Abstract
The present study was aimed to investigate the effect of dihydroartemisinin on the colon cancer cell proliferation and apoptosis. The results from MTT assay revealed a concentration and time dependent relation between the inhibition of SW 948 cell viability and dihydroartemisinin addition. The viability of SW 948 cells was reduced to 45 and 24% on treatment with 30 and 50 µM, respectively concentrations of dihydroartemisinin after 48 h. Morphological examination of SW 948 cells showed attainment of rounded shape and cluster formation on treatment with dihydroartemisinin. Western blot analysis showed a significant increase in the activation of caspase-3 and expression of cleaved PARP by dihydroartemisinin treatment. The activation of PPARγ was increased significantly in SW 948 cells by treatment with dihydroartemisinin. Compared to control, the migration potential of SW 948 cells was reduced significantly (p < 0.005) and the expression levels of MMP-2 and -9 inhibited by dihydroartemisinin at 50 µM concentration. In the dihydroartemisinin treatment group colon tumor formation was significantly inhibited on treatment with 20 mg/kg doses of dihydroartemisinin after 30 days. Therefore, dihydroartemisinin inhibits colon cancer growth by inducing apoptosis and increasing the expression of PPARγ. Thus dihydroartemisinin can be used for the treatment of colon cancer.
Keywords: Dihydroartemisinin, Apoptosis, Condensation, Matrix metalloprotein, Viability
1. Introduction
Colorectal cancer, one of the most commonly detected cancer in the world is responsible for more than 10% of cancer-related deaths (Ferlay et al., 2010). Colorectal cancer has very high rate of metastasis and the most common target of distant metastasis is liver. Among the colorectal cancer patients around 15% have been found to possess liver metastases even at the time of diagnosis (Manfredi et al., 2006). It has been observed that liver resection is followed by recurrence in one-third of the liver metastasis patients (Yamada et al., 2001). Thus the development of the efficient strategy for colon cancer treatment is urgently desired. Peroxisome proliferator-activated receptors γ (PPARγ) present in the various types of cells plays a vital role in the inhibition of inflammatory processes (Bassaganya-Riera et al., 2004). Increase in the expression of PPARγ by various chemotherapeutic agents has been used for the inhibition of inflammation and carcinoma treatment (Bassaganya-Riera et al., 2011). Studies have demonstrated that PPARγ exhibits inhibitory effect on the expression of genes involved in inflammation (8). Its mechanism of action involves interaction with various factors such as NF-κB, p65, p50 or mitogen-activated protein kinase (Ricote and Glass, 2007).
Metabolites recovered from medicinal plants have wide level of biological properties (Antonisamy et al., 2015, Balamurugan, 2015, Rathi et al., 2015, Nandhini and Stella Bai, 2015). Artemisinin isolated from a Chinese herb Artemisia annua is a well-known anti-malarial drug which has been used from very long time in China (Meshnick, 2002, O'Neill, 2004). Various other analogs of artemisinin such as dihydroartemisinin, artesunate, etc. have also shown promising anti-malarial activity and are therefore used for the clinical treatment of malaria. Screening of the artemisinin and its analogs against various types of cancers including, breast and ovarian cancers led to a marked reduction in the rate of carcinoma cell proliferation and metastasis (Chen et al., 2009, Kalaiselvi et al., 2016, Neelamkavil and Thoppil, 2016, Valsan and Raphael, 2016). Use of dihydroartemisinin against various normal cell lines showed no cytotoxicity (Chen et al., 2009). The promising anti-proliferative activity of dihydroartemisinin against cancer cell lines and its safety against normal cells makes it a potential candidate for cancer therapy. The present study was performed to investigate the effect of dihydroartemisinin on the colon cancer cell growth. The results demonstrated that dihydroartemisinin inhibited the viability and induced apoptosis through increase in the expression of PPARγ.
2. Materials and methods
2.1. Chemicals
Dihydroartiminisin and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich Co. (St. Louis, MO).
