Abstract
The degradation process of acephate in aqueous solution with •OH and produced by 60Co-γ irradiation and electron pulse radiolysis was studied in the present paper. In the aqueous solution, acephate reacted with and transformed to transient species which can absorb weakly in the wavelength range of 300–400 nm and decay very fast. According to the decay of hydrated electron, the reaction rate constant of and acephate is (3.51 ± 0.076) × 109 dm3·mol−1·s−1. The transient species produced in the reaction of •OH and acephate do not distinctly absorb the light in the wavelength range of 300–700 nm, so the decay and kinetics of the transient species cannot determinedirectly. The competing reaction of KSCN oracephate with •OH were studied to obtain the reaction rate constant of •OH and acephate, which is (9.1 ± 0.11) × 108 dm3·mol−1·s−1. Although acetylamide and inorganic ions were determined in the products of the reaction of acephate with •OH or , the concentration of inorganic ions in the products of the reaction of acephate with •OH is higher than that in the product of the reaction of acephate with . Moreover, there were sulfide in the products of the reaction of acephatewith . The degradation pathways of acephate by •OH and were also proposed based on the products from GC-MS.
Keywords: Acephate, Electron pulse radiolysis, Reaction kinetics, Degradation pathway
1. Introduction
The molecular formula of acephate, is C4H10NO3SP, and the molecular structure is shown in Fig. 1 (Raghu et al., 2012)
Fig. 1.
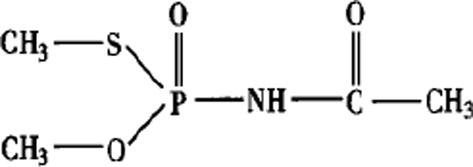
The molecular structure of acephate.
The pure product of acephate is a white crystalline phase, and has pungent odour. It is readily soluble in water (the solubility is 790 g·L−1 in water at 20 °C). As a low-toxicity organophosphate insecticide, acephate has been widely used on food crop and no-food crop to control chewing and sucking insects (Sharmaa et al., 2015, Joshi and Sharma, 2012, Arshadullah et al., 2017). After the acephate is applied to plants, it is absorbed by roots or foliage of plant and transferred into the plants. When chewing and sucking insects feed on those plants, the acephate attacks the nervous system of insects and kill them. However, the acephate have high to medium acute oral or medium inhalation toxicity to mammals (Sasikala and Barathi, 2014, Raghu et al., 2014, Suemizu et al., 2014, Yang et al., 2014). It will hurt the nervous and respiratory system, and cause eye and gastrointestinal problem in human (Satoh and Jokanovic, 2014, Kumruzzaman and Sarker, 2017). Therefore, it is a concerning issue to degrade and remove acephate in the plants and environment. Many studies have done to degrade and remove acephate, most of which involve biodegradation method (Zhao et al., 2010), acid hydrolysis (Szeto et al., 1979), photocatalytic decomposition (Echavia et al., 2000), electrolyzed water treatment (Hao et al., 2011), ozone method (Wang et al., 2015), supercritical carbon dioxide method (Yu, 2002), and γ-irradiation methods (Chen et al., 2008, Yang et al., 2015, Gao et al., 2015).
When acephate was degraded in some above methods, the N—C, P—N, P—S or P—O bonds would be broken, and some of compounds, such as methamidophos which is higher toxic than acephate, were produced. In the acid or base solution, the P—N bond was broken and O, S-dimethyl phosphorothioate was produced. Methamidophos and O-methl N-acetylphosphoramidate were identified as the major soil metabolites via cleavage of C—N or P—S bonds. In the plants, 10% of acephate was degraded to methamidophos by the broken of N—C bond. In the mammals, the metamorphose, S-methyl acetyl phosphoramidothioate and O, S-dimethyl phosphorothioate were also found in the urine when the acephate enter the body (Gammon et al., 2008). Pan et al. investigated acephate and methamidophos which was the metabolite of acephate, during tea cultivation, manufacturing, and infusion. They found that methamidophos changed from 0.576–0.630 to 0.568–0.645 from fresh tea leaves to made tea. Hence it is very important to know the degrading pathway of acephate when the methods are applied to reduce the toxic product.
60Co-γ irradiation, which could produce free radicals, such as •H and •OH, can decomposed many organic compounds (Qinet al., 2006). The active radicals can have reacted with substances which have chemical stability and are not easy to be degrade (Sun et al., 2015, Anbar and Neta, 1967, Gao et al., 2017). Hence, 60Co-γ irradiation methods were used to degrade acephate (Chen et al., 2008, Yang et al., 2015, Gao et al., 2015, Ong et al., 2017, Samad et al., 2017, Zaidi et al., 2017). Chen research the degradation rate of acephate by 60Co-γ irradiation in apple juice, but the degradation pathway of acephate and the degradation compounds were not mentioned. Yang et al., 2015, Gao et al., 2015 researched the reductive and oxidative radicals which influence the degradation of acephate by 60Co-γ irradiation, and determined inorganic ions in the solution, but the reaction kinetics and the degradation pathway were not discussed. For this reason, some experiments have been done in this work to found out the reaction kinetics of •OH or with acephate, and the degradation pathway of acephate in aqueous solution by •OH (oxidation process) and (reductive process) were also discussed. A lot of degradation processes, involved in the oxidation or reduction, are applied to degrade the acephate in the environment and food, and will product some high toxic compound, such as methamidophos. This study will systematically evaluate the degradation process of acephate in oxidation and reductive process, including the reaction rate constant. It is important for us to know if the processes will produce new pollutants to environment or new harmful by-products, which will be a benefit added to the already versatile use of various degradation methods.
