Abstract
This study aims at distinguishing honey based on botanical and geographical sources. Different floral honey samples were collected from diverse geographical locations of Saudi Arabia. UV spectroscopy in combination with chemometric analysis including Hierarchical Cluster Analysis (HCA), Principal Component Analysis (PCA), and Soft Independent Modeling of Class Analogy (SIMCA) were used to classify honey samples. HCA and PCA presented the initial clustering pattern to differentiate between botanical as well as geographical sources. The SIMCA model clearly separated the Ziziphus sp. and other monofloral honey samples based on different locations and botanical sources. The results successfully discriminated the honey samples of different botanical and geographical sources validating the segregation observed using few physicochemical parameters that are regularly used for discrimination.
Keywords: UV spectroscopy, Chemometrics, Honey, Saudi Arabia
1. Introduction
Honey is an important food that is categorized as a functional food i.e. a class of foods that has health promoting and disease preventing properties in addition to multiple nutritional values. Honey is acknowledged as a natural product with tremendous medicinal values. Its properties can only be guaranteed if the honey is authentic. Many documented biological activities of honey include antioxidant, immunomodulatory, cancer prophylactic and curative properties (Al-yahya et al., 2013). Experimental evidence indicate that honey from variety of floral and geographical sources like Manuka, Saudi Ziziphus, Toulang, Chestnut, Rhododendron, Pasture, Jelly bush, Blossom, Sage and Neem may exert several beneficial health effects. These include gastroprotective, hepatoprotective, reproductive, hypoglycemic, antioxidant, antihypertensive, antibacterial, antifungal and anti-inflammatory effects (Erejuwa et al., 2012, Ansari et al., 2013, Noori et al., 2013, Can et al., 2015). Honey varies in its composition depending on several factors such as botanical source, geographical origin, and storage conditions (Gheldof et al., 2002, Yao et al., 2004, Khan et al., 2016, Kaygusuz et al., 2016). Bees forage different plants, and due to different proportions of the possible sources of nectar, honey is always a mixture of different sources (Oddo and Bogdanov, 2004). Monofloral honey is produced from nectar that mainly originates from a single plant species and possesses distinctive organoleptic characteristics. These honey types of distinct botanical origin are often traded at a higher price than honeys from mixed botanical origins and can thus be considered premium products (Donarski et al., 2010, Can et al., 2015).
In Saudi Arabia, honey is a highly regarded product. Honey is widely consumed in Saudi Arabia as a curative agent either alone or as a carrier for medicinal herbal mixtures and is used as the main constituent in several traditional foods throughout the country. The annual consumption of honey in the country is very high, over 39,000 tons. Honey produced in Saudi Arabia sells for 10–20 times more money than imported honeys. Moreover, limited availability and high pricing of monofloral honey especially Ziziphus honey in Saudi Arabia are probably the biggest temptations for its adulteration or admixture with other Kashmiri Ziziphus honey types. Hence, identification of authenticity is important for financial reasons in addition to consumer and producer protection. Honey authenticity is defined by the Codex Alimentarius standards, the European Union (EU) honey directives, and different national legislations. The authenticity of honey has two aspects: The first aspect concerns production, i.e. ensuring that the natural constituents of honey are not adulterated or altered during processing. The second aspect of authenticity pertains to its geographical and botanical origin.
Many different techniques are employed in authenticity testing of honey including physical and chemical parameters to characterize honeys (Alqarni et al., 2014, Khan et al., 2016). Pollen analysis (melissopalynology) was used as the traditional method to determine the honey’s botanical origin (Arvanitoyannis et al., 2005, Adgaba et al., 2017b). In the past few decades, more recent analytical techniques were implemented for the determination of honey’s botanical origin in an effort to find alternative methods for honey authentication. These methods were based on statistical evaluation of their physicochemical data (Marini et al., 2004, Ruoff et al., 2007) or the determination of certain chemical constituents to be used as biomarkers by applying various chromatographic and spectroscopic techniques (Anklam, 1998, Yao et al., 2004, Kaskoniene and Venskutonis, 2010, Kaygusuz et al., 2016).