2.2. Cell culture
The SW 948, human colon cancer cell line was purchased from the Cell Bank of the Chinese Academy of Sciences, Shanghai, China. The cells were cultured in RPMI-1640 medium (Gibco, Shanghai, China), supplemented with 10% FBC (Gibco) and penicillin/streptomycin (1:100, Sigma) in an incubator with humid atmosphere of 5% CO2 at 37 °C.
2.3. Analysis of cell proliferation
The cell viability was determined by suing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, SW 948 cell suspension was prepared by stirring the cells with PBS after washing. The concentration of cells was determined and the cells were then seeded at a density of 2 × 106 per well into the 96-well plates. The plates were cultured overnight and subsequently treated with various concentrations of the dihydroartiminisin. After incubation for 24, and 48 h, MTT solution (Sigma-Aldrich, St. Louis, MO, USA) was added to each for the well and incubated for 4 h. The medium was removed and DMSO (150 µL) was added to the wells. The absorbance for each well was measured in triplicates at 570 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
2.4. Western blot analysis
SW 948 cells were seeded at a density of 2 × 106 cells per well 6-well plates and incubated with various concentrations of dihydroartiminisin for 48 h. The cells were harvested and washed twice with phosphate-buffered saline. RIPA lysis buffer kit (Best Bio, shanghai, China) was used for the extraction of total proteins from the cells on ice for 45 min. The cell lysates were collected and then centrifuged at 4 °C for 20 min at 12,000×g to remove the debris. Bicinchoninic acid protein assay kit (Multi sciences, Hangzhou, China) was used for the detection of concentration of proteins. The proteins were separated by electrophoresis using 10% SDS-PAGE gels and transferred to a PVDF membrane (Merck Millipore, Darmstadt, Germany). The membrane blocking was performed using BSA for 1 h followed by incubation with primary polyclonal antibodies overnight 4 °C. The membranes were the washed again with PBS before incubation with goat-anti-rabbit secondary antibodies at room temperature for 1 h. The protein bands were detected by using enhanced chemiluminescence Western blotting detection kit (Santa Cruz Biotechnology, Inc.).
2.5. Analysis of apoptosis
Apoptosis induction in SW 948 cells by dihydroartiminisin treatment was measured by the Annexin V-FITC Apoptosis Detection kit (BD Bioscience, San Jose, CA, USA). The cells after incubation for 24 h in 6-well plates at a density of 2.5 × 106 cells per ell were treated with various concentrations of dihydroartiminisin for 48 h. Following incubation, the cells were washed two times with ice cold PBS and subsequently re-suspended in 100 µl binding buffer. The cells were treated with 3 µl of Annexin V-FITC (BD Bioscience) and 10 µl propidium iodide (PI; BD Bioscience) at room temperature for 15 min. Flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used for the fluorescent intensity determination three times.
2.6. Wound healing assay
The SW 948 colon cancer cells at a density of 2 × 105were seeded into 6-well plates and allowed to attain confluent monolayers. The cells were then cultured for 12 h in FBS free RPMI-1640 medium. In the center of each well a line was drawn using a 200 µl pipette tip for producing wound area. The cells were washed with PBS two times to remove the non-adherent cells. Various concentration of dihydroartiminisin were added to each of the well in medium containing 1% FBS. The images were captured using digital photography at a magnification of ×200.
2.7. Migration assay
For migration assay a 24-well Trans-well chamber (Corning Life Sciences, Corning, New York, NY, USA) bearing an 8-µm-pore PET membrane was used. Briefly, into the lower chamber 600 µl RPMI 1640supplemented with 10% FBS was placed. In the upper chamber, SW 948 colon cancer cells at a density of 2 × 105 cells per mL into the medium containing dihydroartiminisin were added and allowed to migrate. After 24 h, the cells in the upper chamber were cleaned using cotton-tipped swab and the migrated cells in the lower chamber were washed with PBS and subjected to crystal violet staining after fixation. The optical density was recorded at 570 nm for quantification of the migrated cells.
