Abstract
Heavy alcohol consumption is associated with deleterious changes in the brain but associations of moderate alcohol intake are not well understood. We examined the association of alcohol consumption with brain white matter health in 377 middle-aged men (56–66 years old; mean 61.8 ± 2.6 years) who were participants in the Vietnam Era Twin Study of Aging (VETSA). T1-, T2-, proton density-, and diffusion-weighted magnetic resonance images were obtained. Diffusion measures were quantified from 12 major white matter tracts. Global white matter lesion (WML) burden was also quantified. Mixed effects linear models examined differences in diffusivity and WMLs by amount of alcohol intake. Analyses adjusted for numerous demographic, health, and lifestyle variables. An inverted-U association was found between alcohol intake and fractional anisotropy (FA) in several tracts, including the inferior-frontal-occipital fasciculus, uncinate fasciculus, superior longitudinal fasciculus, the forceps minor and the anterior thalamic radiations. In these tracts, FA increased with increasing alcohol intake, peaking with moderate alcohol intake (9–28 drinks in 14 days), and declining with heavier intake. Associations remained significant after exclusion of individuals with diabetes or hypertension. There was a U-shaped association in WML burden with highest burden among never drinkers and heavy drinkers (>28 drinks in 14 days). This association was no longer significant after exclusion of individuals with hypertension, since WML burden among heavy drinkers no longer differed from that of other drinkers. This suggests that hypertension related to heavy alcohol intake may contribute to WML burden observed among heavy drinkers. Together, these correlational results suggest that among middle-aged men, moderate drinking may be associated with metrics of better white matter health, particularly microstructural measures, whereas drinking beyond recommended guidelines may be associated with both microstructural and macrostructural white matter damage.
Keywords: Neuroimaging, Diffusion-weighted imaging, Fractional anisotropy, Aging, White matter lesion, White matter hyperintensity, DTI, Ethanol
Highlights
-
•
In middle-aged men brain white matter metrics differed by amount of alcohol intake.
-
•
Fractional anisotropy (FA) was highest among moderate drinkers.
-
•
FA decreased with heavier drinking (>2 drinks/day, on average).
-
•
Never drinkers and heavy drinkers had highest white matter lesion burden.
-
•
Light to moderate drinking is associated with indices of better white matter health.
1. Introduction
Many epidemiological studies have indicated that moderate alcohol intake is associated with preserved cognitive function in aging and reduced risk of dementia (Beydoun et al., 2014; Neafsey and Collins, 2011; Richard et al., 2017). In contrast, human neuroimaging studies have generally found that alcohol is associated with deleterious changes in the brain including global and regional brain shrinkage and white matter damage, with frontal lobes being particularly affected (Oscar-Berman and Marinkovic, 2007; Sullivan et al., 2010). However, most neuroimaging studies have focused on individuals with risky drinking patterns or alcohol use disorders. Relatively few have looked at associations of neuroimaging measures with alcohol use among community-based samples. Of those that have, some have found suggestions of a protective association of moderate drinking with white matter health (Mukamal et al., 2001; den Heijer et al., 2004), but others have not (Anstey et al., 2006; Ding et al., 2004; Topiwala et al., 2017). Suggestions of a protective association of light-to-moderate alcohol intake on white matter lesions (WMLs) have been found for older adults (den Heijer et al., 2004; Mukamal et al., 2001), but not for middle-aged adults (Anstey et al., 2006; Ding et al., 2004). It is possible that moderate alcohol intake may protect against the development of WMLs with advancing age, becoming evident only at older ages.
White matter is highly vulnerable to aging. WMLs, evidenced as areas of abnormal signal intensity within the white matter on magnetic resonance imaging (MRI), increase in prevalence with age (Gunning-Dixon et al., 2009; Jernigan et al., 2001) and are often of vascular origin (Bernbaum et al., 2015; Fazekas et al., 1993). Moderate alcohol intake reduces the risk of cardiovascular morbidity and mortality through multiple potential pathways, including anti-atherosclerotic, anti-thrombotic and anti-inflammatory mechanisms (Bau et al., 2007; Collins et al., 2009; Rimm et al., 1999). It is plausible that moderate alcohol intake may also protect against cerebrovascular dysfunction that contributes to white matter damage in aging.
Diffusion-weighted imaging, which is sensitive to microscopic changes in the brain that affect the free flow of water molecules, enables sensitive assessment of aging-related and health-related changes in brain white matter microstructure. White matter microstructure is often probed through the use of fractional anisotropy (FA) – a measure of the degree to which water motion is restricted along a single direction. In white matter tracts, diffusion occurs most strongly along the tract length (axial diffusion), with axonal walls and myelin sheaths serving as barriers to the flow of water across the tract (radial diffusion). Axonal and myelin loss alter diffusion, as do other pathological changes such as increased fluid and loss of intracellular structures. Decreases in FA and increases in mean diffusivity occur with aging (Sullivan et al., 2008), and in individuals with alcohol use disorders (Pfefferbaum et al., 2009). Alterations in white matter diffusion have been reported to precede development of WMLs (de Groot et al., 2013), and thus may be a more sensitive index of white matter health than macroscopic lesions.
Here, we investigated the association between alcohol intake and white matter health in a cohort study of middle-aged men from across the USA, the Vietnam Era Twin Study of Aging (VETSA, (Kremen et al., 2013; Kremen et al., 2006). Based on prior findings of a positive association between moderate alcohol intake and cognitive function in aging (Neafsey and Collins, 2011; Richard et al., 2017), and on findings of cardioprotective effects of moderate drinking (Xi et al., 2017), we hypothesized that individuals who consume moderate amounts of alcohol would show indices of healthier white matter relative to those who do not drink or who drink more heavily, and that associations would be stronger for measures of white matter diffusion than for WMLs.
2. Material and methods
2.1. Participants
VETSA participants were recruited from the Vietnam Era Twin Registry, a nationally distributed sample of male-male twin pairs who served in the United States military at some point between 1965 and 1975 (Goldberg et al., 2002). Participants are similar in health and lifestyle characteristics to American men in their age range (Schoenborn and Heyman, 2009). Although all VETSA participants are veterans, the majority (~80%) did not experience combat situations.
The current study focused on 377 participants (including 132 twin pairs) who completed the wave 2 MRI visit between 2009 and 2013, whose anatomical and diffusion-weighted imaging data passed quality control. The study was conducted under institutional review board supervision at the participating institutions; all participants provided signed informed consent.
