Abstract
Introduction
Suspected non-Alzheimer's pathophysiology (SNAP) is a biomarker driven designation that represents a heterogeneous group in terms of etiology and prognosis. SNAP has only been identified by cross-sectional neurodegeneration measures, whereas longitudinal measures might better reflect “active” neurodegeneration and might be more tightly linked to prognosis. We compare neurodegeneration defined by cross-sectional ‘hippocampal volume’ only (SNAP/L−) versus both cross-sectional and longitudinal ‘hippocampal atrophy rate’ (SNAP/L+) and investigate how these definitions impact prevalence and the clinical and biomarker profile of SNAP in Mild Cognitive Impairment (MCI).
Methods
276 MCI patients from ADNI-GO/2 were designated amyloid “positive” (A+) or “negative” (A−) based on their florbetapir scan and neurodegeneration ‘positive’ or ‘negative’ based on cross-sectional hippocampal volume and longitudinal hippocampal atrophy rate.
Results
74.1% of all SNAP participants defined by the cross-sectional definition of neurodegeneration also met the longitudinal definition of neurodegeneration, whereas 25.9% did not. SNAP/L+ displayed larger white matter hyperintensity volume, a higher conversion rate to dementia over 5 years and a steeper decline on cognitive tasks compared to SNAP/L− and the A- CN group. SNAP/L− had more abnormal values on neuroimaging markers and worse performance on cognitive tasks than the A- CN group, but did not show a difference in dementia conversion rate or longitudinal cognition.
Discussion
Using a longitudinal definition of neurodegeneration in addition to a cross-sectional one identifies SNAP participants with significant cognitive decline and a worse clinical prognosis for which cerebrovascular disease may be an important driver.
Keywords: Suspected non-AD pathology, Mild cognitive impairment, Neurodegeneration, Longitudinal, Cross-sectional
Highlights
-
•
74.1% of SNAP subjects also met the criteria for longitudinal neurodegeneration (L+).
-
•
SNAP/L+ had a larger WMH volume compared to the SNAP/L− group and the A- CN group.
-
•
SNAP/L+ showed a higher conversion rate and steeper cognitive decline than A- CN.
-
•
SNAP/L− showed similar conversion rate and cognitive decline as A- CN.
1. Introduction
Biomarkers of Alzheimer's disease (AD) have generally been divided into two classes: molecular (e.g. amyloid PET, cerebrospinal fluid (CSF) Aβ) and neurodegenerative (e.g. volumetric MRI and FDG PET). Neurodegeneration is by definition a dynamic process; however, most studies classify individuals on this dimension with cross-sectional measures. While a static measure captures past neurodegeneration, other factors may confound these measurements, for example, some individuals may have smaller hippocampal volumes for developmental or other reasons not related to neurodegeneration. A measure of declining volume over time, on the other hand, is likely a more specific indicator of a neurodegenerative process.
How we define neurodegeneration is gaining importance, as neurodegeneration is often used for classification in staging models. For example, the presence of atrophy or hypometabolism in the absence of cerebral amyloid defines the recently labeled category of suspected non-Alzheimer's pathophysiology (SNAP). A significant proportion of patients with Mild Cognitive Impairment (MCI) have received this classification based on cross-sectional measures of neurodegeneration (Caroli et al., 2015; Petersen et al., 2013; Prestia et al., 2013). However, the clinical implication of SNAP-MCI status remains unclear, as previous reports have shown widely varying results with regard to progression to dementia and cognitive decline. The reported progression vary from 0% to 56% in 2–3 years of follow-up (Caroli et al., 2015; Prestia et al., 2013; Wisse et al., 2015; Schreiber et al., 2017) and one study even reported a higher dementia progression rate in SNAP than in an amyloid and neurodegeneration positive, or prodromal AD (pAD), group (Petersen et al., 2013). Similar inconsistent findings are present for cognitive decline in these groups, with some studies showing almost similar cognitive decline in SNAP and pAD (Caroli et al., 2015), whereas others showed significantly less cognitive decline in SNAP as compared to pAD (Schreiber et al., 2017; Knopman et al., 2015; Chung et al., 2017). It is possible that these mixed results are partly attributable to heterogeneity in underlying ‘active’ neurodegeneration in SNAP, but also in pAD. While extant studies have investigated longitudinal change in neurodegeneration markers in SNAP ((Knopman et al., 2015), also note (Burnham et al., 2016; Gordon et al., 2016) in CN older adults), no prior study has utilized a longitudinal measure of neurodegeneration to define this group.
