Abstract
Drought, one of the environmental stresses, plays crucial role in reduction in plant production on majority of agricultural fields of world, In order to evaluate drought stress on RNA content Relative water content (RWC), and chlorophyll content, Water deficit was induced by Polyethylene glycol (PEG) in peanut (Arachis hypogaea), accession number ICGV 91114. In this current study we evaluate RNA content and Relative water content (RWC) both in leaves and roots and chlorophyll content in leaf. The present study was undertaken with the aim to investigate the effect of water deficit imposed by PEG-6000, 40 old day seedlings were treated with varying concentrations of polyethylene glycol-6000 (PEG-6000; w/v-5%, 10%, 15% & 20%) for 24 h. The results showed that RNA content and Relative water content (RWC) content was significantly reduced in both leaves and roots with increased concentration of PEG, In leaves, a concentration dependent decline in chlorophyll content with increasing concentration of polyethylene glycol-6000 (PEG-6000). Reduction in chlorophyll ‘a’ level was to a greater extent than the chlorophyll ‘b’. Thus, this attributes can be used as screening tool for drought tolerance in peanut.
Keywords: Relative water content, RNA content chlorophyll content, PEG-6000, Peanut
1. Introduction
Due to unfavorable environmental conditions plants are subjected to various abiotic and biotic stresses affecting their growth, metabolism and yield (Kaur and Gupta, 2005). Drought is one of major abiotic stresses constraining crop productivity worldwide, it reduces plant productivity by inhibiting growth and (Singh et al., 2014) slows growth, induces stomatal closure, and therefore reduces photosynthesis (Németh et al., 2002). Extensive field studies have been conducted for understanding the plant tolerance and oxidative stress in response to water deficit. The stress caused due to water creates senescence and abscission in the plants (Karamanos, 1978). The effect of water stress in the leaf of the plant mainly reduces the bulk production of the biomass (Rawson and Turner, 1982, Saxena, 1993). The relative amount of Chlorophyll is directly connected to the photosynthetic capacity of the major plants (Fotovat et al., 2007). Besides chlorophyll content, drought stress play a major role in affecting the enzymes involved in the Calvincycle (Monakhova and Chernyadev, 2002). It is reported that the production of plants also affected by showing to reactive oxygen species (Horling et al., 2003). Polyethylene glycol(PEG-6000) generates osmotic stress which reduces photosynthetic rate, which later effects chlorophyll-a and chlorophyll-b contents, any stress to the plant effects mechanism of photosynthesis at cellular level which includes pigments, photosystems, the electron transport system and co2 reduction pathways and reduce photosynthesis.
PEG is mainly used for the determination of the drought stress related information’s from the plants (Turkan et al., 2005, Landjeva et al., 2008). It is known that PEG does not enter the cell wall space (Rubinstein, 1982) and PEG molecules with a molecular weight greater than 3000 are apparently not absorbed (Tarkow et al., 1996). In the present study, PEG-6000 was used for drought. Simulation of drought stress by polyethylene glycol (PEG) induces drought stress on the plants (Jiang et al., 1995). It is reported that PEG induced significant water stress in plants and not having any toxic effects (Emmerich and Hardegree, 1990). The objective of this research was to determine, relative water content(RWC) and RNA content in leaves and root, chlorophyll content in leaves of peanut (Arachis hypogaea)ICGV 91114 under drought stress.
2. Materials and methods
2.1. Sample collection
For carrying out the above three experiments, ICGV 91114 were obtained from the International Crops Research Institute for the Semi–Arid Tropics (ICRISAT), Patancheru, Hyderabad, Telangana, India. Four seeds per pot in the suitably sized pots filled with mixture of soil were sown to raise the seedlings plants, the seedlings/plants were maintained in the net house covered with water proof sheets during the experiment. They were watered regularly thrice a week for 40 days until water stress was carried out at the flowering stage.
2.2. Drought treatment
40 old days seedlings were plucked from the pots and treated with different concentrations of PEG 6000 (Polyethylene Glycol) 100 ml of 5%, 10%, 15% and 20% along with controls (100 ml of water) for 24 h, later the leaf and root were harvested separately, Leaf Samples were named as 1(control) 2 (5% PEG treated) 3(10% PEG treated) 4 (15% PEG treated) & 5 (20% PEG treated), Root Samples were named as A (control) B (5% PEG treated) C (10% PEG treated) D (15% PEG treated) E (20% PEG treated). After naming, leaf and root were kept frozen under liquid N2, and stored at −80 °C, until later experiment to measure chlorophyll, relative water content and RNA content.