2. Material and methods
2.1. Chemical reagent
Acephate, O, S-dimethyl acetic phosphoramidothioate, was provide by Beijing Pesticide Verification Place. AllChemical (HCl, CH3COOCH2CH3, KCl, NaSO4, CH3COONa, Na2HPO3, KH2PO3, NaOH, t-CH3CH2OH, NaHCO3, CH3CH2OOH) were obtained from Pengyu Company and all of an analytical grade. The oxygen (99%), nitrogen (99%) and nitrous oxide (95%) were provided by ERHUAN Company.
2.2. Apparatus
2.2.1. 60Co-γ radiant source
The Cobalt-60 gamma-rays source was providing by HELIYUAN Company of Baoding, Hebei, China. The irradiation dose rate was 119.6 Gy·min−1, which was calibrated using a Silver dichromate dosimeter.
2.2.2. Pulse radiolysis experiment
Pulse radiolysis experiments were performed using a 10 MeV linear accelerator delivering an electron pulse with duration of 8 ns, and a dosage is between 10 and 50 Gy per pulse, at the Shanghai Institute of Applied Physics. The pulse electricity current is 2–3 A. The source of analyzing light was a 500 W xenon lamp. The electron beam and analyzing light beam passed perpendicularly through a quartz cell with optical length of 20 mm. The transmitted light entered a monochromat and determined by a photomultiplier. The electron pulse dosimetry was determined using a thiocyanate dosimeter with G[(CNS)2(SCN)2·−] = 6.0 in a 100 mmol·L−1 KSCN solution saturated with N2O by taking ε480 nm = 7600 mol−1·L·cm−1.
2.2.3. Gas chromatograph
Routine analyses were carried out on FULI gas chromatography (FULI Ltd., China) equipped with Flame Photometric Detector. The fused silica capillary column is AE. FEAP-(30 m × 0.32 mm i.d., 0.25 μm film thickness).
The temperatures of the injector, oven, and the detector were set at 250, 120 and 250 °C, respectively. The nitrogen carrier gas velocity was 40 cm·s−1. The splitter pressure was set OFF. The pre-column pressure was set at 0.8 kg·cm−2. The concentration of acephate was calculated according to relative standard curve method.
Identification of peaks was performed in a GC (7890A, Agilent Corporation, USA) with a mass spectrometry detector (5975C). Compounds were separated on HP-35 fused-silica capillary columns (30 m length × 0.25 mm i.d., 0.25 μm film thickness).
The temperature of injector, ion source and interface was all set at 250 °C. The oven program was 2 min at 50 °C, 30 °C·min−1 to 170 °C (2min), and 10 °C·min−1 to 260 °C (20 min). Ions were formed for mass spectrometric detection using ion electron impact ionization mode scan. The total flow of helium carrier gas was 7.0685 mL·min−1, with 2.0343 mL·min−1 injected split flow.
2.2.4. Ion chromatograph
The concentration of ion in the aqueous solution was carried on a Dionex DX-600IC ion chromatograph (Dionex, USA) equipped with conductivity detector. The anion and cation chromatographic column is Ionpac® AS14 (4 × 250 mm) and Ionpac® CS12A (4 × 250 mm), respectively.
20 mmol·min−1 methane sulfuric acid and 50 mmol·min−1 sodium hydroxide solution were used as eluent solution to determine the cation and anion, respectively.
2.3. Degradation methods
2.3.1. Acephate reacted with •OH
The acephate was dissolved in triply distilled water to prepare the desired concentrations. The prepared solution was added into 10 mL Vials, followed by purging for 30 min with nitrous oxide, and then sealed by rubber closure. After that the samples were irradiated by 60Co-γ source or Pulse radiolysis.
2.3.2. Acephate reacted with
Acephate with tert-butyl alcohol were dissolved in triply distilled water to prepare the desired concentrations. After that the pieces were added into 10 mL vials and purged for 30 min with nitrous oxide, and then sealed by rubber closure. Finally, the samples were irradiated by 60Co-γ Radiant Point or Pulse radiolysis system.
Immediately after irradiation, the pH of solution was determined by a pHS-3C pH meter (Shanghai Exact Science Instrument Corporation, China), 20 μL aliquot was injected into ion chromatography to determine the ions, and 1 mL aliquot was used for the parent compound analysis and degradation by-products which were carried immediately on gas chromatography.
2.4. Residue determination
2.4.1. Gas chromatography analysis
A 1 mL portion of the sample and 4 g of anhydrous sodium sulfate were added and mixed in a 50 mL beaker. The solid was washed with the mixture of 2 mL of acetone and 2 mL of acetic ether at first, and then washed twice by the mixture of 1 mL of acetone and 1 mL of acetic ether. The organic extract was made to 10.00 mL with acetic ether, and 1 μL aliquot was analyzed by GC.
3. Results and discussion
3.1. Analysis of the product of acephate reacted with •OH or
The primary active radicals, , •OH, and •H, formed by decomposed of water molecules by irradiation energy is given by following equation (Miller et al., 2004):
| H2 O → eaq−•H, •OH | (1) |
The concentration of primary active radicals increased with the increase of the absorbed dose. In the N2O-saturated aqueous solutions, (G = 2.7) can be transferred to •OH, and the concentration of •OH is double (G = 5.4) (Qin et al., 2006):
| eaq− + N2 O + H2 O → •OH + N2 + OH− | (2) |
In this condition, the concentration of •H (G = 0.55) is near10 percent of that of •OH, and •OH as the main radical, was reacted with acephate.
