Significance
The major histocompatibility complex (MHC) is one of the most polymorphic gene families in the vertebrate genome, with natural selection actively promoting and maintaining variability. The exact mechanism/mechanisms responsible for these characteristics remain unclear, but identifying them is fundamental to our understanding of host–pathogen dynamics. Using targeted crosses of the model Trinidadian guppy, a tractable parasite, and exposure-controlled infection trials, we show that novel MHC variants are associated with less severe infections. Uniquely, our experimental design separates novel variant advantage from other modes of selection and confounding variables, such as individual MHC variability and genomic background. We thus demonstrate a fundamental process driving evolution of the vertebrate immune system, which helps explain the unique features of MHC genes.
Keywords: host–pathogen coevolution, Red Queen coevolution, major histocompatibility complex, Poecilia reticulata, frequency-dependent selection
Abstract
The major histocompatibility complex (MHC) is crucial to the adaptive immune response of vertebrates and is among the most polymorphic gene families known. Its high diversity is usually attributed to selection imposed by fast-evolving pathogens. Pathogens are thought to evolve to escape recognition by common immune alleles, and, hence, novel MHC alleles, introduced through mutation, recombination, or gene flow, are predicted to give hosts superior resistance. Although this theoretical prediction underpins host–pathogen “Red Queen” coevolution, it has not been demonstrated in the context of natural MHC diversity. Here, we experimentally tested whether novel MHC variants (both alleles and functional “supertypes”) increased resistance of guppies (Poecilia reticulata) to a common ectoparasite (Gyrodactylus turnbulli). We used exposure-controlled infection trials with wild-sourced parasites, and Gyrodactylus-naïve host fish that were F2 descendants of crossed wild populations. Hosts carrying MHC variants (alleles or supertypes) that were new to a given parasite population experienced a 35–37% reduction in infection intensity, but the number of MHC variants carried by an individual, analogous to heterozygosity in single-locus systems, was not a significant predictor. Our results provide direct evidence of novel MHC variant advantage, confirming a fundamental mechanism underpinning the exceptional polymorphism of this gene family and highlighting the role of immunogenetic novelty in host–pathogen coevolution.
Host–pathogen coevolution is thought to drive the maintenance of genetic variation in immune genes, with consequences for important evolutionary processes including the evolution of virulence, the maintenance of sex, and sexual selection (1–4). One of the most striking examples of genetic polymorphism thought to be maintained by such processes is the vertebrate major histocompatibility complex (MHC), where dozens to hundreds of alleles may segregate in natural populations (5–7). The high polymorphism of this important immune gene family, which codes for proteins that present pathogen-derived antigens to T cell receptors, has been a subject of research for decades (8), and understanding the processes that maintain this diversity has implications for areas outside evolutionary biology, from human health (9) to conservation biology (10, 11).
Despite almost 50 y of investigation, the processes driving evolution at the MHC are not fully understood (12). At the molecular level, the exceptionally high ratio of nonsynonymous (protein-altering) to synonymous nucleotide substitutions in MHC genes suggests that selection is not only maintaining polymorphism (“balancing selection”), but is also actively promoting new polymorphism [“positive selection”; refs. (13 and 14)]. Several mutually nonexclusive mechanisms may contribute to these selective pressures: heterozygote advantage (recognizing a wider spectrum of antigens); frequency-dependent selection from fast-evolving pathogens that favors rare or novel MHC variants; and variable selection in space and time (12). Recent theoretical work has suggested that frequency-dependent selection resulting from Red Queen dynamics may be the more important process, with the advantage conferred by novel alleles being particularly important in generating the patterns of allelic diversity observed at the MHC (15, 16). Novel allele advantage is an old hypothesis in MHC research, dating to the earliest days of observing the MHC’s extreme polymorphism (17). The mechanistic potential for novel MHC variants to confer adaptive advantage against pathogens has been demonstrated experimentally only relatively recently, using congenic mice and artificially selected virus lineages (18, 19). However, the number of MHC alleles segregating in wild populations can be upwards of two orders of magnitudes higher than that of the mouse-virus system. It may be more difficult for pathogens to adapt to specific local variants, and novel variants will be competing in a much larger pool of alleles with potentially a wide range of antigen-binding properties. Experimentally testing novel variant advantage in more natural, ecological contexts is much harder as the potential selective pressures acting on the MHC are notoriously hard to disentangle (12).
Here, we used direct experimentation to investigate how novel MHC class II alleles (which recognize extracellular pathogens) in tropical freshwater guppies (Poecilia reticulata) affected the infection trajectory of their monogenean parasite Gyrodactylus turnbulli. These ectoparasites are widespread across guppy populations and exert significant selective pressure (20, 21). Heavy infections can kill hosts (22, 23), and some MHC class II genotypes have been linked to gyrodactylid infection in the wild (ref. 21; see also ref. 24). This host–parasite system is highly tractable: Host exposure is easy to control, and infections can be monitored through time without killing host or parasite (22, 23). Hosts in our experiments were F2 descendants of crosses between guppy populations that shared no MHC alleles (Materials and Methods), and gyrodactylid worms for each replicate cross were wild-caught and came from one of the populations used to found the respective cross (Fig. 1). In addition to MHC novelty defined by amino acid sequences, we also considered novelty based on MHC “supertypes,” where MHC alleles are grouped into clusters with similar physicochemical properties (25–27). We predicted that hosts carrying MHC variants that were novel with respect to parasite origin would perform better in controlled gyrodactylid infection trials than hosts carrying “local” MHC variants.
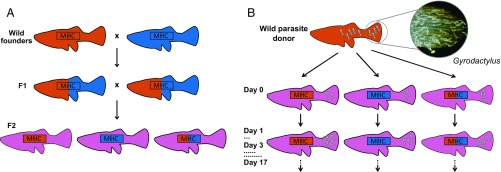
Fig. 1.