2.2. Alcohol intake
Alcohol use was assessed as part of a structured medical history interview at each visit. Participants were asked if they had consumed ≥ 20 alcoholic drinks in their life. Those who responded ‘Yes’ were asked the number of days in the past 2 weeks in which they consumed beer, and how many beers they had on days in which they drank beer. These questions were repeated for wine and hard liquor. We summed across beverage types to yield the total number of drinks in the past 14 days. Participants also completed the Cage questionnaire, a 4 question interview designed to detect problematic drinking; scores >1 are indicative of potential for alcohol use disorder (Ewing, 1984).
In primary analyses, alcohol was treated as a categorical variable to enable separation of current nondrinkers from never drinkers (those who reported not consuming 20 drinks in their life). To examine dose effects, current alcohol consumers were divided into approximate quartiles, and groups are referred to as very light drinkers (1–3 drinks in the past 14 days); light drinkers (4–8 drinks); moderate drinkers (9–28 drinks); and heavier drinkers (>28 drinks in the past 14 days; a level of drinking that may increase risk for alcohol-related problems according to the National Institute on Alcohol Abuse and Alcoholism, 2005).
In secondary analyses, we examined the association of alcohol with white matter metrics when alcohol was treated as a continuous variable (number of drinks in the past 14 days). Due to the non-normal distribution, values were log transformed after adding a constant (1) to avoid values of zero.
2.3. Health, behaviors, and socioeconomic covariates
We examined demographic, health, and behavioral variables that may be associated with alcohol intake, including age, race/ethnicity, indicators of socioeconomic status, adiposity, smoking, blood pressure, cholesterol, diabetes, and inflammation.
Information on educational attainment, family income, cigarette smoking and medical history were obtained from structured interviews. During the research visit, height, weight, and waist circumference were measured. Systolic and diastolic blood pressure (SBP and DBP) were measured as the average of 2 morning and 2 afternoon seated readings. Participants were classified as having hypertension based on SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, self-report of a physician diagnosis, or self-reported use of antihypertensive medication for cardiovascular health.
Diabetes status was ascertained from self-report of a doctor's diagnosis or reported use of a diabetes-related medication. Fasting morning blood samples were obtained. Low- and high-density lipoprotein (LDL and HDL) cholesterol and triglycerides were assayed as part of a lipid panel via spectrophotometry. C-reactive protein (CRP) levels were assessed with nephelometry. Due to non-normal distributions, triglyceride, cholesterol, and CRP values were log-transformed prior to analyses. Assays were conducted by Quest Diagnostics Inc./Nichols Institute, San Juan Capistrano, CA.
2.4. Image acquisition and processing
T1-, T2-, proton-density- (PD) and diffusion-weighted images were obtained using standardized protocols on 3T scanners at two sites (University of California San Diego and Massachusetts General Hospital). Image acquisition details have been previously described (Fennema-Notestine et al., 2016; McEvoy et al., 2015). Image processing was performed at UC San Diego, using previously described procedures (Fennema-Notestine et al., 2016; McEvoy et al., 2015), and briefly summarized below.
2.5. White matter microstructure
Diffusion-weighted images were corrected for distortions and registered to the T1-weighted image (Hagler Jr et al., 2009). Diffusion images were rigidly resampled, using cubic interpolation, into a standard orientation with 2 mm isotropic resolution. Conventional DTI methods were used to model the diffusion tensor as an ellipsoid where eigenvalues λ1, λ2, and λ3 define the three primary axes (Basser, 1995; Basser et al., 1994; Le Bihan et al., 2001; Pierpaoli et al., 1996). Derived measures included FA, a scalar value of the degree of anisotropic diffusion within the voxel; axial diffusivity, the average diffusion along the primary axis; and radial diffusivity, the average diffusion along the two non-primary axes. Average values for specific fiber tracts were obtained using a probabilistic atlas of fiber tract locations and orientations (Hagler Jr et al., 2009). T1-weighted images were used to nonlinearly register the brain to a common space, and diffusion tensor orientation estimates were compared to the AtlasTrack atlas to obtain a map of the relative probability that a voxel belonged to a particular fiber given the location and similarity of diffusion orientations. These probability values were used to calculate weighted averages of the diffusion measures for each fiber tract. A fiber probability threshold of 0.08 was used to ensure that voxels with very low probability of belonging to a given fiber did not contribute to average values.
FreeSurfer software (Fischl et al., 2002) was used to automatically segment gray matter, white matter and cerebral spinal fluid (CSF) on the high resolution structural image, and this information was used to identify and exclude voxels in fiber tract regions of interest that were primarily gray matter or CSF. Diffusion metrics in 12 major fiber tracts were analyzed: uncinate fasciculus (UF), inferior frontal-occipital fasciculus (IFOF), inferior and superior lateral fasciculi (ILF, SLF), cingulum and parahippocampal portions of the cingulum bundle (CgC, CgH), fornix, anterior thalamic radiations (ATR), corticospinal tract (CST), corpus callosum (CC), and forceps minor and forceps major (Fmin, Fmaj). Because a prior study indicated no hemispheric differences in the association of alcohol with diffusion metrics (Pfefferbaum et al., 2009), right and left hemisphere measures were averaged.
2.6. White matter lesions
A multi-channel segmentation approach, which leverages complementary information in T1- T2- and PD-weighted volumes, was used to quantify WML volume. These measures were available for 348 participants. Details of the approach have been previously described (Fennema-Notestine et al., 2016). Briefly, T2 and PD volumes were registered to the T1 volume, all volumes were bias corrected, and then a 3-class tissue segmentation was performed (white matter, gray matter, CSF). WMLs were classified using morphological operators to identify voxel clusters originally segmented as gray matter that fell within anatomically-defined white matter regions, excluding partially-volumed voxels along ventricles. Results were visually reviewed and edited when necessary to correct misclassifications. Total white matter volume and WML volume were log-transformed due to non-normal distributions. The proportion of log total white matter that was accounted for by log WML volume was calculated to determine WML burden.
2.7. Statistical analysis
To determine whether demographic and clinical characteristics differed across alcohol groups, we used linear mixed-effect models (Proc Mixed SAS, version 9.4) for continuous variables, and chi-square tests for categorical variables. To assess stability of alcohol use, we computed the Pearson correlation between alcohol intake reported at the current visit with that reported in the visit that occurred 5 years previously (VETSA wave 1).
