Abstract
The probiotic effects of seven newly isolated gut bacteria, from the indegenous honey bees of Saudi Arabia were investigated. In vivo bioassays were used to investigate the effects of each gut bacterium namely, Fructobacillus fructosus (T1), Proteus mirabilis (T2), Bacillus licheniformis (T3), Lactobacillus kunkeei (T4), Bacillus subtilis (T5), Enterobacter kobei (T6), and Morganella morganii (T7) on mortality percentage of honey bee larvae infected with P. larvae spores along with negative control (normal diet) and positive control (normal diet spiked with P. larvae spores). Addition of gut bacteria to the normal diet significantly reduced the mortality percentage of the treated groups. Mortality percentage in all treated groups ranged from 56.67% up to 86.67%. T6 treated group exhibited the highest mortality (86.67%), whereas T4 group showed the lowest mortality (56.67%). Among the seven gut bacterial treatments, T4 and T3 decreased the mortality 56.67% and 66.67%, respectively, whereas, for T2, T6, and T7 the mortality percentage was equal to that of the positive control (86.67%). Mortality percentages in infected larval groups treated with T1, and T5 were 78.33% and 73.33% respectively. Most of the mortality occurred in the treated larvae during days 2 and 3. Treatments T3 and T4 treatments showed positive effects and reduced mortality.
Keywords: Honey bee larvae, Mortality, Probiotics, Gut bacteria, Apis mellifera jemenitica
1. Introduction
Probiotics are live microorganisms, when supplemented with food and ingested in adequate amounts, provide health benefits to the host by improving the intestinal microbial balance (Fontana et al., 2013, Fuller, 1989). Gut bacteria are widely used as probiotics and are included in many functional foods and dietary supplements (Gourbeyre et al., 2011). Since antibiotics are prohibited in animal feed in many countries, natural substances are being explored to improve animal health. The use of beneficial bacteria is very important tool against pathogens, and is commonly implemented in agriculture, aquaculture, and in human and animal health care (Balcázar et al., 2006, Wang et al., 2008, Andrearczyk et al., 2014).
Use of probiotics for health benefits is very common not only in human diet but also in the forage of various vertebrates and invertebrates (Patterson and Burkholder, 2003, Talpur et al., 2012). Probiotic bacteria are more beneficial, when supplemented in the diet of the organisms from which they had been isolated (Ptaszyńska et al., 2016). Probiotic bacteria, especially, lactic acid and acetic acid bacteria, have intriguing characteristics, such as tolerance to acidic pH, metabolism of various sugars, and production of organic acid as an end product. These unique characteristics facilitate colonization of the sugar rich digestive tract of honey bees and inhibition of the invasion and growth of acid sensitive pathogenic bacteria (Hamdi et al., 2011). The development of hypopharyngeal glands and fat bodies in honey bees was stimulated, and protein utilization was increased when probiotic bacteria were added to pollen substitutes (Kazimierczak-Baryczko and Szymaś, 2006).
Gut bacteria stimulate the immunity of honey bee larvae (the stage, where organism is vulnerable to infection by different pathogens) and promote mounting of anti-pathogen immune responses. Feeding larvae with a diet supplemented with non-pathogenic gut bacteria stimulated the transcription of genes involved in immune response (Evans and Lopez, 2004). Addition of probiotic bacteria into the honey bee larval food decreased the number of larvae infected by Paenibacillus larvae (Forsgren et al., 2010). Therefore, addition of probiotic bacteria will ultimately improve the honey bee immune response against pathogens. The aim of this study was to evaluate the effect of isolated gut bacteria from local honey bee Apis mellifera jemenitica of Saudi Arabia, on the mortality of honey bee larvae infected with P. larvae.