Schematic of the experiment. (A) Breeding design. Wild fish from two populations (P-generation) were crossed to produce F1s that were heterozygous across the genome with respect to population of origin. These were allowed to mate at random to produce F2s that segregated into heterozygotes and two types of homozygotes at the focal MHC class II locus, while having, on average, “mixed” genetic backgrounds [23 chromosome pairs (40), plus crossing-over when F1s reproduce]. (B) Controlled experimental infections. Two gyrodactylid worms from one of the P-generation source streams were inoculated on to the caudal fin of each F2 fish. Each infected fish was then kept in isolation and its infection monitored every other day for 17 d (22).
Results
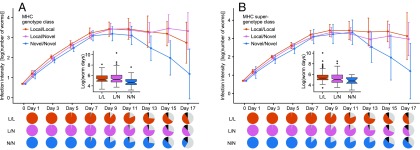
Among guppies that survived to the end of the experiment (n = 209), fish carrying only novel alleles or supertypes (designated as N/N genotypes and N/N supergenotypes, respectively; Materials and Methods) experienced G. turnbulli infections that were significantly less severe than fish carrying only local alleles or supertypes (L/L) (Fig. 2). Infection severity was measure in “worm days” (the area under a graph of number of worms against time), and analyses used AICC-based multimodel inference (Materials and Methods). The N/N genotypes (n = 44) and N/N supergenotypes (n = 14) respectively experienced 35.1% and 34.7% fewer worm days compared with the L/L genotype and supergenotype fish (n = 59 and 88, respectively; P = 0.003 and 0.012; SI Appendix, Tables S2.1 a and b and S2.2 a and b). L/N genotypes (n = 106; i.e., fish carrying both novel and local alleles) experienced infections of comparable intensity to L/L genotypes (P = 0.65; SI Appendix, Table S2.1b), whereas L/N supergenotypes (n = 107) experienced intermediate infection intensities that were only marginally nonsignificant relative to L/L (P = 0.055; SI Appendix, Table S2.2b). Direct comparison of the best allele-based and supertype-based models of worm days, using a constant set of covariates, indicated that allele-based groupings produced the better fit (ΔAICC = 4.22; SI Appendix, Tables S2.1a and S2.2a); however, fish carrying at least one novel supertype (n = 121) experienced 27.1% fewer worm days than fish with no novel supertypes but at least one novel allele (n = 29; top-ranked model; P = 0.02; SI Appendix, Tables S2.3 a and b and Fig. S2.1). We did not detect a significant interaction between replicate population and MHC genotype/supergenotype class (ΔAICC = +7.37/+5.71, P ≥ 0.13/0.24). Neither the number of alleles nor the number of supertypes carried by a host—measures used as analogs of heterozygosity – were significantly associated with the number of worm days experienced by hosts (ΔAICC = +1.82/+1.28, P = 0.52/0.32; SI Appendix, Tables S2.1 a and b and S2.2 a and b). No genetic variables were significant predictors of host mortality (SI Appendix, Tables S2.5 a–c and S2.6 a–c), despite worm load itself being a significant predictor of mortality from infection day 3 onwards (SI Appendix, Table S2.4).
Fig. 2.
Summary of G. turnbulli trajectory data for different MHC genotypes (i.e., allele-based; A) and supergenotypes (B). Points and lines apply only to fish that survived the experiment, and represent the mean infection intensity for each MHC genotype/supergenotype group on the focal day, controlling for other factors affecting infection intensity. Local/local = all MHC variants from the same stream as the worms; novel/novel = all variants from the “other” stream; local/novel = mixed. Inset boxplots summarize the worm-day data as analyzed in SI Appendix, Tables S2.1 a and b (genotypes) and S2.2 a and b (supergenotypes). Pie charts show the proportion of fish of each MHC group that are dead (black), still infected (colored), or have cleared their infection (gray) at each infection day. In both graphs, the area under the novel/novel curve is significantly smaller than that for local/local, but death rate (black area of pies) does not differ significantly by genotype/supergenotype class. Error bars are 95% CIs of infection intensity on the focal day.
Discussion
Our results support the novel MHC variant hypothesis: N/N hosts experienced parasite infections that were significantly less severe than those of L/L hosts. This was the case whether novelty was defined by amino acid sequences (alleles) or by physicochemical functional groups (supertypes). Differences in parasite burden between the genotype classes did not translate into a detectable effect on host survival. However, this may reflect the relatively benign and stable conditions of the experiment: In the wild, fish weakened by infection may be more susceptible to predation (28) and secondary infections (29), and to environmental stressors such as river spates (30)—even one additional worm can reduce a wild guppy’s survival probability (30). Furthermore, besides reducing survival, parasites may reduce host fitness by affecting reproductive potential, as has previously been demonstrated in guppies (31).
The novel variant advantage that we observed could, in theory, result in either balanced polymorphism or fixation of the novel variant—i.e., it is consistent with both balancing and positive selection. When a novel variant is introduced into a natural population (by point mutation, microrecombination, or introgression), both processes are cooccurring and indistinguishable, likely resulting in an increase of the novel allele’s frequency. We explored this potential using computer simulations parameterized from our current data on the effects of novel alleles/supertypes on gyrodactylid load, and from the effect of gyrodactylid load on the survival of guppies in the field from a mark-recapture study (30). These simulations show that upwards of 11% of novel amino acid variants should successfully establish in a population, and upwards of 54% if the variant is a novel supertype (compared with <0.1% in neutral simulations; SI Appendix, Appendix S10).
In the long term, the same process that leads to novel variant advantage—adaptation of parasites to local MHC genotypes—should diminish the advantage of the variant, leading to balanced polymorphism via negative frequency-dependent selection (12, 16, 17, 32). Such dynamics, whereby a novel (or rare) allele increases in frequency, loses its advantage, and decreases in frequency again, have yet to be demonstrated. Alternatively, in the absence of balancing selection, novel variants could spread to fixation in a population, but such a scenario is inconsistent with the high MHC polymorphism observed in most study systems being coupled with strong signatures of positive selection.
