Significance
Women are much more likely to develop autoimmune diseases, such as systemic lupus erythematous, rheumatoid arthritis, and multiple sclerosis. Sex hormones, including estrogen and testosterone, clearly influence disease susceptibility, but the precise cellular and molecular targets of these hormones have remained unexplained. While most studies have focused on what causes the damaging inflammation in females, there is also much to be learned by studying the factors that confer protection to males. Using a mouse model of multiple sclerosis, a CNS demyelinating disease, we identified a testosterone-driven pathway mediated by mast cell-dependent IL-33 expression that limits the development of a destructive immune response in males. The identification of such pathways has important therapeutic implications.
Keywords: sex-dimorphic EAE, ILC2, IL-33, mast cells, testosterone
Abstract
The cellular and molecular basis of sex-dimorphic autoimmune diseases, such as the CNS demyelinating disease multiple sclerosis (MS), remains unclear. Our studies in the SJL mouse model of MS, experimental autoimmune encephalomyelitis (EAE), reveal that sex-determined differences in Il33 expression by innate immune cells in response to myelin peptide immunization regulate EAE susceptibility. IL-33 is selectively induced in PLP139–151-immunized males and activates type 2 innate lymphoid cells (ILC2s), cells that promote and sustain a nonpathogenic Th2 myelin-specific response. Without this attenuating IL-33 response, females generate an encephalitogenic Th17-dominant response, which can be reversed by IL-33 treatment. Mast cells are one source of IL-33 and we provide evidence that testosterone directly induces Il33 gene expression and also exerts effects on the potential for Il33 gene expression during mast cell development. Thus, in contrast to their pathogenic role in allergy, we propose a sex-specific role for both mast cells and ILC2s as attenuators of the pathogenic Th response in CNS inflammatory disease.
There are well-established differences in the immune responses of females and males. These discrepancies are perhaps best exemplified by the three- to ninefold increase in the incidence of autoimmune diseases, such as systemic lupus erythematous, Grave’s disease, and rheumatoid arthritis in females (1–3). In multiple sclerosis (MS), a T cell-mediated demyelinating disease of the CNS, not only is the incidence three to four times higher in women, there are also sex-determined differences in the average age of onset and in the clinical course (4). Women generally present at a younger age and preferentially exhibit a relapsing-remitting course, whereas men develop disease later in life and more often develop chronic progressive disease. Although the molecular underpinnings of such sex dimorphism are still largely undefined, the interplay between X chromosome dosage, distinct microbiota, and sex hormones likely contribute (5, 6).
The SJL mouse model of MS, experimental autoimmune encephalomyelitis (EAE), recapitulates several features of the human disease. Similar to MS, myelin-reactive helper T (Th) cells gain access to the CNS and orchestrate local inflammatory damage to the myelinated neurons, leading to variable neurological deficits (7). Female mice exhibit higher incidence, more severe disease, and a more consistent relapsing pattern than their male counterparts (8). This sex-determined disease susceptibility corresponds to differences in myelin-specific T cell cytokine responses. Whereas females generate proinflammatory IFN-γ–dominant responses, the response in males is skewed toward the production of IL-4 and IL-10 and is nonpathogenic (9–11).
Sex hormones, particularly testosterone, a steroid hormone primarily secreted by the testes, can alter T cell responses in immunized mice. Testosterone treatment of SJL females attenuates EAE by shifting the pathogenic IFN-γ–dominated anti-myelin response to a nonpathogenic IL-4 and IL-10 response. Expression of other proinflammatory cytokines, including TNF and IL-1β (11–14), is suppressed as well. Conversely, castration or treatment of male mice with flutamide, an androgen receptor (AR) antagonist, results in increased disease severity (13, 15). Male recipients develop EAE after adoptive transfer of primed T cells from female donors, indicating that testosterone exerts a protective influence during T cell priming (12). However, the precise mechanisms of these disease-attenuating effects have not been clearly defined.
In humans, testosterone is present at levels seven to eight times greater in adult men than women and is also associated with protection (16, 17). The delayed onset of MS and more severe disease course in men correlates with the physiologic age-related decline in testosterone (17). Limited studies also demonstrate that testosterone treatment in male patients improves MS outcomes (18, 19). For example, in a cohort of 10 men with relapsing-remitting MS, daily testosterone therapy for 12 mo reversed gray matter atrophy and improved cognitive performance (19).
Our previous studies of EAE susceptibility in c-kit mutant (KitW/Wv) SJL mice implicated type 2 innate lymphoid cells (ILC2s) in the male-specific Th2-mediated protection from EAE (20). ILC2s are a subset of CD45+ Lineage-negative (Lin−), IL-7Rα+ c-kit+/− ILCs, cells that share functional characteristics with conventional T cells but lack antigen receptors (21). These cells respond quickly and vigorously to a variety of cytokines and lipid mediators, including IL-33, IL-25, IL-2, and prostaglandin D2 (PGD2) (21–23). ILC2s are distinguished from other ILC subsets (CD45+ Lin− IL-7Rα+) by the expression of the IL-33 receptor, a heterodimeric cell surface receptor composed of IL-1 receptor accessory protein (IL-1RAcP) and ST2, as well as the production of IL-4, IL-5, IL-9, and IL-13. ILC2s are reported to reside primarily in mucosal-associated tissues, such as the lung and gut, where they contribute to tissue repair and homeostasis (21). ILC2s are essential for initiating and maintaining Th2 responses and play a critical role in both protective Th2 immunity to helminth infections and in the pathologic Th2-mediated inflammation of allergic disease.
We previously reported that ILC2s accumulate in the draining lymph nodes (LNs) and CNS of EAE-resistant wild-type SJL males after PLP139–151 immunization, corresponding with a nonpathogenic Th2 anti-myelin response (20). Male SJL-KitW/Wv mice, which have profound defects in both mast cell and ILC2 development, lack this ILC2 response, generate a robust Th17 response, and develop EAE. Because mast cell reconstitution does not restore protection, we proposed that the absence of Th2-promoting ILC2s is responsible for the differences in anti-myelin responses by male wild-type and KitW/Wv mice. In the present study, we asked whether the Th17-dominant anti-myelin response in females also corresponds to a deficit in ILC2 development or function. We demonstrate that ILC2s are not strongly activated in immunized females. This deficit in ILC2 activity is not the result of altered development or an intrinsic inability of this population to respond to inflammatory cues, but rather to significantly reduced production of IL-33, a cytokine that promotes type 2 immunity, in part through its ability to activate ILC2s (24). Mast cells are one important source of IL-33 in vivo and although there is no sex-dependent difference in AR expression, testosterone selectively induces Il33 in male-derived bone marrow mast cells (BMMCs). We propose a previously unknown and sex-specific role for both mast cells and ILC2s as important attenuators of the proinflammatory Th17 response in EAE. Furthermore, these data define a cellular and molecular target of testosterone and identify a mechanism of action for testosterone-mediated protection in an autoimmune disease of the CNS.
Results
Protection from EAE in Male SJL Mice Corresponds to a Dominant Th2 Anti-myelin Response in both the Periphery and CNS.