Recently, analytical techniques in conjunction with multivariate analysis and chemometrics have been widely implemented in the quality control of various foods and herbal drugs (Tistaert et al., 2011, Gad et al., 2013a). The application of UV spectroscopy in the analysis of food products has increased during the past decade, probably due to its advantage of being simple, quick, nondestructive, and relatively inexpensive to carry out (Souto et al., 2010, Gad et al., 2013b).
In this study, the validation of honey samples authenticity was based on chemometric analysis of UV spectroscopic data, to confirm the botanical source of Saudi honey samples. The model reported by Roshan et al. (2013) was employed for detection of authenticity of honey samples. The results obtained by this study will protect local honey producers and consumers from fraudulent honeys. The authentication of local honeys will promote the production and marketing of local honeys, and this in turn will encourage local beekeepers to increase their production.
2. Materials and methods
2.1. Sample collection
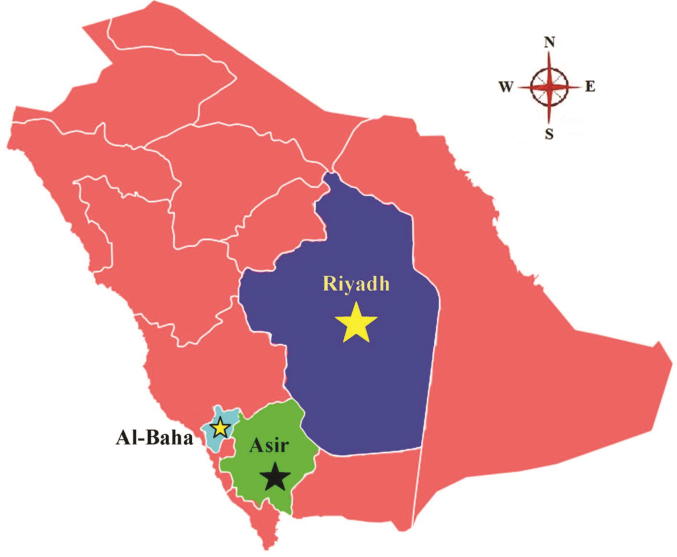
A total of eighteen honey samples were used in this study. Eight samples belonged to Ziziphus (Sidr) honey of which five (K7, K13, K17, K21 and K30) represented Ziziphus spina-christi while, the remaining three (K37, K45 and K61) were from Z. nummularia. Similarly, nine samples were Acacia honey, of which four samples (K49, K57, K85, and K97) represented Acacia gerrardii (Talah), three sample (K4, K26, and K28) belonged to A. tortlis (Sumra), and two samples (K9, K23) were from A. origena (Talah). Only one sample (K150) represented multifloral honey. All samples were collected from different geographical locations of Saudi Arabia. Sample codes, botanical and geographical origins, and collection season are shown in Table 1. A map of Saudi Arabia showing collection sites of different honey samples is represented in Fig. 1. Physicochemical (refractive index, water content, pH, free acid content, and electrical conductivity) and sensory analyses were carried out at Bee research chair, King Saud University Riyadh. The physicochemical data for all samples (unpublished) show that all the honey sample are genuine and fall in the criteria set by codex Alimentarius.
Table 1.
Plant origin, area of collection, area description, harvesting season, and code designated for collected honey samples.
| Sample code | Plant origin | Area of collection | Area description | Harvesting season (2016) |
|---|---|---|---|---|
| K4 | Acacia tortolis | Mashooqa, Al-Baha | Mountainous wild forest | March |
| K7 | Ziziphus spina-christi | Al-Baha | Mountainous wild forest | October |
| K9 | A. origena | Al-Baha | Mountainous wild forest | June |
| K13 | Z. spina-christi | Asir | Mountainous wild forest | October |
| K17 | Z. spina-christi | Asir | Mountainous wild forest | October |
| K21 | Z. spina-christi | Mashooqa, Al-Baha | Mountainous wild forest | September |
| K23 | A. origena | Baljurashi, Al-Baha | Mountainous wild forest | June |
| K26 | A. tortolis | Al-Baha | Mountainous wild forest | April |
| K28 | A. tortolis | Al-Baha | Mountainous wild forest | April |
| K30 | Z. spina-christi | Al-Baha | Mountainous wild forest | September |
| K37 | Z .nummularia | Rawdhat-Khoraim | Subtropical oasis | June |
| K45 | Z .nummularia | Rawdhat-Khoraim | Subtropical oasis | July |
| K49 | A. gerrardii | Rawdhat-Khoraim | Subtropical oasis | June |
| K57 | A. gerrardii | Rawdhat-Khoraim | Subtropical oasis, irrigated fields | August |
| K61 | Z .nummularia | Rawdhat-Khoraim | Subtropical oasis | July |
| K85 | A. gerrardii | Rawdhat-Khoraim | Subtropical oasis, irrigated fields | August |
| K97 | A. gerrardii | Rawdhat-Khoraim | Subtropical oasis | July |
| K150 | Multifloral | Al-Kharj | Plains with irrigated field | July |
Fig. 1.