Novel variant advantage may also explain the striking transspecies polymorphism observed at the MHC (33): If such polymorphisms are derived from hybridization (as opposed to being ancestral; ref. 34), novel variant advantage may accelerate introgression and promote interspecies sharing of polymorphism. Furthermore, novel allele advantage might also affect the evolution of MHC-based mating preferences, an important factor in shaping MHC diversity (35–37). Our results suggest that preferences for partners with MHC alleles that are novel in a population, rather than those that just differ from self MHCs, should be strongly favored.
Negative frequency-dependent dynamics [favoring both novel and (sufficiently) rare alleles] and heterozygote advantage are two important types of balancing selection maintaining MHC diversity (12). Although frequency dependence and heterozygote advantage are not mutually exclusive, their relative influences are notoriously hard to test independently (12) and may be impossible to separate by observational studies alone (38). Other processes that can contribute to MHC diversity further complicate the separation (2, 12, 39). However, the crosses in our experiment produced genotypes/supergenotypes that would not normally be found in natural populations at the time at which novelty enters/arises. In particular, we generated hosts that were “homozygous” with respect to MHC novelty (N/N) while controlling for genetic background. Hence, we were able to separate the effects of novelty from the effects of simply carrying more MHC variants, and this showed that the number of alleles or supertypes carried by an individual was not significantly associated with infection intensity (SI Appendix, Tables S2.1 a and b and S2.2 a and b). Furthermore, we did not detect an overdominance-type advantage for L/N fish (SI Appendix, Tables S2.1b and S2.2b). The simplicity of the guppy-Gyrodactylus system may explain the lack of heterozygote advantage, which previous work has shown to be particularly important in multipathogen systems (40). Importantly, however, our results show that, against a natural host–pathogen genetic background, novel MHC variants can be selectively advantageous by virtue of properties arising from their novelty/extreme rarity (17, 32), rather than by simply being present in heterozygotes.
Direct comparison of the best allele-based and supertype-based models of infection intensity indicated that allele-based groupings produced the better fit (SI Appendix, Tables S2.1a and S2.2a). However, fish with at least one novel supertype experienced infections that were significantly less severe than fish with no novel supertypes but at least one novel allele (i.e., a novel amino acid sequence variant within a shared supertype). This suggests that functional novelty may be more important than simple allelic novelty, whereby the unique binding properties of the novel supertypes are more likely to fill an immune response void (41, 42). The disparity between the statistical model and empirical observations on parasite loads of guppies with and without novel supertypes highlights that the relative fitness contributions of novel supertypes and novel alleles within supertypes remain to be determined. Despite this uncertainly, our experiment demonstrates a general advantage of novel MHC variants.
While the breeding design of our study controls for population-level linkage between MHC class II genes and other genes that may affect immune responses [28 chromosome pairs (43), plus recombination when F1s reproduce], we cannot exclude possible effects from genes that may be in close physical linkage with the MHC without knockdown experiments or isogenic guppy lineages. Unlike for tetrapods, linkage with MHC class I can be ruled out for teleost fish (44)—a pertinent point because, although MHC class I usually targets intracellular pathogens, these genes have been coopted into roles more typical of class II in some teleosts (45). Concerning the exact mechanism by which the MHC may influence responses to skin ectoparasites, one possibility is through antigen-presenting skin cells (dendritic cells) mediating production of proinflammatory cytokines. This has been demonstrated in vitro with zebrafish skin tissue (46) and implied by gene expression studies on salmonids infected with sea lice (47–49). That supertypes are associated with lower infection intensities also suggests a functional rather than linkage-based influence of MHC.
Our results suggest that novel variants rather than locally adapted variants are associated with lower levels of parasite infection, and the lack of a significant interaction with replicate population suggests this finding may be generalizable. This contrasts with several studies on stickleback (Gasterosteus aculeatus) MHC–parasite interactions. When comparing or crossing lake and river stickleback populations, these studies have variously shown local adaptation (24), immigrant advantage (50), and a mixture of signals (51). This variation likely reflects the highly divergent ecologies and parasite faunas of lake and river sticklebacks, which may exert a complex suite of selection pressures (24, 50–54). Our study builds on the important insights from these studies by overcoming some of their limitations, such as the use of only a single population pair (24, 50); no experimental control of population-level linkage (ref. 50; although this study’s use of statistical control means it is uniquely able to demonstrate effects of such linkage); and the stock caveats of snapshot observational studies (ref. 51; e.g., uncertainty regarding where an allele may be in a frequency-dependent dynamic, and the difficulty of separating effects of rare alleles from heterozygosity; ref. 12). Also, these previous studies did not test for effects of supertypes. The most pertinent stickleback experiment to our result presents the simplest finding: MHC variants that confer resistance, irrespective of being rare or common, tend to increase in frequency (55). Many traits and circumstances may make a variant resistant; our study shows that novelty is likely to be one of them.
Based on our results, we predict that novel variants, including those coming from immigrants, have a reasonable probability of establishing and spreading. However, inferring the consequences of novel variant advantage from MHC-based population genetic structure is more challenging, especially in populations connected by gene flow (42). Furthermore, genuine novelty of a variant can only be ascertained by exhaustively sampling a population’s MHC diversity over an extended period of time. Introgression of novel alleles may be easier to observe if it occurs among well-diversified populations coming into secondary contact, including between species (e.g., ref. 56) or between allopatric populations (such as those we investigated). We suspect that the small number of alleles shared between Trinidad and Tobago (SI Appendix, Appendix S5) might come from fish introduced to Tobago by humans, as most of these alleles were found in a population close to human settlements and communication hubs. If so, our simulations suggest that these alleles are likely to spread across Tobago in the nearby future, subject to migration rate to other Tobagonian populations.