We therefore compare cross-sectional evidence of neurodegeneration using hippocampal volume only (L−) versus evidence of both cross-sectional and longitudinal neurodegeneration using hippocampal atrophy rate (L+) in the classification of MCI patients. As a conceptual study, we aim to examine the impact of these definitions on progression to dementia and cognitive decline. We hypothesize that the SNAP/L+ group will be enriched in individuals with a higher rate of progression to dementia and more cognitive decline than the SNAP/L− group. Additionally, we compare these groups on a number of biofluid and imaging markers aiming to gain understanding in the underlying pathology, e.g. the role of subthreshold amyloid or vascular pathology. We hypothesize that these groups have different biomarker profiles reflecting differences in the presence of more rapid neurodegeneration.
2. Materials and methods
2.1. Participants
We used data from ADNI-GO/2 (see Supplementary material) from 276 MCI participants with florbetapir and MRI scans at baseline and within 7–18 months after.
To establish cut-off points for neurodegenerative measures, we used data from amyloid positive (A+) participants (see Group definitions) with AD dementia for whom a baseline and follow-up (7–18 months after baseline) MRI scan were available (n = 66). Additionally, amyloid negative (A−) cognitively normal older adults with MRI scans at these points (n = 76) were used as a reference for the analyses in the different SNAP- and pAD-defined groups.
The study was approved after ethical review of each site's local review board and all participants provided informed written consent.
2.2. Imaging and biofluid markers
For hippocampal volume, baseline 3T T1-MRI scans were used. Hippocampal volume was measured using a previously published multi-atlas segmentation method (Wang et al., 2011). Hippocampal atrophy rates were computed with an unbiased deformation-based morphometry technique (described in (Yushkevich et al., 2010)) that measures change in hippocampal volume between baseline and follow-up MRI. See details in Supplementary material. Hippocampal volume at baseline was corrected for intracranial volume (ICV), obtained as described below. The difference in hippocampal volume between the two time points was expressed as percentage volume loss per year. An average over the two hemispheres was used for both measures.
ICV, white matter hyperintensity (WMH) volume, standardized uptake value ratio (SUVR) for the florbetapir and FDG-PET images, SPARE-AD (Spatial pattern of Abnormality for Recognition of Early AD) – an index derived from imaging data to quantify brain atrophy patterns typical of AD (Davatzikos et al., 2009), APOE-ɛ4 carrier status and CSF levels of Aβ42 came from publicly available processed data on the ADNI website. See Supplementary material for details.
2.3. Clinical and neuropsychological assessment
The Clinical Dementia Rating sum of boxes score (Morris, 1993) was obtained for all subjects during screening and diagnosis up to 5 years after baseline was analyzed. All participants underwent the Mini Mental Status Examination (MMSE) (Folstein et al., 1975) and tests of specific cognitive domains at baseline. A composite score was calculated for delayed recall, based on the 5- and 30-min trial of the Auditory Verbal Learning Test (Rey, 1964) and the Delayed Recall Task of the Alzheimer's Disease Assessment Scale-Cognitive (Rosen et al., 1984). Given the potential role of vascular disease in SNAP and its potential impact on executive functioning, we also examined the Trail Making Test B (TMT-B), which was log-transformed before conversion to z-score and inverted so that lower values represent worse performance. Z-scores were calculated using the means and standard deviations of the A- CN group at baseline. We also analyzed change over time using data from the 1, 2, 3 and 4 year visits.
2.4. Group definitions
Amyloid status was defined by a florbetapir SUVR value of 1.11 (Landau et al., 2012). Neurodegeneration status was defined by two different measures: baseline hippocampal volume (corrected for ICV) and annual hippocampal atrophy rate. As done previously (Petersen et al., 2013; Jack Jr et al., 2012; Knopman et al., 2013), the cut-off point for the cross-sectional measure was obtained by taking the 90th percentile of the A+ participants with AD dementia. The 90th percentile for the longitudinal measure provided a cut-off point of −0.22, which is not reflective of active neurodegeneration. We therefore chose a stricter cut-off point at the 80th percentile. A cut-off point of 2044 mL for ICV-corrected hippocampal volume and −0.80%/year for hippocampal atrophy rate was established with this approach.