2.3. RNA isolation and quantification
RNA was isolated from leaves and root by Trizol reagent. The Trizol reagent was developed by Chomczynski and Sacchi (1987) is a mixture of phenol and guanidine isothiocyanate. The freshly cultivated plants were used for the extraction of the total RNA. The standard extraction methodology were followed for the the extraction of the total RNA. Total RNA was quantified using micro-spectrophotometry (NanoDrop Technologies, Inc.). DNA was removed with Turbo DNA-free (Ambion, Inc.) using the rigorous protocol. RNA integrity was measured using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.).
2.4. Relative water content
To determine relative water content, 20 leaves from each group were weighed immediately (FW) after harvesting the plant. Leaves were then placed in distilled water for 4 h and then turgid weight (TW) was measured. Then the leaves were dried in oven at 80 °C for 24 h to obtain their dry weight (DW). The method proposed by Sharp et al. (1990) Relative water content was calculated by the following formula.
2.5. Chlorophyll estimation
Total Chlorophyll content, content of Chlorophyll ‘a’ and Chlorophyll ‘b’ were extracted and quantified the modified method of Arnon (1949). After the extraction and analysis, the relative amount of Chlorophyll ‘a’, Chlorophyll ‘b’ and the total content of Chlorophyll were calculated using the following formulae:
where
A = Absorbance at specific wavelengths
V = final volume of chlorophyll extract in 80% Acetone
W = fresh weight of tissue extracted 12.7, 2.69, 22.9, 4.68, 20.2 & 8.02 are the constants
In the present experiment the volume (V) of 10 ml 80% Acetone and weight (W) of 500 mg fresh leaf tissue was used in all the 5 water stressed samples and also the control (Arnon, 1949).
3. Results and discussion
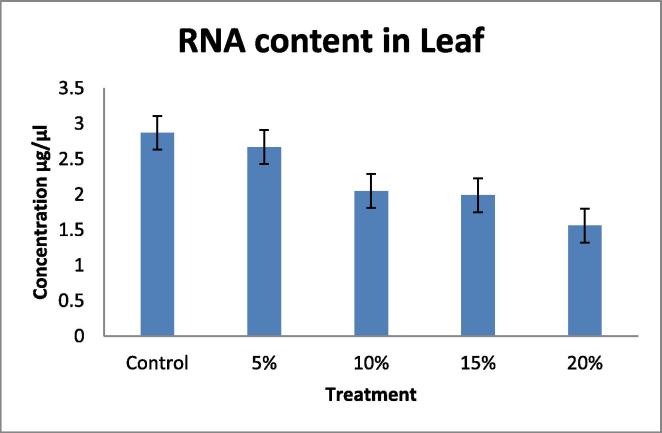
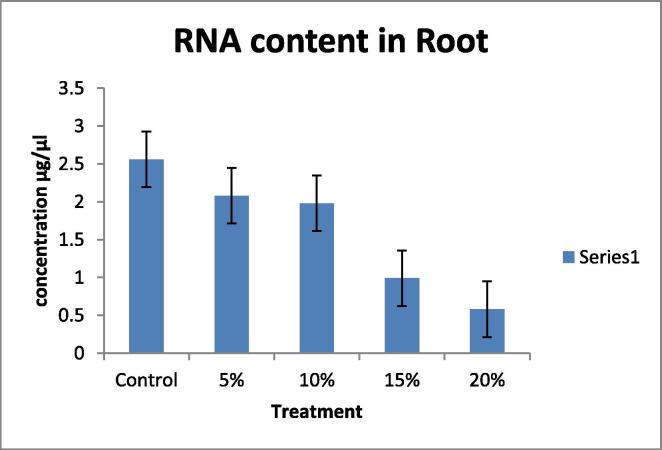
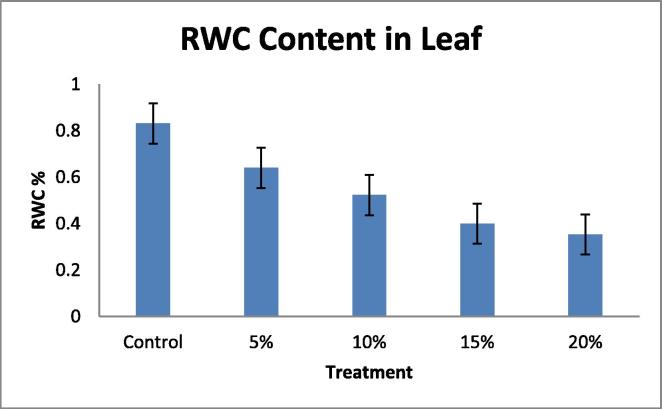
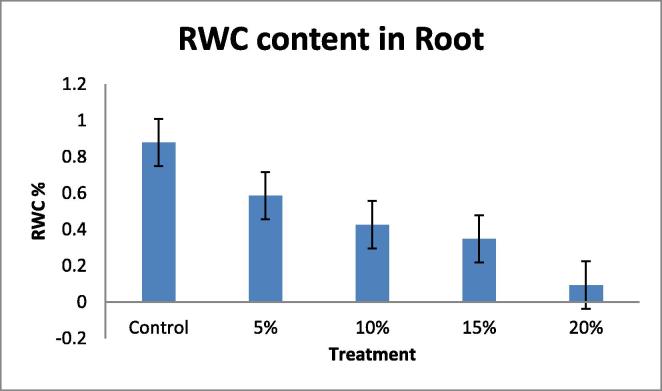
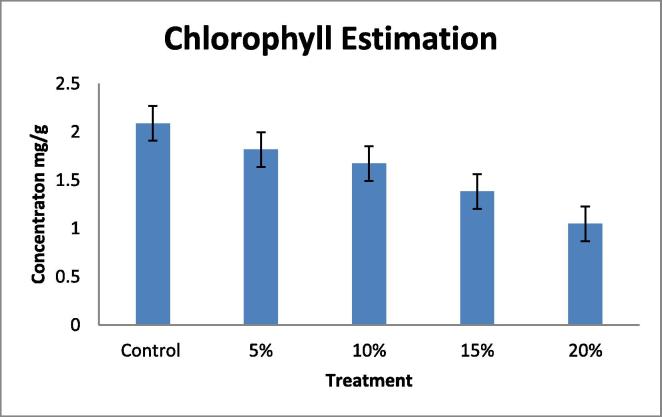
Drought tolerant with a high yield potential under drought stress; currently, drought is a major limiting factor in peanut cultivation, making irrigation necessary. However, peanut crop plants can adapt to water stress in various ways, Many agro-physiological parameters related to drought tolerance have been established, RNA content, Relative water content (RWC) and chlorophyll content with decrease in water supply (Deblonde et al., 1999). A fast screening tool would be helpful in selecting valuable genotypes with defined growth strategies that translate to drought tolerance and are suitable for experiments and/or breeding. In this present investigation the plants were short term drought-stressed by PEG 6000 (Polyethylene Glycol) for 24 h. In this experiment 40 day old seedlings were imposed