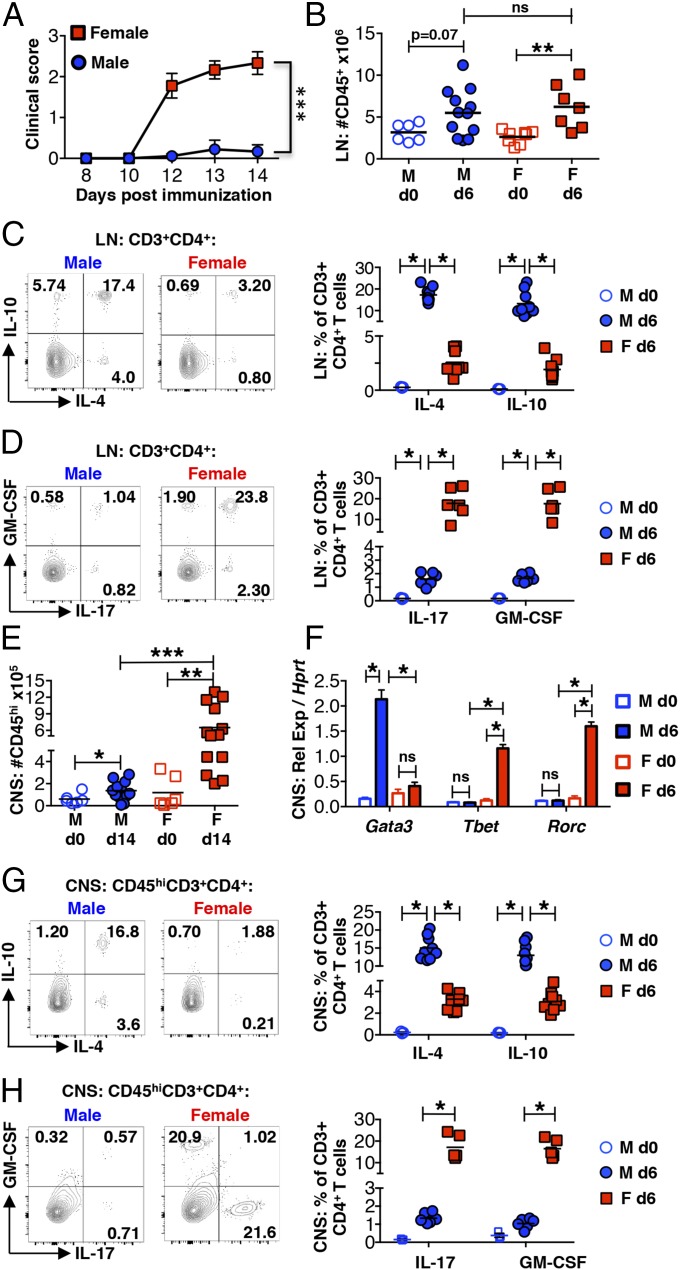
Previous reports provided evidence of a Th2 bias in myelin peptide-immunized SJL male mice (9–11). However, these studies were performed before the discovery of Th17 cells and only compared IL-4, IL-10, and IFN-γ mRNA expression and secretion by total leukocytes. To further characterize the sex-specific Th cell responses, mice were immunized with PLP139–151 and disease course (Fig. 1A) and T cell cytokine responses were evaluated by flow cytometry in both the draining LNs 6 d post immunization (dpi) and CNS 14 dpi. Although the total numbers of CD45+ immune cells in the LNs are similar between protected males and susceptible females 6 dpi (Fig. 1B), there is a striking difference in Th responses. The proportion of myelin-specific IL-4– and IL-10–producing Th2 cells is significantly higher in males. In contrast, Th17 cells are most prevalent in females (Fig. 1 C and D).
Fig. 1.
Immunized male SJL mice exhibit a dominant Th2 anti-myelin response. (A) Clinical scores of age-matched 4- to 6-wk-old SJL mice after immunization with PLP139–151. ***P < 0.0001 by two-way ANOVA. (B) Number of CD45+ cells in the draining inguinal LNs of naive mice (d0) and at 6 dpi detected by flow cytometry. ns, not significant: P > 0.05 by Student’s t test and **P < 0.01 by Student’s t test. (C and D) Percentage of IL-4– and IL-10–producing (C) or IL-17– and GM-CSF–producing (D) CD3+CD4+ T cells in the draining LNs 6 dpi. Cells were analyzed after 5 h of restimulation with PLP139–151. *P < 0.0001 by Student’s t test. (E) Number of CD45hi cells in the CNS (combined brains and spinal cords) of naïve mice (d0) and at 14 dpi. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t test. (F) Lineage-determining gene expression in the CNS of naïve mice (d0) and at 6 dpi assessed by RT-PCR and shown relative to the housekeeping gene Hprt. ns, not significant: P > 0.05, *P < 0.0001 by Student’s t test. n = 3 mice for naïve mouse analyses and n = 6 for immunized mouse analyses. (G and H) Percentage of IL-4– and IL-10–producing (G) or IL-17– and GM-CSF–producing (H) CD45hiCD3+CD4+ T cells in the CNS 14 dpi restimulated as in C and D above. *P < 0.01 by Student’s t test. Representative flow cytometry plots are shown. Gating is based on fluorescence-minus-one (FMO) controls.
Consistent with less-severe EAE, CD45hi CNS-infiltrating cells are significantly reduced in male mice 14 dpi (Fig. 1E). However, the same Th response dichotomy is observed. At 6 dpi, Gata3, a Th2 and ILC2 lineage-determining gene, is induced in males and Tbet and Rorc, which specify Th1/ILC1 and Th17/ILC3 differentiation, respectively, are induced in females (Fig. 1F). The majority of CNS-infiltrating Th cells are also Th2-skewed in males and Th17-skewed in females (Fig. 1 G and H) 14 dpi. Thus, protection in males is not due to a reduced or absent anti-myelin Th cell response but results from the generation of qualitatively distinct anti-myelin T cells that fail to efficiently enter the CNS.
Immunization-Induced ILC2 Accumulation Differs in Immunized Male and Female Mice.