2.5. Statistical analyses
Cross-sectional cognitive and biomarker profile for the differentially defined SNAP groups was analyzed using analyses of variance for normally distributed data, Mann-Whitney U tests for non-normally distributed data and Pearson χ2 tests for categorical data. In a second analysis, we corrected for age, gender and education for the cognitive tests in cases where there was a significant group difference. Moreover, we performed linear mixed-effects models (Laird and Ware, 1982) with group, time and a group*time interaction term to assess a group difference in cognitive decline over time. The fixed effects in the mixed-effects model included the above three terms and covariates (the specific cognitive task at baseline, age, gender and education). Subject-specific random intercept and slope for time were included in the mixed-effects model to account for correlations among repeated measures of the cognitive outcomes.
3. Results
3.1. Cross-sectional characterization of the SNAP groups
3.1.1. SNAP/L− vs SNAP/L+
Fifty-five MCI patients were considered SNAP with 25.9% receiving this designation based on only the cross-sectional measure (SNAP/L−) and 74.1% also meeting the longitudinal definition of neurodegeneration (SNAP/L+) (Table 1). SNAP/L− had a larger percentage of males, more years of education both at a trend level, and, interestingly, a higher percentage of APOE-ɛ4 carriers. SNAP/L+ by definition showed a higher atrophy rate than SNAP/L− and was also characterized by a larger WMH volume. The groups did not differ on any of the other neuroimaging biomarkers, including baseline hippocampal volume. With regard to clinical and cognitive status, the groups did not differ except for a slightly lower MMSE score in the SNAP/L− group.
Table 1.
Description of the two SNAP groups.
| SNAP/L− | SNAP/L+ | A- CN group | |
|---|---|---|---|
| Number (%) | 14 (25.9%) | 40 (74.1%) | 76 |
| Age (years) | 71.6 (7.6) | 74.0 (6.8) | 72.4 (6.0) |
| Gender (% men) | 12 (85.7%)a*,b* | 23 (57.5%) | 45 (59.2%) |
| Education (years) | 17.7 (2.0)a* | 16.3 (2.6) | 16.9 (2.6) |
| APOE-ɛ4 (%) | 6 (42.9)a,b | 6 (15.0) | 13 (17.1) |
| HV (mL) | 1769 (210)b | 1743 (249)c | 2126 (230) |
| HV rate (%/year) | 1.02 (2.27)a,b | −2.90 (2.07)c | −1.31 (1.66) |
| Follow up time MRI (days) | 317 (95) | 330 (89) | 304 (96) |
| FDG-PET◊ | −0.14 (1.12) | −0.39 (0.89)c | 0.00 (1.00) |
| SPARE-AD score | −0.65 (1.00)b | −0.41 (1.00)c | −1.34 (0.46) |
| Florbetapir (SUVR) | 1.02 (0.04) | 1.00 (0.06) | 1.01 (0.05) |
| CSF Aβ pg/mL | 207 (45)b | 205 (40)c | 236 (27) |
| WMH◊ | −0.10 (1.22)a | −0.86 (0.89)c | 0.00 (1.00) |
| MMSE score | 27.4 (1.7)a,b | 28.5 (1.5)c | 29.2 (1.1) |
| Delayed memory at baseline◊ | −1.22 (1.02)b | −1.11 (1.15)c | 0.00 (1.00) |
| TMT-B◊ | −0.78 (1.04)b | −0.91 (0.78)c | 0.00 (1.00) |
| CDR SUM | 1.5 (0.7)b | 1.3 (0.7)c | 0.0 (0.1) |
| Conversion to dementia over 5 years (%) | 0 (0) | 5 (12.5)c | 2 (2.6) |
Significant differences between: a) SNAP/L- and SNAP/L+; b) SNAP/L- and A- CN group; c) SNAP/L+ and A- CN group ⁎p < 0.10; ◊ z-score. Results reported in the table are not corrected for age, gender and education (for the cognitive tasks), see the Results section for corrected results.