Whereas in the N2-saturated (pH = 6.9) aqueous solutions in the presence of 0.1 mol·L−1 of tert-butyl alcohol, all of •OH (G = 2.7) was reacted with tert-butyl alcohol (Anbar and Neta, 1967):
| (CH3)3 COH + •OH → •CH2 C(CH3)2 OH + H2O k3 = 6 ×108 L·mol-1·s-1 | (3) |
Thus, the acephate reacted with reductive radicals, (G = 2.7) and •H (G = 0.55) in the solutions.
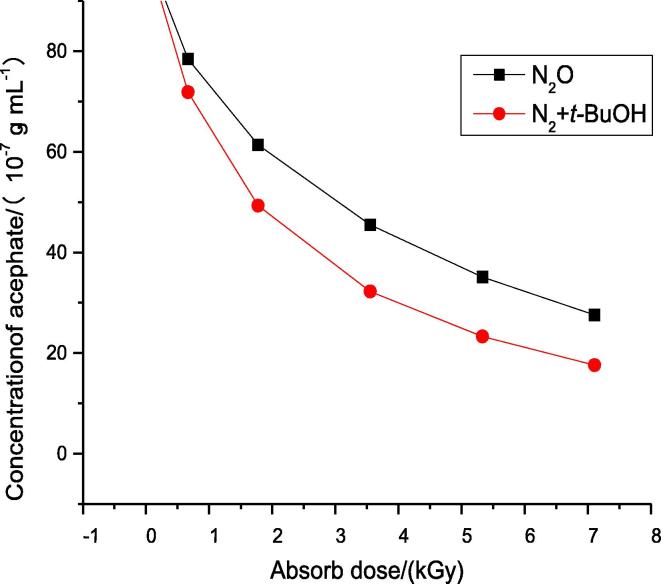
In both of the saturated solutions, the conentration of acephate decrease with the increase of the absorbed dose (as shown in Fig. 2). Hence, both •OH and can react with acephate and cause the concentration of acephate decrease. At the same absorbed dose, the •OH yield (G = 5.4) in the nitrous oxide- saturated solution is double than the yield (G = 2.7) in the N2-saturated solutions in the presence of tert-butyl alcohol, which means that more radicals in nitrous oxide-saturated solution will react with the acephate. However, after the reaction, the concentration of acephate in nitrous oxide-saturated solution is higher than that in nitrogen-saturated solution in the presence of tert-butyl alcohol. Hence, could degrade acephate more efficiently than •OH.
Fig. 2.
The concentration of acephate changing with absorbed dose in nitrous oxide-saturated solutions or nitrogen-saturated with in the presence of tert-butyl alcohol.
After irradiation, the inorganic ions in both saturated solutions were determined by ion chromatographic analysis, and the concentrations of NH4+, SO42− and H2PO4− were presented in Table 1. At the same absorbed dose, the concentrations of NH4+, SO42− and H2PO4− in nitrous oxide-saturated solution were all higher than that in the N2-saturated solution in the presence of tert-butyl alcohol, even though the NH4+ could be oxidized by •OH and converted to NO3−. Thus, could degrade acephate easier than •OH do, but the mineralization degree of acephate by was lower than that by •OH.
Table 1.
The change of inorganic ions with absorbed dose in nitrous oxide-saturated solutions and nitrogen-saturated solution in the presence of tert-butyl alcohol.
| Absorbed dose | [SO42−] (10−4 mol·L−1) |
[PO43−] (10−4 mol·L−1) |
[NH4+] (10−4 mol·L−1) |
|||
|---|---|---|---|---|---|---|
| N2+t-BuOH | N2O | N2+t-BuOH | N2O | N2+t-BuOH | N2O | |
| 1.6 kGy | 0.025 | 0.31 | 0.054 | 0.08 | 0.061 | 0.063 |
| 3.2 kGy | 0.043 | 0.74 | 0.034 | 0.16 | 0.075 | 0.089 |
| 5.2 kGy | 0.064 | 1.36 | 0.026 | 0.31 | 0.087 | 0.095 |
| 7.0 kGy | 0.088 | 1.89 | 0.061 | 0.46 | 0.090 | 0.11 |
During the degradation process of acephate, some of products were formed according to the result of GC–MS. When the absorbed dose was below 6 kGy, there were chromatographic peaks, which belonged to acetamide, with a retention time of 4.19 min in both the nitrous oxide-saturated solutions and nitrogen-saturated solution containing tert-butyl alcohol. In N2-saturated solution containing tert-butyl alcohol, the height of acetamide peak elevated with the absorbed dose from 0 to 9 kGy. It meant that the concentration of acetamide increased with the rise of absorbed dose. However, in the nitrous oxide-saturated solution, when the absorbed dose arrived at 9 kGy, the peak of acetamide disappeared, which meant that the acetamide was degraded completely. Furthermore, in the N2-saturated solution containing tert-butyl alcohol, when the absorbed dose reached 6 kGy, there was a chromatographic peak with a retention time of 11.52 min. The chromatographic profile exhibited molecular ion peak at 135.9, which was 100 percent similar to organic sulfur compound. When absorbed dose reached 9 kGy, the chromatographic peak with a retention time of 11.53 min disappeared, and a new chromatographic peak with a retention time of 8.77 min was showed. The new molecular ion peak at 93.9 (m/e) was like thiol compound. Therefore, in N2-saturated solution containing tert-butyl alcohol, the sulfur compound which was produced at low absorbed dose was further reduced to thiol compound with the rise of absorbed dose. In the solution saturated with nitrous oxide, except acetamide, other organic compound, such as sulfur compound and thiol compound, were not determined by gas chromatography 1 and gas chromatography 2 at the whole absorb dose. Hence, when the acephate was degraded by the •OH radical, the intermediate products, including acetamide, were also oxidized by •OH radical to change into inorganic ions. It can be deduced that more •OH radical was needed to participate further in several steps of degradation of acephate. So it was clear that why at the same absorbed dose, although the yield of •OH in nitrous oxides-saturated solution was double of yield of in the N2-saturated solution containing tert-butyl alcohol, the degradation rate of acephate in nitrous oxides-saturated solution is relatively low, compared to the N2-saturated solution containing tert-butyl alcohol. However, the concentration of ions in the former was higher than that in the latter, which meant the acephate was degraded more completely in nitrous oxide-saturated solution than that in N2-saturated solution containing tert-butyl alcohol.
