Abstract
Potassium bromate (KBrO3) is widely used as a food additive and is a major water disinfection by-product. The present study reports the side effects of KBrO3 administration in Swiss mice. Animals were randomly divided into three groups: control, low dose KBrO3 (100 mg/kg/day) and high dose KBrO3 (200 mg/kg/day) groups. Administration of KBrO3 led to decreased white blood corpuscles (WBCs), red blood corpuscles (RBCs) and platelets count in the animals of both the high and the low dose groups. Altered lipid profile represented as low density lipoprotein (LDL), high density lipoprotein (HDL) and cholesterol levels were observed in plasma samples of both KBrO3 treated groups of mice. Also, an increased plasma level of LDH was detected in both KBrO3 treated groups. Histological investigations showed impaired renal and hepatic histology that was concomitant with increased plasma Creatinine level in both of KBrO3-treated groups. Nevertheless, decreased glutathione (GSH) level in both renal and hepatic tissue of mice after KBrO3 intake was detected. These results show that KBrO3 has serious damaging effects and therefore, its use should be avoided.
Keywords: Platelets, Lipid profile, LDH, Creatinine, Reduced glutathione
1. Introduction
Potassium bromate (KBrO3) is a well-known flour improver that acts as a maturing agent (Vadlamani and Seib, 1999). It has been in use as a food additive for the past 90 years (Oloyede and Sunmonu, 2009). It acts principally in the late dough stage giving strength and elasticity to the dough during the baking process while also promoting the rise of bread. KBrO3 is also used in beer making, cheese production and is commonly added to fish paste products (Ahmad and Mahmood, 2014). Additionally, it is used in pharmaceutical and cosmotic industries and is a constituent of cold wave hair solutions (Oloyede and Sunmonu, 2009). Moreover, KBrO3 can appear as a byproduct in an ozonization of water containing bromide. As a result of KBrO3 biotransformation, free radicals generation can cause oxidative damage to essential cellular macromolecules, leading to marked nephrotoxicity and cancer in experimental animals (Chipman et al., 1998). International Agency for Research on Cancer (IARC) has classified KBrO3 as a possible human carcinogen (group 2B) and its application in food processing was restricted. Indeed, many previous reports has documented that KBrO3 can induce multiple organ toxicity in humans and experimental animals (Farombi et al., 2002, Kujawska et al., 2013, Ahmad et al., 2015) and that kidney is considered to be the primary target organ of these dangerous compound (Kurokawa et al., 1990, Ahmad et al., 2013). KBrO3 is extremely irritating and injurious to tissues especially those of the central nervous system (CNS) and kidneys. The pathological findings included renal tissue damage and haemolysis (Robert and William, 1996). Carcinogenic and mutagenic effects of KBrO3 have been also reported in experimental animals (Kurokawa et al., 1987). Several cases of accidental poisoning in children resulting from ingestion of bromate solution and sugar contaminated with bromate were reported as the source of an outbreak of mild poisoning in New Zealand (Paul, 1966). Consequently, KBrO3 has been banned in several countries including the United Kingdom in 1990, Nigeria in 1993 and Canada in 1994 (Oloyede and Sunmonu, 2009). Toxicological studies have convincingly shown that KBrO3 affects the nutritional quality of bread as the main vitamins available in bread are degraded (Sai et al., 1992). It is known that KBrO3 induces oxidative stress in tissues (Sai et al., 1991, Watanabe et al., 1992, Parsons and Chipman, 1992, Parsons and Chipman, 2000) that could be the basis of bromate-induced carcinogenesis (Chipman et al., 2006). The present study attempts to assess the effects of oral administration of KBrO3 on the lipid profile in plasma, oxidative stress, hepatic and renal histomorphology of Swiss mice using two different doses of KBrO3 to compare their effects.
2. Materials and methods
2.1. Animals
Forty five (45) Swiss Webster (SW) mice were obtained from animal house-College of pharmacy-king Saud University and maintained and monitored in a specific pathogen-free environment. All animal procedures were performed in accordance with the standards set forth in the Guidelines for the Care and Use of Experimental Animals issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The study protocol was approved by the Animal Ethics Committee at King Saud University. All animals were allowed to acclimatize in plastic cages inside a well-ventilated room for one week prior to the experiment. The animals were maintained under standard laboratory conditions (temperature of 23 °C, relative humidity of 60–70% and a 12-h light/dark cycle), fed a diet of standard commercial pellets and given water ad libitum.
2.2. KBrO3 preparation and dosing schedule
Potassium bromate salt, a product of British drug home limited, Poole England was supplied in its white crystalline form by ASILA chemicals (Saudi Arabia). It was then dissolved in water to prepare the 100 mg/kg dose (0.5 gm/L) and the 200 mg/kg dose (1 gm/L). Animals were divided into 3 groups as follows: Group (I) control group (was given distilled water); Group (II) Low dose KBrO3 group (was given 100 mg/kg); Group (III) High dose KBrO3 group (was given 200 mg/kg). KBro3 was orally administered daily through oral intubation at the two doses of 100 and 200 mg/kg/day for 42 days.