For our primary analyses we used linear-mixed effect models to determine whether white matter metrics differed between groups. Base models included fixed effects of scanner (one per site) and age. A “family ID” variable was included as a random effect to control for non-independence of twin data. Adjusted models included potentially confounding covariates that differed across groups at p < 0.10, and included years of education, family income (categorized into ≤$39,999; $40,000–$89,999, ≥$90,000), SBP, HDL, smoking (never, current, former) and diabetes status (yes/no). When significant differences in FA were observed, secondary analyses examined whether diffusion differences occurred primarily along radial or axial directions. To assess the association between alcohol and WMLs, analyses were repeated using WML burden as the outcome variable. A probability value of p < 0.05 was considered significant. We corrected for multiple comparisons across the 12 tracts using a false discovery rate approach taking into account the correlation between tracts to determine the effective number of independent tests (Li and Ji, 2005). When significant associations of alcohol with DTI metrics were observed, following adjustment for multiple comparisons across the 12 tracts, post-hoc comparisons were performed to assess significance of differences between the moderate drinking group and the other 5 groups, using the Sidak adjustment for multiple comparisons.
In secondary analyses, we repeated the analyses treating alcohol as a continuous measure of log-transformed number of drinks over the past 14 day, and including alcohol as a non-linear variable.
We performed sensitivity analyses to determine whether outliers in the heavy drinking group accounted for observed decreases in FA among the heavy drinking group, by excluding 18 individuals who reported drinking >56 drinks in the past 14 days (equivalent to >4 drinks/day). We examined whether the inclusion of individuals with potential alcohol use disorders may have affected the results by excluding individuals with Cage scores > 1. We also examined the influence of cardiometabolic disease on the results by repeating the analyses after excluding individuals with diabetes or with hypertension.
3. Results
Participants were 61.8 (±2.62, range 56–66) years old, on average, predominantly white, non-Hispanic (88.6%), with an average education of 13.8 years (SD = 2.1, range 5–20 years). The majority (346/377; 92%) reported having consumed at least 20 alcoholic beverages in their life, and most (248/377, 66%) reported drinking in the past 14 days. Beer was the most frequently consumed beverage (197/248, 79% of current drinkers), followed by wine (118/248, 48%) and hard liquor (108/248, 44%). Many (134/248, 54%) reported drinking more than one type of alcohol. Among the 318 participants with data at both waves, number of drinks reported in wave 2 correlated with number of drinks reported 5 years previously in wave 1 at r = 0.67; p < 0.001.
Table 1 shows the demographic, behavioral, and clinical characteristics of each group. Groups did not differ in age. Moderate drinkers had significantly higher family income than other groups. Heavy drinkers were more likely to be current smokers than other groups. HDL levels increased with increasing alcohol intake, and SBP was highest in the heavy drinking group. Rates of diabetes were highest among current non-drinkers and very light drinkers. The proportion of individuals with potential alcohol use problems (Cage questionnaire score > 1) was highest among the heaviest drinking group, followed by current non-drinkers.
Table 1.
Participant characteristics by alcohol group. Values shown are mean (standard deviation), unless otherwise indicated.
| Never drinker (N = 31) | Current non-drinker (N = 98) | Very light, 1–3 drinks (N = 69) | Light, 4–8 drinks (N = 59) | Moderate, 9–28 drinks (N = 68) | Heavy, > 28 drinks (N = 52) | Statistic | |
|---|---|---|---|---|---|---|---|
| Age, years | 62.3 (2.3) | 61.3 (2.6) | 61.9 (2.6) | 62.0 (2.4) | 61.5 (2.8) | 62.4 (2.6) | F(5,127) = 0.68; p = 0.64 |
| Education, years | 14. 5 (2.1) | 13. 5 (2.1) | 13. 8 (2.0) | 13.7 (2.0) | 14.1 (2.0) | 13.5 (2.3) | F(5,127) = 1.94; p = 0.09 |
| White non-Hispanic, No. (%) | 26 (84) | 78 (80) | 61 (88) | 65 (92) | 62 (91) | 47 (90) | χ2 (5) = 6.842; p = 0.25 |
| Drinks in past 14 days | 0 | 0 | 1.9 (0.8) | 5.5 (1.4) | 16.5 (5.7) | 57.0 (26.4) | |
| Family income, no. (%) | χ2 (10) = 32.67; p < 0.001 | ||||||
| ≤$39,999 | 5 (16) | 30 (31) | 16 (23) | 3 (5) | 4 (6) | 10 (19) | |
| $40,000–$89,999 | 20 (65) | 47 (48) | 37 (54) | 35 (61) | 34 (50) | 27 (52) | |
| ≥$90,000 | 6 (19) | 20 (21) | 16 (23) | 19 (33) | 30 (44) | 15 (29) | |
| Smoking status, no. (%) | χ2(10) = 45.90; p < 0.001 | ||||||
| Never | 27 (87) | 42 (43) | 31 (45) | 23 (39) | 27 (40) | 9 (17) | |
| Current | 2 (7) | 15 (15) | 14 (20) | 11 (19) | 10 (15) | 19 (37) | |
| Former | 2 (7) | 41 (42) | 24 (35) | 25 (37) | 31 (46) | 24 (46) | |
| BMI, kg/m2 | 28.6 (3.2) | 28.8 (4.4) | 27.9 (4.8) | 29.6 (4.4) | 28.7 (4.5) | 27.9 (3.7) | F(5,127) = 1.17; p = 0.33 |
| Waist, inches | 39.4 (3.4) | 39.9 (4.5) | 39.1 (4. 8) | 40.8 (4.0) | 38.9 (4.3) | 38.9 (4.2) | F(5,126) = 1.17; p = 0.19 |
| Systolic BP, mm Hg | 123 (13) | 127 (17) | 125 (14) | 130 (16) | 128 (18) | 134 (16) | F(5,127) = 2.56; p = 0.03 |
| Diastolic BP, mm Hg | 77.2 (9) | 78.5 (10) | 77.3 (8) | 78.3 (11) | 77.7 (10) | 81.0 (8) | F(5,127) = 0.88; p = 0.50 |
| Log HDLa | 3.76 (0.28) | 3.81 (0.27) | 3.80 (0.23) | 3.83 (0.30) | 3.91 (0.29) | 4.06 (0.28) | F(5,119) = 11.62; p < 0.001 |
| Log LDLb | 4.66 (0.36) | 4.62 (0.35) | 4.65 (0.34) | 4.64 (0.29) | 4.66 (0.434) | 4.56 (0.36) | F(5,113) = 0.80; p = 0.55 |
| Log triglyceridesa | 4.84 (0.50) | 4.79 (0.61) | 4.71 (0.55) | 4.74 (0.55) | 4.64 (0.55) | 4.66 (0.55) | F(5,118) = 0.79; p = 0.56 |
| Log CRPc | 0.21 (1.00) | 0.41 (1.10) | 0.41 (1.08) | 0.57 (1.15) | 0.16 (1.03) | 0.34 (0.98) | F(5,121) = 0.78; p = 0.57 |
| HTN no. (%) | 17 (55) | 54 (55) | 31 (45) | 35 (59) | 27 (40) | 36 (69) | χ2(5) = 13.29; p = 0.02 |
| HTN Med no. (%) | 16 (52) | 53 (54) | 32 (46) | 28 (48) | 24 (35) | 29 (56) | χ2 (5) = 7. 34; p = 0.20 |
| Statin Med no. (%) | 8 (26) | 32 (33) | 28 (41) | 28 (48) | 24 (35) | 24 (46) | χ2 (5) = 7. 24; p = 0.20 |
| Diabetes no. (%) | 1 (3) | 18 (18) | 16 (23) | 9 (15) | 4 (6) | 5 (10) | χ2(5) = 14.0; p = 0.02 |
| Cage score > 1 no. (%) | 0 | 36 (37) | 12 (17) | 10 (17) | 16 (23.5) | 32 (62) | χ2(5) = 52.74; p < 0.001 |
BMI = body mass index; HTN = hypertension; HDL = high density lipoprotein cholesterol LDL = low density lipoprotein cholesterol; CRP = C-reactive protein; BP = blood pressure, Med = medicine. Analyses showing significant differences across groups are shown in bold.