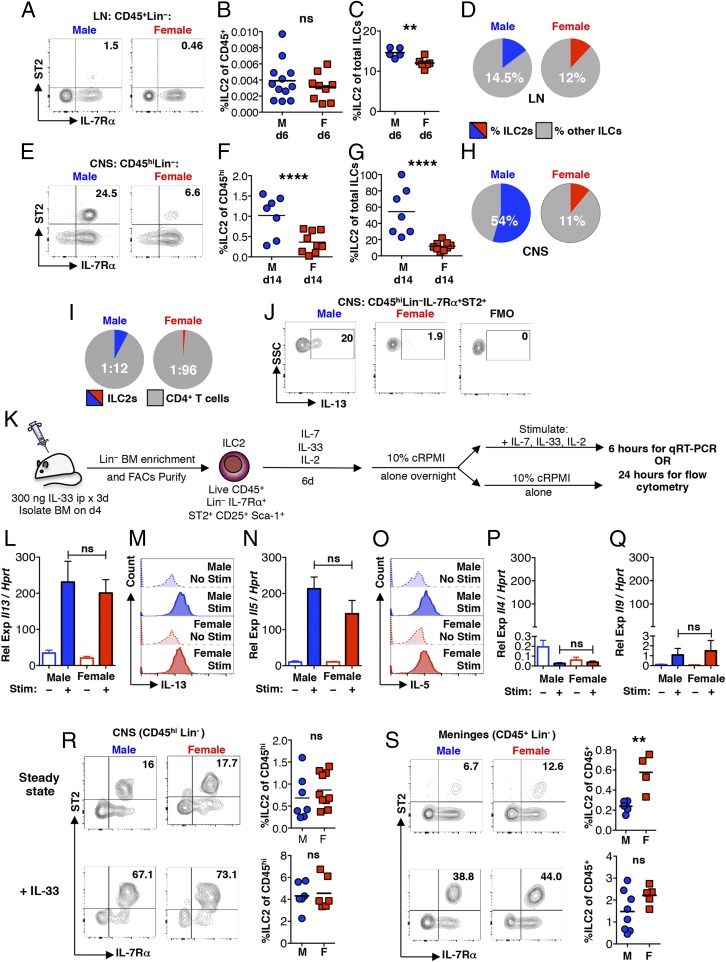
We asked whether ILC2 (CD45+ Lin− IL-7Rα+ ST2+) (Fig. S1) populations in the LNs and CNS show sex-related differences in immunized mice. In the draining LNs, ILC2s comprise a very rare population of cells at day 6 in both sexes, making up only ∼0.0004% of total CD45+ cells in males and ∼0.0003% in females (Fig. 2 A and B). However, there is a slight but significant increase in the proportion of ILC2s compared with other proinflammatory ILC subsets (CD45+ Lin− IL-7Rα+ ST2−) in males (Fig. 2 C and D). These likely include the IFN-γ–producing ILC1s and IL-17– and GM-CSF–producing ILC3s, which promote the differentiation of Th1 andTh17 cells, facilitate T cell migration to the CNS, and have been implicated in the pathogenesis of EAE (21, 25, 26).
Fig. 2.
Despite similar steady-state ILC2 populations, ILC2s show higher accumulation in immunized males. (A) ILC2s (CD45+ Lin− IL-7Rα+ ST2+) in the draining LNs at 6 dpi. Numbers represent percentage of IL-7Rα+ ST2+ cells of the CD45+ Lin− population. (B) LN ILC2s at 6 dpi expressed as a proportion of the total CD45+ population. ns, not significant: P > 0.05 by Student’s t test. (C and D) LN ILC2s expressed as a proportion of total ILCs (CD45+ Lin− IL-7Rα+). **P < 0.01 by Student’s t test. (E) ILC2s in the CNS (pooled brain and spinal cord of individual mice) at 14 dpi. Numbers represent the percentage of IL-7Rα+ ST2+ cells of the CD45hi Lin− population. (F) CNS ILC2s at 14 dpi expressed as a proportion of the total CD45hi population. ****P < 0.0001 by Student’s t test. (G and H) CNS ILC2s at 14 dpi expressed as a proportion of total ILCs (CD45hi Lin− IL-7R+), ****P < 0.0001 by Student’s t test. (I) Average ratio of CNS ILC2s to T cells (CD45hi CD3+ CD4+) (ILC2:Th) 14 dpi. n > 10 mice per group. *P < 0.05 by Fisher’s exact test. (J) ILC2 IL-13 expression in the CNS at 14 dpi measured directly ex vivo. (K) Schematic depicting enrichment, in vitro expansion and activation of bone marrow-derived ILC2s. (L–Q) Il13 (L), Il5 (N), Il4 (P), and Il9 (Q) expression (relative to Hprt) assessed by qRT-PCR by resting (–) or IL-2–, IL-7–, and IL-33–stimulated (+) ILC2 cultures, ns: P > 0.05 by Student’s t test. (M and O) IL-13 (M) and IL-5 (O) expression in resting (no stim) or stimulated (stim) ILC2 cultures. In L–Q, all data are representative of four independent cultures. (R and S, Upper) ILC2s (CD45+/hi Lin− IL-7Rα+ ST2+) in the CNS (pooled brain and spinal cord) and meninges isolated from individual naive mice. Numbers represent percentage of the CD45hi/+ Lin− population. Compilation of percentage of ILC2s (of CD45+/hi) cells from individual mice is also shown. (R and S, Lower) Representative flow cytometry analyses of ILC2s in the CNS (pooled brain and spinal cord) and meninges isolated from IL-33–treated mice. Mice were given 300 ng IL-33 intraperitoneally for 3 consecutive days before analysis. Numbers represent percentage of the CD45+/hi Lin− population. Compilation of percentages of ILC2s (of CD45+/hi) from individual mice is shown. n ≥ 3 for all tissues. ns: P > 0.05 by Student’s t test. **P < 0.01 by Student's t test. Representative flow cytometry plots are shown. Gating is based on FMO controls.
At day 14 dpi, the differences in these populations in the CNS are more dramatic. ILC2s comprise ∼1.0% of the total CD45hi population in males and ∼0.36% in females (Fig. 2 E and F). ILC2s were not detected in the CD45mid CNS cell population. There is also preferential accumulation of ILC2s, which average ∼54% of total ILCs in males. In females, ILC2s comprise only ∼11% of total ILCs (Fig. 2 G and H).
ILC2s can induce Th2 cell polarization through a contact-dependent mechanism by acting as antigen-presenting cells and expressing IL-13 (27–29). Thus, it is notable that although ILC2s comprise a small proportion of the total CNS CD45hi cell population, their frequency is much higher relative to Th cells in immunized males (1:12) than in females (1:96) (Fig. 2I). Furthermore, although expression is variable between mice, ILC2s from both sexes express IL-13 (Fig. 2J), detected by intracellular staining.
No Sex-Related Differences Exist in Basal and Exogenously Activated ILC2s.
The frequencies of ILC2 precursors in the bone marrow (Lin−CD45+α4β7+CD25+) and thymus (Lin−CD4−CD8−CD44+c-kit+CD25+) (30, 31) are similar between naïve males and females (Fig. S2 A and B). Proportions of mature resident ILC2 populations (CD45+ Lin− IL-7Rα+ST2+) are also the same (Fig. 2R, Upper, and Fig. S2 C–F), with the exception of the meninges, which show an average 2.4-fold higher frequency in females (Fig. 2S, Upper). Compared with other tissues examined, ILC2s are most prevalent in the CNS and meninges at steady state (Fig. 2 R and S).
