Abstract
The current study reports rapid and easy method for synthesis of eco-friendly silver nanoparticles (AgNPs) using Coriandrum sativum leaves extract as a reducing and covering agent. The bio-reductive synthesis of AgNPs was monitored using a scanning double beam UV-vis spectrophotometer. Transmission electron microscopy (TEM) was used to characterize the morphology of AgNPs obtained from plant extracts. X-ray diffraction (XRD) patterns of AgNPs indicate that the structure of AgNPs is the face centered cubic structure of metallic silver. The surface morphology and topography of the AgNPs were examined by scanning electron microscopy and the energy dispersive spectrum revealed the presence of elemental silver in the sample. The silver phyto nanoparticles were collected from plant extract and tested growth potential and metabolic pattern in (Lupinus termis L.) seedlings upon exposure to different concentrations of AgNPs. The seedlings were exposed to various concentrations of (0, 0.1, 0.3 and 0.5 mg L−1) AgNPs for ten days. Significant reduction in shoot and root elongation, shoot and root fresh weights, total chlorophyll and total protein contents were observed under the higher concentrations of AgNPs. Exposure to 0.5 mg L−1 of AgNPs decreased sugar contents and caused significant foliar proline accumulation which considered as an indicator of the stressful effect of AgNPs on seedlings. AgNPs exposure resulted in a dose dependent decrease in different growth parameters and also caused metabolic disorders as evidenced by decreased carbohydrates and protein contents. Further studies needed to find out the efficacy, longevity and toxicity of AgNPs toward photosynthetic system and antioxidant parameters to improve the current investigation.
Keywords: Silver nanoparticles, XRD, Lupinus termis, Growth potential
1. Introduction
Recently nanotechnology progress and silver nanoparticles (AgNPs) have been connected in different industries, including daily and medical products. Present production of AgNPs, worldwide, is estimated to be around 500 tons per year and increase over the next few years as expected. (Mueller and Nowack, 2008). By several routes, silver nanoparticles may be released into the environment during their synthesis, incorporation of the AgNPs into other goods, and recycling or disposal of these goods and nanoparticles corresponding to their bulk materials have been shown high and unique toxicity (Shi et al., 2011). Consequently, is necessary to understanding nanomaterial mode of interaction, uptake, accumulation and impact on the biosystems. It is of concern and increasing importance to control measures to avoid nano pollution in ecological systems (Navarro et al., 2008).
Several pathways exist or are predicted for NP association and uptake in plants (Dietz and Herth, 2012). Prakash et al. (2014) have been examined the impact of various types of NPs on higher plants. Toxicity studies of NPs are still emerging and have shown negative effects on the growth and plants development (Qian et al., 2013).
Lin and Xing (2007) analyzed the phytotoxicity of five types of multiwalled NPs (MWCNT, Al, Al2O3, Zn and ZnO) on seed germination and seedling root growth in six higher plant species (Raphanussativus, Lolium perenne, Lactuca sativa, Brassica napus, Zea mays and Cucumis sativus). The biosystems impact at various levels is expected to device and implement proper mitigation or control measures to avoid nano pollution turning out to be a serious ecological apprehension (Navarro et al., 2008). To indicate most of the toxicological studies of AgNPs it have been conducted on microbial and animal cells; however studies which focused on plant cells was restricted (Navarro, et al., 2008). Recently, Ma et al. (2010) had shown that different sizes of AgNPs (20–80 nm) were toxic to Arabidopsis thaliana seedlings and they caused at very low concentration a stunted growth, indicating an increase in toxicity after increasing AgNPs concentration.
Most of the phytotoxicity studies reported so far have used chemically synthesized nanoparticles, whose synthesis and fabrications utilize toxic chemicals (Lin and Xing, 2007). Henceforth, it is accepted that the harmful impressions initiated by AgNPs to the plant systems may be because of the toxic compounds adsorbed on the nanomaterials surface.
From this point forward, an effort was made, in the present study, to investigate the possible effects of green AgNPs on growth and metabolism of Lupinus termis L plant.
Through green chemistry approach, silver nanoparticles were synthesized using Coriandrum sativum shoot extract which is an annual important spice annual crop of the Apiaceae and is one of valuable medicinal plants which grown all over the world and comes from the Mediterranean region. (Renata, 2013).
Bitter lupine (Lupinus termis L.) is considered one of the family Fabaceae (Leguminosae), it is a valuable ancient legume which has a high adaptation to poor soils and dry climates and contains high amount of protein, dietary fiber, oil, minerals and different functional components (Ismail et al., 2014). Earlier in Egypt and other countries for thousands of years, Lupine seeds have been used for human feed and as a medication (ARC, 1994). Across Egypt, Lupinus termis is cultivated in a wide range of environments and its seed have a nutritional quality superior to other legumes seed and similar to soybean seed (Raza and Jrnsgard, 2005).