HDL and triglyceride values were unavailable for 24 participants;
LDL levels were unavailable for 27 participants;
CRP levels were unavailable for 12 participants.
3.1. Association between alcohol and white matter microstructure
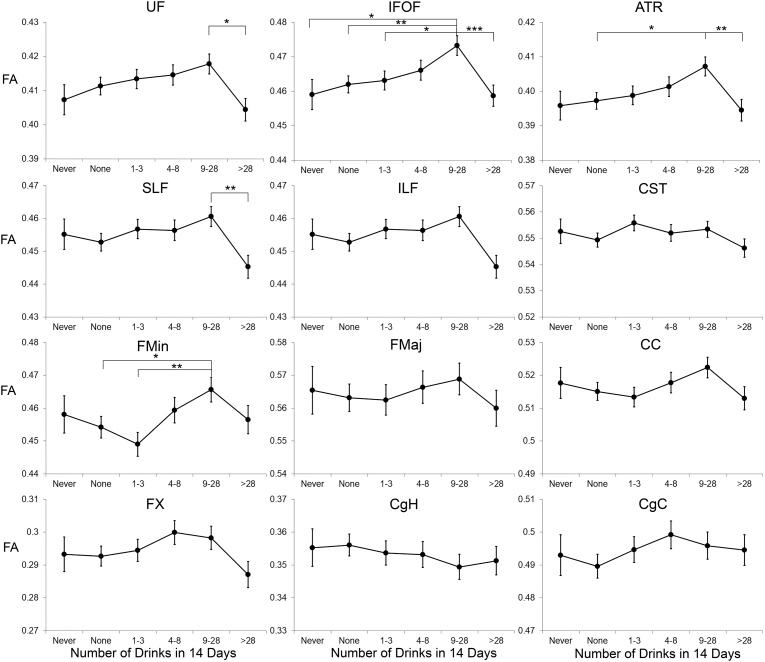
Fig. 1 shows mean FA in the 12 white matter tracts as a function of group. In base models, controlling for age, scanner and family-relatedness, differences in FA by alcohol group were significant after correction for multiple comparisons for the IFOF only (Table 2), with highest FA among moderate drinkers. After adjusting for potentially confounding covariates that differed across groups (education, family income, SBP, HDL, smoking and diabetes status) significant effects were additionally observed for the UF, ATR, SLF, and Fmin, after correction for multiple comparisons (Table 2). For all these tracts, average FA was highest for the moderate drinking group (9–28 drinks in 14 days) with lower FA for the heavier drinking group. Post-hoc pairwise comparisons comparing the moderate drinking group to the other groups indicated that FA among moderate drinkers was significantly higher than among never drinkers and non-drinkers for the IFOF; higher than among non-drinkers for the ATR and Fmin, and higher than among heavier drinkers for the UF, IFOF, ATR, and SLF (see Fig. 1).
Fig. 1.
Fractional Anisotropy (FA) as a function of alcohol intake group for 12 white matter tracts. Estimated means are adjusted for scanner and age. Error bars indicate standard error of the mean. In fully adjusted models, FA differed significantly by alcohol group for the UF, IFOF, ATR, SLF, and Fmin. Significant post-hoc comparisons, after adjustment for multiple comparisons, are shown. UF = uncinate fasciculus; IFOF = inferior fronto-occipital fasciculus; ATR = anterior thalamic radiations; SLF = superior longitudinal fasciculus; ILF – inferior longitudinal fasciculus; Fmin = forceps minor; Fmaj = forceps major; CC – corpus callosum; FX = fornix, CgH = cingulum, parahippocampus; CgC = cingulum, cingulate; CST = corticospinal tract. *p < 0.05; **p < 0.01; ***p < 0.001.
Table 2.
Results of the base and fully-adjusted fixed effects linear models of differences in FA across alcohol groups.
| Tract | Base modela | Fully adjusted modelb |
|---|---|---|
| Uncinate Fasciculus | F(5, 126) = 2.63; p = 0.03 | F(5, 110) = 2.87; p = 0.02 |
| Inferior fronto-occipital fasciculus | F(5, 126) = 4.23; p = 0.001 | F(5, 110) = 4.59; p < 0.001 |
| Anterior-thalamic radiations | F(5, 126) = 2.75; p = 0.02 | F(5, 110) = 3.16; p = 0.01 |
| Superior longitudinal fasciculus | F(5, 126) = 2.86; p = 0.02 | F(5, 110) = 3.03; p = 0.01 |
| Inferior longitudinal fasciculus | F(5, 126) = 2.18; p = 0.06 | F(5, 110) = 2.33; p = 0.05 |
| Corticospinal tract | F(5, 126) = 1.20; p = 0.31 | F(5, 110) = 1.12; p = 0.35 |
| Forceps minor | F(5, 126) = 2.61; p = 0.03 | F(5, 113) = 3.07; p = 0.01 |
| Forceps major | F(5, 126) = 0.45; p = 0.81 | F(5, 110) = 1.06; p = 0.39 |
| Corpus callosum | F(5, 126) = 1.50; p = 0.20 | F(5, 110) = 2.49; p = 0.04 |
| Fornix | F(5, 126) = 1.68; p = 0.15 | F(5, 110) = 2.51; p = 0.03 |
| Cingulum - parahippocampus | F(5, 126) = 0.44; p = 0.82 | F(5, 110) = 0.15; p = 0.98 |
| Cingulum - cingulate | F(5, 126) = 0.74; p = 0.60 | F(5, 110) = 0.97; p = 0.44 |
Significant effects after correction for multiple comparisons, are shown in bold.
Base models include age and site as covariates, and family-relatedness as a random effect.