2. Materials and methods
2.1. Collection of first instar larvae
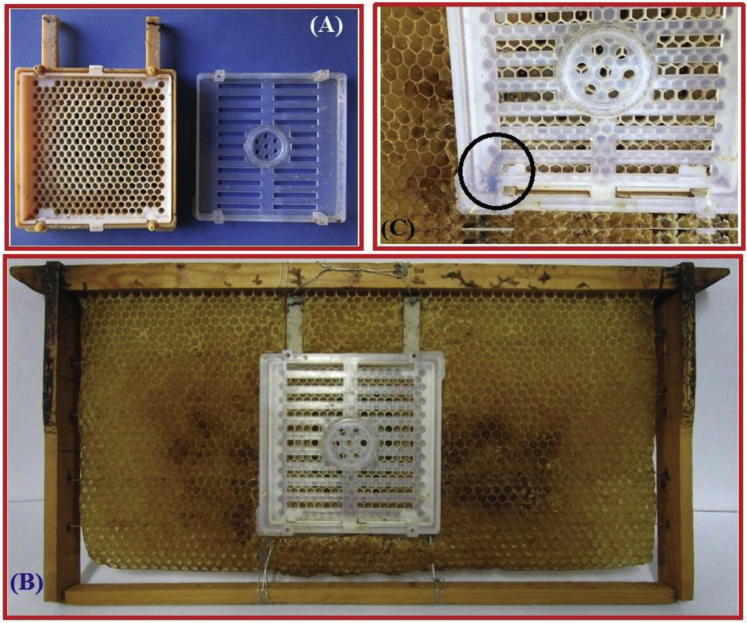
Honey bee (A. m. jemenitica) larvae were collected from an apiary maintained at Bee Research Chair, Plant Protection Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia. The method described by Aupinel et al. (2005) was used with certain modifications to obtain larvae within the same age range. A frame was taken out from a healthy bee colony, and the Jenter cage (Hammann, Hassloch-Germany) was fixed in the frame by cutting a part of the wax comb. The cage was designed to permit free movement of the worker bees across the cage, which is essential for stimulating queen for egg laying, and nursing bee larvae (Fig. 1). The queen was confined in the Jenter cage and the frame was placed in the bee colony to allow the queen to lay eggs. After approximately 30 h, when a large number of eggs was present in the cage cells, the queen was uncaged. The frame, along with the Jenter cage containing the eggs, was kept in the middle of the bee colony for three days for incubation. The Jenter cage allowed the worker bees to feed the newly hatched larvae, but prevented the queen from entering and laying new eggs. After 3 days, the frame containing the 1st instar larvae were brought to the laboratory and placed in an incubator (Binder, Tutlingen, Germany) adjusted at 34 °C and 80% relative humidity.
Fig. 1.
Methodology and apparatus used for collection of first instar honey bee larvae (A) The Jenter cage (B) Frame installed with the Jenter cage (C) Queen caged (in circle) into the Jenter cage.
2.2. Paenibacillus larvae and gut bacterial isolates
The reference strain of P. larvae ATCC 9545 was used in this experiment and spore suspension was prepared according to methodology used by Shimanuki and Knox (2000). Seven gut bacteria, which had already shown strong antagonistic activity against P. larvae (Data not Published), were evaluated for their probiotic effect on bee health. These isolates were Fructobacillus fructosus KY027123; Proteus mirabilis KY027132; Bacillus licheniformis KY027142; Lactobacillus kunkeei KY027158; Bacillus subtilis KY027169; Enterobacter kobei KY027178; and Morganella morganii KY027186. A single colony of each gut bacterium was inoculated in tubes containing 10 mL of selective broth and incubated at 36 °C overnight. The bacterial suspensions were diluted to approximately 1 × 106 CFU per mL in sterilized distilled water and used to spike normal diet (NDT) with respective gut bacterium.
2.3. Diet preparation
NDT was prepared according to Vandenberg and Shimanuki (1987). The ingredients of NDT in percentage weight were: royal jelly-RJ (50%), D-glucose (6%), D-fructose (6%), yeast extract (1%) and sterilized autoclaved water (37%). The experiments were conducted in three replicates for six days. Diets used in different treatments on Day-I were as following: T1 (NDT + F. fructosus KY027123 + P. larvae spores); T2 (NDT + Proteus mirabilis KY027132 + P. larvae spores); T3 (NDT + B. licheniformis KY027142 + P. larvae spores); T4 (NDT + L. kunkeei KY027158 + P. larvae spores); T5 (NDT + B. subtilis KY027169 + P. larvae spores); T6 (NDT + E. kobei KY027178 + P. larvae spores); T7 (NDT + M. morganii KY027186 + P. larvae spores). Negative control (C1) was NDT alone while, the positive control (C2) composed of NDT + P. larvae spores (Table 1). From Day-II onwards, treatments (T1-T7) constituted NDT plus respective gut bacteria without P. larvae spores by adding bacterial suspensions with the ratio 1:10 (O’Callaghan et al., 2012) in NDT (Ami et al., 2010) and treatments C1 and C2 were NDT only. The fresh diets were prepared and stored at 4 °C for less than two days during the experiment. Prior to feeding, diets were pre warmed at 34 °C and provided to the larvae according to their age using a micro pipette. Daily diet quantity for each bee larvae was 5, 10, 20, 20, 30, 40 µL on day I, II, III, IV, V, and VI respectively.