Map of Saudi Arabia showing the regions Al-Baha, Asir, and Riyadh used to collect honey samples.
2.2. Sample preparation
The method reported by Roshan et al. (2013) was followed in the preparation of the samples. To dissolve most of the honey components, an expanded polarity range was obtained by dissolving each tested honey sample (2.50 g) into 20 mL of 80% ethanol solution (Carlo Erba anhydrous HPLC grade ethanol diluted with distilled water). After filtering by grade 1 Whatman filter paper, these samples were transferred into a 25 mL volumetric flask, and completed to volume with 80% ethanol to be kept as stock solution.
2.3. Ultraviolet spectroscopy
From each stock solution, 1 mL honey solution was taken into a 10 mL volumetric flask and completed with 80% ethanol. All the samples were analyzed by UV spectroscopy using a UV-1601 PC UV–visible spectrophotometer (Shimadzu, Japan) equipped with a quartz cell with an optical path of 1 cm and spectral resolution of 1 nm in the range 200–400 nm. Absorption readings on spectral points of all the samples were converted into a data matrix using Microsoft Excel 2013 (Microsoft, Redmond, WA, USA) with the spectral points as variables represented by columns and their corresponding spectral absorption measurements of different samples represented by rows. For each honey sample, five replicates were prepared.
2.4. Chemometric analysis
The data measured were represented in a matrix consisting of the total number of samples and their replicates multiplied by 200 variables in MS Excel and exported to the appropriate software for chemometric analysis of the spectra. The UV spectral data were subjected to unsupervised recognition techniques of data analyses performed by applying hierarchical cluster analysis (HCA) using Hierarchical Clustering Explorer 3.5 (Human computer interaction laboratory, University of Maryland, College Park, MD, USA) and principal component analysis (PCA) using Unscrambler 9.7 (CAMO SA, Oslo, Norway). The UV data matrix pre-processing was performed prior to data analysis by mean centring of the raw spectral data matrix of all the samples, a default option in the software. Both HCA and PCA methods aim to reduce the multivariate space in which objects (samples) are distributed but are complementary in their ability to present results. HCA was used to sort the sample into groups using average linkage method for cluster building, and the distance between clusters was computed by Pearson’s correlation method as a measure of similarity. PCA was utilized as a data reduction technique to generate a visual plot of the samples and their distribution on a score plot often showing trends that, despite having to be interpreted and explained. HCA and PCA were followed by the supervised pattern recognition technique of soft independent modeling of class analogy (SIMCA) using Unscrambler 9.7, which is considered a key chemometric approach for classification. This technique allows for the classification of the samples into an already existing group, assigning new objects to the class to which they show the largest similarity. SIMCA is strongly based on PCA, because each class is defined by an independent PCA.
3. Results and discussion
The UV absorption bands of the presented samples are usually associated with the presence of different chromophores exemplified in various components as phenolics, flavonoids, and conjugated systems as well as other UV-absorbing systems, (Andersen and Markham, 2006) and recently these compounds have been used as markers for the determination of the botanical origin of honey (Bertoncelj et al., 2011). The UV spectrum of each of the studied honey samples was recorded (5 replicates for each of the honey samples) versus 200 variables representing the absorbencies in the region between 200 and 400 nm.
