaysia. Malay. J. Sustain. Agri. 2017;1(1):08–10. [Google Scholar]
- Rosatella A.A., Simeonov S.P., Frade R.F.M., Afonso C.A.M. Cheminform abstract: 5-hydroxymethylfurfural (hmf) as a building block platform: biological properties, synthesis and synthetic applications. Cheminform. 2011;13(4):754–793. [Google Scholar]
- Sahari M.A., Ataii D., Hamedi M. Characteristics of tea seed oil in comparison with sunflower and olive oils and its effect as a natural antioxidant. J. Am. Oil Chem. Soc. 2004;81(6):585–588. [Google Scholar]
- Sharma D., Yadav K.D. Vermicomposting of flower waste: optimization of maturity parameter by response surface methodology. Malay. J. Sustain. Agri. 2017;1(1):15–18. [Google Scholar]
- Shen, J.F., Chen, Q.P., Wu, X.Q., Jiang, T.J., Wang, X.Q., Kang, H.Q., Inventors; Zhejiang Univ., Assignee. 2008 Dec. 19. Use and Preparation Method of Fruit Hull of Camellia Oleifera Abel Extract. CN101744948A.
- Wang X.N., Chen Y.Z., Li-Qi W.U., Liu R.K., Yang X.H., Wang R. Oil content and fatty acid composition of camellia oleifera seed. J. Central South Univ. Forest. Technol. 2008;71(3):161. [Google Scholar]
- Yoshida T., Nakazawa T., Hatano T., Yang R.C., Yang L.L., Yen K.Y. A dimeric hydrolysable tannin from camellia oleifera. Phytochemistry. 1994;37(1):241–244. doi: 10.1016/0031-9422(94)85033-x. [DOI] [PubMed] [Google Scholar]
- Zhang, D.L., Stack, L., Chen, Y.Z., Zhang, R.Q., Yu, J.F., Xie, B.X., Ruter, J.M., 2008. Teaoil camellia—Eastern “olive” for the world. In: Proceedings of the International Symposium on Asian Plants with Unique Horticultural Potential, vol. 769, pp. 43–48.
- Zhang L.L., Wang Y.M., Wu D.M., Xu M., Chen J.H. Comparisons of antioxidant activity and total phenolics of Camellia oleifera Abel fruit hull from different regions of China. J. Med. Plants Res. 2010;4(14):1420–1426. [Google Scholar]
- Zhang Y., Chen Q., Luo X., Dai T., Lu B., Shen J. Mutagenicity and safety evaluation of the water extract of Camellia oleifera Abel. J. Food Sci. 2011;76(3) doi: 10.1111/j.1750-3841.2011.02101.x. [DOI] [PubMed] [Google Scholar]
- Zhu B.F., Peng L., Luo L.F., Liu J.H., He Q.D. The analysis of exploitive value and edible secuirty in fleshy fruit and fleshy leaf of Camellia oleifera Abel. J. Food Sci. Biotechnol. 2007;26(2):1–6. [Google Scholar]
- Zhu B.F., Peng L., Luo L.F. Study on health care functions of extract from fleshy fruit and fleshy leaf of Camellia oleifera Abel. J. Food Sci. Biotechnol. 2007;26(1):46–50. [Google Scholar]
- Zhu J., Zhu Y., Jiang F., Xu Y., Ouyang J., Yu S. An integrated process to produce ethanol, vanillin, and xylooligosaccharides from Camellia oleifera shell. Carbohy. Res. 2013;382:52–57. doi: 10.1016/j.carres.2013.10.007. [DOI] [PubMed] [Google Scholar]