2.3. Sample collection
Blood was collected from the heart in heparinized tubes and plasma was obtained for biochemical investigations. Plasma was stored at −80 °C until use. Small pieces of liver and kidneys were removed, cut and put in sterile saline. The pieces were then fixed in 10% neutral buffered formalin and then embedded in paraffin.
2.4. Histological analysis of hepatic and renal tissues
The preparation of tissues for histological examination was done as described by Krause (2001); the photomicrographs were observed using the Leitz, DIALUX research microscope at x200. Pathological evaluation in H/E stained tissue sections was done by a pathologist blinded for the experimental regimen.
2.5. Cell blood count (CBC)
Whole blood samples were analyzed with an automatic Vet abc™ Animal Blood Counter (Horiba ABX, Montpellier, France) using the hematology kits specified for that instrument (Horiba ABX, France) according to the manufacturer’s instructions.
2.6. Determination of creatinine level in plasma
Plasma samples were analyzed using commercial kits (bioMerieux, Marcy I’Etoile, France) for Creatinine according to the instructions of the manufacturer. Absorbance was measured with an Ultrospec 2000 U/V spectrophotometer (Amersham Pharmacia Biotech, Cambridge, England).
2.7. Lactate dehydrogenase (LDH)
Lactate dehydrogenase was determined using specified kits LiquiUV Test (Human, Germany) according to the manufacturer’s instructions. Briefly, 20 μl of plasma was added to 1000 μl buffer solution (provided by in the kit) then incubated in cuvettes for 5 min at 30 °C. After that, 250 μl of the substrate was mixed with the solution and the absorbance was monitored after 1, 2 and 3 min. The colour development was detected at 340 nm in a spectrophotometer.
2.8. Lipid profile in plasma
LDL, HDL and total cholesterol levels were measured by an enzymatic colorimetric kit (Wako Chemicals USA, Inc.). Briefly, 10 μl of plasma were put into tubes and 1 ml of colour reagent solution was then added. 10 μl of standard solution (provided by in the kit), were put into tubes and 1 ml of colour reagent solution was then added. The solution was mixed well and incubated at 37 °C for 5 min. The colour development was detected at 500 nm in a spectrophotometer.
2.9. Glutathione (GSH) assay
Glutathione content was determined according to the procedure of Beutler et al. (1963) with some modification. Briefly, 0.20 ml of tissue supernatant was mixed with 1.5 ml precipitating solution containing 1.67% glacial metaphosphoric acid, 0.20% Na-EDTA and 30% NaCl. The mixture was allowed to stand for 5 min at room temperature and centrifuged 1000g for 5 min. One ml clear supernatant was mixed with 4 ml 0.30 M Na2HPO4 and 0.50 ml DTNB reagent (40 mg 5, 5′dithiobis-(2-nitrobenzoic acid dissolved in 1% sodium citrate). A blank was similarly prepared in which 0.20 ml water was used instead of the brain supernatant. The absorbance of the color was measured at 412 nm in a spectrophotometer.
2.10. Statistical analysis
Prior to further statistical analysis, the data were tested for normality using the Anderson-Darling test, as well as for homogeneity variances. The data was normally distributed and is expressed as the mean ± standard error of the mean (SEM). Significant differences among the groups were analysed by one- or two-way ANOVA followed by Bonferroni’s test for multiple comparisons using PRISM statistical software (GraphPad Software). The data was also reanalysed by one- or two-way ANOVA followed by Tukey’s post-test using SPSS software, version 17. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Decreased WBCs, RBCs and platelets count after KBrO3 intake
WBCs count was significantly decreased in both of KBrO3 treated groups. As shown in Table 1, the low dose of KBrO3 was associated with a lower number of WBCs in comparison to the control group. Additionally, the high dose of KBrO3 was accompanied with a much more decrease in the WBCs count in comparison to either the control or the low dose group. RBCs count was also decreased in the low dose KBrO3 group in comparison to the control group. Similarly, the decrease in RBCs count was higher in the high dose KBrO3 group in comparison to both the low dose and the control one. Platelets count was having a similar pattern of decrease in both of KBrO3 treated groups. A significantly decreased Platelets count was detected in the low dose KBrO3 group. On the other hand, the high dose KBrO3 group has showed a lower platelets count in comparison to either the control group or the low dose KBrO3 group.
Table 1.
Effect of KBrO3 treatment on the RBCs, WBCs and platelets count RBCs, WBCs and platelets count were measured in the three groups of mice, and the results are presented as the means ± SEM (n = 10), *P < 0.05 for low dose KBrO3 group vs. control; #P < 0.05 for high dose KBrO3 group vs. control.
| Mean total leukocyte count (×109/L) | Mean total reticulocyte count (×109/L) | Mean platelet count (×109/L) | |
|---|---|---|---|
| Control | 11.3 ± 0.62 | 8.3352 ± 0.13200 | 875 ± 38 |
| KBrO3 (100 mg/dl) | 10.0 ± 0.58* | 7.2550 ± 0.3294* | 423 ± 42* |
| KBrO3 (200 mg/dl) | 9.5 ± 0.93# | 7.1375 ± 0.4019# | 405 ± 63# |
3.2. Altered lipid profile in plasma of KBrO3 treated mice
Disturbance in lipid profile in plasma is considered as an indicator for many physiological disorders. LDL, HDL and cholesterol levels in plasma samples of the three experimental groups were investigated. Both of the low and the high doses of KBrO3 were accompanied with a significant reduction in the plasma concentrations of HDL (Fig.1a). Conversely, the plasma level of LDL was increased in both of the low and the high dose KBrO3 groups. A significant increase was detected in the high dose KBrO3 group in comparison to the control group (Fig.1b). Nevertheless, the change in the plasma level of cholesterol (Fig.1c) in both of KBrO3 groups was not significant in comparison to the control group.