Our data constitute empirical demonstration of a long-posited, fundamental model of the evolution of MHC variability: Novel immune variants confer a selective advantage, consistent with Red Queen scenarios in which parasites adapt to local host immune genotypes (8, 15, 16). We show this effect using wild-sourced host and pathogen genetic variation, indicating that demonstrations of novel MHC advantage in congenic laboratory systems (18, 19) may be applicable in the context of natural MHC diversity. Furthermore, because we show this with replicate population crosses while controlling for population genetic background, our finding should be pertinent regardless of the source of novelty (e.g., mutation, recombination, introgression) or the rarity with which novelty enters a system. Looking beyond the MHC, although Red Queen dynamics have been shown in several nonvertebrate host–pathogen systems (57–59), examples of experimentally tested molecular mechanisms are rare, even for simple systems such as bacteria–phage interactions (60). In contrast, we explicitly link phenotype (infection intensity) to genotype (novel/local MHC) with an a priori hypothesis, using a gene family with a well-characterized immunological function. Overall, our results show that the advantage of immunogenetic novelty is a key selective agent underpinning Red Queen coevolution, and that, despite skepticism (1, 61), Red Queen coevolution may be an important force in shaping the immune genes of complex organisms.
Materials and Methods
All methods are described in more detail in SI Appendix, Appendix S1. At our field laboratory in Tobago, we conducted controlled gyrodactylid infection experiments on five replicate guppy populations. Each population was the F2 descendant of a cross between a wild Trinidad guppy population and a wild Tobago guppy population (Fig. 1). Sampling locations and numbers of founding pairs per replicate are given in SI Appendix, Appendix S3. We reared the founding females for each replicate from wild-caught juveniles to ensure they were (i) virgins and (ii) free from gyrodactylids before being used for breeding (husbandry/screening details in SI Appendix, Appendix S1.1). The males were freshly caught wild adults. To ensure a 1:1 sex ratio at foundation, and to minimize the risk of parasite transfer from males to females, we used artificial insemination (62) to make the crosses. Between-island crosses allow a more powerful proof-of-principle test of the novel variant hypothesis than within-island crosses because they minimize the risk of crossed guppy populations having shared/exchanged MHC alleles or parasites in the recent past (between-island population structure is stronger than within-island structure; refs. 42 and 63; SI Appendix, Appendix S5). Between-island crosses also removed a hierarchical factor (“island”) that we would have struggled to achieve adequate replication to control. All males and females were fin-clipped for DNA (caudal fin, 2–4 mm2; preserved in 0.3 mL of 97% ethanol).
After insemination, we released the females to 800-L mesocosms (one per crossed population) and provided supplementary feeding (SI Appendix, Appendix S1.1). One month after observing the first F1s in each mesocosm, we removed all surviving founding females and allowed the F1s to mature and mate among themselves. One month after observing the first F2s in each mesocosm, we removed and fin-clipped all F1s. We did not attempt to control the mating of F1s because of logistical constraints and because population-level replication coupled with the testing of a very general allelic property (novelty) should minimize potential family effects. However, as a precaution, we tested whether FIS among F2s differed significantly from zero (see below). At both removal stages, we verified that the fish were free from ectoparasites (64). We found no endoparasites in a subsample of four to eight fish per mesocosm that were dissected fresh after the experiment.
Three months after observing the first F2s, we began controlled gyrodactylid (“gyros”) infection experiments on these parasite-naïve fish. For each experimental population, we caught fresh wild guppies from one founder population (SI Appendix, Table S3.2), screened these for gyros, and used fish with suitably large infections as parasite donors. Heavier donor infections are both easier to work with (gyros jump to new hosts more readily when the donor has a higher gyro load) and likely to be important sources of new infections in the wild. To minimize the handling of infected fish and the time for which gyros were held in captivity before making experimental infections, we did not attempt to identify gyros to species level before the experiments, despite the likelihood of multiple species being present (see molecular identification below). We did not captive-breed individual lineages because we wanted wild parasite genetic variation, free from artificial selection (65). Under weak anesthesia (MS-222), each recipient F2 fish was infected with two gyros. Full details of the infection protocol are given in SI Appendix, Appendix S1.2. After being revived, each recipient fish was transferred to a separate 400-mL isolation container. After infection on day 0, we briefly anesthetized all infected fish and counted the number of gyros on day 1, and then every other day thereafter for 17 d (22, 23). Isolation containers were kept at ambient outside temperature in shade (husbandry details in SI Appendix, Appendix S1.2). Any fish found dead (checked every 3–12 h, depending on infection intensity) was promptly preserved whole in 1 mL of 97% ethanol, with the ethanol replaced after 6 h and again after 24 h. Fish that survived until day 17 were fin-clipped.
The work conducted in these experiments was approved by Cardiff University’s animal ethics committee and covered by UK Home Office License PPL 302876. All Tobago-sourced wild fish were collected with permission from the Tobago House of Assembly (permit 004/2014). No specific permits are required on Trinidad, but we collected only from areas where guppies were reasonably abundant.
From genomic DNA extracted from fin clips, we genotyped all P generation and F2 fish at a 217-bp fragment of the MHC class II that codes for the highly polymorphic β-chain of the MHC molecule’s antigen binding groove (66). All genotypes used in downstream analyses had ≥300 allelic reads (median = 1,042 reads). Full details of primers, PCR conditions, and genotyping bioinformatics (67, 68) parameters are given in SI Appendix, Appendix S1.3.