water stress with different concentration; 100 ml of 5, 10, 15, 20%, of PEG 6000 (Polyethylene Glycol). The present data indicates the significant differences in RNA content, Relative water content (RWC) in leaf and root, chlorophyll contents in leaf when drought- stressed was induced by PEG 6000 (Polyethylene Glycol). A pronounced reduction in, RNA content (Table 1, Table 2; Fig. 1, Fig. 2). Relative water content (RWC) (Table 3, Table 4; Fig. 3, Fig. 4). And chlorophyll contents (Table 5; Fig. 5) with increasing water deficit was observed. There is reduced RNA synthesis with increased water stress, (He et al., 1999) chloroplast RNAase was upregulated which causes degradation of RNA during water stress. It was also been reported the decrease of ribosomes and polyribosomes during water stress ribosomes get cluttered on mRNAs to protect them from degradation, therefore ribosomes get disrupted hence another reason for mRNAs degradation (Mason et al., 1988) (Scott et al., 1979) This data indicates a pronounced reduction in RNA content in leaf and root with increasing water deficit (Table 1, Table 2 Fig. 1, Fig. 2). To understand the dehydration tolerance which shows the metabolic activities in the tissues of plant, RWC is considered to measure water status in plant (Sinclair and Ludlow, 1986) it was also observed the decline of RWC with increased water stress was also observed in barley (Yuan et al., 2005) and tomato (Zgallaï et al., 2005) and pigeonpea plants (Kumar et al., 2011). It clearly evidence that severe stress clearly affect the relative water content as compare to the control of same age group plant the significant differences in RWC was observed as compare to control and stressed of leaf and root (40 days old). The sharp decrease in RWC with the increased PEG concentration was noted of same age group plants. As water stress effects photosynthesis, The highest content of chlorophyll ‘a’ and ‘b’ was observed in control leaves while both progressive stresses of PEG concentration. Chlorophyll content was also affected during the present investigation which shows that long progressive stress along with some other environmental factor may affect photosynthetic ability of the plant system. Water stress imposed by PEG-6000 effects Enzymes of chlorophyll metabolism and photosynthetic pigments, In our present report it was observed that Chla is more sensitive than Chlb to PEG induced water stress (Hsu and Kao, 2003). Also demonstrated that PEG induced water stress cause decrease in total chlorophyll content in rice leaves. Hassanzadeh et al. (2009) revealed decrease in Chla but increase in Chlb content under drought stress in seasame. Decrease in the total chlorophyll content by PEG 6000 has also been noticed by Pratap and Sharma (2010) in black gram and Guo et al. (2013). A