Fig. 1.
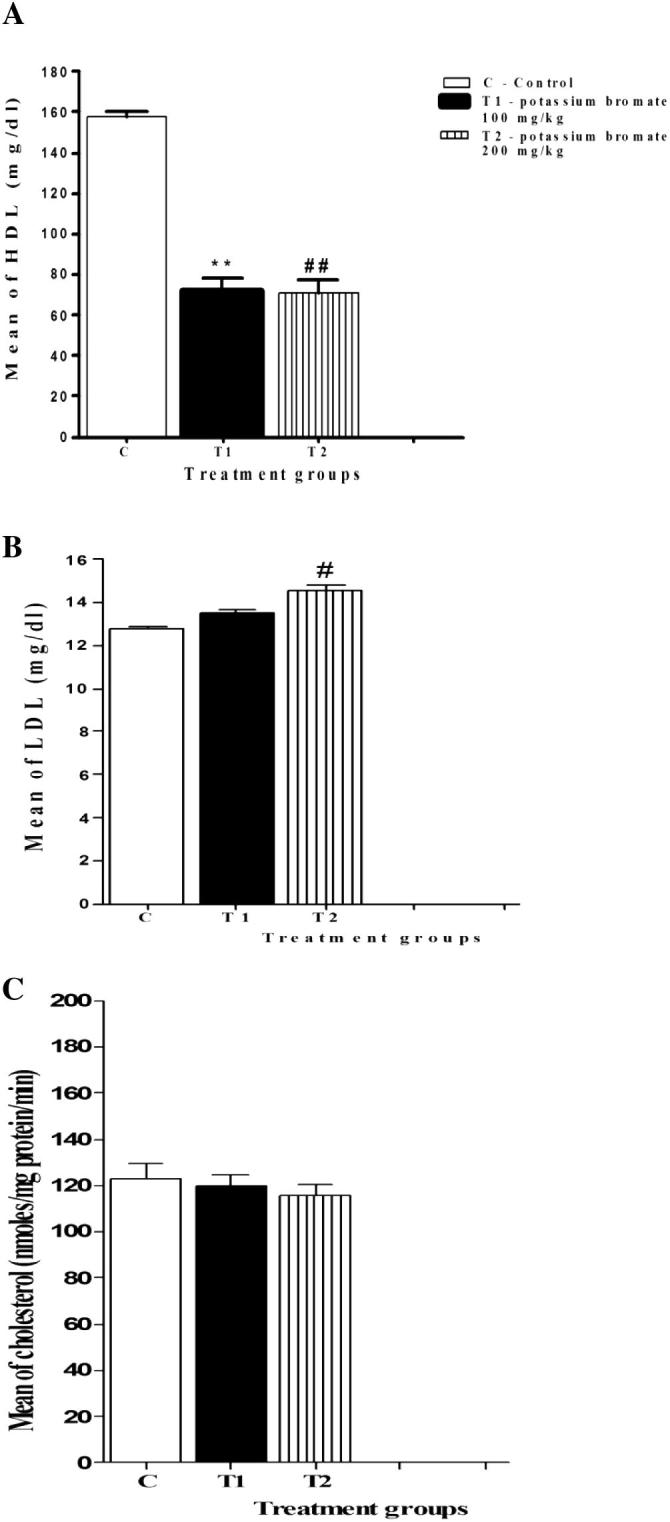
Effect of KBrO3 on the lipid profile in plasma samples of treated mice using two different doses of KBrO3. (a) Plasma level of high density lipoprotein, (b) Plasma level of low density lipoprotein, (c) Plasma level of cholesterol. The data are the mean ± SEM for 10 mice per group. *P < 0.05 for low dose KBrO3 treated group vs. control group; #P < 0.05 for high dose KBrO3treated group vs. control group; +P < 0.05 for high dose KBrO3 treated group vs. low dose KBrO3 treated group.
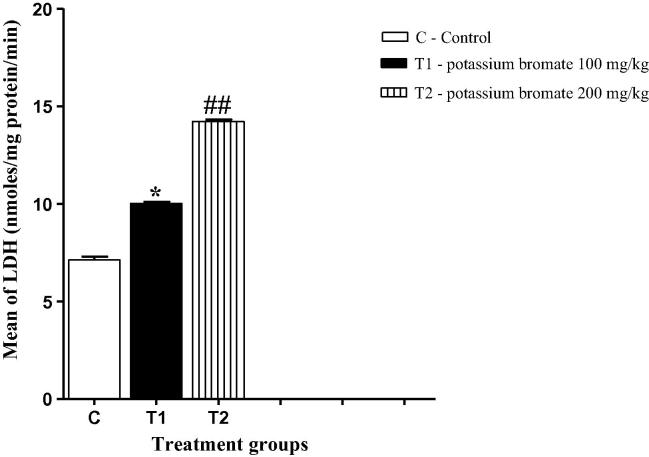
3.3. KBrO3 treatment was associated with an increased plasma level of LDH
LDH is often used as a marker of tissue breakdown and can function as an indicator for liver toxicity. Fig. 2 illustrates that both groups of the high and the low doses of potassium bromate were associated with a significant increase in the plasma level of LDH in comparison to the control group. However, the high dose of potassium bromate was accompanied with a much more increase in the LDH level in comparison to either the control or the low dose group.