3.2. Analysis of intermediate reaction of acephate with hydrated electron
3.2.1. The decay track of hydrated electron
3.0 × 10−3 mol·L−1 or 6.0 × 10−3 mol·L−1 of acephate in the N2-saturated solutions containing 0.1 M tert-butyl alcohol were irradiated by pulse radiolysis system. In those solutions, the acephate were reacted with as the following:
| (4) |
The decay track of hydrated electron observed at the wavelength of 640 nm was showed in Fig. 3. The decay of hydrated electron with acephate in the solution (Fig. 3(b) and (c)) was faster than the hydrated electron without acephate in the solution (Fig. 3(a)). With the increasing concentration of acephate, the decay rate of hydrated electron was accelerated. Hence, hydrated electron was reacted with acephate, and the reaction rate was related to the concentration of acephate.
Fig. 3.
The decay track of hydrated electron observed at wavelength of 640 nm. Dose per pulse = 40G, optical length = 1 cm, saturated with nitrogen containing 0.1 M tert-butyl alcohol. The concentration of acephate (mol·L−1): a = 0; b = 3.0 × 10−3; c = 6.0 × 10−3.
3.2.2. The transient optical absorption spectra of transient product
The N2-saturated solutions containing 0.1 M tert-butyl alcohol in the presence of 6.0 × 10−3 mol·L−1 of acephate was irradiated by pulse radiolysis system, and the optical absorption spectra of transient product was showed in Fig. 4. There was an absorption peak with maximum absorption at 700 nm. The absorption peak was characteristic of absorption of , and decay fast with the time. At the same time, there was a weak absorption band with the wavelength range of 300–400 nm. According to the result from UV spectrometry, the acephate did not have characteristic absorption peak with the wavelength range of 300–400 nm. So, it could be deduced that the absorption peak is ascribe to transient product from the reaction of with acephate. The transient absorption spectra were determined from 300 nm to 450 nm in detail, and was showed in Fig. 5. We can see from Fig. 5, the transient species with strong absorption at wavelength of 300–400 nm was build-up after pulse radiolysis within 0.01 μs. The absorption turns relatively strong within 0.05 μs and then turn weak within 0.5 μs. It meant that the concentration of transient species was highest at the time of 0.05 μs and then decayed after that. The concentration of the transient species was low after 0.5 μs.
Fig. 4.
The transient absorption spectra after pulse radiolysis. Dose per pulse = 40 G, optical length = 1 cm, saturated with nitrogen containing 0.1 M tert-butyl alcohol. The concentration of acephate is c = 6.0 × 10–3 mol·L−1.
Fig. 5.
The detail of transient absorption spectra at 300–450 nm after pulse radiolysis. Dose per pulse = 40G, optical length = 1 cm, saturated with nitrogen containing 0.1 M tert-butyl alcohol. The concentration of acephate is c = 6.0 × 10−3 mol·L−1.
To find out the relation of and the transient species, the decay spectra was determined at 325, 370 and 640 nm, respectively, and was showed in Fig. 6. As Fig. 6 showed, with the decay of hydrated electron, new compound which had absorption at 325 and 370 nm was produced. The generation and decay rate of new compound was slower than that of .
Fig. 6.
The decay spectra at 325, 370 and 640 nm. Dose per pulse = 40 G, optical length = 1 cm, saturated with nitrogen containing 0.1 M tert-butyl alcohol. The concentration of acephate is c = 6.0 × 10−3 mol·L−1.
3.2.3. Determination of reaction rate of hydrated electron with acephate
The reaction rate of hydrated electron with acephate was given by the following:
| (5) |
where ν is the reaction rate of hydrated electron with acephate; [] is the concentration of hydrated electron; [Acephate] is the concentration of acephate.
In order to obtain a pseudo-first-order reaction, a large excess of acephate was used ([Acephate] ≫ []). So that, as the reaction progressed, only a small amount of acephate was consumed, and its concentration could be considered to stay constant. So Eq. (5) could be changed to:
| (6) |
where kobs represented k5 [acephate] as appearance rate constant. The concentration of hydrated electron could be calculated using the following Eq. (7):
| (7) |
Here, c was the concentration of hydrated electron (mol·L−1); D was absorbed dose (Gy); G was the yield of hydrated electron.