reason for decrease in chlorophyll content as affected by water deficit is that drought or heat stress by producing reactive oxygen species (ROS) such as O2 and H2O2, can lead to lipid peroxidation and consequently, chlorophyll destruction also, with decreasing chlorophyll content due to the changing green color of the leaf into yellow, Several methods which range from withdrawal of water to plants to the use of chemicals such as polyethylene glycol, mannitol etc., have been employed to create water stress in plants. Plant exposes their root system to this solution and no other toxicities were observed at plant level following the addition of PEG-6000 (Scott et al., 1979). It is reported that PEG induced significant water stress in plants and not having any toxic effects (Emmerich and Hardegree, 1990). RNA content, Relative water content (RWC) and chlorophyll content parameter can be used to select high yielding genotypes that maintain cell turgor under water stress environment to give relative high yield. A decrease in the RWC observed in both progressive mild and severe water stress. Relatively higher RWC was noted in progressive mild stress than severe stress indicating that plants have the ability to sustain their water content under mild stress, whereas this ability lost under severe stress treatment. Decrease in the RWC in PEG induced water stress was also reported in rice leaves (Hsu and Kao, 2003) and in Tomato (Zgallaï et al., 2005). According to results of (Bayoumi et al., 2008), RWC involved in absorbing more amount of water from the soil and/or the ability to control water loss through stomata and RWC parameter can be used to select high yielding genotypes that maintain cell turgor under water stress environment to give relative high yield.
Table 1.
Effect of water deficit on RNA content in Leaf.
| S. no | Samples | PEG concentration (%) | A260/A280 | Concentration (µg/µl) |
|---|---|---|---|---|
| 1 | Leaf 1 (Control) | 00 | 1.85 | 2.87 ± 0.005 |
| 2 | Leaf 2 | 05 | 1.86 | 2.67 ± 0.01 |
| 3 | Leaf 3 | 10 | 1.89 | 2.05 ± 0.01 |
| 4 | Leaf 4 | 15 | 1.89 | 1.99 ± 0.01 |
| 5 | Leaf 5 | 20 | 1.87 | 1.56 ± 0.01 |
Table 2.
Effect of water deficit on RNA content in Root.
| S. no | Samples | PEG concentration (%) | A260/A280 | Concentration (µg/µl) |
|---|---|---|---|---|
| 1 | Root A (Control) | 00 | 1.88 | 2.56 ± 0.025 |
| 2 | Root B | 05 | 1.81 | 2.08 ± 0.01 |
| 3 | Root C | 10 | 1.86 | 1.98 ± 0.01 |
| 4 | Root D | 15 | 1.89 | 0.99 ± 0.005 |
| 5 | Root E | 20 | 1.83 | 0.58 ± 0.005 |
Fig. 1.
Figure showing the RNA Concentration in Leaf.
Fig. 2.
Figure showing the RNA Concentration in Root.
Table 3.
Effect of water deficit on relative water content (RWC) in Leaf.
| PEG | RWC% |
|---|---|
| Control | 0.831 |
| 5 | 0.64 |
| 10 | 0.523 |
| 15 | 0.4 |
| 20 | 0.35 |
Table 4.