Fig. 2.
Effect of KBrO3 on the lactate dehydrogenase level in plasma samples of treated mice. The data are the mean ± SEM for 10 mice per group. *P < 0.05 for low dose KBrO3 treated group vs. control group; #P < 0.05 for high dose KBrO3treated group vs. control group; +P < 0.05 for high dose KBrO3 treated group vs. low dose KBrO3 treated group.
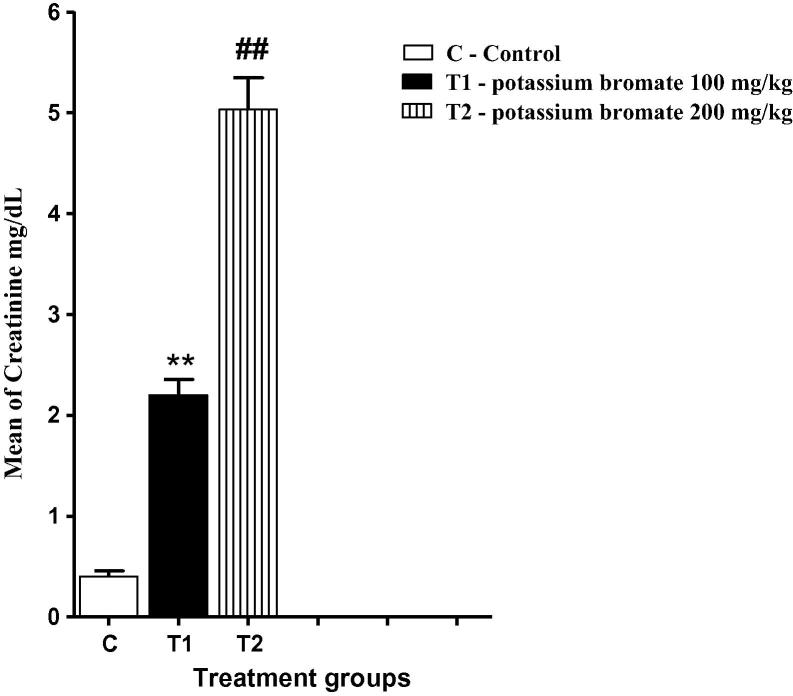
3.4. Impaired hepatic and renal histology concomitant with increased plasma Creatinine in both KBrO3 groups
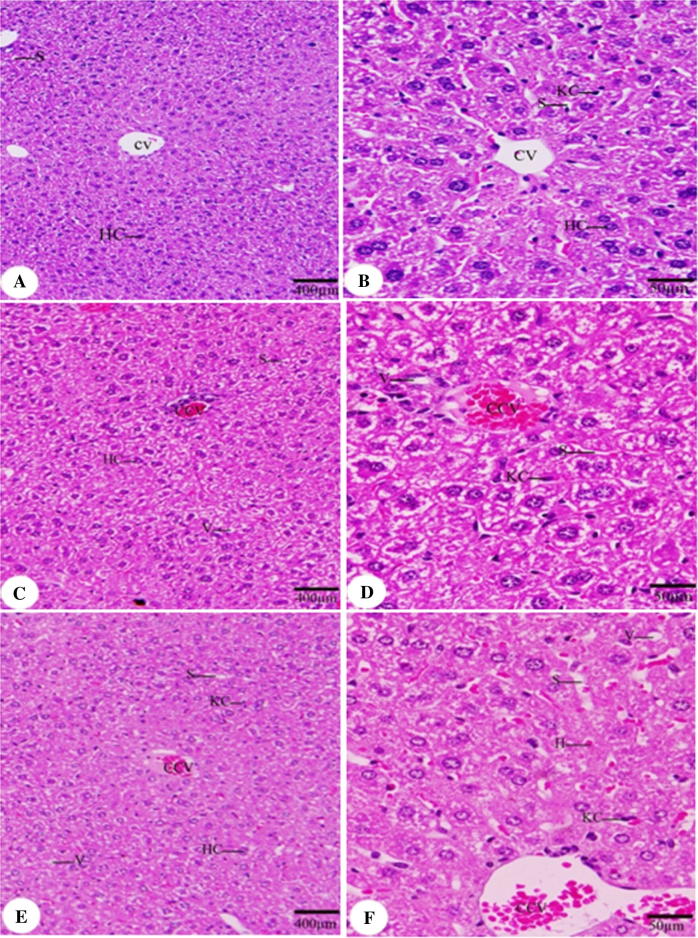
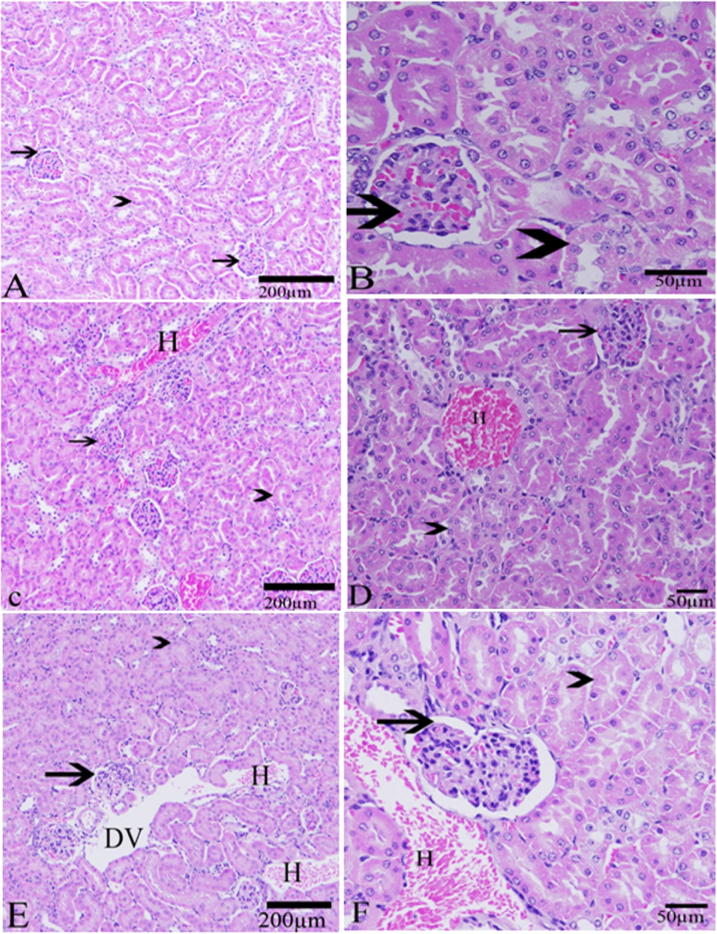
As a result of KBrO3 treatment, hepatic tissue sections of KBrO3 treated mice have showed a congestion of central vein with a relative increase in Kuppfer cells (KCs) in comparison to the control group (Fig. 3). Renal tissue of KBrO3 treated group of mice was largely affected in comparison to the control group. Hemorrhage was seen in both KBrO3 treated groups. Dilated blood vessels were seen in the high dose KBrO3 treated group (Fig. 4). To confirm these findings, Creatinine level in plasma, which is considered as an important indicator for kidney function, was determined. As illustrated in Fig. 5, a significant increase in the plasma level of Creatinine in both of KBrO3 treated groups was