Table 1.
The experimental plan to assess the probiotic effects of gut bacteria (isolated from Apis mellifera jemenitica) on honey bee larvae.
| Experimental Group | Number of larvae (n) | Treatments (Day-I) | Treatments (Day-II to Day-VI) | CFU/mL (P. larvae spores/ gut bacteria) |
|---|---|---|---|---|
| C1 | 20 | Normal diet only | NDT | – |
| T1 | 20 | NDT + F. fructosus + P. larvae spores | NDT + F. fructosus | 1 × 106 |
| T2 | 20 | NDT + Proteus mirabilis + P. larvae spores | NDT + Proteus mirabilis | 1 × 106 |
| T3 | 20 | NDT + B. licheniformis + P. larvae spores | NDT + B. licheniformis | 1 × 106 |
| T4 | 20 | NDT + L. kunkeei + P. larvae spores | NDT + L. kunkeei | 1 × 106 |
| T5 | 20 | NDT + B. subtilis + P. larvae spores | NDT + B. subtilis | 1 × 106 |
| T6 | 20 | NDT + E. kobei + P. larvae spores | NDT + E. kobei | 1 × 106 |
| T7 | 20 | NDT + M. morganii + P. larvae spores | NDT + M. morganii | 1 × 106 |
| C2 | 20 | NDT + P. larvae ATCC 9545 spores | NDT | 1 × 106 |
Abbreviations:Negative control (C1); Positive control (C2) Treatment (T); Normal diet (NDT); colony forming units (CFU).
2.4. Exposure bioassays
The first instar larvae were reared in the cells of the Jenter cage and kept in an incubator at 34 °C and 80% relative humidity. Each experimental group consisted of 20 larvae. The negative control (C1) group consisted of NDT only, while the positive control group (C2) contained NDT spiked with P. larvae spores on Day-I. The other experimental groups were provided with NDT containing P. larvae spores and respective gut bacteria on the Day-I only.
From Day-II onwards, the larvae in the experimental groups T1-T7 were provided NDT supplemented with respective gut bacteria to assess their probiotic effects against infection while the experimental group C1 and C2 were provided NDT only (Table 1). Diet was placed next to the mouth of each larva by using a micropipette so that it may consume easily. Daily mortality of larvae in each experimental group was observed until five days, post treatments and dead larvae were adequately removed using sterilized forceps. Dead larvae had no movement when observed under cold light while the live larvae showed respiratory movement.
2.5. Statistical analysis
The mortality of honey bee larvae was analyzed in three replicates. The Statistix 8.1 (2005) software was used to analyze the data with mortality as the response variable and different diets (normal as well as supplemented with gut bacteria and P. larvae spores) as the main effect. All pairwise comparison of means was performed using Tukey's Honest Significant Difference (HSD) test at p < 0.05.