An in vitro ILC2 culture system was used to determine if there are intrinsic differences in responses to ILC2-activating factors (Fig. 2K). IL-33 was administered on 3 consecutive days by intraperitoneal injection. On day 4, ILC2s (CD45+Lin−IL-7Rα+ST2+CD25+Sca-1+) were isolated from the bone marrow by magnetic bead-enrichment and subsequent flow cytometry sorting (Fig. S3A). After culture with IL-33, IL-2, and IL-7 for 6 d, ILC2s derived from both male and female mice were greater than 94% pure (Fig. S3B), showed similar c-kit expression (Fig. S3B), and expanded equivalently by greater than 10-fold (Fig. S3C). Upon restimulation with the same cytokine mixture (Fig. 2K), female- and male-derived ILC2s exhibit comparable robust induction of IL-13 and IL-5 mRNA and protein (Fig. 2 L–O), and a similar but smaller induction of Il4 and Il9 (Fig. 2 P and Q).
The in vivo expansion of ILC2s 4 d after IL-33 administration was also evaluated. Although there is variable induction between tissues, the expansion of ILC2s is similar in males and females (Fig. 2 R and S, Lower, and Fig. S2 G–J). Thus, female-derived ILC2s develop normally and, if provided with appropriate factors, their response is indistinguishable from male-derived ILC2s both in vitro and in vivo.
Mast Cells Are a Source of Sex-Determined Il33 Expression.
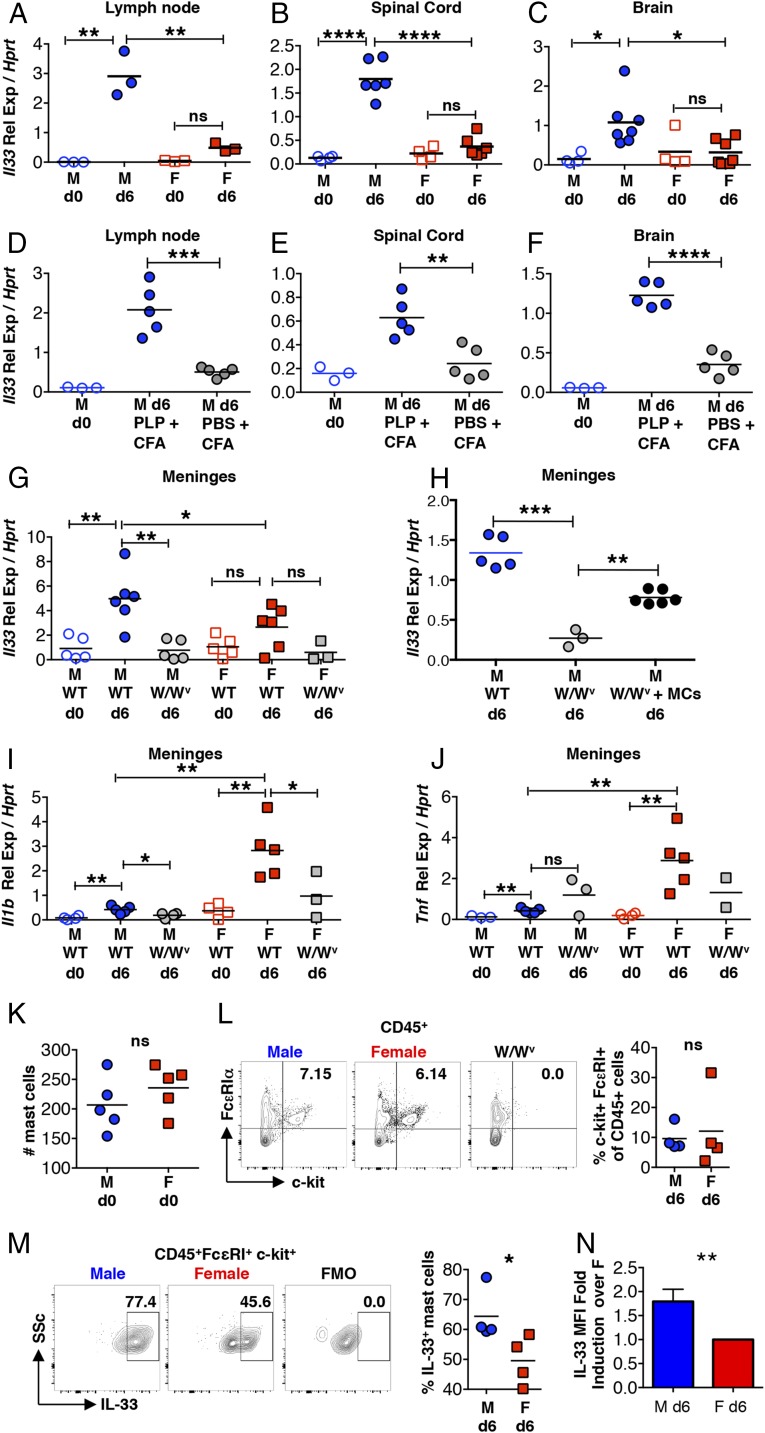
We asked if reduced expression of IL-33, a potent ILC2 activator (32), could account for the relative inactivity of ILC2s in females. As shown in Fig. 3 A–C, Il33 expression is negligible in the LNs, spinal cord, and brain of naïve mice but shows significant increases 6 dpi in male but not female mice. This Il33 response is only observed in mice immunized with PLP139–151 but not in those given Mycobacterium tuberculosis (Mtb)-containing CFA alone (Fig. 3 D–F), demonstrating that Il33 induction is dependent on a functional autoreactive T cell response and is not solely due to microbial-derived signals.
Fig. 3.
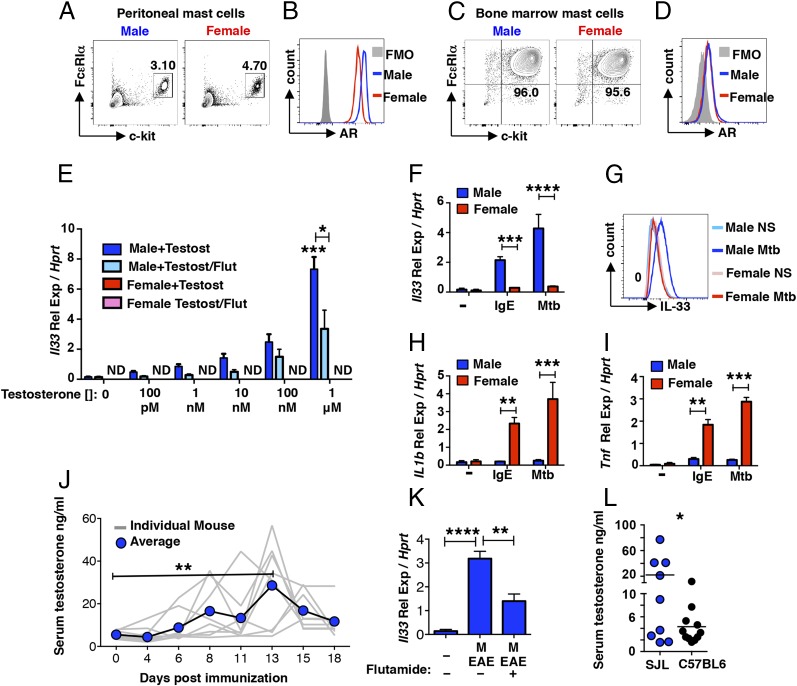
Sex-dimorphic Il33 expression by mast cells in immunized SJL mice. (A–F) Il33 expression detected by qRT-PCR in the inguinal LNs (A and D), spinal cord (B and E), and brain (C and F) of naïve, PLP139–151 + CFA-immunized (6 dpi), and PBS + CFA-treated mice (6 dpi). (G) Il33 expression in the meninges of naïve or immunized (6 dpi) wild-type and mast cell-deficient KitW/Wv (W/Wv) male and female mice. (H) Il33 expression in the meninges of immunized (6 dpi) wild-type, mast cell-deficient KitW/Wv (W/Wv) and W/Wv male mice intracranially reconstituted with 1 × 106 BMMCs (MCs). (I and J) Il1b (I) and Tnf (J) expression in the meninges of naïve and immunized (6 dpi) WT and KitW/Wv (W/Wv) male and female mice. (K) Mast cell numbers in the meninges of naïve mice detected by Toluidine blue staining. (L) Mast cells in the meninges of immunized mice 6 dpi detected by flow cytometry. Each data point represents the analysis of meninges pooled from five mice. Numbers represent the percent mast cells (c-kit+FcεRIα+) of the CD45+ high side scatter cell population. (M) IL-33 production by meningeal mast cells (gated on CD45+ FcεRIα+ c-kit+) 6 dpi. (N) MFI of IL-33–expression by meningeal mast cells 6 dpi expressed as fold-change over the average MFI for all female mice analyzed. n = 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by Student’s t test. Representative flow cytometry plots are shown. Gating is based on FMO controls.