SNAP = suspected non-AD pathology; L−/+ = neurodegeneration negative/positive as defined by a longitudinal measure; A- = amyloid negative; CN = cognitively normal; HV = hippocampal volume; FDG-PET = Fludeoxyglucose Postron Emission Tomography; SPARE-AD = Spatial Pattern of Abnormality for Recognition of Early Alzheimer's Disease; SUVR = standardized uptake value ratio; CSF = cerebrospinal fluid; WMH = white matter hyperintensity; MMSE = Mini Mental Status Examination; TMT = Trail Making Test; CDR = Clinical Dementia Rating.
3.1.2. SNAP vs A- CN
SNAP/L− had a larger percentage of males, at a trend level, and APOE-ɛ4 carriers than A- CN, smaller hippocampal volume, by definition, higher SPARE-AD score (higher is more abnormal) and lower CSF Aβ values. The atrophy rate in SNAP/L− was, interestingly, less than in A- CN. Additionally, compared to A- CN, SNAP/L− performed worse on the selected cognitive tasks. SNAP/L+ was not different from A- CN with regard to demographics, but showed more abnormal values on most of the biomarkers, including FDG-PET and WMHs, and worse performance on all selected cognitive tasks. Notably, neither group differed from A- CN on florbetapir SUVR.
Correcting above analyses for age, gender, and education (for the cognitive tasks) did not notably change the results.
3.2. Longitudinal characterization of the SNAP groups
3.2.1. SNAP groups vs each other and A- CN
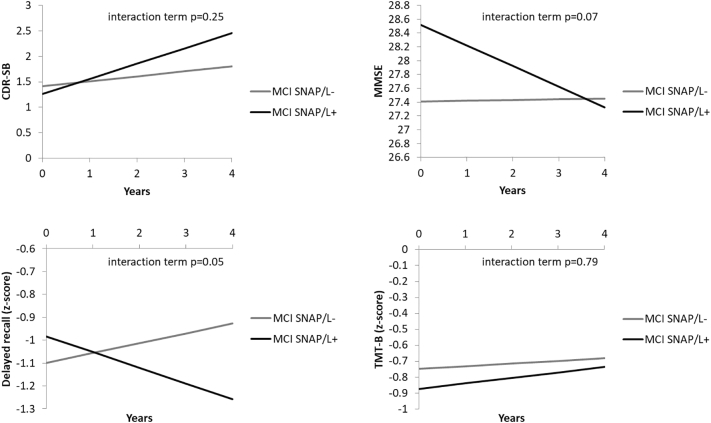
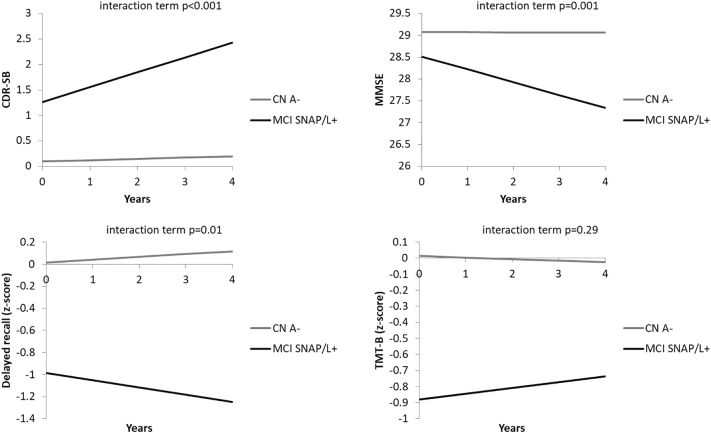
SNAP/L+ qualitatively, though not significantly, has a higher, although modest, conversion rate (12.5% versus 0.0%), which is significantly different from A- CN (and at a trend level after correcting for age and gender; Table 1). Although we found no significant differences between the two SNAP groups, SNAP/L+ trended towards more decline on the MMSE and delayed memory as compared to SNAP/L− (Fig. 1). SNAP/L− did not differ from A- CN, whereas SNAP/L+ showed a steeper increase on the CDR and a steeper decline on the MMSE and delayed memory, but not the TMT-B (Fig. 2).
Fig. 1.
Comparison of longitudinal performance of the SNAP/L− and SNAP/L+ group on the CDR-SB, MMSE, delayed recall and TMT-B. The group*time interactions reached a trend level for MMSE and delayed recall (p = 0.0501). SNAP/L−/+ = Suspected Non-Alzheimer's pathophysiology with or without additional longitudinal evidence of neurodegeneration; CDR-SB=Clinical Dementia Rating Sum of Boxes; MMSE = Mini Mental Status Examination; TMT-B = Trail Making Test B.