2.8. In vivo tumorigenicity assay
Twenty pathogen-free male Balbc-nu mice (age, 8 weeks) were obtained from the Animal Institute of the Chinese Academy of Medical Science (Guangzhou, China). All the procedures on animals were performed in accordance with standard protocols approved by the Ethics Committee of Hunan Normal University (Changsha, China). The animals were given colonic carcinogen (Sigma-Aldrich) and DSS (MP Biomedicals, LLC, Aurora, OH) for producing colon cancer mice model. The mice in the treatment group were then given dihydroartiminisin at a dosage of 20 mg/kg body weight daily for one month intra-peritoneally. After completion of the treatment the animals were sacrificed to extract the colon cancer tissues.
2.9. Statistical analysis
The data presented are the mean of ±SD and were analyzed using SPSS software, version 15.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was also used for the data analysis. P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Effect of dihydroartemisinin on SW 948 cell viability
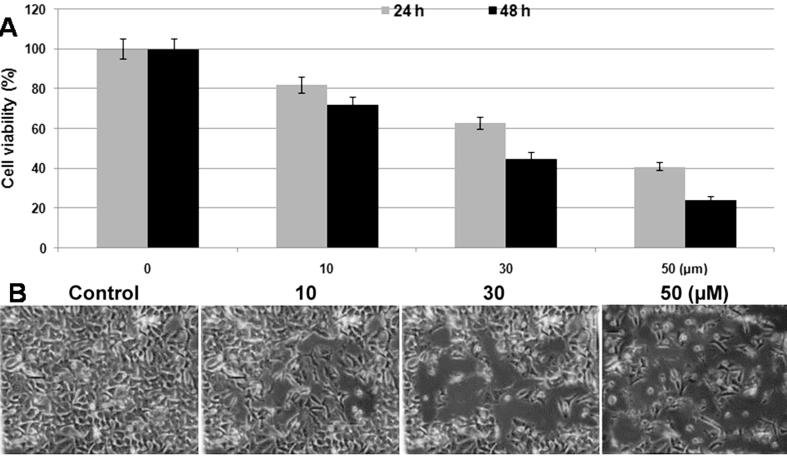
The MTT assay revealed a concentration and time dependent relation between the inhibition of SW 948cell viability and dihydroartemisinin addition (Fig.1A). Treatment with various concentrations of dihydroartemisinin revealed significant inhibition from 10 µM after 48 h. The viability of SW 948cells was reduced to 45 and 24% on treatment with 30 and 50 µM, respectively concentrations of dihydroartemisinin after 48 h. However, the inhibition in viability was 37 and 59% on treatment with 30 and 50 µM, respectively concentrations after 24 h (Fig.1A). Morphological examination of SW 948 cells showed attainment of rounded shape and cluster formation on treatment with dihydroartemisinin at 50 µM concentration (Fig.1B).
Fig. 1.
Dihydroartemisinin inhibits SW 948 cell viability in concentration based manner. (A) SW 948cells were incubated for 24 and 48 h with various concentrations of dihydroartemisinin and then analyzed by MTT assay. For each concentration the absorbance was measured in triplicates. (B) Morphological examination of SW 948 cells after treatment with dihydroartemisinin for 48 h showed apoptotic features. Magnification ×220.
3.2. Effect of dihydroartemisininon apoptosis induction in SW 948 cells
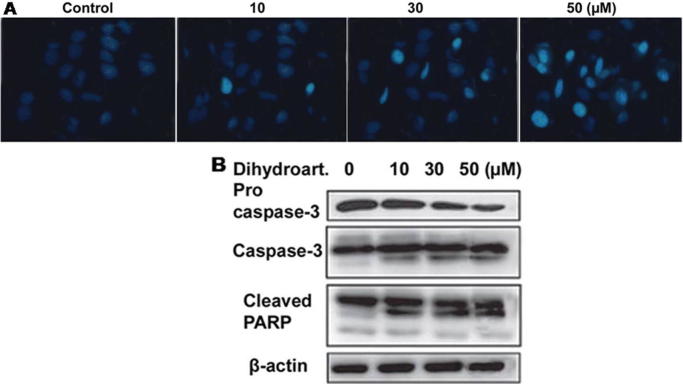
Hoechst 33342 staining revealed apoptosis induction in SW 948 cells on treatment with various concentrations of dihydroartemisinin for 48 h. Compared to the control cells, dihydroartemisinin treatment increased the population cells with condensation of nuclear material in concentration dependent manner (Fig.2A). Western blot analysis showed a significant increase in the activation of caspase-3 and expression of cleaved PARP on treatment with dihydroartemisinin for 48 h in SW 948 cells (Fig.2B).