This research aims to characterize green silver nanoparticles through high resolution transmission electron microscopy (HRTEM) and X-ray diffraction analysis. Germination, morphological and anatomical changes in leaves of Lupinus termis were studied in response to different concentration of green AgNPs. Biochemical responses were emphasized as chlorophyll content, florescence, protein, total soluble sugar, total phenol contents and activities of total antioxidant enzymes in AgNPs treated seedlings.
2. Materials and methods
2.1. Materials
The healthy leaves of Coriandrum sativum were collected from the botanical garden of faculty of science, Alexandria University, Alexandria, Egypt. Seeds of Lupinus termis (Var. Giza 2) were obtained from the Agricultural Research Center, Ministry of Agriculture, Giza, Egypt. Ultra purified water was used for experiment and all the chemicals were obtained from Sigma Aldrich, Alexandria, Egypt.
2.2. Preparation of plant extract
Fresh leaves of Coriandrum sativum (100 g) were chopped into fine pieces and transferred to sterile 250 mL conical flask. Aqueous leaf extract of Coriandrum sativum was prepared by heating the chopped fresh leaves in 100 mL of distilled water on sand bath for 30 min to facilitate the formation of aqueous extract and the extract was filtered through Whatmann No.1 filter paper to remove insoluble fractions and macromolecules. The filtrate was mixed with equal volume of acetone and make centrifugation for the resultant precipitate at 1500 rpm for 10 min and keep it in refrigerator at 4 °C. Use this extract as stabilizing and reducing agent.
2.3. Synthesis of silver nanoparticles
In the typical synthesis of silver nanoparticles, 12 mL of the aqueous extract of Coriandrum sativum was added to 88 mL of 1 mM (10−3 M) solution of silver nitrate in 250 mL Erlenmeyer flask. The resulting greenish mixture was incubated for 24 h in a rotary shaker (200 rpm) at 26 °C. The reaction was started at room temperature and in absence of light to minimize photo-activation of silver nitrate. The appearance of brown color indicate the completion of Ag reduction and the synthesis of CSL-AgNPs. The solution obtained was transferred to an amber colored bottle to prevent autoxidation of silver. Distilled water was used as controls throughout the experiment.
2.4. Characterization of silver nanoparticles
Indication of the bio-reduction of AgNPs in the aqueous C. sativum extract was evidenced by monitoring the absorption spectra in the range between 200 and 800 nm using double beam UV-vis spectrophotometer (T70/T80 series UV/Vis spectrophotometer PG Instrument Ltd., England). A spectrum of silver nanoparticles was plotted with wavelength nm on x-axis and absorbance on y-axis. XRD was recorded in the 2q range of 30–80° using XRD Brukerd 8 Advance CuKa Target, Germany, the energy of which was 8.04 keV and wavelength was 1.54 A°. The applied voltage was 40 kV and current was 25 mA. Scherer equation was used to estimate the crystallite size of the AgNPs. The morphology of silver nanoparticles was confirmed by Transmission Electron Microscopy (TEM). Analysis of silver nanoparticles were performed using a TEM (Joel JEM CX100, Japan) with an acceleration voltage of 80 kV. Silver nanoparticles were imaged after air drying of a droplet on a carbon-coated 200-mesh copper grid.
2.5. Germination experiments
For the study, Lupinus termis L (Giza 2) seeds were surface sterilized using 1% mercuric chloride and 70% ethanol for 5 min and were thoroughly washed with deionized water then soaked in sterilized distilled water for 24 h. Triplicate experiments were conducted in which each plate contained 10 seeds and placed equidistantly into 100 mm × 15 mm Petri dish containing 10 ml (2%) agar supplemented with different concentrations (0–900 ppm) AgNPs. Dishes were maintained under regulated conditions for 3 more days. After the completion of incubation period the germination was recorded 3 days after treatment and the effect of AgNPs on seed germination were evaluated according to the parameters described by Vashisth et al. (2010). The following equations were used to calculate germination percentage (%G) and relative germination (RG),
| (1) |
| (2) |
2.6. Influence of CSL-AgNPs on seedling growth
In another experiment with a certain selected concentrations of AgNPs (0, 100, 300 and 500 ppm) for the study of germination were selected considering that the worst case situation at which plants could be exposed is that 500 ppm. Seeds were allowed to germinate in each pot (15 cm diameter × 20 cm height), filled with pre acid washed sand for 5 days. All pots were placed in a growth chamber and seedlings were further grown under the following conditions: 16/8-h day/night period, controlled temperature 25/20 °C day/night and 80% relative humidity under photon flux density of 340 μmol m−2 s−1 light intensity. Aliquots of fifty milliliters of AgNPs suspension were administered to each pot day after day. After 10 days of plant growth, seedlings were harvested and separated into root and shoot and used immediately for root length, shoot length, number of leaves, fresh and dry weight analysis and biochemical parameters.