Although epithelial cells are the best-studied source of IL-33 (33), mast cells are reported to show both constitutive and inflammation-induced expression (34–37). To assess Il33 expression by mast cells in vivo, we first compared responses in wild-type and mast cell-deficient KitW/Wv mice 6 dpi in the meninges, mast cell-rich tissues that surround the brain and spinal cord. Il33 transcripts are significantly increased in wild-type males compared with naïve mice or immunized KitW/Wv males (Fig. 3G). Selective mast cell reconstitution of the meninges of KitW/Wv males was performed as previously reported (38, 39), and partially restores the response (Fig. 3H). The inducible expression of Il1b and Tnf, genes encoding inflammatory cytokines, is significantly higher in the meninges of immunized wild-type female SJL mice compared with males. As previously shown, this response in female mice is mast cell-dependent (Fig. 3 I and J) (38–40).
We next isolated meningeal tissues and compared mast cell IL-33 protein production in mice by flow cytometry 6 dpi. As shown in Fig. 3K, naïve males and females have similar numbers of mast cells and mast cell frequencies are similar after immunization (Fig. 3L). While IL-33 protein was detected in mast cells derived from both sexes, the percentage of IL-33–expressing mast cells is higher in males (Fig. 3M). There is also a significant increase in the median fluorescence intensity (MFI) of IL-33–expressing cells in males at 6 dpi (Fig. 3N). The differences in IL-33 protein expression between males and females are not as dramatic as those observed at the RNA level, suggesting that steady-state levels in mast cells may be masking the ability to quantify differences in inducible IL-33.
IL-33 Expands ILC2s, Drives a Th2 Response, and Diminishes Clinical Signs of EAE in Females.
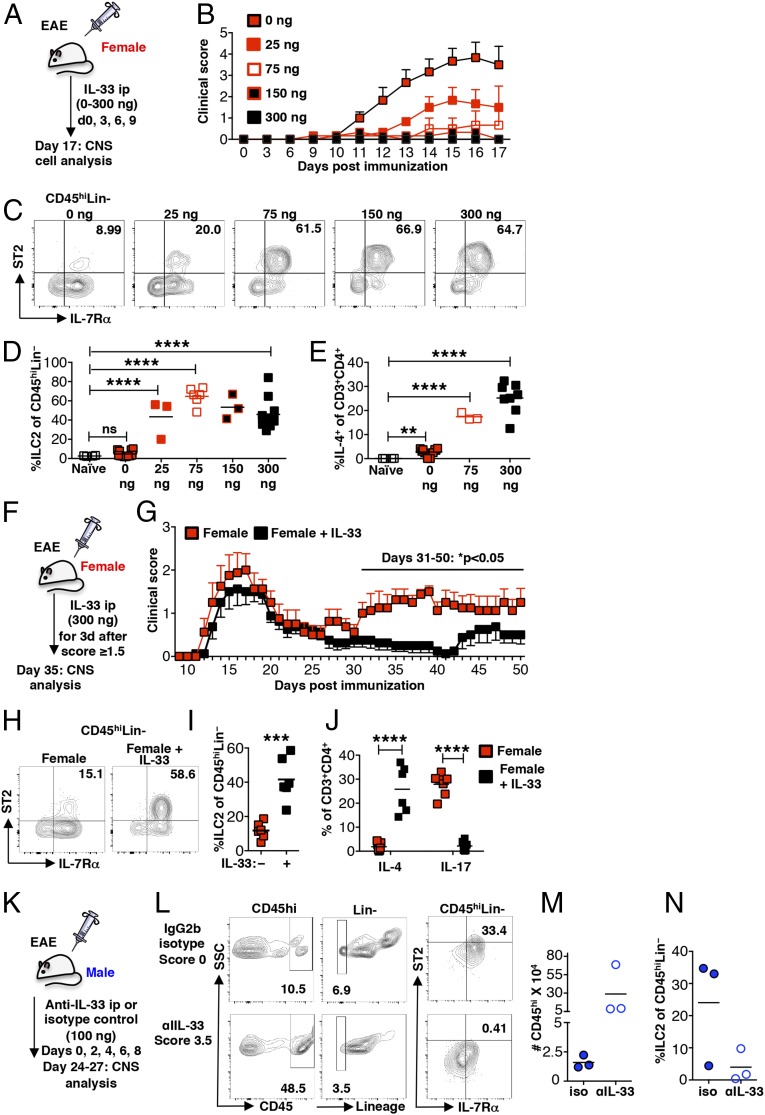
If reduced expression of IL-33 leading to a diminished ILC2 response in females underlies sex-dimorphic EAE susceptibility, IL-33 treatment of SJL females should confer protection by activating ILC2s and shifting the pathogenic anti-myelin response to one that is Th2 skewed. To test this hypothesis, female mice were treated with increasing doses of IL-33 during the preclinical phase of EAE (Fig. 4A). As shown in Fig. 4B, the clinical signs of EAE were significantly diminished in a dose-dependent manner, with no disease evident in the 300-ng–treated cohort. This protection corresponds with dose-dependent increases of ILC2s and Th2 cells in both the LNs (Fig. S4) and CNS (Fig. 4 C–E).
Fig. 4.