3. Results
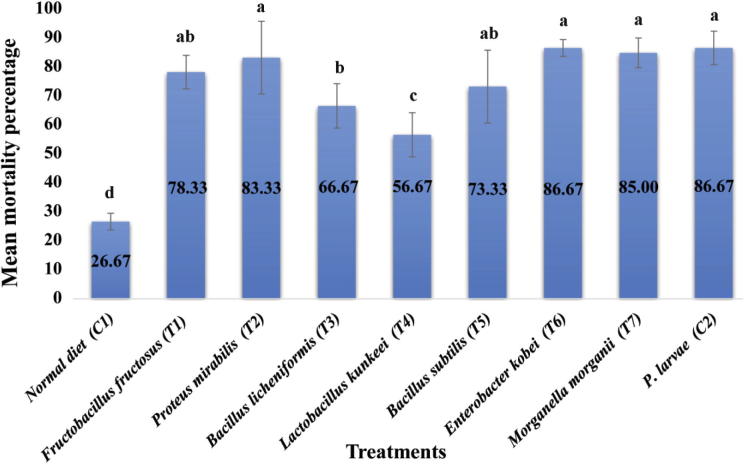
The effects of gut bacteria on the mortality of honey bee larvae were evaluated to determine their probiotic role. Significant differences in the mortality percentage of the treated groups were observed. Values ranged from 56.67% up to 86.67% (Table 2). The positive control (C2; bee larvae fed with normal diet [NDT] containing P. larvae spores) exhibited the highest mortality (86.67%), whereas, the larvae fed with NDT alone (C1; negative control) showed the lowest mortality (26.67%). Of the seven gut bacterial treatments, T4 (bee larvae fed with NDT containing L. kunkeei), and T3 (bee larvae fed with NDT containing B. licheniformis) decreased mortality (56.67%, and 66.67%, respectively). In contrast, the mortality percentages in T2 (bee larvae fed with NDT containing Proteus mirabilis), T6 (bee larvae fed with NDT containing E. kobei), T7 (bee larvae fed with NDT containing M. morganii) were near to that of the positive control (Fig. 3).
Table 2.
Mean mortality percentage of honey bee Apis mellifera jemenitica larvae infected (in vivo) with Paenibacillus larvae, treated with control and different gut bacterial supplemented diets.
| Experimental groups | Treatments | Mean mortality (%) |
|---|---|---|
| C1 | NDT only | 26.67 ± 2.89d |
| T1 | NDT + F. fructosus + P. larvae spores | 78.33 ± 5.77ab |
| T2 | NDT + Proteus mirabilis + P. larvae spores | 83.33 ± 12.58a |
| T3 | NDT + B. licheniformis + P. larvae spores | 66.67 ± 7.64b |
| T4 | NDT + L. kunkeei + P. larvae spores | 56.67 ± 7.64c |
| T5 | NDT + B. subtilis + P. larvae spores | 73.33 ± 12.58ab |
| T6 | NDT + E. kobei + P. larvae spores | 86.67 ± 2.89a |
| T7 | NDT + M. morganii + P. larvae spores | 85.00 ± 5.10a |
| C2 | NDT + P. larvae ATCC 9545 spores | 86.67 ± 5.7a |
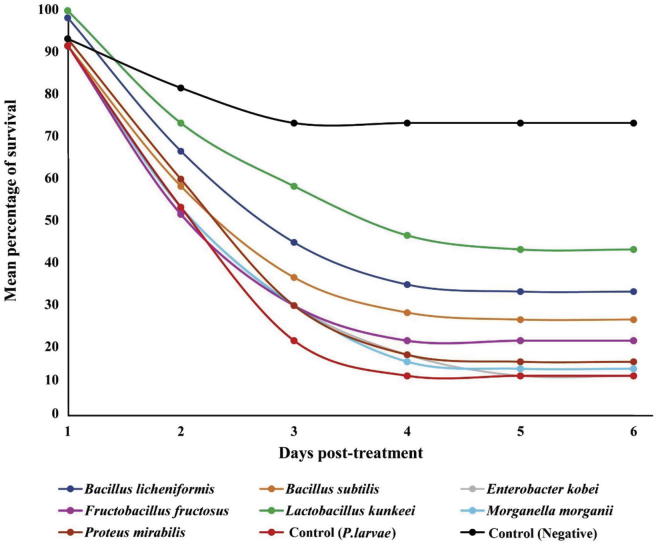
Fig. 3.
Survivorship of honey bee (Apis mellifera jemenitica) larvae infected (in vivo) with Paenibacillus larvae treated with controls and different gut bacteria supplemented in normal diet.
Similar mortality percentages 78.33%, and 73.33% were observed for T1 (bee larvae fed with NDT containing F. fructosus), and T5 (bee larvae fed with NDT containing B. subtilis), respectively (Fig. 2). The relative zones of inhibition (ZOI) of seven gut bacteria against P. larvae were shown in Table 2 for comparative purpose. Survivorship of honey bee (A.m. jemenitica) larvae infected (in vivo) with P. larvae treated with controls and different gut bacteria supplemented in normal diets is shown in Fig. 3. B. licheniformis (T3) and L. kunkeei (T4) among the supplemented gut bacteria enhanced the survival percentage of treated larvae. Most of the mortality occurred in the treated larvae on days 2 and 3. The negative control (C1; bee larvae fed with NDT only) and T4 (bee larvae fed with NDT containing L. kunkeei) showed lower mortality on these two days compared to other treatments (Fig. 3).