We used custom Python scripts to assign MHC alleles in each replicate as coming from the maternal or paternal founding populations. Assignment was unambiguous—there was only one linkage block (one to two allelic variants per haplotype), all variants within population crosses differed by at least one nonsynonymous mutation, and we observed no allele sharing between any crossed population pair (SI Appendix, Appendix S5). We then designated alleles as either “L” (local) when they belonged to the same host population as the worms used for that replicate, or “N” (novel) when detected only in the “other” founding population, and allocated F2s to three genotype groups based on these designations: N/N (two novel haplotypes); L/L (two local haplotypes); and L/N (mixed/“heterozygous”). Although a nominally novel allele could be present in the local population at a frequency too low for our sample to detect, population genetic analyses on a larger dataset suggested that this is likely to be extremely rare (e.g., only 8/214 alleles in our dataset were present on both islands; SI Appendix, Appendix S5). Because of the breeding design, all three genotype groups should have the same average genetic background with respect to population hybridization and heterozygosity [23 chromosome pairs (43), plus recombination when F1s reproduce].
Amino acid (AA) substitutions vary in their functional consequences for an MHC molecule’s antigen binding profile, such that alleles with different AA sequences may be functionally similar. These MHC supertypes are predicted to bind similar antigenic “supermotifs” and may better characterize the breadth of host defense than alleles (25–27). Using 15 guppy MHC codons previously identified as being under positive selection (69), five physicochemical descriptors of each AA (70), and discriminant analysis of principal components (71, 72), we reduced the list of allele sequences to 14 supertype clusters (full description in SI Appendix, Appendix S1.3). Supertype designations were used to assign fish into L/L, N/N, and L/N “supergenotypes” analogous to allele-based groupings, except that supertypes shared between a pair of crossed populations (all pairs shared at least one supertype; SI Appendix, Appendix S5) were treated as local. Supergenotypes thus do not describe the haplotype makeup of individuals—multiallelic novel haplotypes may contain one or more shared supertypes. Only nine individuals, over three replicate populations, had a number of supertypes lower than their number of alleles (i.e., they carried 2+ alleles of the same supertype). Of these, only one changed novel/local categorization between allelic and supertype-based analyses (from L/N to L/L). Novel and local alleles did not differ systematically in the supertype physicochemical parameter space, and we thus concluded that the only “special” property of novel variants was their novelty (details in SI Appendix, Appendix S6).
We used mitochondrial barcoding to identify two to four gyros per replicate to species level (full details in SI Appendix, Appendix S1.3). All sequences showed their strongest matches (98–100% identity) against published G. turnbulli (Gt) sequences, except for those representing 35 fish from the AV/SS population, all infected on the same day, which matched G. bullatarudis (Gb; 98–100%). These fish also showed markedly different pathology, consistent with Gb (SI Appendix, Appendix S1.3). Given the taxonomical and pathological differences between Gt and Gb and the absence of indications of Gb elsewhere in the experiment, we excluded these fish from our analyses.
The analyses described in the next two paragraphs were performed separately for alleles and supertypes. We first tested if MHC group (L/L, L/N, N/N) predicted whether individual fish survived the experiment. We used corrected Akaike information criterion (AICC) ranking (73) of logistic regressions to explore all combinations of the following main effects: MHC group; number of MHC variants (alleles/supertypes; continuous, 1–4); standard length (continuous, z-transformed); age/sex (one variable: “male” if in possession of a fully shaped gonopodium, i.e., “hook and hood” visible; “female” if length >13.0 mm and no gonopodium evident; “juvenile” for all others); temperature (mean daily maximum over monitored period, z-transformed); and experimental population. We also included interactions between experimental population and both MHC group and number of MHC variants. We then examined models comprising the top two units of ranked AICC. For clarity of presentation in the main text, we only report the ΔAICC of the highest ranked model to include MHC group and the P values (two-tailed) for certain contrasts, but in SI Appendix, Appendix S2, we give a fully nuanced account of the analysis.
We used a similar process to test whether MHC group predicted infection burden among fish that survived the experiment. We used worm days as the response variable, calculated as the total area under a fish’s 17-d infection trajectory line. Worm days are tractable to analyze (no zero-inflation or random effects) and provide an ecologically relevant summary metric of an infection trajectory: Fish that experience more worm days can reasonably be considered to have endured a greater parasite burden, and, in the wild, be more vulnerable to decreased condition and associated consequences (28–31). For this analysis, we used generalized linear models with negative binomial errors (log link function), and the same AICC approach and set of predictors used for analyzing probability of death. As a follow-up test of the relative importance of AA sequence novelty versus putative functional novelty, we used model ranking to test whether worm days differed significantly between fish carrying at least one novel supertype and fish carrying no novel supertypes but at least one novel AA variant (the latter are novel alleles that belong to shared supertypes; SI Appendix, Tables S2.3 a and b and Fig. S2.1).
Twenty-eight fish from replicate population DR/SC were missing gyro counts for either day 7 or day 9 because of a 36-h power cut that rendered microscope work impossible. We dealt with this in the main analysis by dropping these same days from the worm-day calculation for all fish, rather than excluding the affected fish. However, N/N fish still experienced significantly fewer worm days than L/L fish when we used the full area data restricted to only complete cases (SI Appendix, Appendix S7).
FIS was significantly different from zero (0.216, SE = 0.060, P = 0.001) in HC/Guan, suggesting family effects may be distorting haplotype frequencies in this replicate population. Repeating the main analyses without this replicate did not result in a different interpretation (SI Appendix, Appendix S8).
To visualize average infection trajectories for each MHC genotype/supergenotype, we analyzed the number of gyros per fish for each infection day separately, using AICC to find the highest ranked model to include MHC group as a predictor. From these models, we generated predicted daily worm loads for each MHC group that we then used (i) to plot Fig. 2; (ii) to estimate the percentage reduction in number of worm days of N/N fish relative to L/L fish that was minimally affected by the incomplete cases; and (iii) as a post hoc exploration of which MHC groups had the lowest/highest loads on which days, and which fish were the most likely to clear their infections (full details in SI Appendix, Appendix S9).