Under our experimental condition, the dose of per pulse and the yield of hydrated electron were 40 Gy and 2.7, respectively, and substituted in Eq. (7). The concentration of was obtained as 1.1 × 10−5 mol·L−1, and the concentration of acephate used in the experiment was 6.0 × 10−3 mol·L−1. Hence, the concentration of acephate was a large excess ([Acephate] ≫ []), and could work for Eq. (5). The integrated Eq. (6) was following:
| (8) |
The concentration of was measured from the optical density (OD) valued at 640 nm (not 700 nm) (Joshua et al., 1965), because acephate did not absorb the light in the wavelength range of 300–700 nm, and had absorption peak around 700 mm. After the pulse radiolysis, the pseudo-first-order kinetic equation was used to fit the decrease of . Plot the ln(OD) versus time t to get a line as Fig. 7 showed and the line slope was just the appearance rate constant kobs.
Fig. 7.
The pseudo-first-order reaction of absorption decay of hydrated electron at 640 nm. Dose per pulse = 40G, optical length = 1 cm, saturated with nitrogen containing 0.1 M tert-butyl alcohol. The concentration of acephate is c = 6.0 × 10−3 mol·L−1.
By changing the concentration of acephate, a series of appearance rate constant kobs could be obtained. Plot the series of kobs again the concentration of acephate to get the slope and the intercept of the line in Fig. 8. The line slope was absolute rate constant k5 as (3.50 ± 0.086) × 109 dm3·mol−1·s−1.
Fig. 8.
The relation of appearance rate constant with the concentration of acephate.
3.3. Analysis of intermediate reaction of acephate with hydroxyl radical
The aqueous N2O-saturated solution containing 6.0 × 10−3 mol·L−1 acephate was irradiated by pulse radiolysis system. The transient species only have a weak absorption spectrum. Moreover, like acephate, the hydroxyl radical did not absorb the light in the wavelength range of 300 nm to 700 nm. In order to determine the reaction rate of hydroxyl radical with acephate, a competitive kinetics was used (Buxton et al., 1988, Kujawa et al., 1988).
When the N2O-saturated solution containing KSCN in the presence of acephate was irradiated, some reactions would happen as the following:
| •OH + acephate → product k10 | (9) |
| •OH + SCN− → OH− + •SCN k11 | (10) |
| •SCN + SCN− → (SCN)2− k12 | (11) |
Thiocyanate ion was used as competitive scavenger of hydroxyl radical (reaction (10) and reaction (11)). The thiocyanate radical anion had a strong absorption at 480 nm. When acephate was added into the N2O-saturated solution containing KSCN, reaction (9) would occur, which decreased the concentration of transient (SCN)2•−. Hence, we could obtain the k10 if we compare the different concentration of the only (SCN)2•− and the (SCN)2•− with acephate. The kinetic decay tract of (SCN)2•− in solution with different concentration of acephate after pulse radiolysis was showed in Fig. 9. As Fig. 9 showed, with the increase of concentration of acephate, the OD of (SCN)2•− decreased.
Fig. 9.
The absorption decay of (SCN)2·−.
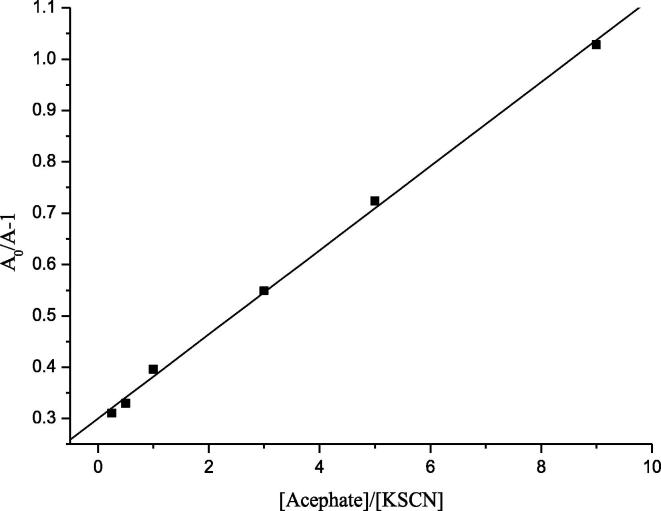
The competition for the hydroxyl radicals followed the Eq. (12):
| (12) |
where A0 was peak transient absorption of the only (SCN)2•− and Ai was peak transient absorption of (SCN)2•− with acephate.
The plot as given in Fig. 10, and from the slop of this linear plot, the rate constant k9 for hydroxyl radical reaction with acephate could be calculated as (9.1 ± 0.11) × 108 dm3·mol−1·s−1, based upon = 1.1 × 1010 dm3·mol−1·s−1. Hence, the acephate reaction with (k5 = (3.51 ± 0.076) × 109 dm3·mol−1·s−1) was faster than with •OH (k9 = 9.1 ± 0.11) × 108 dm3·mol−1·s−1).
Fig. 10.
The linear relation of [Acephate]/[SCN−] and A0/A-1.
The concentration of KSCN is 5.0 × 10−3 mol/L. The concentration of acephate (mol·L−1): a = 0; b = 0.005; c = 0.045.