Fig. 2.
Bar graph showing the effects of gut bacteria and control treatments on the mortality percentage of honey bee (Apis mellifera jemenitica) larvae infected (in vivo) with Paenibacillus larvae. Letters on error bars represent standard deviations. Means with same superscript letters are not significantly different (p < 0.05).
4. Discussion
The mortality of in vivo infected bee larvae was studied to investigate whether, the gut bacteria (L. kunkeei, P. mirabilis, E. kobei, M. morganii, F. fructosus, B. licheniformis, and B. subtilis) are capable of reducing the lethal effects of P. larvae infection. The results show that there was a significant decrease in the mortality of infected bee larvae fed with NDT supplemented with gut bacteria (Table 2). The bee larvae were fed with the NDT-only, negative control (C1) and NDT spiked with P. larvae spores, as a positive control (C2); both treatments affected the mortality percentage as expected (Table 2, Fig. 1). The C1-treated larvae showed less mortality as they were not infected by P. larvae spores, and continued to grow normally. In contrast, the C2-treated larvae were infected with P. larvae spores, showed highest mortality indicating that P. larvae spores were able to thrive in the bee larvae guts and caused high mortality.
This can be explained by the findings of Yue et al. (2008), who found that P. larvae spores germinate (about 12 h after ingestion), colonize, and proliferate massively in the midgut and subsequently destroy the epithelium layer to enter the hemocoel and cause larval death. Larvae fed with the T4 (NDT supplemented with L. kunkeei), and T3 (NDT supplemented with B. licheniformis) showed the lower mortality 56.67%, and 66.67% respectively after that of the negative control.
The possible reason behind the low mortality in the T4 treated larvae may be the presence of L. kunkeei, which is a members of the lactic acid bacteria (LAB) family. LAB produce organic acids, antibacterial compounds, hydrogen peroxide, diacetyl, benzoate, bacteriocins and proteins with putative antimicrobial functions, which protect the host from invading pathogens (Olofsson and Alejandra Vázquez, 2008, Butler et al., 2013). These results are in accordance with those of Forsgren et al. (2010), who demonstrated that LAB, including L. kunkeei from honey bee gut had strong inhibitory effect on the in vitro growth of P. larvae.
Larval mortality in the T3 treatment which comprised NDT supplemented with B. licheniformis, was comparable to that of the T4 treatment. This showed that B. licheniformis provided immunity against the pathogens, which decreased the mortality percentage of the treated honey bee larvae. Bacillus spp. produce bacteriocins, stimulate immunity, and possess adhesion abilities (Duc et al., 2004, Barbosa et al., 2005). The results obtained with B. licheniformis are consistent with Evans and Armstrong (2006), who found that members of the genus Bacillus isolated from honey bee larvae showed strong inhibitory activity against P. larvae in vitro. B. licheniformis, has also proven to be a good probiotic in the aquaculture industry. Merrifield et al. (2010) demonstrated that when fish were fed on a diet supplemented with B. licheniformis, their feed conversion ratio and growth rate improved significantly.
Larval mortality in treatments T1 and T5, which were supplemented by F. fructosus and B. subtilis gut bacteria, respectively, were statistically similar. T1 and T5 treatments showed decreased mortality in infected larvae, but their effect was lower than that observed for the C1, T3, and T4 treatments. F. fructosus is a special type of LAB that prefers fructose sugars to glucose for their growth (Endo, 2012). This could possibly explain, why the effect of F. fructosus on larvae mortality was lower than that with L. kunkeei as NDT does not contain abundant fructose. The growth of Bacillus in the honey bee gut is pH sensitive (Mohr and Tebbe, 2006). The intestinal pH of honey bee larvae mostly remains at 6.8 (Colibar et al., 2010) and optimum pH for antibiotic activity in B. subtilis is pH-8 (Jamil et al., 2007). This could possibly explain the poor effect of B. subtilis on reduction of mortality in bee larvae.