IL-33 treatment ameliorates EAE, expands ILC2s, and establishes a Th2 response in preclinical and established EAE. (A) Schematic of treatment of wild-type female mice with increasing doses of recombinant IL-33 during preclinical EAE. (B) Clinical scores of IL-33–treated age-matched female SJL mice. n = 3 per cohort. (C) ILC2s (CD45hiLin−IL-7Rα+ST2+) in the CNS of immunized and IL-33–treated female mice as in A, representative of three independent experiments. Numbers depict the percentage of ILC2s of the CD45hiLin− population. (D) Percentage of ILC2s of the CD45hiLin− population in the CNS of immunized, IL-33–treated mice as in A. ns: P > 0.05 and ****P < 0.0001 by Student’s t test. (E) Percentage of IL-4–expressing CD45hiCD3+CD4+ T cells in the CNS of immunized and IL-33–treated mice as in A. **P < 0.01 and ****P < 0.001 by Student’s t test. (F) Schematic showing IL-33 treatment (300 ng) of wild-type female mice after established disease. Mice were treated when they exhibited a score of ≥1.5. (G) EAE scores of female SJL mice treated with IL-33 after establishment of clinical disease (F). n = 8 per cohort, three independent experiments. *P < 0.05 by two-way ANOVA with multiple comparisons. (H) ILC2s (CD45hiLin−IL-7Rα+ST2+) in the CNS of immunized and IL-33 treated female mice as in F, representative of three independent experiments. Numbers depict the percentage of ILC2s of the CD45hiLin− population. (I) ILC2s, expressed of the CD45hiLin− population, in the CNS of immunized and IL-33–treated female mice as in F. ***P < 0.001 by Student’s t test. (J) IL-4– and IL-17–expressing CD45hiCD3+CD4+ T cells in the CNS of immunized, IL-33–treated female mice as in F. ****P < 0.001 by Student’s t test. (K) Schematic depicting treatment of male mice with anti–IL-33 or an IgG2b isotype control. (L) Flow cytometric analysis of CD45hi Lin− IL-7Rα+ ST2+ populations in the CNS: brain and spinal cord combined from individual mice treated with IL-33 blocking antibody (clinical score: 3.5) or isotype control (clinical score: 0) at 23 dpi. (M) CD45hi cells infiltrating the CNS of control male mice showing no clinical disease (three of four) and anti–IL-33–treated mice with clinical signs (three of four) at 23 dpi. (N) ILC2s detected in the CNS of control mice showing no clinical disease (three of four) and anti–IL-33–treated mice with clinical signs (three of four). Representative flow cytometry plots are shown. Gating is based on FMO controls.
IL-33 treatment also reverses established disease. IL-33 (300 ng) was administered for 3 consecutive days when individual mice exhibited a score ≥1.5, usually between 14 and 18 dpi (Fig. 4F). Although both treated and untreated mice exhibit similar remission kinetics, the treated cohort does not relapse before day 40 (Fig. 4G). This protection corresponds to a significant increase in ILC2 frequency in the CNS and a shift from a Th17- to a Th2-dominated response at 33 dpi, more than 2 wk after the last IL-33 injection (Fig. 4 H–J). Thus, IL-33 has efficacy in preventing both disease onset and relapse.
Finally we tested the effects of treatment with anti–IL-33 antibody and an IgG2b isotype control in a small cohort of SJL males. Mice were administered 100 μg of antibody intraperitoneally on days 0, 2, 4, 6, and 8 post immunization (Fig. 4K). One of four control mice (maximum score: 0.5) and three of four anti–IL-33–treated mice (maximum score: 3.5) showed signs of clinical disease by day 26 post immunization. The loss of disease protection in anti–IL-33–treated mice was associated with increased CD45hi cell infiltration of the CNS (Fig. 4M) and reduced ILC2 accumulation (Fig. 4N), further supporting the idea that IL-33 protects and acts by inducing ILC2 activation.
Testosterone Induces Il33 Expression by SJL-Derived Mast Cells.
Mast cells are reported to express the AR (41, 42), raising the possibility that male hormones influence differential IL-33 expression in these cells. AR expression was detected in peritoneal and BMMCs isolated from male and female SJL mice (Fig. 5 A–D). Although higher in peritoneal cells from males (Fig. 5 A and B), AR expression is indistinguishable in male- and female-derived BMMCs (Fig. 5 C and D). To determine if testosterone can directly induce Il33 expression by mast cells, BMMCs were treated with increasing concentrations of purified testosterone in the presence and absence of flutamide, an AR antagonist. Neither flutamide nor its solvent affects BMMC viability (Fig. S5). Testosterone directly induces Il33 in a dose-dependent manner in male-derived, but not female-derived, mast cells (Fig. 5E). The addition of flutamide significantly reduces Il33 expression resulting in an approximate 10-fold shift in the testosterone dose–response (i.e., compare 10 nM with 100 nM ± flutamide), demonstrating that AR engagement is required for this response.
Fig. 5.
Testosterone induces mast cell Il33 expression. (A and B) AR expression by peritoneal mast cells of naïve mice (CD45+c-kit+FcεRIα+). Numbers represent percentage of the total CD45+ population, n = 9 mice. (C and D) AR expression by BMMCs. Numbers denote percentage of c-kit+FcεRIα+ cells after 6–8 wk of culture. (E) Il33 expression by BMMCs from male and female donor mice after a 6-h culture with testosterone at indicated concentrations in the presence or absence of 100 nM flutamide. ***P < 0.001 over nonstimulated and *P < 0.05 ± flutamide by Student’s t test. Representative results from three independent cultures. (F) Il33 expression by resting (−), IgE- or Mtb-activated BMMCs after 6 h. (G) IL-33 protein expression by resting and Mtb-activated BMMCs after 24 h, representative of results from three independent cultures. (H) Il1b and (I) Tnf gene expression by resting (−), IgE-activated, or Mtb-activated BMMCs after 6 h. **P < 0.01 and ***P < 0.001 by Student’s t test. Data are representative of experiments with five independent cultures derived from both sexes. (J) Testosterone concentrations in the serum of male mice at the indicated days post immunization detected by ELISA. **P < 0.01 by two-way ANOVA with multiple comparisons. (K) Il33 expression by BMMCs from male donor mice after a 6-h stimulation period with serum from naïve male mice or male mice at 14 dpi in the presence or absence of 100 nM flutamide. **P < 0.01 and ****P < 0.0001 by Student’s t test. Data are representative three independent cultures and activation experiments. (L) Comparison of serum testosterone levels in 5- to 8-wk-old naive SJL and C57BL/6 mice, *P < 0.05. Representative flow cytometry plots are shown. Gating is based on FMO controls.
Surprisingly, this male-specific IL-33 expression pattern is also evident after activation through FcεRI cross-linking or with Mtb, where inducible IL-33 mRNA and protein is only observed in male-derived BMMCs (Fig. 5 F and G). Similar to the pattern observed in the meninges, female-derived BMMCs exhibit higher expression of Il1b and Tnf, compared with male-derived cells (Fig. 5 H and I). Thus, other mechanisms, perhaps those that regulate chromatin state, are also operating to regulate sex-specific cytokine expression.
A kinetic analysis of serum testosterone concentrations in male mice revealed testosterone increases at early time points post immunization, with average maximum increases between days 13 and 14 followed by decreases through day 18 (Fig. 5J), indicating early testosterone induction occurs in response to inflammatory signals and may contribute to increases in IL-33. Serum from immunized male, but not female mice, induces ll33 expression in BMMCs, a response that is inhibited by flutamide (Fig. 5K) in support of this idea.