Fig. 2.
Dihydroartemisinin induces apoptosis in SW 948 cells. (A) SW 948 cells after incubation for 48 h with dihydroartemisinin were examined by fluorescent microscopy. (B) The cells after treatment with various concentrations of dihydroartemisinin for 48 h were subjected to western blot analysis.
3.3. Effect of dihydroartemisininon activity of PPARγ
Western blot analysis showed that the activation of PPARγ was increased significantly in SW 948 cells on treatment with dihydroartemisinin for 48 h (Fig. 3). Increase in the concentration of dihydroartemisinin from 30to 50 µM also increased the expression level of PPARγ.
Fig. 3.
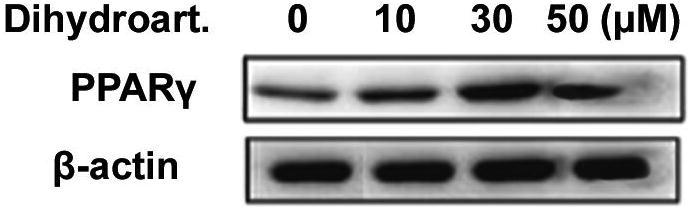
Dihydroartemisinin treatment increases the activation of PPARγ in SW 948 cells. After incubation for 48 h, the cells were analyzed for the activation of PPARγ using western blot assay. Β-actin was used as the internal loading control.
3.4. Effect of dihydroartemisinin on SW 948 cell migration
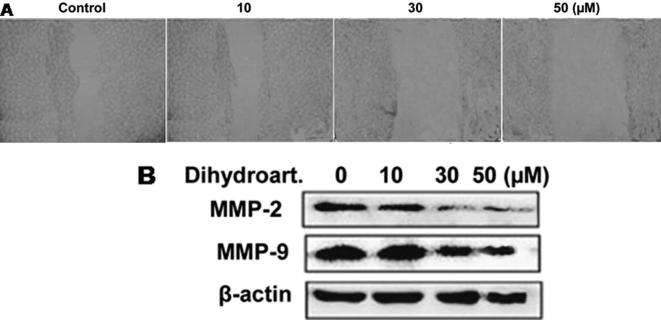
Analysis of the migration potential of SW 948 cells using Matrigel chamber revealed a significant decrease on treatment with dihydroartemisinin. Compared to control, the migration potential of SW 948 cells was reduced significantly (p < 0.005) by 50 µM concentration of dihydroartemisinin (Fig.4A). Dihydroartemisinin treatment at a concentration of50 µM markedly inhibited the expression levels of MMP-2 and -9 activities in SW 948 cells (Fig.4B).
Fig. 4.
Dihydroartemisinin treatment inhibited the migration potential of SW 948 cells. (A) The after attaining confluence were wounded using pipette tip, treated with dihydroartemisinin and then analyzed for migration potential. (B) The cells treated with dihydroartemisinin for 48 h were analyzed for the expression of MMP-2 and -9 using western blot analysis.
