Dear Editor,
Skeletal muscle weakness and physical disability are common in critically ill patients. After ICU admission, noticeable reduction in muscle mass and attendant functional disabilities start within 3 days and thereafter worsen progressively [1]. Ultrasonography is noninvasive and easily available at the bedside, and a useful tool for evaluating muscle atrophy [2]. Typically, ultrasonographic assessment of muscle mass is carried out on lower limbs. Faced with limited and seemingly anomalous data for upper-limb muscle atrophy in bed-ridden patients [3, 4], we investigated whether critical illness was associated with upper-limb muscle atrophy. The study was approved by the clinical research ethics committee at Tokushima University Hospital (approval number 2593). At enrolment, written informed consent was obtained from patients or from an authorized surrogate.
We consecutively recruited adult patients who were expected to require mechanical ventilation for longer than 48 h and to remain in the ICU more than 5 days. All scanning was done with patients in supine and elbows and knees in passive extension. The transducer was placed perpendicular relative to the long axis of the limbs. The muscle mass of the biceps brachii and rectus femoris were evaluated on days 1, 3, 5, and 7 with serial ultrasonagraphic measurements of thickness and cross-sectional area.
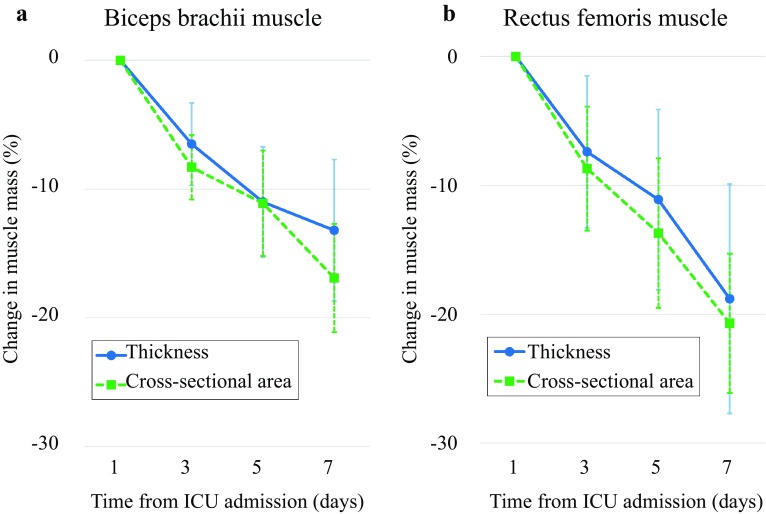
Twenty-eight patients were enrolled, and all patients remained in the study on day 3, 23 on day 5, and 21 on day 7. The mean age was 68 ± 9 years, 18 males, and median APACHE II score 27.5 (23.0–29.3). Biceps brachii thickness and cross-sectional area decreased by 6.5, 11.0, and 13.2% (p < 0.01), and by 8.3, 11.1 and 16.9% on day 3, 5, and 7 (p < 0.01), respectively (Fig. 1). Rectus femoris thickness and cross-sectional area decreased by 7.4, 11.1, and 18.8%, and by 8.7, 13.7, and 20.7% on days 3, 5, and 7 (p < 0.01), respectively. Intra- and inter-observer reproducibility was 0.96–0.99 and 0.98–0.99, respectively.
Fig. 1.
Change in muscle mass of biceps brachii and rectus femoris. Muscle mass of both biceps brachii and rectus femoris progressively decreased. a Biceps brachii thickness and cross-sectional area statistically significantly (p < 0.01) decreased. b Rectus femoris thickness and cross-sectional area statistically significantly (p < 0.01) decreased. p values were derived from a generalized linear mixed model. Data are expressed as means and 95% confidence intervals. CSA cross-sectional area
Turton et al. found that the muscle thickness of the upper limbs of ICU patients remained unchanged during the first 10 days, while APACHE II score was lower and their patients were younger than in the present study [3]. de Boer et al. investigated voluntarily immobilized normal patients [4]. Healthy volunteers used their arms actively during bed rest, and this may have counteracted the tendency to atrophy. Measurement protocols may also be attributable to the disparity between our results and previous studies. We measured both muscle thickness and cross-sectional area, and regard our measurement as more precise and accurate, or, at the very least, less susceptible to measurement bias [5].
This study is limited by the small sample size. Meanwhile, we were unable to evaluate muscular strength and function. We still do not know to what extent muscle atrophy can be reversed; inability to restore muscle mass, especially in elderly patients, to previous levels can negatively affect patient long-term outcome.
Our findings show both upper and lower limbs wasted in critically ill patients. It is prudent to monitor upper-limb muscle atrophy as well as lower-limb muscle atrophy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
References
- 1.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selby A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SD, Hart N, Montgomery HE. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 2.Bunnell A, Ney J, Gellhorn A, Hough CL. Quantitative neuromuscular ultrasound in intensive care unit-acquired weakness: A systematic review. Muscle Nerve. 2015;52:701–708. doi: 10.1002/mus.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turton P, Hay R, Taylor J, McPhee J, Welters I. Human limb skeletal muscle wasting and architectural remodeling during five to ten days intubation and ventilation in critical care—an observational study using ultrasound. BMC Anesthesiol. 2016;16:119. doi: 10.1186/s12871-016-0269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer MD, Seynnes OR, di Prampero PE, Pisot R, Mekjavic IB, Biolo G, Narici MV. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol. 2008;104:401–407. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- 5.Puthucheary ZA, McNelly AS, Rawal J, Connolly B, Sidhu PS, Rowlerson A, Moxham J, Harridge SD, Hart N, Montgomery HE. Rectus femoris cross-sectional area and muscle layer thickness: comparative markers of muscle wasting and weakness. Am J Respir Crit Care Med. 2017;195:136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.