Fully adjusted models additionally included education, family income, smoking status, average systolic blood pressure, diabetes status, and high density lipoprotein level as covariates.
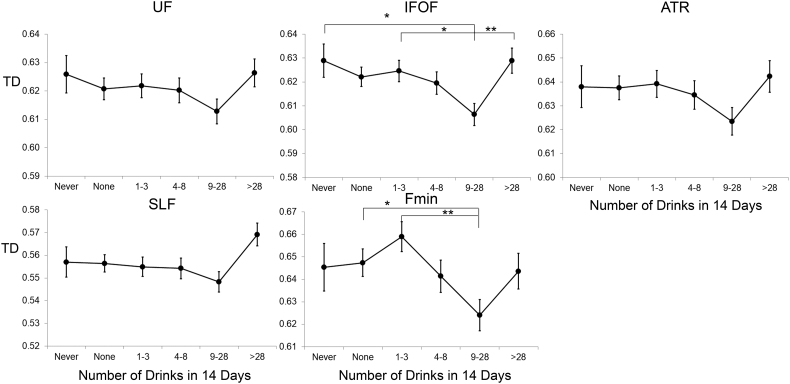
Examination of radial and axial diffusivity in tracts showing significant association with FA revealed no differences across groups in axial diffusivity (all p values > 0.1; see Table 3), but significant group differences for radial diffusivity in the IFOF and Fmin (Table 3), with lowest diffusivity among moderate drinkers, and higher diffusivity among heavy drinkers (Fig. 2).
Table 3.
Results of the fixed effects models of differences in radial and axial diffusivity across alcohol groups for the tracts showing significant differences in FA.
| Tract | Radial diffusivity | Axial diffusivity |
|---|---|---|
| Uncinate | F(5, 126) = 1.27; p = 0.28 | F(5, 126) = 0.94; p = 0.46 |
| Inferior fronto-occipital fasciculus | F(5, 126) = 3.29; p = 0.008 | F(5, 126) = 0.95; p = 0.45 |
| Anterior thalamic radiations | F(5, 126) = 1.35; p = 0.25 | F(5, 126) = 0.54; p = 0.75 |
| Superior longitudinal fasciculus | F(5, 126) = 2.29; p = 0.05 | F(5, 126) = 0.71; p = 0.62 |
| Forceps minor | F(5, 126) = 3.17; p = 0.01 | F(5, 126) = 0.79; p = 0.56 |
*Models adjusted for age, scanner, education, family income, smoking status, average systolic blood pressure, diabetes status, and high density lipoprotein level as covariates. Family-relatedness was included as a random effect. Significant effects after correction for multiple comparisons are shown in bold.
Fig. 2.
Radial diffusivity (RD) as a function of alcohol intake group for the five tracts that showed significant FA differences. Estimated means are adjusted for scanner and age. Error bars show standard error of the mean. Significant differences in RD were found for the IFOF and Fmin. Significant posthoc comparisons, after correction for multiple comparisons, are shown. For abbreviations, see legend for Fig. 1. *p < 0.05; **p < 0.01.
Sensitivity analyses focused on the IFOF because this tract showed the strongest associations with alcohol intake. Sensitivity analyses were performed with adjustment for the same potential confounders included in the primary analyses. When alcohol was modeled as a continuous rather than categorical variable, there was a significant inverted-U association of alcohol with IFOF FA in base (F(1,129) = 11.80; p = 0.001) and fully adjusted models (F(1,113) 0 = 12.4; p = 0.001). When the primary analyses, treating alcohol as a categorical variable were repeated with exclusion of individuals who consumed >56 drinks in the past 14 days, FA continued to be lower among the heaviest drinking group than among the moderate drinking group (Sidak adjusted p = 0.005). Excluding individuals with possible alcohol use disorders (Cage score > 1), did not change the results: the main effect of alcohol remained significant F(5,58) = 5.73; p < 0.001, and post-hoc comparisons showed greater FA among moderate drinkers than among current non-drinkers (Sidak adjusted p = 0.005), and heavier drinkers (Sidak adjusted p < 0.001). Excluding individuals with diabetes (n = 53) did not change the results (base model, F(5,100) = 3.34, p = 0.008; fully adjusted model F(5,86) = 3.90, p = 0.003; raw p values). Although excluding individuals with hypertension (n = 200) reduced sample size by more than half, FA continued to differ across groups (base model: F(5,33) = 3.49; p = 0.012; fully adjusted model (F(5,23) = 3.38; p = 0.02), with highest FA among light and moderate drinkers and lowest FA among the never drinkers. Similar results were obtained after exclusion of individuals with either hypertension or diabetes (excluding 216 individuals; base model: F(5,28) = 2.73; p = 0.04; fully adjusted model (F(5,19) = 2.57; p = 0.061)).
3.2. Association between alcohol and white matter lesions
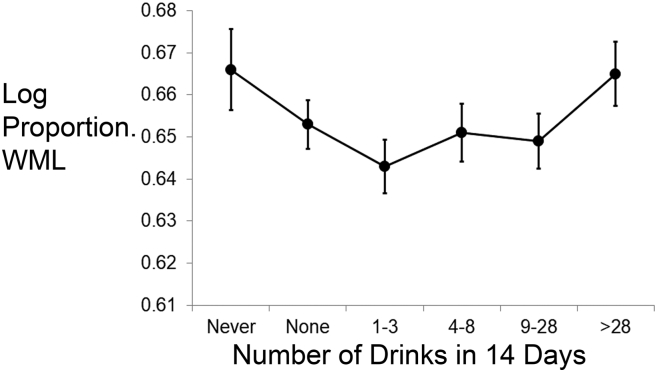
The linear mixed effect model showed no significant differences in WML burden as a function of alcohol group (F(5,113) = 1.50; p = 0.20; Fig. 3). This was unchanged after adjustment for covariates (F(5,98) = 1.72; p = 0.14). However, when alcohol was treated as a continuous variable, there was evidence for a U-shaped association (base model: F(1,116) = 5.08; p = 0.026; fully adjusted model: F(1,101) = 4.50; p = 0.036). These results were unchanged after exclusion of heaviest drinkers (base model: F(1, 103) = 6.71; p = 0.01, fully adjusted model: (F(1,89) = 5.16; p = 0.026); Although the effect was attenuated, similar results were observed when restricted to those with Cage Score of 1 or less (base model (F(1,66) = 3.78; p = 0.056; fully adjusted model: F(1,53) = 3.27; p = 0.076). Exclusion of individuals with diabetes did not change the results (base model (F(1,92) = 5.16 p = 0.026; fully adjusted model: (F(1,79) = 4.34; p = 0.041), but the quadratic association was no longer significant after excluding individuals with hypertension (base model (F(1,32) = 0.27 p = 0.61); fully adjusted model: (F(1,22) = 0.29; p = 0.59). Exclusion of individuals with hypertension reduced WML burden among the heaviest drinking group but not among never drinkers, who continued to show highest WML burden.