2.7. Germination indices
The seedling vigor indices (I&II) were calculated based on Vashisth and Nagarajan (2010) equations
| (3) |
| (4) |
Other parameters such as Plant height stress tolerance index (PHSI), Root length stress tolerance index (RLSI) and Dry matter stress tolerance index DMSI) are calculated by using the following formula
Plant height stress tolerance index was calculated by using the following formula
(PHSI) = Plant height of stressed plant /Plant height of control plant × 100
Root length stress tolerance index was calculated by using the following formula
(RLSI) = Root length of stressed plant / Root length of control plant × 100
Dry matter stress tolerance index was calculated by using the following formula
(DMSI) = Dry matter of stressed plant /Dry matter of control plant × 100
2.8. Statistical method
Statistical analysis was done using Statistical Package for Social Sciences (SPSS/version 20) software. The statistical test used as follow: Arthematic mean, standard deviation, for compare between more than two groups ANOVA-test was used for parametric data, followed by post hoc test by Duncan method Sokal and Rohlf (1995).
3. Results
3.1. Characterization of silver nanoparticles
The leaf extract of Coriandrum sativum was used as a reducing and stabilizing agents for the synthesis of C. sativum leaves silver nanoparticles (CSL-AgNPs). Rapid appearance of a yellowish-brown color in the reaction mixture as a result of surface plasmon, suggested the formation of colloidal AgNPs. The color noted by visual observation increased in intensity giving brown color after 24 h of incubation and hence confirmed the completion of reaction between leaf extract and AgNO3 (Fig. 1).
Fig. 1.

C. sativum leaves extract with AgNO3 before and after the synthesis of CSL-AgNPs.
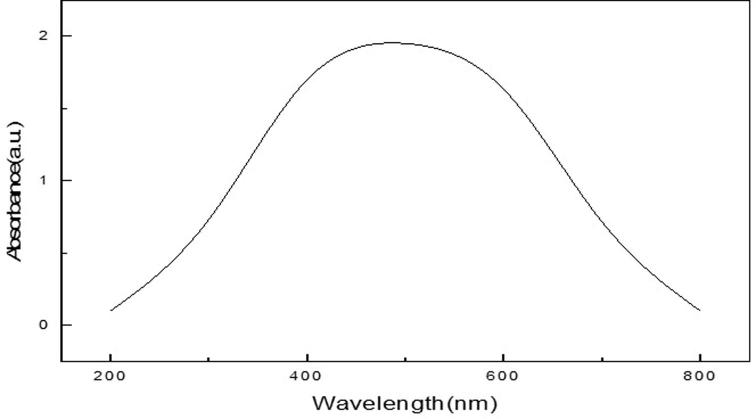
The analysis of silver nanoparticles by using UV–Visible spectrometric analysis showed absorption spectra at 450 nm and this suggesting the occurrence of bio-reduction for the silver nitrate forming silver nanoparticles (Fig. 2).
Fig. 2.
Absorbance peak of C. sativum leaf AgNPs at 450 nm of silver nanoparticles recorded by UV-visible spectra.
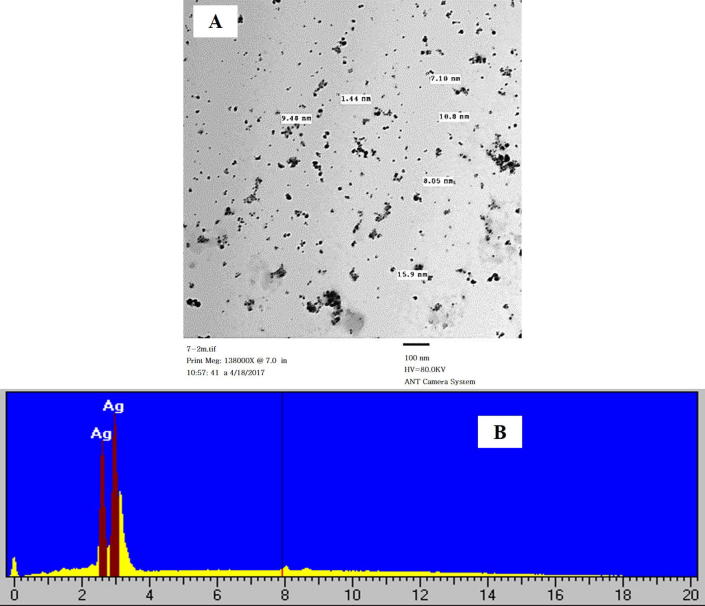
The examination of particle size of CSL-AgNPs by TEM showed that, the particle size ranged between 1 and 16 nm silver nanoparticles and the average size of the CSL-AgNPs ranged between 5 and 10 nm (Fig. 3A). The size and shape of CSL-AgNPs were uniform and are with varying shapes (Fig. 3). A significant content of AgNPs was recognized after quantitative analysis for GSL-AgNPs by energy dispersive X-ray analysis EDX (Fig. 3B).