3.4. The degradation path of acephate
In the N2-saturated and N2O-saturated solution, there were different radical’s reaction with acephate, and caused the difference of degradation ratio and degradation product. degraded acephate more quickly, but less inorganic ions in solution than •OH. The •OH took part in more steps of degradation of acephate then . The rate constant k5 of acephate reacted with was (3.51 ± 0.076) × 109 dm3·mol−1·s−1, and with •OH was 9.1 ± 0.11 × 108 dm3·mol−1·s−1.
3.4.1. The degradation path of acephate reaction with
The N2-saturated aqueous solution containing 0.1 M tert-butyl alcohol and acephate, the concentration of NH4+ was higher than SO42−, and SO42− was higher than PO43−. Compared to •OH, the could degrade acephate more efficiently. However, the oxidative radical, such as •OH, could degrade methamidophos, while the reductive radicals, and •H, had no contribution to the degradation (Zhao et al., 2009). The reductive and oxidative radicals play the different role in the degradation of methamidophos and acephate. Hence, the different molecular construct of compound, the reductive and oxidative radicals have different contribution.
Singh et al. (1998) had researched the physical chemistry, molecular orbital and electrical properties of acephate, and give the bond order and charge distributions of every bond in Fig. 11.
Fig. 11.

The bond order and partial charge density of chemical bond of acephate.
As Fig. 11 showed, in the molecular of acephate, the partial charge density of P and C (C O bond) was 2.563–2.662 and 0.314–0.350, respectively, and there are partial negative charge on other atoms. , a reducing agent, could be a nucleophilic reagent, and likely to attack the double bond on P or C, and more likely to attack the double bond on P. When attacked acephate, a transient species was formed, and the electron delocalize in O—P—N—C—O bond forming a conjugated system which had a weak absorption peak in the wavelength range of 300–400 nm. When the P—N bond was broken in the transient species, the acetamide was formed, and a little of acetamide was reacted further to produce NH4+, which was the highest concentration in the inorganic ions. Hence, the possible reaction pathway of with acephate was showed in Fig. 12.
Fig. 12.
The possible degradation pathway of acephate reacted with hydrated electron.
3.4.2. The degradation path of acephate reaction with •OH
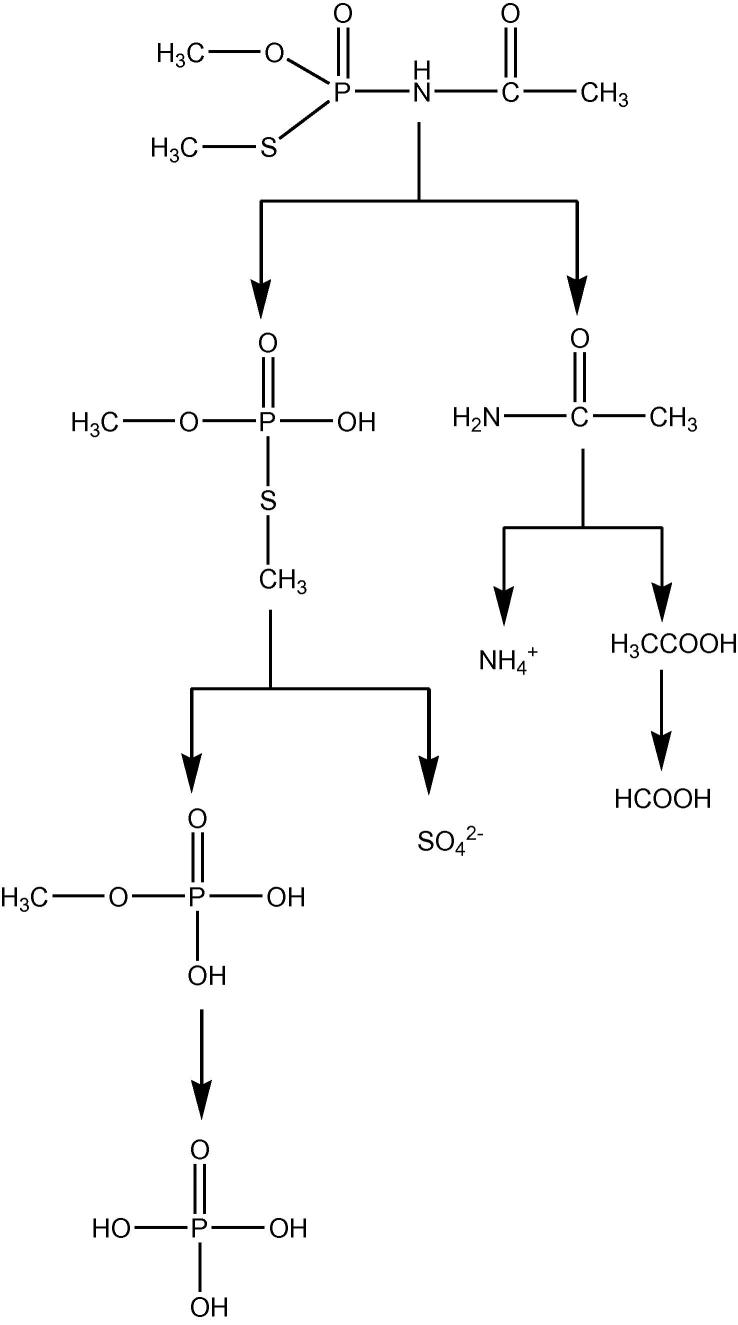
Hydroxyl radicals is strongly oxidative (E0 = 2.8 v), and a strong electrophilic reagent. Hence, •OH would attached the atoms which had high partial negative charge, such as S, N and O (Fig. 11). Glory et al. (2009) induced that •OH reaction with acephate had two paths as Fig. 13 showed: the oxidative radicals, such as •OH and •O2−, would attach bond P—N in acephate first, and break the bond. The acetamide and phosphoric acid, and the acetamide was degraded into carbon dioxide, nitrate and other inorganic compounds; the oxidative radicals would attach the bond P—S and P—O, and make methoxyl and methylthio groups which were connected with phosphorus hydroxylated. And then the bond N—C was broken, and the products were turned into inorganic ions. According to the result of our experiment from the analysis of products of •OH reaction with acephate, the degradation path of photocatalytic degradation of acephate using TiO2 was suitable to the degradation of achephate by •OH. However, some of NH4+ was determined in the solution, because some of N atom was not oxidized into NO3−. The degradation pathway was showed in Fig. 13.