Fig. 3.
White matter lesion (WML) burden as a function of alcohol intake group. WMLs were calculated as the proportion of log abnormal white matter volume to log total white matter volume. Estimated means from the base model, which adjusted for scanner and age, are shown. Error bars indicate the standard error of the mean.
4. Discussion
Numerous studies have indicated that chronic alcoholism is associated with detrimental changes in the brain (Harper, 2009; Oscar-Berman and Marinkovic, 2007; Sullivan et al., 2010). However, few studies have focused on effects of moderate drinking on healthy brain aging. We found evidence for an inverted-U shaped association between alcohol intake and white matter microstructure and gross WMLs, with stronger associations observed for white matter microstructure. For both types of measures, light to moderate alcohol intake was associated with indices of better white matter health than non-drinking or heavier drinking.
Several white matter tracts that travel through the frontal lobe showed signs of better white matter microstructural integrity among moderate drinkers, with most pronounced associations in the IFOF, a major white matter tract that extends from occipital cortex to the inferior frontal lobe. FA in the IFOF increased with increasing alcohol intake, reaching a maximum at the moderate drinking level (9–28 drinks in the prior 14 days), then decreasing with greater alcohol intake. A similar pattern was also observed in the anterior projections of the thalamus and the forceps minor - the anterior fibers of the corpus callosum that connect left and right frontal cortex. Significantly decreased FA in heavy drinkers (>28 drinks in 14 days) relative to moderate drinkers was also observed in the UF, a fiber tract that connects anterior temporal to prefrontal cortex, and the SLF, a fiber tract that connects posterior temporal and parietal areas to frontal cortex.
The finding of reduced FA with heavier drinking is consistent with results from studies of individuals with alcohol use disorders or risky drinking patterns, who have shown decreased FA and increased radial diffusivity in frontal and superior white matter tracts (Harris et al., 2008; Pfefferbaum et al., 2009; Yeh et al., 2009). Animal models of binge drinking and human neuropathological studies on individuals with alcohol use disorders have demonstrated neuronal loss, primarily in frontal association cortex, and alterations in white matter, including myelin damage (Samantaray et al., 2015; Wiggins et al., 1988).
Given the multitude of factors that can affect alterations in diffusion, we cannot be certain of the neurobiological basis of the differences observed here (Beaulieu, 2002; Wheeler-Kingshott and Cercignani, 2009). However, animal and human histopathological studies indicate that myelin damage increases radial diffusivity and reduces FA, and that FA primarily reflects radial rather than axial diffusivity (Chang et al., 2017; Klawiter et al., 2011; Song et al., 2005). Thus, reduced FA and increased radial diffusivity among the heavier drinking group may reflect myelin damage related to heavy alcohol exposure, whereas the increased FA and decreased radial diffusivity among moderate drinkers may reflect better-preserved myelin.
WMLs are common among older adults, particularly those with hypertension, and are commonly associated with ischemic damage (Bernbaum et al., 2015; Fazekas et al., 1993). We previously reported that in this middle-aged sample, WMLs were predominantly located in periventricular and deep frontal and parietal white matter, and were positively associated with age and hypertension (Fennema-Notestine et al., 2016). The U-shaped association between alcohol and WMLs observed here is consistent with findings from two studies of older adults, the Cardiovascular Health Study (Mukamal et al., 2001) (aged 65+ years) and the Rotterdam study (den Heijer et al., 2004) (aged 60–90 years) in which light-to-moderate drinkers showed fewer WMLs than did abstainers or heavy drinkers. Our findings differ from the null results reported in two studies of middle-aged adults, the ARIC (Ding et al., 2004) (mean age 57 years) and PATH Through Life (60–64 years) studies (Anstey et al., 2006). However, the ARIC study (Ding et al., 2004) contained too few heavy drinkers to examine WMLs separately in heavy drinkers, and the PATH Through Life study (Anstey et al., 2006) did not differentiate between never drinkers and current non-drinkers. Additionally, our multi-channel segmentation method for quantifying WMLs is likely more sensitive than semi-quantitative methods for grading WMLs (Ding et al., 2004), or than methods that rely on less information (Anstey et al., 2006). It may, thus, improve ability to detect subtle differences in WMLs in our middle-aged sample. Our results suggest that putative effects of alcohol on WMLs are already present in midlife (ages 56–66 years). Because alterations in white matter diffusion appear to precede development of WMLs (de Groot et al., 2013), our results also suggest that associations of alcohol with white matter microstructure may be evident even earlier in life and may be more reliably detected than WMLs in midlife adults. Given that white matter microstructure deteriorates with age (Gunning-Dixon et al., 2009), the observed associations will likely increase with age. Ongoing follow-up will enable us to address this issue.
There are several pathways by which alcohol may exert protective effects. Alcohol intake increases HDL levels, which in turn, are associated with reduced risk of atherosclerosis (Rimm et al., 1999). HDL levels increased systematically across our alcohol groups, with highest levels among the heaviest drinking group. Inclusion of HDL in the adjusted models did not attenuate the observed associations, suggesting that differences in HDL do not underlie the associations of alcohol with white matter microstructure. Alcohol also has anti-inflammatory properties (Collins et al., 2009; Crews and Nixon, 2009); however, CRP levels did not differ across groups. Other mechanisms not assessed here, such as alcohol's anticoagulant effects or possible direct neuroprotective effects (Collins et al., 2009), may underlie the observed association of higher FA among moderate drinkers.
A frequently-cited concern regarding studies that report health benefits of alcohol is the use of non-drinking comparison groups that mix former drinkers with abstainers. Former drinkers may have poorer health than current drinkers (Rehm et al., 2008; Wannamethee and Shaper, 1997) and lifetime abstainers may differ from drinkers in numerous factors that may also affect health (Bondy and Rehm, 1998). The finding that approximately 30% of our current non-drinking group had a Cage score > 1 suggests that this group contained individuals with a history of prior heavy drinking. However, excluding these individuals from the analyses did not materially change the results. Furthermore, our finding that moderate drinkers showed higher IFOF FA than lighter drinkers also suggests that the protective associations of alcohol observed here do not arise from confounds related to non-drinkers. Moderate drinking was associated with higher family income, which may be associated with increased access to health care and ability to pursue a healthy lifestyle. Including income and education as covariates in the analyses did not attenuate the associations, but residual confounding cannot be ruled out.