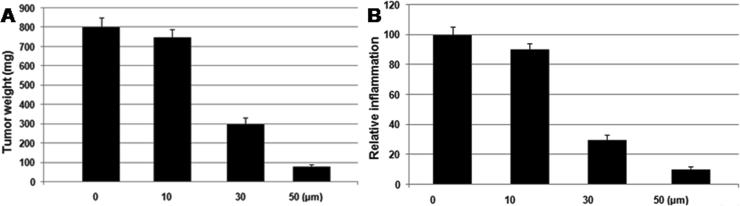
3.5. Dihydroartemisinin inhibits tumor formation inmice model
Treatment of the AOM/DSS mice model with dihydroartemisinin inhibited the colon tissue inflammation markedly compared to the control (Fig. 5). In the dihydroartemisinin treatment group colon tumor formation was significantly inhibited on treatment with 20 mg/kg doses of dihydroartemisinin after 30 days (Fig. 5).
Fig. 5.
Dihydroartemisinin treatment for 30 days inhibits the colon cancer growth and inflammation of colon in the mice model. The mice after treatment with 20 mg/kg doses of dihydroartemisinin for 30 days were sacrificed to extract the colon tissues for histopathological examination.
4. Discussion
Dihydroartemisinin is a potent anti-cancer candidate because of its marked potential to inhibit cancer cell viability without inducing any toxicity in the normal cells (Chen et al., 2009). In the present study effect of dihydroartemisinin on the colon cancer cell viability was investigated. Dihydroartemisinin inhibited the viability and induced apoptosis in colon cancer cells through increase in the expression of PPARγ. Breast and ovary cancer cell proliferation is inhibited significantly by treatment of the cells with dihydroartemisinin (Singh and Lai, 2001, Noorudheen and Chandrasekharan, 2016, Santhosh et al., 2016, Sreeshma et al., 2016). In the present study dihydroartemisinin treatment inhibited the viability of SW 948 colon cancer cells markedly in concentration and time dependent manner compared to the control cells. The inhibition of cell viability was marked by dihydroartemisinin treatment after 48 h. Apoptosis, programmed death of cells is very important for removal of unwanted cells from the body. The mechanism of apoptosis can involve either mitochondrial non-mitochondrial pathways (Lorenzo and Susin, 2007, Puthur, 2016). The results from present study demonstrated that dihydroartemisinin treatment induced apoptosis in the SW 948 cells. Treatment of the colon cancer cells with dihydroartemisinin significantly increased the expression of activated caspase-3 and cleaved PARP. The cells became rounded and shrunken in size following treatment with dihydroartemisinin. PPARγ present in the various types of cells plays a vital role in the inhibition of inflammatory processes. Increase in the expression of PPARγ by various chemotherapeutic agents has been used for the inhibition of inflammation and carcinoma treatment (Serasanambati and Chilakapati, 2016). The colorectal cancer has been suppressed by the use of chemotherapeutic agents activating PPARγ which in turn activate tumor suppressor genes (Yamaguchi et al., 2008). In the current study dihydroartemisinin treatment of SW 948 cells caused a marked increase in the expression of PPARγ. PPARs plays an important role in the suppression of NF-κB which is involved in the generation of reactive oxygen species and tumor necrosis factor (Surh, 2008). In the present study dihydroartemisinin treatment of the mice with colon cancer inhibited the formation of colon inflammation. The migration of cancer cells to adjacent and distant organs if facilitated by the expression of matrix metalloproteins (Chiu et al., 2011). In the present study treatment of colon cancer cells with dihydroartemisinin marked inhibited the expression of MMP-2 and MMP-9. In the mice model of colon cancer, dihydroartemisinin treatment reduced tumor growth and inhibited the formation of inflammation.
5. Conclusion
In summary, dihydroartemisinin inhibits colon cancer growth by inducing apoptosis and increasing the expression of PPARγ. Thus dihydroartemisinin can be used for the treatment of colon cancer.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgement
The funding is Natural Science Foundation of Xinjiang Province (No: 2016D01C015).