Fig. 2.
Comparison of longitudinal performance of the MCI SNAP/L+ and CN A- group on the CDR-SB, MMSE, delayed recall and TMT-B. The group*time interactions reached significance for CDR-SB, MMSE and delayed recall. SNAP/L + =Suspected Non-Alzheimer's pathophysiology with additional longitudinal evidence of neurodegeneration; CN A- = Amyloid negative Cognitively Normal older adults; CDR-SB = Clinical Dementia Rating Sum of Boxes; MMSE = Mini Mental Status Examination; TMT-B = Trail Making Test B.
3.2.2. SNAP vs second reference group MCI A-C-L-
We also compared the two SNAP groups to those MCI patients who were amyloid negative without evidence of neurodegeneration using either measures (Amyloid Negative, Cross-sectional neurodegeneration negative and Longitudinal neurodegeneration negative; A-C-L-), as a second reference group (n = 44; mean age = 67.3 ± 7.0 years; 40.9% male; mean MMSE = 28.9 ± 1.1). There were no differences between A-C-L- and SNAP/L-, except for CDR-SB which was due to a significant decrease in CDR-SB in A-C-L- (i.e. improvement) as compared to SNAP/L- (Supplementary Fig. 1). SNAP/L+ displayed a significantly steeper increase on the CDR-SB, decline on the MMSE and, at a trend level, on delayed recall as compared to A-C-L- (Supplementary Fig. 2). No differences were found for the TMT-B.
3.3. Cross-sectional and longitudinal characterization of the pAD groups
3.3.1. Cross-sectional characterization
pAD was defined as A+ with evidence of neurodegeneration [either cross-sectional alone (pAD/L−) or both cross-sectional and longitudinal (pAD/L+) evidence of neurodegeneration]. pAD/L+ and pAD/L− do not differ on any of the biomarkers, nor on any of the cognitive tasks, with the notable exception of a higher score on the CDR-SB, a higher percentage of APOE-ɛ4 carriers, both at a trend level, a higher atrophy rate, by definition, and smaller hippocampal volume in pAD/L+ (Supplementary Table 1). Both pAD groups differ significantly from the A- CN group on all neuroimaging and biofluid markers and all cognitive tasks. Correcting above analyses for age, gender, and education (for the cognitive tasks) did not notably change the results.
3.3.2. Longitudinal characterization
pAD/L+ displayed a significantly higher conversion rate (55.8% versus 26.3%; also after correcting for age and gender), a significant steeper increase in CDR-SB and a slightly steeper decline at a trend level delayed recall than pAD/L- (Supplementary Fig. 3). Both groups show a significantly worse prognosis than the A- CN group with regard to conversion to dementia (also after correcting for age and gender), the CDR-SB and all selected cognitive tasks, except for the comparison of the pAD/L− group with the A- CN group for delayed recall which reached a trend level.
3.4. Comparison of SNAP and pAD groups on longitudinal cognition
3.4.1. Comparison of the SNAP/L− and pAD/L− groups
The pAD/L− shows a significantly steeper increase on the CDR-SB and a steeper decline on MMSE and TMT-B, as well as a trend level on delayed recall as compared to the SNAP/L−, indicating worse progression in the context of amyloid even in the absence of longitudinal neurodegeneration.
3.4.2. Comparison of the SNAP/L+ and pAD/L+ groups
The pAD/L+ shows a significantly steeper increase on the CDR-SB and a steeper decline on all cognitive tests as compared to the SNAP/L+, again, indicating worse progression in the context of amyloid.
4. Discussion
We showed that approximately 3/4 of the subjects with SNAP, defined according to a cross-sectional neurodegeneration measure of hippocampal volume, also met our criteria of neurodegeneration based on a longitudinal measure of hippocampal atrophy rate while 1/4 did not. Further examination of these two groups showed that both had abnormal values on other cross-sectional neurodegeneration biomarkers, as well as cognitive impairment as compared to the A- CN group. However, SNAP/L+ had a larger WMH volume as compared to the SNAP/L− group and the A- CN group. Most importantly, SNAP/L+ had a worse prognosis with a higher conversion rate and steeper decline on cognitive tasks. The SNAP/L− group showed no differences in terms of conversion rate or cognitive decline as compared to the A- CN group.