Hypertension and diabetes are also important potential confounders, since these diseases are associated with increased risk of white matter damage (Bernbaum et al., 2015; Fazekas et al., 1993; Gouw et al., 2008), and moderate drinkers showed lowest prevalence of these disorders. Although it is possible that this may be a manifestation of cardiovascular benefits of a history of moderate drinking, particularly with regard to diabetes (Matsumoto et al., 2014), sensitivity analyses excluding individuals with diabetes or hypertension continued to show higher FA among light and moderate drinkers than among non-drinkers and very light drinkers. However, the U-shaped association of WML burden with alcohol intake was no longer significant after excluding individuals with hypertension. After exclusion of individuals with hypertension, the heaviest drinking group no longer showed higher WML burden than other drinkers. Because heavy alcohol intake is a well-known risk factor for hypertension (Matsumoto et al., 2014), and hypertension is one of the strongest predictors of white matter damage (Skoog, 1998), this suggests that alcohol-related hypertension may underlie the higher WML burden observed among heavy drinkers.
This study has other limitations. Self-reported alcohol use is prone to under-estimation (Devos-Comby and Lange, 2008). However, this would not affect the rank-order of alcohol intake groups, but does limit our ability to determine thresholds at which associations change from beneficial to harmful. Additionally, we used single time-point assessment of alcohol use, which may not adequately reflect an individual's history of alcohol exposure. However, studies have shown that alcohol use tends to remain stable in middle and older age (Bobo et al., 2010; Brennan et al., 2011; McEvoy et al., 2013; Moore et al., 2005), and we observed a high correlation (r = 0.67) between self-reported alcohol use in VETSA waves 1 and 2. Finally, the sample was restricted to primarily white men. Thus, findings may not generalize to women or other races or ethnicities.
5. Conclusions
Among middle-aged men, moderate alcohol intake appears to be associated with signs of healthier brain white matter, whereas heavier alcohol intake is associated with signs of white matter damage. Given the correlational nature of this study, caution is warranted in interpreting the findings to suggest a causal effect of alcohol on brain white matter health. Our analyses did adjust for multiple health and demographic factors, thus minimizing their potential as alternative causal factors. Nevertheless, other factors, not assessed here, may underlie the observed associations. Our findings extend previously reported possible beneficial associations of alcohol with cardiovascular health to measures of brain health. They show that these associations occur in middle age, prior to the age at which substantial age-related cognitive decline typically manifests. Longitudinal follow-up is needed to determine whether moderate alcohol intake during middle life is related to rate of white matter decline in older age.
Acknowledgments
Acknowledgements
Funding
This work was supported by the National Institutes of Health (grant numbers AA021187, AG018386, AG022381, AG022982 and AG018384). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA, NIA/NIH, or the VA. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interest
LKM holds stock in CorTechs Laboratories, Inc.
AMD is a founder and holds equity in CorTechs Laboratories, Inc., and also serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity, Inc. and receives funding through research agreements with General Electric Healthcare and Medtronic, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
All other authors declare no competing financial interests.
References
- Anstey K.J., Jorm A.F., Reglade-Meslin C., Maller J., Kumar R., von Sanden C., Windsor T.D., Rodgers B., Wen W., Sachdev P. Weekly alcohol consumption, brain atrophy, and white matter hyperintensities in a community-based sample aged 60 to 64 years. Psychosom. Med. 2006;68:778–785. doi: 10.1097/01.psy.0000237779.56500.af. [DOI] [PubMed] [Google Scholar]
- Basser P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bau P.F., Bau C.H., Rosito G.A., Manfroi W.C., Fuchs F.D. Alcohol consumption, cardiovascular health, and endothelial function markers. Alcohol. 2007;41:479–488. doi: 10.1016/j.alcohol.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bernbaum M., Menon B.K., Fick G., Smith E.E., Goyal M., Frayne R., Coutts S.B. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J. Cereb. Blood Flow Metab. 2015;35:1610–1615. doi: 10.1038/jcbfm.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun M.A., Beydoun H.A., Gamaldo A.A., Teel A., Zonderman A.B., Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14(643) doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo J.K., Greek A.A., Klepinger D.H., Herting J.R. Alcohol use trajectories in two cohorts of U.S. women aged 50 to 65 at baseline. J. Am. Geriatr. Soc. 2010;58:2375–2380. doi: 10.1111/j.1532-5415.2010.03180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S., Rehm J. The interplay of drinking patterns and other determinants of health. Drug Alcohol Rev. 1998;17:399–411. doi: 10.1080/09595239800187241. [DOI] [PubMed] [Google Scholar]
- Brennan P.L., Schutte K.K., Moos B.S., Moos R.H. Twenty-year alcohol-consumption and drinking-problem trajectories of older men and women. J. Stud. Alcohol Drugs. 2011;72:308–321. doi: 10.15288/jsad.2011.72.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E.H., Argyelan M., Aggarwal M., Chandon T.S., Karlsgodt K.H., Mori S., Malhotra A.K. The role of myelination in measures of white matter integrity: combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. NeuroImage. 2017;147:253–261. doi: 10.1016/j.neuroimage.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.A., Neafsey E.J., Mukamal K.J., Gray M.O., Parks D.A., Das D.K., Korthuis R.J. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol. Clin. Exp. Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F.T., Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos-Comby L., Lange J.E. “My drink is larger than yours?” A literature review of self-defined drink sizes and standard drinks. Curr. Drug Abuse Rev. 2008;1:162–176. doi: 10.2174/1874473710801020162. [DOI] [PubMed] [Google Scholar]
- Ding J., Eigenbrodt M.L., Mosley T.H., Jr., Hutchinson R.G., Folsom A.R., Harris T.B., Nieto F.J. Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2004;35:16–21. doi: 10.1161/01.STR.0000105929.88691.8E. [DOI] [PubMed] [Google Scholar]