Fig. 3.
Transmission electron microscope (TEM) micrograph of CSL-AgNPs spread in cooper grid (A) and Energy dispersive X-ray characterization spectrum of CSL-AgNPs. Visible peaks approve the presence of silver in the sample (B).
X-ray Diffraction Spectroscopy data showed the broad diffraction peaks of the CSL-AgNPs and are marked by their Bragg indices. Peaks indicating the presence of face centered unit cells of CSL-AgNPs. The dots mark the measured diffraction data, the continuous red and blue lines represent the calculated profile and difference between the observed and calculated intensities, respectively. The diffracted peaks at 2θ 38.2, 44.3, 64.7 and 77.4 positions were indexed to 111, 200, 220 and 311 planes of pure silver and that indicated the synthesis of CSL-AgNPs (Fig. 4).
Fig. 4.
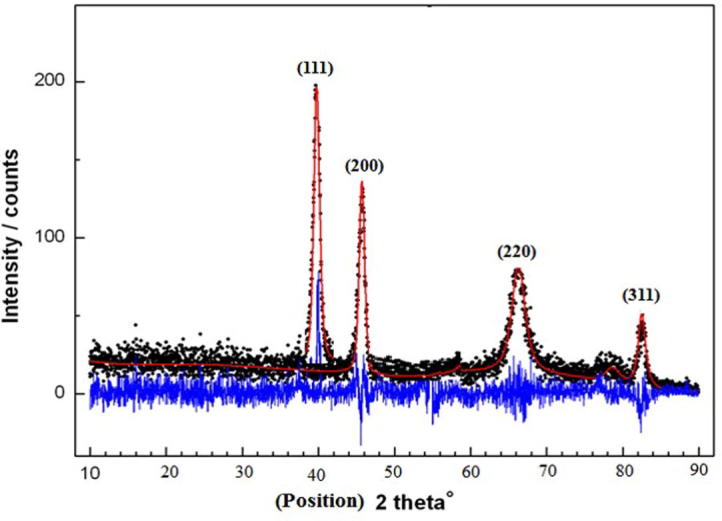
X-ray diffraction pattern of the synthesized C. sativum leaf extract -AgNPs.
3.2. Effect of CSL-AgNPs on Lupinus termis L. seed germination and seedling growth parameter
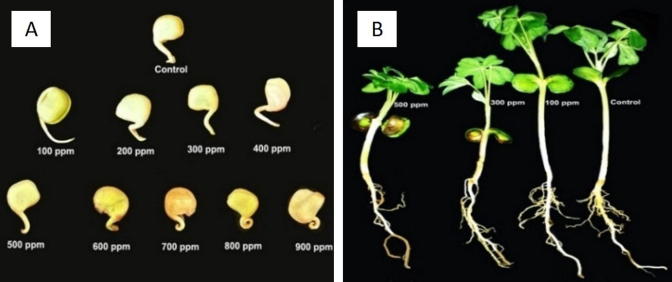
The data presented in Table 1 and showed in Fig. 5 showed that treatment of Lupinus termis L. seeds with different concentrations of AgNPs ranged from 100 ppm to 900 ppm acquired varied germination percentages and their germination percentage decreased with increasing the AgNPs concentration over 100 ppm. Also, the relative growth (RG) decreased to one third of the control by 900 ppm. The produced radicles were sensitive to higher concentrations of AgNPs as they are coiled and appeared brown from 600 ppm to 900 ppm as shown in Fig. 5 and Table 1. The relative germination decreased gradually with increasing AgNPs concentration more than 100 ppm (Table 1).
Table 1.