4.1. Snap
As has been pointed out previously, SNAP is likely a heterogeneous group with regard to their etiology (e.g. (Mormino et al., 2016)). This heterogeneity is reflected in the wide range of reported outcomes in those with SNAP-MCI (Caroli et al., 2015; Prestia et al., 2013; Wisse et al., 2015; Schreiber et al., 2017). We hypothesized that utilizing a longitudinal definition of neurodegeneration in addition to a cross-sectional one might partly explain this observed heterogeneity. Indeed the SNAP/L+ group had a poorer prognosis while there was no clear difference between SNAP/L− and the A- CN group. This may indicate that part of the “noise” in the SNAP construct may be due to individuals who do not have evidence of ‘active’ neurodegeneration and their structural abnormalities (e.g. small hippocampi) may be related to developmental factors or slow aging effects that may have made their memory vulnerable to fall within the MCI category (see below). In that context, this group would not be expected to demonstrate clinical progression beyond that of controls, as was observed. Alternatively, SNAP/L+ likely represents a group with a more pathological, or active, process and would be expected to be at greater risk for clinical progression. This distinction supports the potential added value of using a dynamic measure of neurodegeneration in addition to a cross-sectional one in staging models (see (Jack Jr et al., 2016)). Alternatively, these findings also may support the idea that one neurodegeneration measure serves as confirmatory test for the other and that having positivity for both is the most rigorous way of testing neurodegeneration with the least likelihood of false positives. It is worth noting that FDG-PET values were also abnormal in SNAP/L+, but less so in the SNAP/L- group. While this difference was not significant, it may suggest that FDG-PET may be somewhat sensitive to individuals with active neurodegeneration as measured by longitudinal hippocampal atrophy rate.
Besides the impact of using these cross-sectional and longitudinal neurodegeneration definitions on outcomes, we also aimed to gain understanding in the potential underlying etiology of SNAP. Interestingly, the SNAP/L+ group had significantly more WMHs compared to the SNAP/L− group. Although cause and consequence cannot be disentangled here, it seems likely that cerebrovascular disease is a major driver of the ongoing hippocampal atrophy and cognitive decline observed in this group. In a posthoc correlation analysis, we found an association of WMHs with hippocampal atrophy rate in A- MCI patients (r = 0.25, p = 0.01; for A+ MCI patients: r = 0.03, p = 0.76), indeed confirming this hypothesis. It would be interesting to see if future larger studies can replicate this finding with other measures of cerebrovascular disease or the degree to which there is a pattern of atrophy that can be distinguished from a more typical one of prodromal AD. Finally, a hint of lower levels of CSF Aβ can be observed with both measures of neurodegeneration as compared to the A- CN group. However, this difference with the A- CN group was not observed for the other measure of amyloid, florbetapir, and it is therefore unclear what the significance is of this finding. Previous findings on the role of subthreshold amyloid in SNAP have also not been straightforward (Wisse et al., 2015; Mormino et al., 2016; Mormino et al., 2014; Vos et al., 2016).
Given that neurodegeneration is generally conceptualized as a dynamic process, what is the explanation for the SNAP group that only showed abnormality on the cross-sectional measure? One possibility is that they simply had developmentally smaller hippocampal volumes which crossed the threshold of abnormality in the context the “normal” volume loss associated with aging. This may make these individuals more susceptible to age-associated memory decline. Alternatively, this group may have experienced previous neuronal injury or a very slow rate of neurodegeneration that – given the relatively short imaging follow-up period (mean ~317 days) – resulted in our longitudinal measure being insensitive. Such a slow rate of progression may be consistent with the hippocampal neurodegeneration expected with primary age-related tauopathy (Jack Jr., 2014) in which neurofibrillary tangle pathology is thought to accumulate at a slower rate than in the setting of cerebral amyloidosis. Tau PET imaging will ultimately be helpful in determining if those with smaller cross-sectional hippocampi are more likely to have tangle pathology in the absence of amyloid. Although it should be noted that one recent study did not find elevated tau levels in CN SNAP participants measured with AV-1451 PET (Mormino et al., 2016). It seems unlikely that vascular pathology explains the neurodegeneration and memory impairments in this group given the normal values on this marker. Interestingly, this group also had a surprisingly high rate of APOE-ɛ4 carriers, which has been associated with smaller hippocampi even in young adults (O'Dwyer et al., 2012), potentially supporting a developmental hypothesis. With regard to the amyloid markers, in this group also a hint of lower CSF Aβ levels was observed as compared to the A- CN group, but with normal florbetapir levels. It therefore remains to be determined if there is a role for subthreshold amyloid deposition in this group as well. Regardless, the finding of a lack of significant progressive atrophy in this group should give pause as to the conceptual meaning of neurodegeneration.