Effect of water deficit on relative water content (RWC) in Root.
| PEG | RWC% |
|---|---|
| Control | 0.879 |
| 5 | 0.586 |
| 10 | 0.426 |
| 15 | 0.348 |
| 20 | 0.093 |
Fig. 3.
Figure showing Relative Water Content (RWC) in Leaf.
Fig. 4.
Figure showing Relative Water Content (RWC) in Root.
Table 5.
Effect of water deficit on Chlorophyll ‘a’ ‘b’ and total Chlorophyll.
| PEG | Chlorophyll “a” | Chlorophyll “b” | Total |
|---|---|---|---|
| Control | 1.606 | 0.474 | 2.08 |
| 5% | 1.34 | 0.47 | 1.817 |
| 10% | 1.1 | 0.56 | 1.673 |
| 15% | 0.874 | 0.528 | 1.38 |
| 20% | 0.66 | 0.386 | 1.048 |
Fig. 5.
Figure showing total chlorophyll content in Leaf.
4. Conclusions
Our present results indicate that a progressive water stress induced PEG-6000 cause significant physiological and biochemical changes in peanut (Arachis hypogaea) ICGV 91114 plant. RNA content, relative water content (RWC) and chlorophyll content, and parameter can be used to select high yielding genotypes that maintain cell turgor under water stress environment. This experiments can be used for other cultivars conditions to have more are more RNA content, relative water content (RWC) and chlorophyll content are more resistant to drought stress and their yield is stable. This attributes can be used as screening tool for drought tolerance in other cultivars. This study was following to find characters of resistant under drought stress and the results showed that RNA content, relative water content (RWC) and chlorophyll content made difference between control and stress of peanut (Arachis hypogaea) ICGV 91114. Thus, this attributes can be used as screening tool for drought tolerance.
Acknowledgements
The authors gratefully thanks the financial support provided by the University Grants Commission (UGC). Author also express deep gratitude to Scientist F of National Institute of Nutrition Dr Bhanu Prakash Reddy. Professor of CPMB (Center for Plant Molecular Biology) V.D Reddy and K.V.Rao.
Footnotes
Peer review under responsibility of King Saud University.
References
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi T.Y., Eid M.H., Metwali E.M. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. Afr. J. Biotech. 2008;7(14) [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Deblonde P.M.K., Haverkort A.J., Ledent J.-F. Responses of early and late potato cultivars to moderate drought conditions: Agronomic parameters and carbon isotope discrimination. Eur. J. Agron. 1999;11:91–105. [Google Scholar]
- Emmerich W.E., Hardegree S.P. Polyethylene glycol solution contact effects on seed germination. Agron. J. 1990;82(6):1103–1107. [Google Scholar]
- Fotovat R., Valizadeh M., Toorehi M. Association between water-use-efficiency components and total chlorophyll content (SPAD)in wheat (Triticum aestivum L.) under well-watered and drought stress conditions. J. Food Agric. Environ. 2007;5(2007):225–227. [Google Scholar]
- Guo R., Hao W.P., Gong D.Z., Zhong X.L., Gu F.X. Effects of water stress on germination and growth of wheat, photosynthetic efficiency and accumulation of metabolites. Soil Processes and Current Trends in Quality Assessment. InTech. 2013 [Google Scholar]
- Hassanzadeh M., Ebadi A., Panahyan-e-Kivi M., Eshghi A.G., Jamaati-e-somarin S.H., Saeidi M., Zabihie-Mahmoodabad R. Evaluation of drought stress on Relative water content and chlorophyll content of Sesame (Sesamum indicum L.) genotypes at early flowering stage. Res. J. Environ. Sci. 2009;3(3):345–360. [Google Scholar]
- He T.X., An L.Z., Lin H.H., Liang H.G. Evidence for transcriptional and post transcriptional control of protein synthesis in water-stressed wheat leaves: a quantitative analysis of messenger and ribosomal RNA. J. Plant Physiol. 1999;155(1):63–69. [Google Scholar]