Supplementary Material
Acknowledgments
We thank Tobago House of Assembly for permission to conduct this project; staff of the Environmental Research Institute Charlotteville, Tobago, for support in the field; P. Turpin for renting us the field station; A. Pilastro for training in guppy artificial insemination; members of the Cardiff University parasite research group who helped with parasite work; J. Lighten for sharing an as-then unpublished manuscript; M. Migalska for Fig. 1; and M. Konczal, M. Migalska, D. S. Richardson, J. Kaufmann, L. G. Spurgin, W. Babik, C. A. Morrison, J. Lighten, W. Smallbone, A. R. Ellison, M. Hammers, M. J. G. Gage, and three anonymous reviewers for comments on earlier manuscript versions. This project was funded by Polish National Science Centre Harmony Grant UMO-2013/10/M/NZ8/00253 (to J. Radwan, C.v.O., and J.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.M. is a guest editor invited by the Editorial Board.
Data deposition: The data files and analysis scripts have been deposited in the Dryad Repository database, datadryad.org/ (doi: 10.5061/dryad.72262).
See Commentary on page 1414.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708597115/-/DCSupplemental.
References
- 1.Woolhouse MEJ, Webster JP, Domingo E, Charlesworth B, Levin BR. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet. 2002;32:569–577. doi: 10.1038/ng1202-569. [DOI] [PubMed] [Google Scholar]
- 2.Milinski M. The major histocompatibility complex, sexual selection, and mate choice. Annu Rev Ecol Evol Syst. 2006;37:159–186. [Google Scholar]
- 3.Brockhurst MA, et al. Running with the Red Queen: The role of biotic conflicts in evolution. Proc Biol Sci. 2014;281:20141382. doi: 10.1098/rspb.2014.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison A, et al. Maintaining functional major histocompatibility complex diversity under inbreeding: The case of a selfing vertebrate. Proc Biol Sci. 2012;279:5004–5013. doi: 10.1098/rspb.2012.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goüy de Bellocq J, Charbonnel N, Morand S. Coevolutionary relationship between helminth diversity and MHC class II polymorphism in rodents. J Evol Biol. 2008;21:1144–1150. doi: 10.1111/j.1420-9101.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 6.Cano P, et al. Common and well-documented HLA alleles: Report of the Ad-Hoc committee of the American Society for Histocompatiblity and Immunogenetics. Hum Immunol. 2007;68:392–417. doi: 10.1016/j.humimm.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Apanius V, Penn D, Slev PR, Ruff LR, Potts WK. The nature of selection on the major histocompatibility complex. Crit Rev Immunol. 1997;17:179–224. doi: 10.1615/critrevimmunol.v17.i2.40. [DOI] [PubMed] [Google Scholar]
- 8.Snell GD. The H-2 locus of the mouse: Observations and speculations concerning its comparative genetics and its polymorphism. Folia Biol (Praha) 1968;14:335–358. [PubMed] [Google Scholar]
- 9.Trowsdale J, Knight JC. Major histocompatibility complex genomics and human disease. Annu Rev Genomics Hum Genet. 2013;14:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radwan J, Biedrzycka A, Babik W. Does reduced MHC diversity decrease viability of vertebrate populations? Biol Conserv. 2010;143:537–544. doi: 10.1016/j.biocon.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool. 2005;2:16. doi: 10.1186/1742-9994-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spurgin LG, Richardson DS. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc Biol Sci. 2010;277:979–988. doi: 10.1098/rspb.2009.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes AL, Nei M. Nucleotide substitution at major histocompatibility complex class II loci: Evidence for overdominant selection. Proc Natl Acad Sci USA. 1989;86:958–962. doi: 10.1073/pnas.86.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrigan D, Hedrick PW. Perspective: Detecting adaptive molecular polymorphism: Lessons from the MHC. Evolution. 2003;57:1707–1722. doi: 10.1111/j.0014-3820.2003.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 15.Borghans JAM, Beltman JB, De Boer RJ. MHC polymorphism under host-pathogen coevolution. Immunogenetics. 2004;55:732–739. doi: 10.1007/s00251-003-0630-5. [DOI] [PubMed] [Google Scholar]
- 16.Ejsmond MJ, Radwan J. Red Queen processes drive positive selection on major histocompatibility complex (MHC) genes. PLoS Comput Biol. 2015;11:e1004627. doi: 10.1371/journal.pcbi.1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodmer WF. Evolutionary significance of the HL-A system. Nature. 1972;237:139–145. doi: 10.1038/237139a0. [DOI] [PubMed] [Google Scholar]
- 18.Kubinak JL, et al. Experimental viral evolution reveals major histocompatibility complex polymorphisms as the primary host factors controlling pathogen adaptation and virulence. Genes Immun. 2013;14:365–372. doi: 10.1038/gene.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubinak JL, Ruff JS, Hyzer CW, Slev PR, Potts WK. Experimental viral evolution to specific host MHC genotypes reveals fitness and virulence trade-offs in alternative MHC types. Proc Natl Acad Sci USA. 2012;109:3422–3427. doi: 10.1073/pnas.1112633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakke TA, Cable J, Harris PD. The biology of gyrodactylid monogeneans: The “Russian-doll killers”. Adv Parasitol. 2007;64:161–376. doi: 10.1016/S0065-308X(06)64003-7. [DOI] [PubMed] [Google Scholar]
- 21.Fraser BA, Neff BD. Parasite mediated homogenizing selection at the MHC in guppies. Genetica. 2010;138:273–278. doi: 10.1007/s10709-009-9402-y. [DOI] [PubMed] [Google Scholar]