Fig. 13.

The degradation pathway of acephate reacted with •OH (Echavia et al., 2009).
4. Conclusion
Both hydroxyl radicals and hydrated electron could decompose the acephate. Although the yield of •OH was double of yield of , the degradation rate of acephate by •OH is relatively lower than that by . However, the acephate was degraded more completely by •OH than that by because there were more inorganic compound in the solution. The reaction rate constant of and •OH reacted with acephate is (3.51 ± 0.076) × 109 dm3·mol−1·s−1 and (9.1 ± 0.11) × 108 dm3·mol−1·s−1, respectively. The degradation path of acephate by is different from by •OH, which participate several steps of degradation.
There are many methods applied to degrade the acephate, such as O3, H2O2, H2O2/UV, were involved in hydroxyl radicals and hydrated electron for oxidative and/or reductive degradation of the acephate. This study will help us to understand the degradation paths and the by-product of acephate which was reacted with and •OH.
Acknowledgement
This work was supported by The National Key R&D Project (2016YFD0401100-1), the Natural Science Foundation of Hebei, China (No. B2012204073) and Science and Technology Support Project of Hebei, China (No. 13273301D and 14236810D-3).
Footnotes
Peer review under responsibility of King Saud University.
References
- Anbar M., Neta P. A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution. Appl. Radiat. Isot. 1967;18:493–523. [Google Scholar]
- Arshadullah M., Suhaib M., Baber R., Usama M., Zaman B.U., Mahmood I.A., Hyder S.I. Growth of chenopodium quiona wild under naturally salt affected soils. Malaysian J. Sustain. Agric. 2017;1(1):01–03. [Google Scholar]
- Buxton G.V., Greenstock C.L., Helman W.P., Ross A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J. Phys. Chem. Data Ref. 1988;17:513–886. [Google Scholar]
- Chen D.M., Yue T.L., Yuan Y.H., Gao Z.P., Liu L.P. Effects of 60Co-γ ray radiation on the degradation of organophosphorus pesticides in apple juice and its quality. Trans. CSAE. 2008;24:270–274. [Google Scholar]
- Echavia G.R.M., Matzusawa F., Negishi N. Photocatalytic degradation of organophosphate and phosphonoglycine pesticides using TiO2 immobilized on silica gel. Chemosphere. 2000;76:595–600. doi: 10.1016/j.chemosphere.2009.04.055. [DOI] [PubMed] [Google Scholar]
- Echavia G.R.M., Matzusawa F., Negishi N. Photocatalytic degradation of organophosphate and phosphonoglycine pesticides using TiO2 immobilized on silica gel. Chemosphere. 2009;76:595–600. doi: 10.1016/j.chemosphere.2009.04.055. [DOI] [PubMed] [Google Scholar]
- Gammon, Derek W., Silva, Marilyn, Carr, Wesley C., 2008. Aephate RISK CHARACTERIZATION DOCUMENT. California Environmental Protection Agency.California, pp. 47–48.
- Gao H., Liu Y., Bao H., Bi Y., Zhao R. 60Co-γray irradiation degradation of Acephate by inN2and N2O saturated aqueous solution. Chem. Res. Appl. 2015;27:45–49. [Google Scholar]
- Gao W., Wang Y., Wang W., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Wu H.J., Liu Y., Chen T., Zhou Y., Su Y., Li L. Reduction of pesticide residues on fresh vegetables with electrolyzed water treatment. J. Food Sci. 2011;76(4):C520–C524. doi: 10.1111/j.1750-3841.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- Joshi Suresh C, Sharma Preeti. Effect of acephate on testicular functions of albino rats. Res. J. Pharm., Biol. Chem. Sci. 2012;3:137–146. [Google Scholar]
- Joshua J., Michael O., Gabriel S. On the photochemistry of aqueous solutions of chloride, and iodine ions. J. Phys. Chem. 1965;68:247–255. [Google Scholar]
- Kujawa P., Mohid N., Zaman K., Manshol W., Ulanski P., Rosiak J.M. Pulse radiolysis of butyl acrylate in aqueous solution. Radiat. Phys. Chem. 1988;53:403–409. [Google Scholar]
- Kumruzzaman D.M., Sarker A. Water requirements for various crops and impact of irrigation in barind area. Malaysian J. Sustain. Agric. 2017;1(1):04–07. [Google Scholar]
- Miller, R.B., 2004. Electronic irradiation of foods: an introduction to the technology. EBM, LLC Albuquerque, New Mexico, pp. 2–3.