Effect of different concentrations of CSL-AgNPs (0–900 ppm) on germination percentage (%G) and relative germination (RG) of Lupinus termis L. after 5 days of treatment.
| 1507807527940Conc. CSL-AgNPs (ppm) | Germination percentage (%G) | Relative germination (RG) |
|---|---|---|
| 0 | 90% | 100% |
| 100 | 100% | 111% |
| 150 | 95% | 106% |
| 200 | 90% | 100% |
| 400 | 70% | 78% |
| 600 | 60% | 66.6% |
| 800 | 50% | 56% |
| 900 | 30% | 33% |
Fig. 5.
(A) Effect of different concentrations of CSL-AgNPs (0–900 ppm) on Lupinus termis L seed germination. (B) Effect of different concentrations of CSL-AgNPs (0, 100, 300 and 500 ppm) on growth parameters of Lupinus termis L.
3.3. Effect of CSL-AgNPs on seedling growth of Lupinus termis L
Table 2, Table 3 showed the diverse seedling growth parameters including fresh weight and dry weight, as well as, root and shoot elongation in L. termis seedlings exposed to various concentrations of CSL-AgNPs at 0, 100, 300 and 500 ppm. The seedling growth parameters have been considered as primary indicator for the phytotoxicity of CSL-AgNPs, especially with higher concentrations. Significant decrease in the fresh and dry weight of both shoot and root was observed upon exposure to higher concentrations of CSL-AgNPs (300 and 500 ppm) (Table 2, Table 3). On the other hand lower dose concentration of CSL-AgNPs (100 ppm) caused a noticeable increase in growth parameters, including fresh and dry weight of both shoot and root.
Table 2.
Effect of CSL-AgNPs on fresh and dry weight of root and shoot; root and shoot length of Lupinus termis L. seedlings after 10 days of treatment.
| Conc. CSL-AgNPs (PPm) | Root fresh weight (g) | Shoot fresh weight (g) | Seedling fresh weight | Root Length (cm) | Shoot Length (cm) | Seedling length (cm) |
|---|---|---|---|---|---|---|
| C | 7.8 ± 0.71a | 18.3 ± 1.66ab | 26.1 ± 2.37a | 8.3 ± 0.75ab | 14.3 ± 1.30a | 22.6 ± 2.05ab |
| 100 | 8.2 ± 0.63 a | 20.1 ± 1.55a | 28.3 ± 2.18a | 9.8 ± 0.75a | 15.6 ± 1.20 a | 25.4 ± 1.95 b |
| 300 | 6.5 ± 0.72ab | 16.1 ± 1.79bc | 22.6 ± 2.51b | 7.3 ± 0.81b | 14.2 ± 1.58 a | 21.5 ± 2.39 a |
| 500 | 5.8 ± 0.05b | 13.0 ± 0.12c | 18.8 ± 0.17c | 5.2 ± 0.05c | 11.7 ± 0.10b | 16.9 ± 0.15b |
| P< | 0.011* | 0.013* | 0.005* | 0.016* | 0.033* | 0.025* |
The same small letters indicate that there was no significant difference, while the different letters indicate that there was a significant difference between the two groups.
Table 3.
Effect of silver nanoparticles on of 10 days old Lupinus termis L. seedlings shoot and root dry mass.
| Conc. CSL-AgNPs (ppm) | Root dry Matter(g) | Shoot dry Matter(g) | Seedlings dry matter(g) |
|---|---|---|---|
| Control | 0.85 ± 0.08a | 1.82 ± 0.17a | 2.67 ± 0.24a |
| 100 | 0.96 ± 0.07a | 2.73 ± 0.21a | 3.69 ± 0.28b |
| 300 | 0.63 ± 0.07b | 1.94 ± 0.22a | 2.57 ± 0.29a |
| 500 | 0.32 ± 0.00c | 1.24 ± 0.01b | 1.56 ± 0.01c |
| P< | 0.002* | 0.0133* | 0.026* |
The same small letters indicate that there was no significant difference, while the different letters indicate that there was a significant difference between the two groups.
Similar pattern was observed for the effect of CSL-AgNPs in root and shoot length under different concentration of CSL-AgNPs. It was observed that, as the concentrations of AgNPs over 100 ppm caused also a statistically significant decline in root and shoot length as shown in Table 2.