- 22.Cable J, van Oosterhout C. The impact of parasites on the life history evolution of guppies (Poecilia reticulata): The effects of host size on parasite virulence. Int J Parasitol. 2007;37:1449–1458. doi: 10.1016/j.ijpara.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Cable J, Van Oosterhout C. The role of innate and acquired resistance in two natural populations of guppies (Poecilia reticulata) infected with the ectoparasite Gyrodactylus turnbulli. Biol J Linn Soc. 2007;90:647–655. [Google Scholar]
- 24.Eizaguirre C, Lenz TL, Kalbe M, Milinski M. Divergent selection on locally adapted major histocompatibility complex immune genes experimentally proven in the field. Ecol Lett. 2012;15:723–731. doi: 10.1111/j.1461-0248.2012.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidney J, Grey HM, Kubo RT, Sette A. Practical, biochemical and evolutionary implications of the discovery of HLA class I supermotifs. Immunol Today. 1996;17:261–266. doi: 10.1016/0167-5699(96)80542-1. [DOI] [PubMed] [Google Scholar]
- 26.Doytchinova IA, Flower DR. In silico identification of supertypes for class II MHCs. J Immunol. 2005;174:7085–7095. doi: 10.4049/jimmunol.174.11.7085. [DOI] [PubMed] [Google Scholar]
- 27.Schwensow N, Fietz J, Dausmann KH, Sommer S. Neutral versus adaptive genetic variation in parasite resistance: Importance of major histocompatibility complex supertypes in a free-ranging primate. Heredity (Edinb) 2007;99:265–277. doi: 10.1038/sj.hdy.6800993. [DOI] [PubMed] [Google Scholar]
- 28.Hatcher MJ, Dick JTA, Dunn AM. How parasites affect interactions between competitors and predators. Ecol Lett. 2006;9:1253–1271. doi: 10.1111/j.1461-0248.2006.00964.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanno T, Nakai T, Muroga K. Mode of transmission of vibriosis among ayu Plecoglossus altivelis. J Aquat Anim Health. 1989;1:2–6. [Google Scholar]
- 30.van Oosterhout C, et al. Selection by parasites in spate conditions in wild Trinidadian guppies (Poecilia reticulata) Int J Parasitol. 2007;37:805–812. doi: 10.1016/j.ijpara.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy CEJ, Endler JA, Poynton SL, McMinn H. Parasite load predicts mate choice in guppies. Behav Ecol Sociobiol. 1987;21:291–295. [Google Scholar]
- 32.Potts WK, Slev PR. Pathogen-based models favoring MHC genetic diversity. Immunol Rev. 1995;143:181–197. doi: 10.1111/j.1600-065x.1995.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 33.Figueroa F, Günther E, Klein J. MHC polymorphism pre-dating speciation. Nature. 1988;335:265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- 34.Wegner KM, Eizaguirre C. New(t)s and views from hybridizing MHC genes: Introgression rather than trans-species polymorphism may shape allelic repertoires. Mol Ecol. 2012;21:779–781. doi: 10.1111/j.1365-294X.2011.05401.x. [DOI] [PubMed] [Google Scholar]
- 35.Penn DJ, Potts WK. The evolution of mating preferences and major hstocompatibility complex genes. Am Nat. 1999;153:145–164. doi: 10.1086/303166. [DOI] [PubMed] [Google Scholar]
- 36.Winternitz JC, et al. Sexual selection explains more functional variation in the mammalian major histocompatibility complex than parasitism. Proc Biol Sci. 2013;280:20131605. doi: 10.1098/rspb.2013.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jan Ejsmond M, Radwan J, Wilson AB. Sexual selection and the evolutionary dynamics of the major histocompatibility complex. Proc Biol Sci. 2014;281:20141662. doi: 10.1098/rspb.2014.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahata N, Nei M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 1990;124:967–978. doi: 10.1093/genetics/124.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Oosterhout C. A new theory of MHC evolution: Beyond selection on the immune genes. Proc Biol Sci. 2009;276:657–665. doi: 10.1098/rspb.2008.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penn DJ, Damjanovich K, Potts WK. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc Natl Acad Sci USA. 2002;99:11260–11264. doi: 10.1073/pnas.162006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakeland EK, et al. Ancestral polymorphisms of MHC class II genes: Divergent allele advantage. Immunol Res. 1990;9:115–122. doi: 10.1007/BF02918202. [DOI] [PubMed] [Google Scholar]
- 42.Lighten J, et al. Evolutionary genetics of immunological supertypes reveals two faces of the Red Queen. Nat Commun. 2017;8:1294. doi: 10.1038/s41467-017-01183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lodi E. Chromosome complement of the guppy, Poecilia reticulata Peters (Pisces, Osteichtyhyes) Caryologia. 1978;31:475–477. [Google Scholar]
- 44.Sato A, et al. Nonlinkage of major histocompatibility complex class I and class II loci in bony fishes. Immunogenetics. 2000;51:108–116. doi: 10.1007/s002510050019. [DOI] [PubMed] [Google Scholar]
- 45.Star B, et al. The genome sequence of Atlantic cod reveals a unique immune system. Nature. 2011;477:207–210. doi: 10.1038/nature10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lugo-Villarino G, et al. Identification of dendritic antigen-presenting cells in the zebrafish. Proc Natl Acad Sci USA. 2010;107:15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braden LM, Barker DE, Koop BF, Jones SRM. Comparative defense-associated responses in salmon skin elicited by the ectoparasite Lepeophtheirus salmonis. Comp Biochem Physiol Part D Genomics Proteomics. 2012;7:100–109. doi: 10.1016/j.cbd.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Braden LM, Barker DE, Koop BF, Jones SRM. Differential modulation of resistance biomarkers in skin of juvenile and mature pink salmon, Oncorhynchus gorbuscha by the salmon louse, Lepeophtheirus salmonis. Fish Shellfish Immunol. 2015;47:7–14. doi: 10.1016/j.fsi.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Braden LM, Koop BF, Jones SRM. Signatures of resistance to Lepeophtheirus salmonis include a TH2-type response at the louse-salmon interface. Dev Comp Immunol. 2015;48:178–191. doi: 10.1016/j.dci.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Bolnick DI, Stutz WE. Frequency dependence limits divergent evolution by favouring rare immigrants over residents. Nature. 2017;546:285–288. doi: 10.1038/nature22351. [DOI] [PubMed] [Google Scholar]