- Ewing J.A. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Kleinert R., Offenbacher H., Schmidt R., Kleinert G., Payer F., Radner H., Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C., McEvoy L.K., Notestine R., Panizzon M.S., Yau W.W., Franz C.E., Lyons M.J., Eyler L.T., Neale M.C., Xian H., McKenzie R.E., Kremen W.S. White matter disease in midlife is heritable, related to hypertension, and shares some genetic influence with systolic blood pressure. Neuroimage Clin. 2016;12:737–745. doi: 10.1016/j.nicl.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Goldberg J., Curran B., Vitek M.E., Henderson W.G., Boyko E.J. The Vietnam era twin registry. Twin Res. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- Gouw A.A., van der Flier W.M., Fazekas F., van Straaten E.C., Pantoni L., Poggesi A., Inzitari D., Erkinjuntti T., Wahlund L.O., Waldemar G., Schmidt R., Scheltens P., Barkhof F., Group L.S. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the leukoaraiosis and disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- de Groot M., Verhaaren B.F., de Boer R., Klein S., Hofman A., van der Lugt A., Ikram M.A., Niessen W.J., Vernooij M.W. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–1042. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Brickman A.M., Cheng J.C., Alexopoulos G.S. Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Jr., Ahmadi M.E., Kuperman J., Holland D., McDonald C.R., Halgren E., Dale A.M. Automated white-matter tractography using a probabilistic diffusion tensor atlas: application to temporal lobe epilepsy. Hum. Brain Mapp. 2009;30:1535–1547. doi: 10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44:136–140. doi: 10.1093/alcalc/agn102. [DOI] [PubMed] [Google Scholar]
- Harris G.J., Jaffin S.K., Hodge S.M., Kennedy D., Caviness V.S., Marinkovic K., Papadimitriou G.M., Makris N., Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol. Clin. Exp. Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T., Vermeer S.E., van Dijk E.J., Prins N.D., Koudstaal P.J., van Duijn C.M., Hofman A., Breteler M.M. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. Am. J. Clin. Nutr. 2004;80:992–997. doi: 10.1093/ajcn/80.4.992. [DOI] [PubMed] [Google Scholar]
- Jernigan T.L., Archibald S.L., Fennema-Notestine C., Gamst A.C., Stout J.C., Bonner J., Hesselink J.R. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Klawiter E.C., Schmidt R.E., Trinkaus K., Liang H.F., Budde M.D., Naismith R.T., Song S.K., Cross A.H., Benzinger T.L. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen W.S., Thompson-Brenner H., Leung Y.M., Grant M.D., Franz C.E., Eisen S.A., Jacobson K.C., Boake C., Lyons M.J. Genes, environment, and time: the Vietnam Era Twin Study of Aging (VETSA) Twin Res. Hum. Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kremen W.S., Franz C.E., Lyons M.J. VETSA: the Vietnam Era Twin Study of Aging. Twin Res. Hum. Genet. 2013;16:399–402. doi: 10.1017/thg.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J.F., Poupon C., Clark C.A., Pappata S., Molko N., Chabriat H. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Li J., Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Matsumoto C., Miedema M.D., Ofman P., Gaziano J.M., Sesso H.D. An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J. Cardiopulm. Rehabil. Prev. 2014;34:159–171. doi: 10.1097/HCR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- McEvoy L.K., Kritz-Silverstein D., Barrett-Connor E., Bergstrom J., Laughlin G.A. Changes in alcohol intake and their relationship with health status over a 24-year follow-up period in community-dwelling older adults. J. Am. Geriatr. Soc. 2013;61:1303–1308. doi: 10.1111/jgs.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy L.K., Fennema-Notestine C., Eyler L.T., Franz C.E., Hagler D.J., Jr., Lyons M.J., Panizzon M.S., Rinker D.A., Dale A.M., Kremen W.S. Hypertension-related alterations in white matter microstructure detectable in middle age. Hypertension. 2015;66:317–323. doi: 10.1161/HYPERTENSIONAHA.115.05336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.A., Gould R., Reuben D.B., Greendale G.A., Carter M.K., Zhou K., Karlamangla A. Longitudinal patterns and predictors of alcohol consumption in the United States. Am. J. Public Health. 2005;95:458–465. doi: 10.2105/AJPH.2003.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamal K.J., Longstreth W.T., Jr., Mittleman M.A., Crum R.M., Siscovick D.S. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: the cardiovascular health study. Stroke. 2001;32:1939–1946. doi: 10.1161/hs0901.095723. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcoholism and Alcohol Abuse . US Department of Health and Human Services, National Institute of Health; 2005. NIAAA report: Helping patients who drink too much.https://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf available at: [Google Scholar]
- Neafsey E.J., Collins M.A. Moderate alcohol consumption and cognitive risk. Neuropsychiatr. Dis. Treat. 2011;7:465–484. doi: 10.2147/NDT.S23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M., Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M., Rohlfing T., Sullivan E.V. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Jezzard P., Basser P.J., Barnett A., Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rehm J., Irving H., Ye Y., Kerr W.C., Bond J., Greenfield T.K. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am. J. Epidemiol. 2008;168:866–871. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E.L., Kritz-Silverstein D., Laughlin G.A., Fung T.T., Barrett-Connor E., McEvoy L.K. Alcohol intake and cognitively healthy longevity in community-dwelling adults: the Rancho Bernardo study. J. Alzheimers Dis. 2017;59:803–814. doi: 10.3233/JAD-161153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm E.B., Williams P., Fosher K., Criqui M., Stampfer M.J. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S., Knaryan V.H., Patel K.S., Mulholland P.J., Becker H.C., Banik N.L. Chronic intermittent ethanol induced axon and myelin degeneration is attenuated by calpain inhibition. Brain Res. 2015;1622:7–21. doi: 10.1016/j.brainres.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn C.A., Heyman K.M. Health characteristics of adults aged 55 years and over: United States, 2004–2007. Natl. Health Stat. Report. 2009:1–31. [PubMed] [Google Scholar]
- Skoog I. A review on blood pressure and ischaemic white matter lesions. Dement. Geriatr. Cogn. Disord. 1998;9(Suppl. 1):13–19. doi: 10.1159/000051184. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Rohlfing T., Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol. Aging. 2008;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Harris R.A., Pfefferbaum A. Alcohol's effects on brain and behavior. Alcohol Res. Health. 2010;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Topiwala A., Allan C.L., Valkanova V., Zsoldos E., Filippini N., Sexton C., Mahmood A., Fooks P., Singh-Manoux A., Mackay C.E., Kivimaki M., Ebmeier K.P. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ. 2017;357 doi: 10.1136/bmj.j2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee S.G., Shaper A.G. Lifelong teetotallers, ex-drinkers and drinkers: mortality and the incidence of major coronary heart disease events in middle-aged British men. Int. J. Epidemiol. 1997;26:523–531. doi: 10.1093/ije/26.3.523. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott C.A., Cercignani M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Wiggins R.C., Gorman A., Rolsten C., Samorajski T., Ballinger W.E., Jr., Freund G. Effects of aging and alcohol on the biochemical composition of histologically normal human brain. Metab. Brain Dis. 1988;3:67–80. doi: 10.1007/BF01001354. [DOI] [PubMed] [Google Scholar]
- Xi B., Veeranki S.P., Zhao M., Ma C., Yan Y., Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J. Am. Coll. Cardiol. 2017;70:913–922. doi: 10.1016/j.jacc.2017.06.054. [DOI] [PubMed] [Google Scholar]
- Yeh P.H., Simpson K., Durazzo T.C., Gazdzinski S., Meyerhoff D.J. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]