Pronounced sex-dimorphic EAE susceptibility is only observed in certain strains of mice (8). Both male and female C57BL/6 mice, which are most commonly used in studies of a monophasic form of EAE, are susceptible and show a similar disease course. Thus, the testosterone-driven IL-33/ILC2 pathway described here is insufficient to confer protection. Variations in serum testosterone levels between strains have been documented (43) and our analysis of of SJL and C57BL/6 males revealed significantly lower levels in C57BL/6 mice (Fig. 5L). We speculate that decreased testosterone levels in susceptible males results in a failure to activate a threshold level of IL-33 required for protection. However, other factors, including strain-specific differences in mast cell numbers and cytokine responses, may also have a role (44, 45).
Discussion
EAE in SJL mice serves as a useful model of MS that recapitulates the female bias for disease development. Here we demonstrate sex-specific IL-33 expression, likely influenced directly by testosterone, underlies disease protection in male SJL mice. IL-33 is selectively induced in immunized males and elicits the activation of ILC2s, a population of cells that are essential for driving Th2 responses (27, 28, 46) (Fig. 6). We hypothesize that under conditions of low testosterone in females, IL-33 is not sufficiently induced, ILC2s are not activated, and a Th17 anti-myelin response predominates. Reduced testosterone levels and an inadequate IL-33 response may also explain the age-related increase in MS and EAE susceptibility observed in males (3, 15), as well as the absence of male protection in strains such as C57BL/6 and NOD/Lt mice (8).
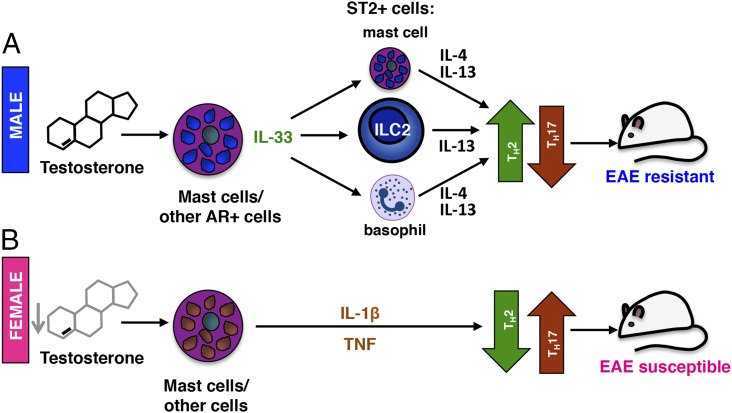
Fig. 6.
A model for sex-dimorphic testosterone-mediated protection in EAE. (A) In males, testosterone concentrations increase upon immunization and together with other inflammatory activators stimulate the production of IL-33 by AR-expressing mast cells or other AR+ cells. IL-33 activates IL-13–producing ILC2s that accumulate in the LNs, meninges, and CNS and promote a protective Th2 response. IL-33–responsive ST2+ mast cells and basophils may amplify the Th2 polarization through production of IL-4 and IL-13. (B) In the absence of sufficiently high testosterone levels, female-derived mast cells express proinflammatory mediators (notably IL-1β and TNF) instead of IL-33, resulting in a pathogenic Th17-dominated anti-myelin response, rendering female mice more susceptible to EAE. The influence of testosterone exposure during development may also confer a higher potential for Il33 gene expression in males.
We previously reported that mast cell–T cell interactions in the meninges induce IL-1β expression by mast cells and GM-CSF production by T cells, and promotes T cell encephalitogenicity (40). These findings demonstrate the importance of immune cell cross-talk in these tissues and suggest that similar cross-talk with T cells may affect mast cell IL-33 expression. We propose that the meninges are also sites where ILC2s regulate Th2 effector function. ILC2s are numerous in the meninges and their proximity to IL-33–producing mast cells can facilitate their activation. Through IL-13–mediated effects on resident dendritic cells or via direct antigen presentation (27–29, 46, 47), ILC2s are well placed to influence the effector phenotype of autoreactive T cells transiting through these tissues before their entry into the CNS parenchyma (48–50).
IL-33 is a mediator belonging to the IL-1 superfamily of cytokines that acts as both a histone binding factor and a pleiotropic cytokine (reviewed in ref. 33). Among its activities, IL-33 can regulate gene transcription, promote tissue homeostasis, fibrosis, and wound healing, as well as directly activate ST2+ immune cells, including ILC2s, T regulatory cells, CD8+ T cells, mast cells, basophils, and Th2 cells. Produced constitutively at relatively low levels by epithelial and endothelial cells, dendritic cells, macrophages, and several CNS resident cells, IL-33 is often localized in the nucleus and is passively released by damaged or necrotic cells, acting as an alarmin. However, IL-33 also exhibits inducible expression and cytoplasmic localization in certain inflammatory settings, and its release can occur independent of cell necrosis via mechanical stress or through autocrine activation of P2 purinergic receptors (33, 51, 52). While it is likely that other cells contribute, we show that mast cells are an important inducible source of this cytokine. Robust Il33 induction is absent in the LNs, meninges, and CNS of PLP131–159-immunized male SJL KitW/Wv mast cell-deficient mice, and this response is largely restored by mast cell reconstitution. IL-33 protein is also detected in meningeal mast cells. Mast cell IL-33 production is of particular interest because the mast cell-specific proteases chymase and tryptase are reported to generate a mature form of IL-33 with significantly increased ability to activate ILC2s (53). Although we have not yet determined how IL-33 is released by mast cells, there is precedence for active transport of cytokines, such as TNF, to cytoplasmic granules and release through degranulation (54, 55).
The male-specific expression of IL-33 in mast cells also reveals a potentially interesting context-dependent function of these cells. Instead of IL-33, immunized females express IL-1β and TNF, inflammatory cytokines that exert a variety of pathogenic effects in EAE, including early and robust neutrophil recruitment, disruption of blood–brain barrier integrity, and promotion of T cell encephalitogenicity (38–40, 56). Even under identical BMMC culture and stimulation conditions, this sex-specific pattern of cytokine expression is evident. These differences are not due to disparate expression of AR or the high affinity IgE receptor, suggesting that there are restraints on the chromatin landscape that limit IL-33 expression in females and IL-1β and TNF expression in males, perhaps imposed by sustained hormone exposure during development.
There is abundant evidence that ILC2s are critical intermediaries in IL-33–mediated EAE protection. ILC2s are among the most robust responders to IL-33 (32), rapidly producing IL-13, a Th2 polarizing cytokine (29, 46). ILC2 depletion studies in mouse models of allergy have clearly established that ILC2s are required for Th2 priming and memory (28, 29, 47). The profound deficits in ST2+ ILC2 activation we observe in immunized female mice that are corrected by IL-33 treatment and the ability of IL-33 blockade to reduce ILC2 activation and reverse protection in males, also support this concept. However, there are several other populations of ST2+ cells that could be targets of IL-33 in this setting, including mast cells and basophils (33). Of particular interest is a subset of ST2+ T regulatory cells that show an IL-33–dependent ability to limit inflammation in a model of inflammatory bowel disease (57). Although IL-33 responsiveness was not assessed, an earlier study by Matejuk et al. (15) shows that decreases in Foxp3+ Tregs correspond with declining testosterone levels and increased EAE severity in aging C57BL/6 mice.