detected. The low dose group had a higher plasma level of Creatinine in comparison to the control. The high dose group has exhibited a much more increase in the plasma level of Creatinine in comparison to both the control and the low dose one.
Fig. 3.
Effect of KBrO3 on the histology of liver. Sections of liver showing central vein (CV), congested central vein (CCV), hepatic sinusoid (S), vacuoles (V), hepatic cell (HC), Kuppfer cell (KC). (A, B) control group, (C, D) potassium bromate 100 mg/kg group, (E, F) potassium bromate 200 mg/kg group. Scale bar = 400 μm in A, C, E and 50 μm in B, D, F.
Fig. 4.
Effect of KBrO3 on the histology of kidney. sections in the kidney showing the kidney tubules (arrow head), Bowman’s capsule (arrow),Hemorrhage (H) and dilated blood vessels (DV). (A, B) control group, (C, D) potassium bromate 100 mg/kg group, (E, F) potassium bromate200 mg/kg group. (H & E stain, Scale bar = 200 μm in A, C, E and 50 μm in B, D, F).
Fig. 5.
Effect of KBrO3 on the plasma level of Creatinine. The data are the mean ± SEM for 10 mice per group. *P < 0.05 for low dose KBrO3 treated group vs. control group; #P < 0.05 for high dose KBrO3 treated group vs. control group; +P < 0.05 for high dose KBrO3 treated group vs. low dose KBrO3 treated group.