3.1. Hierarchical cluster analysis
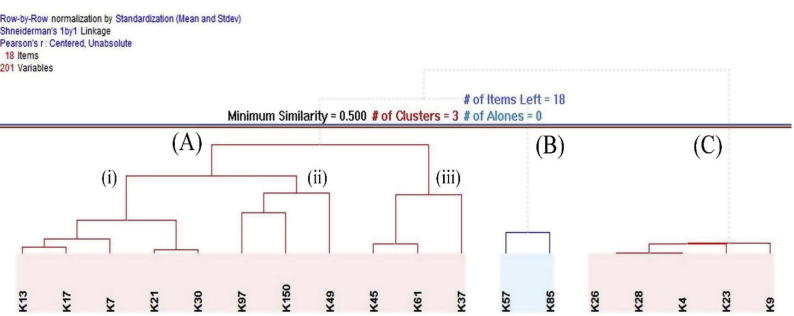
One preliminary way to study data is exploring the natural groupings among the samples. HCA was used to perform a preliminary data scan and to uncover the structure residing in the data. The dendogram in Fig. 2 shows the clustering pattern of the data set constituting the eight Ziziphus sp., nine Acacia sp., and one multifloral sample. Honey samples were segregated into 3 clusters: A, B, and C. The C cluster included the honey samples belonging to A. origena (K9 and K23) and A. tortilis (K4, K26, and K28); while the B cluster included the A. gerradii (K57 and K85) honey samples. However, the A cluster was classified into 3 sub clusters, the first and the third sub clusters included the Ziziphus honey samples. The former on the left represents Z. spina-christi (K7, K13, K17, K21, and K30) while the latter on the right represents Z. nummularia (K37, K45, and K61) honey samples; the second sub cluster in the centre was composed of two of A. gerradii (K49, and K97) honey samples and one multifloral honey sample (K150). The reason behind the splitting of Ziziphus samples into sub clusters may be related to the difference in Ziziphus species and altitudes of collection sites. The samples in first sub cluster of cluster A were collected from Z. spina-christi located in AlBaha at 2200 meters above sea level whereas, samples in third sub cluster of cluster A were collected from Z. nummularia located in Rawdhat-Khoraim at 570 masl. AlBaha is a semi-arid mountainous area with moderate temperature and humidity, whereas Rawdhat-Khoraim is a lowland plain area characterized by high temperature and low humidity.
Fig. 2.
Clustering dendogram of 18 honey samples. A (i) = Ziziphus spina-christi honey samples collected from mountaneous (Al-Baha and Asir) areas, (ii) Acacia gerrardii and multifloral honey samples from lowland (Rawdhat-Khoraimand, Al-Kharj) areas, (iii) Z. nummularia honey samples collected from lowland (Rawdhat-Khoraim) areas; B = A. gerrardii samples collected from lowland (Rawdhat-Khoraim) area; C = A. tortolis and A. origena samples collected from mountainous (Al-Baha) areas.
The chemical compositions of honeys vary according to the constituents of nectars, which in consequence are influenced by the variation in the chemical composition of the same plant species growing at different altitudes (Anklam, 1998). The middle sub cluster of A, constituting, two samples belonging to A. gerradii (K49 and K97) and one multifloral honey (K150) resemble Ziziphus honey samples in their constituents. These samples (K49, K97, and K150) were collected from Al-Kharj and Rawdhat-Khoraim regions in July and August. It is worth noting that Z. nummularia honey samples were collected from Rawdhat-Khoraim in June and July. An overlap in flowering season of A. gerradii and Z. nummularia take place in late July (Adgaba et al., 2017a) which may explain the constituent similarity of A. gerradii (K49 and K97) and multifloral (K150) honey samples produced in Al-Kharj and Rawdhat-Khoraim regions to that of Z. nummularia. The HCA result showing the similarity between the honey samples of A. tortilis (K4, K26, K28) and A. origena (K9 and K23) as they are closely clustered in group C, while the two honey samples of A. gerradii (K57 and K85) seem to be very different (cluster B). Also the results show that we can differentiate between the two types of Ziziphus honey samples even though both had similar organoleptic properties.