4.2. pAD
We also investigated the impact of using a longitudinal measure of neurodegeneration in addition to a cross-sectional one in the context of amyloid. As for SNAP, a considerable portion of ~20% of pAD patients as defined by a cross-sectional measure of neurodegeneration displayed no longitudinal hippocampal atrophy (pAD/L−). This indicates that the pAD group might also be heterogeneous in terms of the driver of current cognitive symptoms or disease stage and prognosis. Indeed, the pAD/L+ group showed a significantly larger conversion rate to dementia over five years as compared to the pAD/L− group, 55.8 vs 26.3%. This was also supported by a steeper increase in CDR and decline on the delayed recall task compared to pAD/L− group. Interestingly, the groups did not differ on most of the other biomarkers, except that the pAD/L− group somewhat paradoxically had smaller cross-sectional hippocampal volumes as compared to the pAD/L+ group. As with SNAP/L−, it is possible that pAD/L− includes individuals with smaller hippocampi due to other factors, such as developmental differences and/or age-related effects that make this group vulnerable to memory loss, but that they have concomitant AD pathology that may reflect an earlier stage of disease pathophysiology than the pAD/L+ group. It is also possible that these groups simply differ in the aggressiveness of the disease or the spatial distribution of neurodegeneration. One possibility that seems unlikely in light of the slower rate of clinical decline is that the pAD/L− group are at a later stage of disease and that we are observing “floor effects”.
Limitations of the current study are the lack of a definitive approach for defining thresholds, the small sample size and the potential differences in the noise of the measures. That is, while both neurodegeneration markers are subject to measurement error, longitudinal measures of neurodegeneration inherently have greater variability due to the additional processing required. This may have affected the precision of the determination of neurodegeneration status. An additional limitation is that the follow up time to determine atrophy rate is somewhat arbitrary (1 year). It is unclear whether atrophy rate is constant over time and whether a longer or shorter follow-up time would have resulted in different group designations. Finally, while this study is investigating the conceptual meaning of neurodegeneration by comparing a cross-sectional measure with a longitudinal one, it might be impractical in clinical practice to obtain longitudinal imaging and certainly less desirable than being able to provide more definitive prediction at baseline. That said, our results indicate that WMH volume is related to higher longitudinal atrophy rates and might potentially be a useful cross-sectional biomarker to aid in predicting which SNAP subjects will worsen over time.
In conclusion, this study showed that defining neurodegeneration by both a cross-sectional and a longitudinal measure of hippocampal volume leads to the identification of a SNAP group enriched in individuals with significant cognitive and clinical decline over time, for which cerebrovascular disease could be an important driver. Indeed, conceptually a longitudinal measure of neurodegeneration is more in keeping with the dynamic and progressive nature of this process. Placed in the context of the recent ATN staging model (Jack Jr et al., 2016; Jack Jr et al., 2017) in which neurodegeneration is generally measured in a cross-sectional manner, the current results suggest that such a designation may be limited and that those with concomitant evidence of both an accelerated rate of atrophy and reduced volume may represent a distinct group in the presence or absence of cerebral amyloid. Future studies will need to confirm these results in larger cohorts and with other measures of neurodegeneration, such as FDG-PET or CSF markers of neurodegeneration.
Disclosures
B. C. Dickerson received an honorarium from Merck and Forum, where he served on a data safety monitoring board and as consultant respectively, and he received royalties from Oxford University Press, where he served as an editor. D.A. Wolk received grant funding from Merck, Biogen, Avid Radiopharmaceuticals and Eli Lilly and personal fees from GE Healthcare, Merck, and Janssen. None of the other authors has anything to disclose.