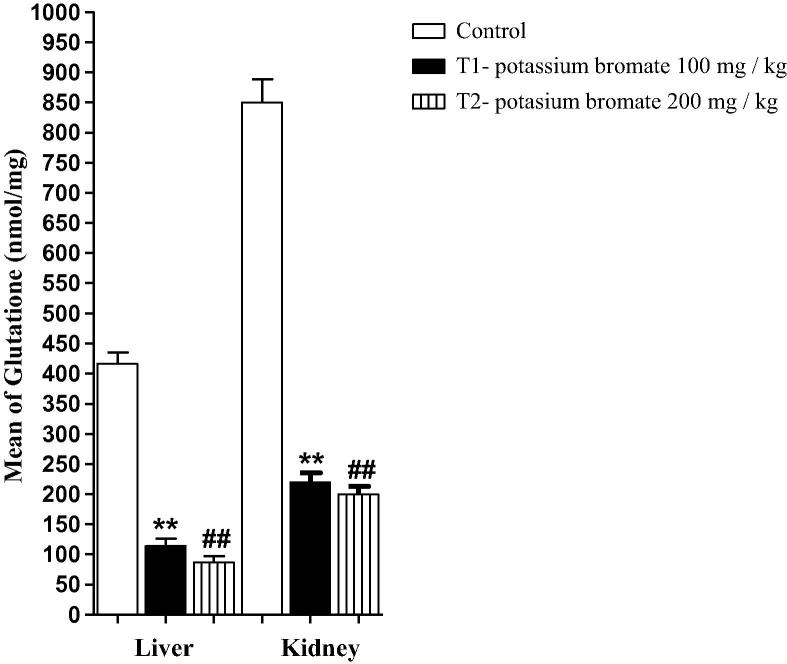
3.5. Decreased glutathione level in both renal and hepatic tissues of KBrO3 treated mice
Reduced glutathione (GSH) is an important antioxidant that plays a crucial role in nearly all living organisms. KBrO3 treatment had a negative impact on both the renal and the hepatic level of this important molecule. In the low dose KBrO3 group, both of the renal and the hepatic levels of GSH were significantly decreased in comparison to that of the control group. At the same time, a significant reduction in the level of this crucial molecule was also monitored in both the renal and hepatic homogenates of the high dose KBrO3 group in comparison to either the control group or the low dose KBrO3 group (Fig. 6).
Fig. 6.
Effect of KBrO3 on the hepatic and renal level of GSH. The data are the mean ± SEM for 10 mice per group. *P < 0.05 for low dose KBrO3 treated group vs. control group; #P < 0.05 for high dose KBrO3treated group vs. control group; +P < 0.05 for high dose KBrO3 treated group vs. low dose KBrO3 treated group.
4. Discussion
Potassium bromate (KBrO3) is widely used as improving additive for bread making (Ahmad et al., 2015) and marketed as a neutralizer in home permanent cold wave hair kits that caused several cases of accidental poisoning in children resulting from the ingestion of this solution (Paul, 1966). Due to its hazardous effects, it has been forbidden in various countries (Oloyede and Sunmonu, 2009). The current study was designed to investigate some of the biochemical changes induced by KBrO3 intake in Swiss Mice. We have observed that the total reticulocyte, leukocyte and platelet counts in the plasma samples of both KBrO3 treated groups have been significantly decreased in comparison to the control group. These reductions in the leukocyte and platelet counts could be due to the DNA strand breakage in these cells induced by the oxidative stress associated with KBrO3, (Chipman et al., 1998, Sai et al., 2000, Parsons and Chipman, 2000, Thompson and Westfall, 1949). Furthermore, there could have been bone marrow suppression with selective megakaryocytic depression (Hoffbrand et al., 2004). So, the reductions in the RBCs, WBCs and platelets could imply selective systemic toxicity effect by KBrO3. Lipid profile represents an important indicator of several pathological conditions. The most common metabolic contributor to the coronary artery disease is the atherogenic lipoprotein profile, characterized by an increased LDL level and a deficiency of HDL level (Superko et al., 2002). Many studies have reported that increased level of LDL is associated with higher risk of atherosclerosis while elevated level of HDL is linked to reduced occurrences of cardiovascular disorders (Grover-Paez and Zavalza-omez, 2009, Olukanni et al., 2013). In the current study, the level of plasma HDL decreased with both doses of KBrO3 leading to elevated atherogenic index which can be used to predict the risk for development of cardiovascular disorders. Therefore, the high ratio of LDL to HDL, caused by KBrO3, may implicate increased tendency for the development of atherosclerosis. LDH is considered as a liver toxicity indicator and the increased LDH level in KBrO3 groups that has been observed in the current study is in accordance with previous reports (Ahmad et al., 2014). In another study, KBrO3 induced chromosomal aberrations (CA) and decreased both the cell proliferation index (PI) and the mitotic index (MI) of human peripheral lymphocytes in vitro (Kaya and Topaktaş, 2007). Histological observation of hepatic tissue sections has confirmed the liver pathology due to KBrO3. Previous studies have illustrated that KBrO3 treatment in wister rats have hepatotoxic effects (Oyewo et al., 2013). KBrO3-mediated renal injury in Wistar rats was also recorded before (Khan and Sultana, 2005). In the current study, histological findings in renal tissue of KBrO3 treated groups were supported by previous reports (Kurokawa et al., 1990). A dose-dependent increase in the numbers of eosinophilic droplets within the proximal tubule epithelium was observed in male F344 rats exposed to 20,100, 200, or 400 mg/L KBrO3 for 12 weeks (Wolf, 1998). Kurokawa et al. (1987) reported similar lesions in proximal renal tubules of male F344 rats following 13 weeks exposure of 500 mg/L KBrO3. In addition to that, Dodd et al. (2013) have reported similar results in the same animal model. So the results in the current study agree very well with the results of these sub chronic studies. Elevated levels of Creatinine in plasma were observed confirming previous reports that KBrO3 ingestion causes acute kidney damage (Kurokawa et al., 1990, Bao et al., 2008). Reduced glutathione is an important antioxidant molecule that can be used by many organs, including kidney and liver, to withstand the induced oxidative stress. Previous studies have illustrated that KBrO3 can decrease the tissue content of this molecule (Chipman et al., 1998, Parsons and Chipman, 2000). In agreement with these previous results, the current study illustrated that renal and hepatic levels of GSH have been reduced after KBrO3 treatment with more reduction in the high dose KBrO3 group in comparison to the low dose one. A similar effect has been observed in our previous study in brain tissue (Ajarem et al., 2016) Taken together, our data reveals that KBrO3 has several harmful effects on the biochemical and histological levels and therefore, its consumption should be prohibited.