3.4. Effect of CSL-AgNPs on growth indices
As shown in Table 4, there was a concentration-dependent reduction in different growth indices including plant height stress tolerance index (PHSI), root length stress tolerance index (RLSI), dry mass stress index (DMSI), vigor indexes I and II especially, upon exposure to the higher concentrations of CSL-AgNPs (Table 4). Seedlings of L. termis exposed to higher concentrations of CSL-AgNPs showed a parallel reduction in all measured growth indices and reached highly significance reduction in seedlings exposed to 300 and 500 ppm in comparison with control. Significant decrease in PHSI, RSLI and DMSI values (74.78, 62.65 and 58.43) was observed in 500 ppm concentrations of CSL-AgNPs. Conversely significant increase in PHSI, RSLI and DMSI values i.e. 112.3, 118.07 and 138.20 were noted in 100 ppm concentrations of CSL-AgNPs respectively (Table 4). Data with respect to the vigor indexes I and II clearly show that both increased up to 100 ppm concentration of CSL-AGNPs and decreased significantly both in 300 and 500 ppm concentrations of CSL-AgNPs (Table 4).
Table 4.
Effect of CSL-AgNPs on growth indices, plant height stress tolerance index (PHSI), root length stress tolerance index (RLSI), dry mass stress index (DMSI), vigor indexes I and II of Lupinus termis L. seedlings after 10 days of treatment growth indices.
| Conc. CSL-AgNPs (ppm) | PHSI% | RLSI% | DMSI% | Vigor index I | Vigor index II |
|---|---|---|---|---|---|
| Control | 100 | 100 | 100 | 2250 ± 204.55a | 213.6 ± 19.42a |
| 100 | 112.3 | 118.07 | 138.20 | 2260 ± 173.85a | 369.0 ± 28.38b |
| 300 | 95.13 | 87.95 | 96.25 | 1720 ± 191.11b | 205.6 ± 22.84a |
| 500 | 74.78 | 62.65 | 58.43 | 1183 ± 10.52c | 109.2 ± 0.97c |
| P< | 0.004* | 0.0113* | 0.022* | 0.001* | 0.001* |
4. Discussion
Nanotechnology applications are currently generally appropriated all through our life, and particularly in agricultural systems. Moreover, nanoparticles physicochemical characteristics, are among the potential possibility for controlling the redox status and changing the development, growth potential, performance, and plant quality. Using plant materials for synthesis of different kinds of nanoparticles has been revolutionized in the last few years (Palanisamy et al., 2014). In the present study, the particle size of the synthesized CSL-AgNPs ranging in between 1 and 16 nm, which may explain the capability of these particles to penetrate the cells. The mechanism of action of different kinds of nanoparticles is unclear in different organisms including plants (Kim et al., 2007, Kim et al., 2011, Mohamed et al., 2014).
Characterization of our synthesized CSL-AgNPs was done using different procedures such as UV–visible spectroscopy, electron microscope equipped with Energy dispersion X-ray diffraction and X-ray diffraction. Absorption spectrum of the prepared CSL-AgNPs showed one sharp peak with surface plasmon transition absorption indicating the synthesis of spherical AgNPs as reported by Forough and Farhadi (2010). This peak is well documented for various metal nanoparticles with particle size ranging from 2 to 100 nm (Suing and Seng, 2013).
The obtained X-ray diffraction peaks corresponded to cubic structure with face centered for Ag ions in its purified form and the size of silver nanoparticles ranging between 1 and 16 nm (Theivasanthi and Alagar, 2012, Arokiyaraj et al., 2014).Transmission electron microscope give us more confirmation, further inputs and more information about the morphology and size of the synthesized AgNPs in presence of C. sativum leaf extract ranging between 1 and 16 nm with spherical morphology (Agnihotri et al., 2014). Analysis of Ag ions by EDX equipped with TEM shows the presence of pure silver approving the synthesis of AgNPs (Ali et al., 2011).
From earlier studies, it has been known that one of the properties that determine the phytotoxicity of AgNPs in plants was the size of the particles, and the smaller particles the larger surface area that could result in higher cellular uptake (Geisler-Lee et al., 2013).
In higher plants, phytotoxicity should be investigated in order to progress a comprehensive nanoparticles toxicity profile (USEPA, 2005). As a rapid and widely acute phytotoxicity test seed germination and root elongation is used with several advantages: sensitivity, simplicity, low cost and suitability for unstable samples or chemicals (Munzuroglu and Geckil, 2002). It was well known that germination is considered as a physiological process beginning with water imbibition by seeds and culminating in the radicle emergence (Kordan, 1992).
The desired concentrations (100, 300 and 500 ppm of CSL-AgNPs) used to testify their effect on growth parameters of Lupinus termis seedlings were selected based on a preliminary test on seed germination and root growth. Maximum decline to only one fifth in germination percentage was recorded at 900 ppm silver nanoparticle concentration.
All mentioned growth parameters increased significantly with low concentration of CSL-AgNPs (100 ppm) signifying a simultaneously effect of this dose, which might direct low dose physiological inductive tolerance in Lupinus termis plant.