Funding
This work was supported by the National Institute on Aging grants: R01 AG037376, P30 AG010124, R01 AG054409; National Institute of Biomedical Imaging and Bioengineering grant: R01 EB017255; and the Berkman Charitable Trust.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.02.008.
Appendix A. Supplementary data
Supplementary material
References
- Burnham S.C., Bourgeat P., Dore V. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. Lancet Neurol. 2016;15(10) doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- Caroli A., Prestia A., Galluzzi S. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology. 2015 doi: 10.1212/WNL.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.K., Plitman E., Nakajima S. Hippocampal and clinical trajectories of mild cognitive impairment with suspected non-Alzheimer's disease pathology. J. Alzheimers Dis. 2017 doi: 10.3233/JAD-170201. [DOI] [PubMed] [Google Scholar]
- Davatzikos C., Xu F., An Y. Longitudinal progression of Alzheimer's-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain. 2009;132(Pt 8) doi: 10.1093/brain/awp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., PR McHugh. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:0022–3956. doi: 10.1016/0022-3956(75)90026-6. 0022–3956; 3. [DOI] [PubMed] [Google Scholar]
- Gordon B.A., Blazey T., Su Y. Longitudinal beta-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non-Alzheimer disease pathophysiology. JAMA Neurol. 2016;73(10) doi: 10.1001/jamaneurol.2016.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Knopman D.S., Weigand S.D. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann. Neurol. 2012;71(6) doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5) doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Wiste H.J., Weigand S.D. Age-specific and sex-specific prevalence of cerebral beta-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16(6) doi: 10.1016/S1474-4422(17)30077-7. S1474-4422(17)30077-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr. PART and SNAP. Acta Neuropathol. 2014;128(6) doi: 10.1007/s00401-014-1362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Jack C.R., Jr., Wiste H.J. Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann. Neurol. 2013;73(4) doi: 10.1002/ana.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Jack C.R., Jr., Lundt E.S. Role of beta-amyloidosis and neurodegeneration in subsequent imaging changes in mild cognitive impairment. JAMA Neurol. 2015;72(12) doi: 10.1001/jamaneurol.2015.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird N.M., Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982;38(4) [PubMed] [Google Scholar]
- Landau S.M., Mintun M.A., Joshi A.D. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 2012;72(4) doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino E.C., Betensky R.A., Hedden T. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71(11) doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino E.C., Papp K.V., Rentz D.M. Heterogeneity in suspected non-Alzheimer disease pathophysiology among clinically normal older individuals. JAMA Neurol. 2016;73(10) doi: 10.1001/jamaneurol.2016.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:0028–3878. doi: 10.1212/wnl.43.11.2412-a. 0028–3878; 11. [DOI] [PubMed] [Google Scholar]
- O'Dwyer L., Lamberton F., Matura S. Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C., Aisen P., Boeve B.F. Mild cognitive impairment due to Alzheimer disease in the community. Ann. Neurol. 2013;74(2) doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestia A., Caroli A., van der Flier W.M. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80(11) doi: 10.1212/WNL.0b013e3182872830. [DOI] [PubMed] [Google Scholar]
- Rey A.L. Presses Universitaires de France; Paris: 1964. examen clinique en psychologie. [Google Scholar]
- Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am. J. Psychiatry. 1984;141(11) doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Schreiber F., Lockhart S.N. Alzheimer disease signature neurodegeneration and APOE genotype in mild cognitive impairment with suspected non-Alzheimer disease pathophysiology. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2016.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos S.J., Gordon B.A., Su Y. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol. Aging. 2016;44 doi: 10.1016/j.neurobiolaging.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Das S.R., Suh J.W. A learning-based wrapper method to correct systematic errors in automatic image segmentation: consistently improved performance in hippocampus, cortex and brain segmentation. NeuroImage. 2011;55(3) doi: 10.1016/j.neuroimage.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse L.E., Butala N., Das S.R. Suspected non-AD pathology in mild cognitive impairment. Neurobiol. Aging. 2015;36(12) doi: 10.1016/j.neurobiolaging.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Wang H., Pluta J. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2-weighted MRI. NeuroImage. 2010;53:1095–9572. doi: 10.1016/j.neuroimage.2010.06.040. 1053–8119; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material