5. Conclusions
KBrO3 treatment in Swiss Mice has several consequences like disturbance in blood biochemistry, renal and hepatic histopathology and decreased antioxidant capacity. These dangerous effects should stop its use in human being.
Competing interests
The authors declare no conflicts of interest. This manuscript has not been published or submitted elsewhere. This work complies with the Ethical Policies of the Journal and has been conducted under internationally accepted ethical standards following relevant ethical review.
Authors’ contributions
NGA put the design of the experiment and carried out all the lab work, preparing the figures and drafted the manuscript. JA participated in the design of the study and helped to draft the manuscript. AA participated in the design of the study and helped to draft the manuscript. SNM participated in the design of the study, participated in the figures preparation and helped to draft and edit the manuscript. MAM participated in the design of the study, helped to perform the statistical analysis and helped to draft the manuscript.
Acknowledgments
This project was supported by King Saud University, Deanship of Scientific Research, college of Science Research Center.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Naif G. Altoom, Email: nalotaiby1@hotmail.com.
Jamaan Ajarem, Email: jajarem@ksu.edu.sa.
Ahmed A. Allam, Email: aallam@ksu.edu.sa.
Saleh N. Maodaa, Email: maodaa_28@yahoo.com.
Mostafa A. Abdel-Maksoud, Email: harrany@gmail.com.
References
- Ahmad M.K., Mahmood R. Protective effect of taurine against potassium bromate-induced hemoglobin oxidation, oxidative stress, and impairment of antioxidant defense system in blood. Environ. Toxicol. 2014 doi: 10.1002/tox.22045. [DOI] [PubMed] [Google Scholar]
- Ahmad M.K., Khan A.A., Mahmood R. Taurine ameliorates potassium bromate-induced kidney damage in rats. Amino Acids. 2013;45:1109–1121. doi: 10.1007/s00726-013-1563-4. [DOI] [PubMed] [Google Scholar]
- Ahmad M.K., Amani S., Mahmood R. Potassium bromate causes cell lysis and induces oxidative stress in human erythrocytes. Environ. Toxicol. 2014;29:138–145. doi: 10.1002/tox.20780. [DOI] [PubMed] [Google Scholar]
- Ahmad M.K., Khan A.A., Ali S.N., Mahmood R. Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA-protein cross-linking and oxidative stress in rat intestine. PLoS One. 2015 doi: 10.1371/journal.pone.0119137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajarem J., Altoom N.G., Allam A.A., Maodaa S.N., Abdel-Maksoud M.A., Chow B.K. Oral administration of potassium bromate induces neurobehavioral changes, alters cerebral neurotransmitters level and impairs brain tissue of swiss mice. Behav. Brain Funct. 2016;12(1):14. doi: 10.1186/s12993-016-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Yao X.S., Tsi D., Yau C.C., Chia C.S., Nagai H. Protective effects of bilberry (Vaccinium myrtillus L.) extract on KBr O3-induced kidney damage in mice. J. Agric. Food Chem. 2008;56:420–425. doi: 10.1021/jf072640s. [DOI] [PubMed] [Google Scholar]
- Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Chipman J.K., Davies J.E., Parsons J.L., Nair J., O’Neill G., Fawell J.K. DNA oxidation by potassium bromate; a direct mechanism or linked to lipid peroxidation? Toxicology. 1998;126:93–102. doi: 10.1016/s0300-483x(97)00174-1. [DOI] [PubMed] [Google Scholar]
- Chipman J.K., Parsons J.L., Beddowes E.J. The multiple influences of glutathione on bromate genotoxicity: implications of dose–response relationship. Toxicology. 2006;221:187–189. doi: 10.1016/j.tox.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Dodd D.E., Layko D.K., Cantwell K.E., Willson G.A., Thomas R.S. Subchronic toxicity evaluation of potassium bromate in Fischer 344 rats. Environ. Toxicol. Pharmacol. 2013;3:1227–1234. doi: 10.1016/j.etap.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Farombi E.O., Alabi M.C., Akuru T.O. Kolaviron modulates cellular redox status and impairment of membrane protein activities induced by potassium bromate KBr O3 in rats. Pharmacol. Res. 2002;45:63–68. doi: 10.1006/phrs.2001.0907. [DOI] [PubMed] [Google Scholar]
- Grover-Paez F., Zavalza omez A.B. Endothelial dysfunction and cardiovascular risk factors. Diab. Res. Clin. Prac. 2009;84:1–10. doi: 10.1016/j.diabres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A.V., Petit J.E., Moss P.A. 4th ed. Blackwell; Oxford: 2004. Essential Haematology; pp. 252–253. [Google Scholar]