3.2. Principal component analysis
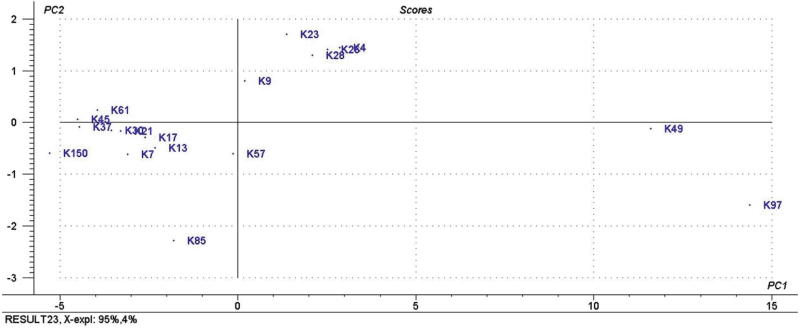
PCA was applied to the matrix formed by the total spectral data corresponding to all of the different honey samples. The maximum number of PCs was set at six; however, the first two components explained almost all of the data variance (95% and 4%). As shown in the score plot of the first two PCs in Fig. 3. The score plot showed that most of the Ziziphus honey samples were clustered in the left lower quarter and Z. nummularia (K37, K45, and K61) took the left side from Z. spina-christi (K7, K13, K17, K21 and K30) honey samples. The Ziziphus honey sample were well separated from the A. gerradii (K57 and K85) and multifloral (K150) honey samples which were also clustered in the lower left quarter of the score plot. The A. tortilis (K4, K26, and K28) and A. origena (K9 and K23) honey samples were clustered on upper right quarter of the score plot. Honey samples of A. tortilis (K4, K26, and K28) were separated from A. origena (K9 and K23) samples, though the both were close to each other. However, the A. gerradii honey samples were scattered. PCA is among the most versatile of all chemometric methods; it involves a mathematical procedure that reduces data dimensionality by performing a covariance analysis between factors and visualizes the hidden trends in a data matrix without much loss of information (Karoui et al., 2007). Even though the results obtained from the PCA revealed obvious clustering of the samples according to their botanical origin, which indicated differences in the honey samples composition, and was in fact mostly coherent with the HCA results. For a more precise demarcation between Ziziphus honey samples, SIMCA technique, which utilizes principal component per category to further confirm the classification obtained by the PCA and HCA.
Fig. 3.
PCA score plot of 18 Saudi honey samples.
3.3. Soft independent modeling of class analogy (SIMCA)
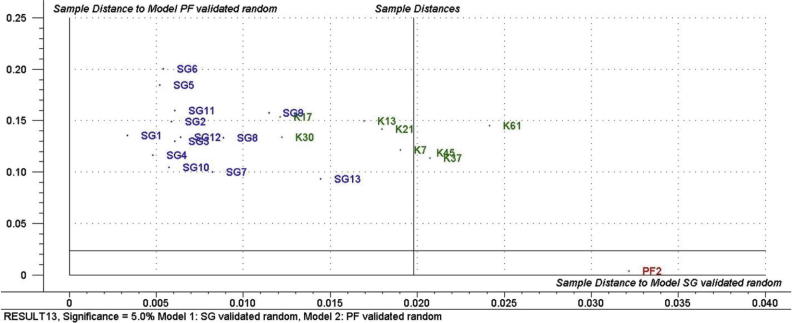
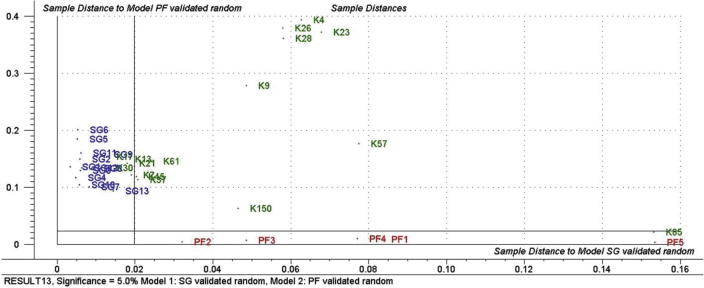
The SIMCA classification process consists of two stages, namely, the training stage, in which the individual models of the data classes are developed, and the testing or validation stage, in which new samples (not used in the training stage) are classified within the established class models to evaluate the model’s efficiency. Classes corresponding to genuine Ziziphus and multifloral honey sample that were constructed in the model by Roshan et al. (2013) were used in this study. Cooman’s plots were used to evaluate the results of classification where an object belonging to a certain class should fall within the membership limit, on the left of the vertical line or below the horizontal line. The SIMCA model designed by Roshan et al. (2013) correctly classified the Ziziphus samples (K7, K13, K17, K21 and K30) as being genuine Ziziphus honey. While the Z. nummularia honey samples (K37, K45, and K61) were closest in distance to the membership limit for genuine Ziziphus honey model, as these honey samples had quite similar organoleptic properties to the Z. spina-christi honey, making it difficult to distinguish between them by sensory analysis. These results were quite promising, as the model clearly split the Z. spina-christi and Z. nummularia honey samples. Z. spina-christi honey samples were collected from Albaha region of Saudi Arabia. This region is located in south of Saudi Arabia and their altitude and geography is close to genuine Ziziphus honey samples that were used in the training stage of the model, therefore the Ziziphus (K7, K13, K17, K21, and K30) honey samples from Albaha were in the membership limit for genuine Ziziphus honey model. While, the Ziziphus (K37, K45, and K61) honey samples from Rawdhat-Khoraim, an area characterized by lowland and arid geographical properties, fall on right side of the membership limit for genuine Ziziphus honey model (Fig. 4). Also, the model showed good specificity as all non-Ziziphus honey samples were not classified into Ziziphus class and fall outside the membership limit (Fig. 5).