Silver nanoparticles was found to interfere with the activities of certain hydrolytic enzymes, required during germination process resulting in a reduction of per cent seed germination. Lin and Xing (2007) have also highlighted that seed germination is inhibited at higher concentrations of nanoparticles.
The present study revealed that the roots were sensitive to the increase in AgNPs concentrations. Morphologically, radicals of the germinated seeds were stunted with brown tips when they treated with high concentration of AgNPs. Correspondingly, Geisler-Lee et al. (2013) have demonstrated that AgNPs-treated seedlings had shorter roots and exhibited visible brown root tips as compared with control plants. As observed in this study, the higher sensitivity of roots to AgNPs might be due to the fact that the roots comes in direct contact with AgNPs in the exposure medium and also, the radicle was not adapted to the treatments (Yin et al., 2012).
Pollutants, in low concentration though having obviously inhibitory effect on root growth, may cannot pass through seed coats and not affect germination. This may clarify that seed germination in this study was not significantly modified by low concentration of nanoparticles, even it was induced by 100 ppm AgNPs treatment.
With regard to the root morphology of Lupinus termis, the control plants had long straight roots, but the plants exposed to AgNPs had much shorter roots regardless of brown and coiled radicles of the germinated seeds. As the concentration of AgNPs increased the browning phenomenon was more apparent in the root. Earlier it was also reported by Geisler-Lee et al. (2013), that exposure to different concentrations of AgNPs has resulted in dark browning of root tip and the inhibition of root hair development on the main seedling roots. Also, While, Yin et al. (2012) observed the damaged cells and broken root cap of L. multiflorum exposed to AgNPs, and wondered that abnormal damaged cells resulted in the loss of root gravitropism which was the reason of radicle coiling of AgNPs treated germinated seeds.
It may be attributed that the toxicity of nanoparticles was due to two different actions, (1) the chemical composition which can caused chemical toxicity. e.g., the release of (toxic) ions and (2) the surface, size and/or shape of the particles which stimuli stress (Bruinink and Stark, 2006). However, shoot fresh weight and shoot and root length increases was recorded at different concentrations of silver nanoparticle treatment, could be mediated via plant growth regulators (cytokinins and gibberellins) which are involved in cell division and cell elongation (Stampoulis et al., 2009). Mena et al. (2016) have been reported that decrease in shoot and root length of seedlings treated with higher silver nanoparticle and the recorded phenomenon is dose dependent. Vigor index represents the combined effect of various external and internal growth regulating factors. It was recorded an increase in vigor index of the treated seedlings at 100 ppm AgNPs treatment but higher concentration inhibited it.
Higher concentrations of CSL-AgNPs showed negative impact on different growth indices such as plant height stress tolerance index (PHSI), root length stress tolerance index (RLSI), dry mass stress index (DMSI), vigor indexes I and II. Our results are consistent with Lee et al., (2008) who examined the impact of Cu-NPs on bean (Phaseolus radiatus) and wheat (T. aestivum) plants. They found the seedling growth parameters of both plants were significantly decrease due to the presence of copper nanoparticles. On the other hand, another study done by Zheng et al. (2005) found that under exposure of spinach plant to low concentrations of TiO2NP a significant improvement in the growth of spinach was exhibited as compared to higher concentrations. According to our results and consistent with others we can concluded that inhibition of plant growth differ significantly among nanoparticles and plants, and it is associated with the nanoparticle concentration.
In conclusion, our results showed that using C. sativum leaf extract could be valuable material in a successful preparation of silver nanoparticles which giving an indication that silver nanoparticle treatment might improve the growth profile at 100 ppm. However, our research indicated that exposure of Lupinus termis seedlings to high concentrations of CSL-AgNPs (300 and 500 ppm) resulted in a highly significant reduction in all growth parameters and growth indices.
Acknowledgement
The authors would like to extend their sincere appreciation to the Dean of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-231.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agnihotri S., Mukherji S., Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014;4:3974–3983. [Google Scholar]
- Ali D.M., Sasikala M., Gunasekaran M., Thajuddin N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, oscillatoriawillei ntdm01. Digest J. Nanomater. Biostruct. 2011;6(2):385–390. [Google Scholar]
- Agricultural Research Contres Min. -Agri. of Egypt. Bull. 226:1–8.