T2, T6, and T7 treatments which comprised NDT supplemented with P. mirabilis, E. kobei, and M. morganii, respectively, did not decrease larval mortality and their effect was similar to that of positive control (C2). The distinct gut environment of honey bee larvae could possibly explain the non-performance of these three gut bacteria against P. larvae (Kwong and Moran, 2016). Since, these gut bacteria were isolated from the worker bees, they performed poorly in larval gut, albeit showing stronger effects on mortality in in vitro cultures of P. larvae.
The maximum mortality of honey bee larvae occurred on the second and third day of the experiment, except for the control and L. kunkeei treatments. The spores of P. larvae start to germinate after 12 h of ingestion, progressively multiply, proliferate, and then destroy the epithelium of the midgut, causing larval death (Yue et al., 2008, Ansari et al., 2017). This could explain the higher mortality in larvae on days 2 and 3. In addition, the low mortality observed on these two days in the bee larvae fed with diet supplemented with L. kunkeei highlighted the probiotic effect of this bacterium.
It was generally observed that total volume of the diet consumed by a larva for six days was 125 µL which is lower than reported by Aupinel et al. (2005), who demonstrated that larva consumes 160 µL of diet. The reason for this difference may be the size of the local honey bees (A. m. jemenitica), which is the smallest bee race of A. mellifera based on morphometric characteristics (Alattal et al., 2014).
5. Conclusion
Probiotic effects of seven gut bacteria isolated from the local honey bees of Saudi Arabia that were selected on the basis of the extent of their inhibitory activity against P. larvae were evaluated. It was concluded that L. kunkeei and B. licheniformis decreased the mortality percentage in bee larvae infected with P. larvae spores. Other bacteria, F. fructosus and B. subtilis were capable of decreasing the mortality to some extent, while Proteus mirabilis, E. kobei, and M. morganii did not perform well in decreasing larval mortality. Bacteria belonging to Lactobacillus and Bacillus and some of their metabolites, are getting significant importance in apiculture industry. Therefore, a more detailed study tailored towards investigating and characterizing the metabolites responsible for inhibition of P. larvae is required or recommended as they are safe to use, ecofriendly, protect bees from pathogen and boost immunity.
6. Declaration of interest
The authors confirm that there is no conflict of interests and are also liable for the content and writing of this article.
Acknowledgement
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alattal Y., Al-Ghamdi A., Al Sharhi M., Fuchs S. Morphometric characterization of the native Honeybee, Apis mellifera Linnaeus, 1758, of Saudi Arabia. Zool. Middle East. 2014;60:226–235. [Google Scholar]
- Ami E.B., Yuval B., Jurkevitch E. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 2010;4:28–37. doi: 10.1038/ismej.2009.82. [DOI] [PubMed] [Google Scholar]
- Andrearczyk S., Kadhim M.J., Knaga S. Influence of a probiotic on the mortality, sugar syrup ingestion and infection of honeybees with Nosema spp. under laboratory assessment. Med. Weter. 2014;70:762–765. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Nuru A., Ahmed A.M., Ayaad T.H., Khan K.A., Al-Waili N. Diagnosis and molecular detection of Paenibacillus larvae, the causative agent of American foulbrood in honey bees in Saudi Arabia. Int. J. Trop. Insect Sci. 2017:1–12. [Google Scholar]
- Aupinel P., Fortini D., Dufour H., Tasei J.N., Michaud B., Odoux J.F., Pham-Delègue M.H. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. B. Insectol. 2005;58:107–111. [Google Scholar]
- Balcázar J., Blas I., Ruizzarzuela I., Cunningham D., Vendrell D., Muzquiz J. The role of probiotics in aquaculture. Vet. Microbiol. 2006;114:173–186. doi: 10.1016/j.vetmic.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Barbosa T.M., Serra C.R., LaRagione R.M., Woodward M.J., Henri- ques A.O. Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005;71:968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler E., Alsterfjord M., Olofsson T.C., Karlsson C., Malmström J., Vásquez A. Proteins of novel lactic acid bacteria from Apis mellifera mellifera: an insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol. 2013;13:1–11. doi: 10.1186/1471-2180-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibar O., Popovici D., Eugeniu C., Korod G. The effect of acidifiant on the development of bee families (Apis mellifica) Med. Vet. 2010;43:296–299. [Google Scholar]