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha lam. in wistar rats. South Ind. J. Biol. Sci. 2015;1:34–37. [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci. 2015;1:47–51. [Google Scholar]
- Bassaganya-Riera J., Reynolds K., Martino-Catt S. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Bassaganya-Riera J., DiGuardo M., Climent M. Activation of PPAR gamma and delta by dietary punicic acid ameliorates intestinal inflammation in mice. Br. J. Nutr. 2011;106:878–886. doi: 10.1017/S0007114511001188. [DOI] [PubMed] [Google Scholar]
- Chen T., Li M., Zhang R., Wang H. Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy. J. Cell Mol. Med. 2009;13:1358–1370. doi: 10.1111/j.1582-4934.2008.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.W., Lin T.H., Huang W.S. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol. Appl. Pharmacol. 2011;255(316–326):2011. doi: 10.1016/j.taap.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Kalaiselvi V., Binu T.V., Radha S.R. Preliminary phytochemical analysis of the various leaf extracts of Mimusops elengi L. South Ind. J. Biol. Sci. 2016;2:24–29. [Google Scholar]
- Lorenzo H.K., Susin S.A. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist. Updat. 2007;10:235–255. doi: 10.1016/j.drup.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Manfredi S., Lepage C., Hatem C., Coatmeur O., Faivre J., Bouvier A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshnick S.R. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Nandhini V.S., Stella Bai G.V. In-vitro Phytopharmacological effect and cardio protective activity of Rauvolfia tetraphylla L. South Ind. J. Biol. Sci. 2015;1:97–102. [Google Scholar]
- Neelamkavil S.V., Thoppil J.E. Evaluation of the anticancer potential of the traditional medicinal herb Isodon coetsa. South Ind. J. Biol. Sci. 2016;2:41–45. [Google Scholar]
- Noorudheen N., Chandrasekharan D.K. Effect of ethanolic extract of Phyllanthus emblica on captan induced oxidative stress in vivo. South Ind. J. Biol. Sci. 2016;2:95–102. [Google Scholar]
- O'Neill P.M. Medicinal chemistry: a worthy adversary for malaria. Nature. 2004;430:838–839. doi: 10.1038/430838a. [DOI] [PubMed] [Google Scholar]
- Puthur J.T. Antioxidants and cellular antioxidation mechanism in plants. South Ind. J. Biol. Sci. 2016;2:14–17. [Google Scholar]
- Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci. 2015;1:60–65. [Google Scholar]
- Ricote M., Glass C.K. PPARs and molecular mechanisms of trans repression. Biochim. Biophys. Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh S.K., Venugopal A., Radhakrishnan M.C. Study on the phytochemical, antibacterial and antioxidant activities of Simarouba glauca. South Ind. J. Biol. Sci. 2016;2:119–124. [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of nuclear factor kappa B (NF-kB) in human diseases-a review. South Ind. J. Biol. Sci. 2016;2:368–387. [Google Scholar]
- Singh N.P., Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70:49–56. doi: 10.1016/s0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- Sreeshma P.S., Raphael K.R., Baby A.A. Pharmacognostic studies of leaves of Naravelia zeylanica (Linn) DC. South Ind. J. Biol. Sci. 2016;2:179–182. [Google Scholar]
- Surh Y.J. NF-kappa B and Nrf2 as potential chemo-preventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac. J. Clin. Nutr. 2008;17(Suppl. 1):269–272. [PubMed] [Google Scholar]
- Valsan A.L., Raphael K.R. Pharmacognostic profile of Averrhoa bilimbi Linn. leaves. South Ind. J. Biol. Sci. 2016;2:75–80. [Google Scholar]
- Yamada H., Katoh H., Kondo S., Okushiba S., Morikawa T. Repeat hepatectomy for recurrent hepatic metastases from colorectal cancer. Hepatogastroenterology. 2001;48:828–830. [PubMed] [Google Scholar]
- Yamaguchi K., Cekanova M., McEntee M.F. Peroxisome proliferator-activated receptor ligand MCC-555 suppresses intestinal polyps in ApcMin/+ mice via extracellular signal regulated kinase and peroxisome proliferator-activated receptor-dependent pathways. Mol. Cancer Ther. 2008;7:2779–2787. doi: 10.1158/1535-7163.MCT-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]