- 51.Stutz WE, Bolnick DI. Natural selection on MHC IIβ in parapatric lake and stream stickleback: Balancing, divergent, both or neither? Mol Ecol. 2017;26:4772–4786. doi: 10.1111/mec.14158. [DOI] [PubMed] [Google Scholar]
- 52.Eizaguirre C, Yeates SE, Lenz TL, Kalbe M, Milinski M. MHC-based mate choice combines good genes and maintenance of MHC polymorphism. Mol Ecol. 2009;18:3316–3329. doi: 10.1111/j.1365-294X.2009.04243.x. [DOI] [PubMed] [Google Scholar]
- 53.Eizaguirre C, et al. Parasite diversity, patterns of MHC II variation and olfactory based mate choice in diverging three-spined stickleback ecotypes. Evol Ecol. 2011;25:605–622. [Google Scholar]
- 54.Kaufmann J, Lenz TL, Kalbe M, Milinski M, Eizaguirre C. A field reciprocal transplant experiment reveals asymmetric costs of migration between lake and river ecotypes of three-spined sticklebacks (Gasterosteus aculeatus) J Evol Biol. 2017;30:938–950. doi: 10.1111/jeb.13057. [DOI] [PubMed] [Google Scholar]
- 55.Eizaguirre C, Lenz TL, Kalbe M, Milinski M. Rapid and adaptive evolution of MHC genes under parasite selection in experimental vertebrate populations. Nat Commun. 2012;3:621. doi: 10.1038/ncomms1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadachowska-Brzyska K, Zieliński P, Radwan J, Babik W. Interspecific hybridization increases MHC class II diversity in two sister species of newts. Mol Ecol. 2012;21:887–906. doi: 10.1111/j.1365-294X.2011.05347.x. [DOI] [PubMed] [Google Scholar]
- 57.Decaestecker E, et al. Host-parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- 58.Koskella B, Lively CM. Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution. 2009;63:2213–2221. doi: 10.1111/j.1558-5646.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 59.Morran LT, Schmidt OG, Gelarden IA, Parrish RC, 2nd, Lively CM. Running with the Red Queen: Host-parasite coevolution selects for biparental sex. Science. 2011;333:216–218. doi: 10.1126/science.1206360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paterson S, et al. Antagonistic coevolution accelerates molecular evolution. Nature. 2010;464:275–278. doi: 10.1038/nature08798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Little TJ. The evolutionary significance of parasitism: Do parasite-driven genetic dynamics occur ex silico? J Evol Biol. 2002;15:1–9. [Google Scholar]
- 62.Evans JP, Zane L, Francescato S, Pilastro A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature. 2003;421:360–363. doi: 10.1038/nature01367. [DOI] [PubMed] [Google Scholar]
- 63.Barson NJ, Cable J, Van Oosterhout C. Population genetic analysis of microsatellite variation of guppies (Poecilia reticulata) in Trinidad and Tobago: Evidence for a dynamic source-sink metapopulation structure, founder events and population bottlenecks. J Evol Biol. 2009;22:485–497. doi: 10.1111/j.1420-9101.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 64.Schelkle B, Shinn AP, Peeler E, Cable J. Treatment of gyrodactylid infections in fish. Dis Aquat Organ. 2009;86:65–75. doi: 10.3354/dao02087. [DOI] [PubMed] [Google Scholar]
- 65.Stewart A, et al. Hook, line and infection: A guide to culturing parasites, establishing nfections and assessing immune responses in the three-spined stickleback. Adv Parasitol. 2017;98:39–109. doi: 10.1016/bs.apar.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 6th Ed Garland Science; New York: 2005. [Google Scholar]
- 67.Biedrzycka A, Sebastian A, Migalska M, Westerdahl H, Radwan J. Testing genotyping strategies for ultra-deep sequencing of a co-amplifying gene family: MHC class I in a passerine bird. Mol Ecol Resour. 2017;17:642–655. doi: 10.1111/1755-0998.12612. [DOI] [PubMed] [Google Scholar]
- 68.Sebastian A, Herdegen M, Migalska M, Radwan J. AMPLISAS: A web server for multilocus genotyping using next-generation amplicon sequencing data. Mol Ecol Resour. 2016;16:498–510. doi: 10.1111/1755-0998.12453. [DOI] [PubMed] [Google Scholar]
- 69.Lighten J, van Oosterhout C, Paterson IG, McMullan M, Bentzen P. Ultra-deep Illumina sequencing accurately identifies MHC class IIb alleles and provides evidence for copy number variation in the guppy (Poecilia reticulata) Mol Ecol Resour. 2014;14:753–767. doi: 10.1111/1755-0998.12225. [DOI] [PubMed] [Google Scholar]
- 70.Sandberg M, Eriksson L, Jonsson J, Sjöström M, Wold S. New chemical descriptors relevant for the design of biologically active peptides. A multivariate characterization of 87 amino acids. J Med Chem. 1998;41:2481–2491. doi: 10.1021/jm9700575. [DOI] [PubMed] [Google Scholar]
- 71.Jombart T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- 72.Jombart T, Ahmed I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics. 2011;27:3070–3071. doi: 10.1093/bioinformatics/btr521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartoń K. 2016 MuMIn: Multi-Model Inference. R package Version 1.15.6. Available at https://CRAN.R-project.org/package=MuMIn. Accessed September 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.