- Ong S.Q., Lee B.B., Tan G.P., Maniam S. Capacity of black soldier fly and house fly larvae in treating the wasted rice in Malaysia. Malaysian J. Sustain. Agric. 2017;1(1):08–10. [Google Scholar]
- Qin H.F., Bao H.Y., Liu A.D., Hou X.G. Photo degradation of 4-chlorobiphenhyl in Hexane by UV Irradiation. Chin. J. Chem. 2006;22:261–265. [Google Scholar]
- Raghu P., Swamy Kumara B.E., Reddy Madhusudana T., Chandrashekar B.N., Reddaiah K. Sol–gel immobilized biosensor for the detection of organophosphorous pesticides: A voltammetric method. Bioelectrochemistry. 2012;83:19–24. doi: 10.1016/j.bioelechem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Raghu P., Reddy Madhusudana T., Reddaiah K., Swamy Kumara B.E., Sreedhar M. Acetylcholinesterase based biosensor for monitoring of Malathion and Acephate in food samples: A voltammetric study. Food Chem. 2014;142:188–196. doi: 10.1016/j.foodchem.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Samad N.S.A., Amid A., Jimat D.N., Shukor N.A.A. Isolation and identification of halophilic bacteria producing halotolerant protease. Galeri Warisan Sains. 2017;1(1):07–09. [Google Scholar]
- Sasikala Ramu, Barathi Seetharaman Biodegradation of acephate and methamidophos by a soil bacterium Pseudomonas aeruginosa strain Is-6. J. Environ. Sci. Health B. 2014;49:23–34. doi: 10.1080/03601234.2013.836868. [DOI] [PubMed] [Google Scholar]
- Satohm, Tetsuo, Jokanović, Milan, 2014. Basic and Clinical Toxicology of Organophosphorus Compounds Toxicity and Novel Biomarkers of OP Exposure, in mahdi balali-mood, mohammad abdollahi, Toxicity and Novel Biomarkers of OP Exposure. Springer, London, pp. 119–139.
- Sharmaa Preeti, Sharmaa Aksha, Jasujaband Nakuleshwar D., Joshi Suresh C. Changes in hematological profile of male albino rats after acephate administration. Toxicol. Environ. Chem. 2015;97:235–242. [Google Scholar]
- Singh A.K., White T., Spassova D., Jiang Y. Physicochemical, molecular- orbital and electronic properties of acephate and methamidophos. Comp. Biochem. Physiol. C: Pharmacol. Toxicol. Endocrinol. 1998;119:107–117. doi: 10.1016/s0742-8413(98)00002-4. [DOI] [PubMed] [Google Scholar]
- Suemizu Hiroshi, Sota Shigeto, Kuronuma Miyuki, Shimizu Makiko, Yamazaki Hiroshi. Pharmacokinetics and effects on serum cholinesterase activities of organophosphorus pesticides acephate and chlorpyrifos in chimeric mice transplanted with human hepatocytes. Regul. Toxicol. Pharm. 2014;70:468–473. doi: 10.1016/j.yrtph.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Sun Weihua, Chen Lujun, Zhang Yongming, Wang Jianlong. Synergistic effect of ozonation and ionizing radiation for PVA decomposition. J. Environ. Sci. 2015;34:63–67. doi: 10.1016/j.jes.2015.01.020. [DOI] [PubMed] [Google Scholar]
- Szeto S.Y., MacCarthy H.R., Oloffs P.C., Shepherd R.F. The fate of acephate and carbaryl in water. J. Environ. Sci. Health B. 1979;B14:635–654. doi: 10.1080/03601237909372157. [DOI] [PubMed] [Google Scholar]
- Wang Bin, Zhu Chang-ping, Gong Run-hang, Zhu Jin, Huang Bo, Xu Fei, Ren Qing-gong, Han Qing-bang, He Zhen-bing. Degradation of acephate using combined ultrasonic and ozonation method. Water Sci. Eng. 2015;8(3):233–238. [Google Scholar]
- Yang J., Gao Y.P., Liu W.H., Ge W., Zhao R.B. The degradation and mineralization of acephate by ionization irradiation. Environ. Prog. Sustain. Energy. 2015;34:324–332. [Google Scholar]
- Yang Xue Qing, Liu Ji Yuan, Li Xian Chun, Chen Mao Hua, Zhang Ya Lin. Key amino acid associated with acephate detoxification by cydiapomonella carboxylesterase based on molecular dynamics with alanine scanning and site-directed mutagenesis. J. Chem. Inf. Model. 2014;54:1356–1370. doi: 10.1021/ci500159q. [DOI] [PubMed] [Google Scholar]
- Yu J.J. Removal of organophosphate pesticides from wastewater by supercritical carbon dioxide extraction. Water Res. 2002;36:1095–1101. doi: 10.1016/s0043-1354(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Zaidi N.A., Hamid A.A.A., Hamid T.H.T.A. Lactic acid bacteria with antimicrobial properties isolated from the intestines of japanese quail (Coturnix Coturnix Japonica) Galeri Warisan Sains. 2017;1(1):10–12. [Google Scholar]
- Zhao R.B., Bao H.Y., Xia L.Y. γ-irradiation degradation of methamidophos. Chin. J. Chem. 2009;27:1749–1754. [Google Scholar]
- Zhao R.B., Bao H.Y., Liu Y.X. Isolation and characterization of penicilliumoxalicum ZHJ6 for biodegradation of methamidophos. Agric. Sci. China. 2010;9:101–105. [Google Scholar]