- Horling F., Lamkemeyer P., Konnig J., Finkemeir I., Kandlbinder A., Baier M., Dietz K. Divergent light, ascorbate and oxidative stressdependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiol. 2003;131:317–325. doi: 10.1104/pp.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.Y., Kao C.H. Differential effect of sorbitol and polyethylene glycol on antioxidant enzymes in rice leaves. Plant Growth Regul. 2003;39(1):83–90. [Google Scholar]
- Jiang Y., Macdonald S.E., Zwiazak J.J. Effects of cold storage and water stress on water relations and gas ex-change of white spruce (Picea glauca) seedlings. Tree Physiol. 1995;15(4):267–273. doi: 10.1093/treephys/15.4.267. [DOI] [PubMed] [Google Scholar]
- Karamanos A.J. Water stress and leaf growth of field beans (Vicia faba L.) in the field: leaf number and total leaf area. Ann. Bot. 1978;42(6):1393–1402. [Google Scholar]
- Kaur N., Gupta A.K. Signal transduction pathways under abiotic stresses in plants. Curr. Sci. 2005;88(11):1771–1780. [Google Scholar]
- Kumar R.R., Karajol K., Naik G.R. Effect of polyethylene glycol induced water stress on physiological and biochemical responses in pigeonpea (Cajanus cajan L. Millsp.) Recent Res. Sci. Technol. 2011;3(1) [Google Scholar]
- Landjeva S., Neumann K., Lohwasser U., Borner M. Molecular mapping of genomic regions associated with wheat seedling growth under osmotic stress. Biol. Plan. 2008;52:259–266. [Google Scholar]
- Mason H.S., Mullet J.E., Boyer J.S. Polysomes, messenger RNA, and growth in soybean stems during development and water deficit. Plant Physiol. 1988;86(3):725–733. doi: 10.1104/pp.86.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monakhova O.F., Chernyad'ev I.I. Protective role of kartolin-4 in wheat plants exposed to soil draught. Appl. Biochem. Microbiol. 2002;38(4):373–380. [PubMed] [Google Scholar]
- Németh M., Janda T., Horváth E., Páldi E., Szalai G. Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci. 2002;162(4):569–574. [Google Scholar]
- Pratap, V., Sharma, Y.K., 2010. Impact of osmotic stress on seed germination and seedling growth in black gram (Phaseolus mungo). [PubMed]
- Rawson H.M., Turner N.C. Recovery from water stress in five sunflower (Helianthus annuus L.) cultivars. II. The development of leaf area. Funct. Plant Biol. 1982;9(4):449–460. [Google Scholar]
- Rubinstein B., Turner N.C. Regulation of H+ excreation. Effects of osmotic shock. Plant Physiol. 1982;99:355–360. doi: 10.1104/pp.69.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M.C., editor. Breeding for Stress Tolerance in Cool-Season Food Legumes (p. 474) Wiley; Chichester, UK: 1993. [Google Scholar]
- Scott N.S., Munns R., Barlow E.W.R. Polyribosome content in young and aged wheat leaves subjected to drought. J. Exp. Bot. 1979;30(5):905–911. [Google Scholar]
- Sharp R.E., Hsiao T.C., Silk W.K. Growth of the maize primary root at low water potentials : II. role of growth and deposition of hexose and potassium in osmotic adjustment. Funct. Plant Biol. 1990;93(4):1337–1346. doi: 10.1104/pp.93.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T.R., Ludlow M.M. Influence of soil water supply on the plant water balance of four tropical grain legumes. Funct. Plant Biol. 1986;13(3):329–341. [Google Scholar]
- Singh N.P., Pal P.K., Vaishali S.K. Morpho-physiological characterization of Indian wheat genotypes and their evaluation under drought condition. Afr. J. Biotech. 2014;13(20) [Google Scholar]
- Tarkow H., Feist W.C., Southerland C.F. Interaction of wood and polymeric materials. Penetration versus molecular size. Forest Prod. J. 1996;16:61–65. [Google Scholar]
- Turkan I., Bor M., Ozdemir F., Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005;168:223–231. [Google Scholar]
- Yuan S., Liu W.J., Zhang N.H., Wang M.B., Liang H.G., Lin H. Effects of water stress on major photosystem II gene expression and protein metabolism in barley leaves. Physiol. Plant. 2005;125(4):464–473. [Google Scholar]
- Zgallaï H., Steppe K., Lemeur R. Photosynthetic, physiological and biochemical responses of tomato plants to polyethylene glycol-induced water deficit. J. Integr. Plant Biol. 2005;47(12):1470–1478. [Google Scholar]