Fig. 4.
Identification of Ziziphus honey samples collected from different Ziziphus plant species and geographical locations using the SIMCA model.
Fig. 5.
Identification of all 18 honey samples collected from different botanical sources and geographical locations using the SIMCA model.
4. Conclusion
Ultraviolet spectroscopy along with chemometric analysis methods successfully differentiate the honey samples of Saudi Arabia that were collected from various botanical sources and geographical locations. HCA along with spectroscopic data were useful to segregate Saudi honey sample. The SIMCA model successfully classified and authenticated the Ziziphus honey samples. Furthermore, it also distinguished the honey samples based on their botanical and geographical sources. Both HCA and PCA showed obvious grouping patterns in 18 tested honey samples. UV spectroscopy coupled with chemometric analysis can be used to measure the authenticity of botanical and geographical origin of Saudi honeys. This approach is simple, inexpensive, and less time consuming.
Declaration of interest
The authors confirm that there is no conflict of interests and are also liable for the content and writing of this article.
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award number (11-AGR2083-02).
Footnotes
Peer review under responsibility of King Saud University.
References
- Adgaba N., Al-Ghamdi A., Tadesse Y., Getachew A., Awad A.M., Ansari M.J., Owayss A.A., Mohammed S.E.A., Alqarni A.S. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. S. J. Biol. Sci. 2017;24:180–191. doi: 10.1016/j.sjbs.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adgaba N., Al-Ghamdi A.A., Getachew A., Tadesse Y., Belay A., Ansari M.J., Radlof S.E., Sharma D. Characterization of honeys by their botanical and geographical origins based on physicochemical properties and chemometrics analysis. Food Measure. 2017 [Google Scholar]
- Alqarni A.S., Owayss A.A., Mahmoud A.A., Hannan M.A. Mineral content and physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014;18:618–625. [Google Scholar]
- Al-yahya M., Mothana R., Al-said M., Al-dosari M., Al-musayeib N., Al-sohaibani M., Parvez M., Rafatullah S. Attenuation of C Cl4 - induced oxidative stressand hepatonephrotoxicity by Saudi Sidr honey in rats. J. Evid. Based Complementary Altern. Med. 2013 doi: 10.1155/2013/569037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O.M., Markham K.R. CRC, Taylor and Francis; Boca Raton, FL: 2006. Flavonoids: Chemistry, Biochemistry, and Applications; p. 1237. [Google Scholar]
- Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998;63:549–562. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Al-Waili N.S., Sharma D., Nuru A., Al-Attal Y. Effect of jujube honey on Candida albicans growth and biofilm formation. Arch. Med. Res. 2013;44(5):352–360. doi: 10.1016/j.arcmed.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Arvanitoyannis I.S., Chalhoub C., Gotsiou P., Lydakis- Simantiris N., Kefalas P. Novel quality control methods inconjunction with chemometrics (multivariate analysis) for detecting honey authenticity. Crit. Rev. Food Sci. Nutr. 2005;45:193–203. doi: 10.1080/10408690590956369. [DOI] [PubMed] [Google Scholar]
- Bertoncelj J., Polak T., Kropf U., Korosec M., Golob T. LC-DAD-ESI/MS analysis of flavonoids and abscisic acid with chemometric approach for the classification of Slovenian honey. Food Chem. 2011;127:296–302. [Google Scholar]
- Can Z., Yıldız O., Sahin H., Turumtay E.A., Silici S., Kolaylı S. An investigation of Turkish honeys; their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180(1):133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Donarski J.A., Jones S.A., Harrison M., Driffield M., Charlton A.J. Identification of botanical biomarkers found in Corsican honey. Food Chem. 2010;118:987–994. [Google Scholar]