- Kaya F.F., Topaktaş M. Genotoxic effects of potassium bromate on human peripheral lymphocytes in vitro. Mutat. Res. 2007;626:48–52. doi: 10.1016/j.mrgentox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Khan N., Sultana M. Inhibition of potassium bromate-induced renal oxidative stress and hyperproliferative response by Nymphaea alba in Wistar rats. J. Enzyme Inhib. Med. Chem. 2005;20:275–283. doi: 10.1080/14756360400028119. [DOI] [PubMed] [Google Scholar]
- Krause W.J. The Parthenon Publishing Group; New York, London: 2001. The Art of Examining and Interpreting Histological Preparations. A Student Handbook; p. 176. [Google Scholar]
- Kujawska M., Ignatowicz E., Ewertowska M., Adamska T., Markowski J., Jodynis-Liebert J. Attenuation of KBr O3-induced renal and hepatic toxicity by cloudy apple juice in rat. Phytother. Res. 2013;27:1214–1219. doi: 10.1002/ptr.4848. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y., Takayama S., Konishi Y. Long term in vivo carcinogenicity tests of potassium bromate, sodium hypochlorite and sodium chlorite conducted in Japan. Environ. Health Prospect. 1987;69:221–236. doi: 10.1289/ehp.8669221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa Y., Maekawa A., Takahashi M., Hayashi Y. Toxicity and carcinogenicity of potassium bromate – a new renal carcinogen. Environ. Health Perspect. 1990;87:309–335. doi: 10.1289/ehp.9087309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oloyede O.B., Sunmonu T.O. Potassium bromate content of selected bread samples in Ilorin, Central Nigeria and its effect on some enzymes of rat liver and kidney. Food Chem. Toxicol. 2009;47:2067–2070. doi: 10.1016/j.fct.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Olukanni O.D., Akande O.T., Alagbe Y.O., Adeyemi O.S., Olukanni A.T., Daramola G.G. Lemon juice elevated level of reduced glutathione and improved lipid profile in Wistar rats. American-Eurasian J. Agric. Environ. Sci. 2013;13:1246–1251. [Google Scholar]
- Oyewo O.O., Onyije F.M., Awoniran P.O. Hepatotoxic effect of potassium bromate on the liver of Wistar rats. J. Morphol. Sci. 2013;30:107–114. [Google Scholar]
- Parsons J.L., Chipman J.K. DNA oxidation by potassium bromate: a direct mechanism or linked to peroxidation. Toxicology. 1992;126:93–102. doi: 10.1016/s0300-483x(97)00174-1. [DOI] [PubMed] [Google Scholar]
- Parsons J.L., Chipman J.K. The role of glutathione in DNA damage by potassium bromate in vitro. Mutagenesis. 2000;15:311–316. doi: 10.1093/mutage/15.4.311. [DOI] [PubMed] [Google Scholar]
- Paul A.H. Chemical food poisoning by potassium bromate. N. Z. Med. J. 1966;65:33–40. [PubMed] [Google Scholar]
- Robert I.A., William B.C. Carcinogenicity of potassium bromate in rabbit. Biol. Edu. 1996;34:114–120. [Google Scholar]
- Sai K., Takagi A., Umemura T. Relation of 8-hydrogen guanosine formation in rat kidney to lipid peroxidation, glutathione level and relative organ weight after a single dose administration of potassium bromate. Jpn. J. Cancer Res. 1991;82:165–169. doi: 10.1111/j.1349-7006.1991.tb01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai K., Hayashi M., Takagi A., Hasegawa R., Sofuni T., Kurokawa Y. Effects of antioxidants on induction of micronuclei in rat peripheral blood reticulocytes by potassium bromate. Mutat. Res. 1992;269:113–118. doi: 10.1016/0027-5107(92)90166-y. [DOI] [PubMed] [Google Scholar]
- Sai K., Kanno J., Hasegawa R., Trosko J.E., Inoue T. Prevention of the down-regulation of gap junctional intercellular communication by green tea in the liver of mice fed pentachlorophenol. Carcinogenesis. 2000;21:1671–1676. doi: 10.1093/carcin/21.9.1671. [DOI] [PubMed] [Google Scholar]
- Superko H.R., Nejedly M., Garrett B. Small LDL and its clinical importance as a new CAD risk factor: a female case study. Prog. Cardiovasc. Nurs. 2002;17:167–173. doi: 10.1111/j.0889-7204.2002.01453.x. [DOI] [PubMed] [Google Scholar]
- Thompson H.C., Westfall S.W. Potassium bromate poisoning. Report of a case due to ingestion of a cold wave neutralizer. J. Paed. 1949;34:362–364. [Google Scholar]
- Vadlamani K.R., Seib P.A. Effect of zinc and aluminium ions in bread making. Cereal Chem. 1999;76:355–360. [Google Scholar]
- Watanabe T., Abe T., Satoh M. Two children with bromate intoxication due to ingestion of the second preparation for permanent hair waving. Act. Paed. Jpn. 1992;34:601–605. doi: 10.1111/j.1442-200x.1992.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Wolf, D.C., 1998. Personal communication from Douglas Wolf, National Health and Environmental Effects Research Laboratory, U.S. EPA to Jennifer Jinot, National Center for Environmental Assessment, U.S. EPA. January 12, 1998.