- Arokiyaraj S., Arasu M.V., Vincent S., Prakash N.U., Choi S.H., Oh Y.K., Choi K.C., Kim K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: an in vitro study. Int. J. Nanomed. 2014;9:379–388. doi: 10.2147/IJN.S53546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K.J., Herth S. Plant nanotoxicology Trends. Plant Sci. 2012;17(3):180. doi: 10.1016/j.tplants.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Forough M., Farhadi K. Biological and green synthesis of silver nanoparticles. J. Eng. Environ. Sci. 2010;4(7):281–287. [Google Scholar]
- Geisler-Lee J., Wang Q., Yao Y., Zhang W., Geisler M., Li K., Huang Y., Chen Y., Kolmakov A., Ma X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology. 2013;7:323–337. doi: 10.3109/17435390.2012.658094. [DOI] [PubMed] [Google Scholar]
- Ismail M.A.S., Gamal M.G., Ghada S.M. Effect of Ascorbic acid and Niacin on Protein, Oil Fatty Acids and Antibacterial Activity of Lupinus termis Seeds. Int. J. Ph. Phyto. R. 15. 2014;6(4):866–873. [Google Scholar]
- Kim J.S., Eunye K., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles nanomedicine: nanotechnology. Biol. Med. 2007;3(2007):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Lee D.S., Ryu S.J., Choi D.S., Lee S.H. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli Korean. J. Microbiol. Biotechnol. 2011;39(1):77–85. [Google Scholar]
- Kordan H.A. Seed viability and germination: a multi-purpose exp. system. J. Biol. Educ. 1992;26:247–251. [Google Scholar]
- Lee W.M., An Y.J., Yoon H., Kweon H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolusradiatus) and wheat (Triticumaestivum): plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008;27:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- Lin D., Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Poll. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Ma X., Geiser-Lee J., Deng Y., Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010;408:3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Mena Z., Armendari R., Jose R., Videa P., Gardea J. Effects of silver nanoparticles on radish sprouts: root growth reduction and modifications in the nutritional value. Front. Plant Sci. 2016;7:1–11. doi: 10.3389/fpls.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.I., Amal A.H., Afaf I.S., Gehan A.E., Abdul-Aziz A., Farid M.A., Humaira H.R., Nadine M. Synthesis of eco-friendly silver nanoparticles using plant extracts and assessment of their antimicrobial activity. Fresenius Environ. Bull. 2014;23(1a):184–189. [Google Scholar]
- Mueller N.C., Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008;42:4447–4453. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- Munzuroglu O., Geckil H. Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticumaestivum and Cucumis sativus. Arch. Environ. Contam. Toxicol. 2002;43:203–213. doi: 10.1007/s00244-002-1116-4. [DOI] [PubMed] [Google Scholar]
- Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Miao A., Quigg A., Santschi P.H., Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology. 2008;17:372–438. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- Palanisamy N.K., Nas F., Amirulhusni A.N., Zaini M.Z., Hussaini J., Liew Jian Ping L.J., Durairaj R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas Aeruginosa. J. Nanobiotechnol. 2014;12:2. doi: 10.1186/1477-3155-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash M., Nair G., Chung M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. 2014;21:8858–8869. doi: 10.1007/s11356-014-2822-y. [DOI] [PubMed] [Google Scholar]
- Qian H., Peng X., Han X., Ren J., Sun L., Fu Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013;25:1947–1956. doi: 10.1016/s1001-0742(12)60301-5. [DOI] [PubMed] [Google Scholar]
- Raza S., Jrnsgard B. screening of white lupin accessions for morphological and yield traits. Afr. Crop Sci. J. 2005;13(2):135–141. [Google Scholar]
- Renata, N.W., 2013. Essential oil composition of the coriander (coriandrum sativum l.) herb depending on the development stage 66 (1), 53–60.
- Shi Q., Li S., Jia J., Jiang J. The Hedgehog-induced Smoothened conformational switch assembles a signaling complex that activates Fused by promoting its dimerization and phosphorylation. Development. 2011;138(19):4219–4231. doi: 10.1242/dev.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampoulis D., Sinha S.K., White J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009;43:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- Suing K.H., Seng T. Rapid and efficient synthesis of silver nanofluid using electrical discharge machining. J. Nanomat. 2013;3:1–7. [Google Scholar]
- Theivasanthi T., Alagar M. Electrolytic synthesis and characterizations of silver nanopowder. Nano Biomed. Eng. 2012;4(2):58–65. [Google Scholar]
- U.S. Environmental Protection Agency, 2005. Nanotechnology White Papere External Review Draft. Available from: <http://www.epa.gov/osa/pdfs/EPA_nanotechnology_white_paper_external_review_draft_12-02-2005>.
- Vashisth A., Nagarajan S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010;167:149–156. doi: 10.1016/j.jplph.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Yin L., Colman B.P., McGill B.M., Wright J.P., Bernhard E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE. 2012;7:1–7. doi: 10.1371/journal.pone.0047674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Hong F., Lu S., Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005;104:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]