- Erejuwa O.O., Sulaiman S.A., Wahab M.S.A. Honey: A novel antioxidant. Molecules. 2012;17:4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H.A., El-Ahmady S.H., Abou-Shoer M.I., Al-Azizi M.M. Application of chemometrics in authentication of herbal medicines: a review. Phytochem. Anal. 2013;24:1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- Gad H.A., El-Ahmady S.H., Abou-Shoer M.I., Al-Azizi M.M. A modern approach to the authentication and quality assessment of thyme using UV spectroscopy and chemometric analysis. Phytochem. Anal. 2013 doi: 10.1002/pca.2426. doi: 10.1002/pca.2426. [DOI] [PubMed] [Google Scholar]
- Gheldof N., Wang X.H., Engeseth N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002;50(21):5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Karoui R., Dufour E., Bosset J.O., De Baerdemaeker J. The use of front face fluorescence spectroscopy to classify the botanical origin of honey samples produced in Switzerland. Food Chem. 2007;101:314–323. [Google Scholar]
- Kaskoniene V., Venskutonis P.R. Floral markers in honey of various botanical and geographic origins: a review. Compr. Rev. Food Sci. Food Saf. 2010;9:620–634. doi: 10.1111/j.1541-4337.2010.00130.x. [DOI] [PubMed] [Google Scholar]
- Khan K.A., Al-Ghamdi A.A., Ansari M.J. The characterization of blossom honeys from two provinces of Pakistan. Ital. J. Food Sci. 2016;28(4):625–638. [Google Scholar]
- Marini F., Magri A.L., Balestrieri F., Fabretti F., Marini D. Supervised pattern recognition applied to the discrimination of the floral origin of six types of Italian honey samples. Anal. Chim. Acta. 2004;515:117–125. [Google Scholar]
- Noori A.L., Al Ghamdi A., Ansari M.J., Al-Attal Y., Al-Mubarak A., Salom K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch. Med. Res. 2013;44(4):307–316. doi: 10.1016/j.arcmed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Oddo L.P., Bogdanov S. Determination of honey botanical origin: problems and issues. Apidologie. 2004;35:S2–S3. [Google Scholar]
- Roshan A.A., Gad H.A., El-Ahmady S.H., Khanbash M.S., Abou-Shoer M.I., Al-Azizi M.M. Authentication of monofloral yemeni sidr honey using ultraviolet spectroscopy and chemometric analysis. J. Agric. Food Chem. 2013;61:7722–7729. doi: 10.1021/jf402280y. [DOI] [PubMed] [Google Scholar]
- Ruoff K., Luginbuhl W., Kilchenmann V., Bosset J.O., von der Ohe K., von der Ohe W., Amado R. Authentication of the botanical origin of honey using profiles of classical measurands and discriminant analysis. Apidologie. 2007;38:438–452. [Google Scholar]
- Souto U.T.C.P., Pontes M.J.C., Silva E.C., Galvao R.K.H., Araujo M.C.U., Sanches F.A.C., Cunha F.A.S., Oliveira M.S.R. UV-vis spectrometric classification of coffees by SPA-LDA. Food Chem. 2010;119:368–371. [Google Scholar]
- Tistaert C., Thierry L., Szandrach A., Dejaegher B., Fan G., Frederich M., Vander Heyden Y. Quality control of Citrireticulataepericarpium: exploratory analysis and discrimination. Anal. Chim. Acta. 2011;705:111–122. doi: 10.1016/j.aca.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Kaygusuz H., Tezcan F., Erim F.B., Yildiz O., Sahin H., Can Z., Kolayli S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT -Food Sci. Tech. 2016;68:273–279. [Google Scholar]
- Yao L., Jiang Y., Singanusong R., Datta N., Raymont K. Phenolic acids and abscisic acid in Australian eucalyptus honeys and their potential for floral authentication. Food Chem. 2004;86:169–177. [Google Scholar]