- Duc L.H., Hong H.A., Barbosa T.M., Henriques A.O., Cutting S.M. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A. Fructophilic lactic acid bacteria inhabit fructose-rich niches in nature. Microb. Ecol. Health Dis. 2012;23:6–9. doi: 10.3402/mehd.v23i0.18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.D., Armstrong T.N. Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecol. 2006;6:1–9. doi: 10.1186/1472-6785-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.D., Lopez D.L. Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae) J. Econ. Entomol. 2004;97:752–756. doi: 10.1603/0022-0493(2004)097[0752:bpiair]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fontana L., Bermudez-Brito M., Plaza-Diaz J., Muñoz-Quezada S., Gil A. Sources, isolation, characterisation and evaluation of probiotics. British J. Nutrition. 2013;109:S35–S50. doi: 10.1017/S0007114512004011. [DOI] [PubMed] [Google Scholar]
- Forsgren E., Olofsson T.C., V́asquez A., Fries I. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie. 2010;41:99–108. [Google Scholar]
- Fuller, R., 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66, 365-378. Gourbeyre, P., Denery, S., Bodinier M.,2011. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J. Leukocyte Biol. 89,685-695. [DOI] [PubMed]
- Gourbeyre P., Denery S., Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011;89:685–695. doi: 10.1189/jlb.1109753. [DOI] [PubMed] [Google Scholar]
- Hamdi C., Balloi A., Essanaa J., Crotti E., Gonella E., Raddadi N., Ricci I., Boudabous A., Borin S., Manino A., Bandi C., Alma A., Daffonchio D., Cherif A. Gut microbiome dysbiosis and honey bee health. J. Appl. Entomol. 2011;135:524–533. [Google Scholar]
- Jamil B., Hasan F., Hameed A., Ahmed S. Isolation of Bacillus subtilis MH-4 from soil and its potential of polypeptidic antibiotic production. Pak. J. Pharm. Sci. 2007;20:26–31. [PubMed] [Google Scholar]
- Kazimierczak-Baryczko M., Szymaś B. Improvement of the composition of pollen substitute for honey bee (Apis mellifera L.) through implementation of probiotic preparations. J. Apic. Sci. 2006;50:15–23. [Google Scholar]
- Kwong W.K., Moran N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016;14:374–384. doi: 10.1038/nrmicro.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield D.L., Bradley G., Baker R.T.M., Davies S.J. Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria post antibiotic treatment. Aquac. Nutr. 2010;16:496–503. [Google Scholar]
- Mohr K.I., Tebbe C.C. Diversity and phylotypes consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 2006;8:258–272. doi: 10.1111/j.1462-2920.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- O’Callaghan, M., Glare, T.R., Lacey, L. A., 2012. Bioassay of bacterial entomopathogens against insect larvae, in: Lacey, L.A. (2nd Eds.), Manual of techniques in invertebrate pathology. IP consul. Int., Washington, pp 101–127.
- Olofsson T.C., Alejandra Vázquez A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr. Microbiol. 2008;57:356–363. doi: 10.1007/s00284-008-9202-0. [DOI] [PubMed] [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Ptaszyńska A.A., Borsuk G., Zdybicka-Barabas A., Cytryńska M., Małek W. Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol. Res. 2016;115:397–406. doi: 10.1007/s00436-015-4761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanuki H., Knox D.A. Diagnosis of Honey Bee Diseases. Agri. Handbook USDA. 2000;690:1–57. [Google Scholar]
- Statistix., 2005. Statistics 8.1. Analytical software for windows.
- Talpur A.D., Memon A.J., Khan M.I., Ikhwanuddin M., Danish D., Abol-Munafi A.B. Inhibition of pathogens by lactic acid bacteria and application as water additive multi isolates probiotics in early stages larviculture of P. pelagicus (Linnaeus, 1758) J. Anim. Plant. Sci. 2012;22:54–64. [Google Scholar]
- Vandenberg J.D., Shimanuki H. Technique for rearing worker honey bees in the laboratory. J. Appl. Res. 1987;26:90–97. [Google Scholar]
- Wang Y., Li J., Lin J. Probiotics in aquaculture: challenges and outlook. Aquaculture. 2008;281:1–4. [Google Scholar]
- Yue D., Nordhoff M., Wieler L.H., Genersch E. Fluorescence in situ- hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera) Environ. Microbiol. 2008;10:1612–1620. doi: 10.1111/j.1462-2920.2008.01579.x. [DOI] [PubMed] [Google Scholar]