Our results support the concept that altering pathogenic Th responses is a viable therapeutic approach for MS. One mechanism of action of the approved MS drugs, glatiramer acetate (Copaxone) and dimethyl fumarate (Tecfidera), is to shift the pathogenic autoreactive Th1/17 autoreactive response to a Th2-dominant one (58, 59). Although testosterone treatment in male MS patients has shown promise, this is not a viable therapy in females, particularly because MS most often strikes young women during their childbearing years. IL-33 treatment or the use of more selective ILC2 activators, as well as strategies to inhibit pathogenic ILC subsets, may provide protection in both early and later stages of MS. While there are no studies of IL-33 efficacy in humans, IL-33 expression is increased in normal-appearing white matter and lesions of MS patients, indicating that perhaps IL-33 is part of a compensatory response to damaging inflammation (60, 61). Studies in models of stroke, spinal cord injury, and Alzheimer’s disease demonstrate that IL-33 reduces CNS tissue loss, reverses astrogliosis and demyelination, and improves cognition (62–67). IL-33 also has a direct effect on oligodendrocyte gene expression and induces p38MAPK phosphorylation these cells, an event linked to myelination (63). Thus, IL-33 therapy has the potential to prevent disease progression by blocking damaging inflammation as well as promoting myelin and neuronal repair in MS and other diseases of the CNS.
Methods
Mice.
Four- to 8-wk-old age-matched SJL/J, SJL/J-KitW/Wv, and C57BL/6J mice were used in all experiments. SJL/J-KitW/Wv mice were derived in our laboratory, as previously described (39). Mice were housed under specific pathogen-free conditions with 12-h light/dark cycles in an Association for Assessment of Accreditation of Laboratory Animal Care-approved facility at Northwestern University. The experiments were carried out under ARRIVE (Animal Research: Reporting of in Vivo Experiments) guidelines and approved by the Northwestern University Animal Care Committee.
EAE Induction.
Four- to 8-wk-old mice were immunized subcutaneously at two injection sites on the posterior flanks with 100 μg PLP139–151 (Genemed Biotechnologies) emulsified in 500 μg CFA (Incomplete Freund’s Adjuvant with desiccated M. tuberculosis H37 RA) (VWR). EAE was scored blindly on a five-point scale: 0, no clinical signs; 1, flaccid tail; 2, ataxia; 3, significant hind limb paralysis; 4, cachexia; 5, death.
Cell Analysis by Flow Cytometry.
Cells were prepared from homogenized tissues. Before staining with indicated antibodies, cells were treated with anti-CD16/32 FcBlock (BioLegend). For intracellular cytokine staining, cells were restimulated for 5 h with 50 ug/mL PLP139–151 (Genemed Biotechnologies). Brefeldin A (eBioscience) added at a concentration of 3 μg/mL for the final 4 h. Intracellular staining for cytokines and the AR was assessed in fixed and permeabilized cells (eBioscience). Monoclonal IL-17–, GM-CSF–, IL-4–, and IL-10–specific antibodies were purchased from eBioscience. The AR-specific rabbit monoclonal antibody was purchased from Abcam and all antibodies directed to extracellular markers, including the lineage stain, were purchased from BioLegend. The lineage cocktail included antibodies to CD3, Ly-6C, CD11b, B220, and Ter-119.
IL-33 expression by meningeal mast cells was assessed using single-cell homogenates prepared from pooled meningeal tissues of five donor mice per sample and stained with c-kit (Brilliant Violet 421, BioLegend) and FcRεIα (APC, BioLegend). After fixation cells were incubated with 1.25 μg polyclonal goat anti-mouse IL-33 antibody (R&D Systems) followed by staining with 5 μL donkey anti-goat IgG PE-conjugated antibody (R&D Systems).
ILC2 FACs Sorting and Expansion in Vitro.
Donor mice were administered 300 ng IL-33 (eBioescience or R&D Systems) via the intraperitoneal route daily for 3 d and killed on day 4. Lin− populations were enriched from bone marrow using the StemCell Hematopoetic Lineage Negative EasySep Kit. Cells were stained with antibodies that detect ILC2 markers [Lineage (Ter-119, Gr-1, B220, CD3, CD11b), CD45, IL-7Rα, Sca-1, CD25] and with the UV live/dead stain (Invitrogen) before FACs sorting (performed in the Robert H. Lurie Cancer Center Flow Cyometry Core). Sorted ILC2s were cultured at 1 × 106 cells/mL in 15% complete RPMI supplemented with IL-33 (12 ng/mL), IL-2 (10 μg/mL), and IL-7 (10 ng/mL) for 6 d with fresh media and cytokine supplementation on days 2, 4, and 6.
Quantitative Real-Time PCR.
RNA was isolated from cultured BMMCs or ILC2s using the Promega SV Total RNA Isolation System. RNA was extracted from pooled meningeal tissues, as well as individual brain, spinal cord, or LN samples with the Qiagen Fibrous Tissue Mini Kit. cDNA was generated using SuperScript III First-Strand Synthesis SuperMix (Invitrogen). Quantification was performed with iCycler iQ5 Real Time PCR Detection System (Bio-Rad) using PerfecTA SYBR Green SuperMix (Bio-Rad). For each sample, the cycle number (Ct) at which the analysis threshold was reached, set in the linear range of the amplification curve [fluorescence = f(cycle)], was calculated. The relative expression of the cDNA template was calculated as the ratio of 2ΔCt for each transcript tested relative to a control housekeeping gene (Hprt). Primers used are listed in Table 1.
Table 1.
PCR primers
| Gene | Forward | Reverse |
| Il33 | 5′ TGAGACTCCGTTCTGGCCTC 3′ | 5′ CTCTTCATGCTTGGTACCCGAT 3′ |
| Hprt | 5′ GTTGGATACAGGCCAGACTTTGTT 3′ | 5′ GAGGGTAGGCTGGCCTATAGGCT 3′ |
| Il4 | 5′ ACAGGAGGGACGCCAT 3′ | 5′ GAAGCCCTACAGACGAGCTCA 3′ |
| IL5 | 5′ AGCACAGTGGTGAAAGAGACCTT 3′ | 5′ TCCAATGCATAGCTGGTGATTT 3′ |
| IL13 | 5′ AGACCAGACTCCCCTGTGCA 3′ | 5′ TGGGTCCTGTAGATGGCATTG 3′ |
| IL9 | 5′CATCAGTGTCTCTCCGTCCCAACTGATG 3′ | 5′ CGGCACACACCACTCCTGGCT 3′ |
| Tbx1 | 5′ GTTCCATGGAGAACGGAGAA 3′ | 5′ CTGTTTGGCTGGCTGTTGTA 3′ |
| Rorc | 5′ CTTCCATTGCTCCTGCTTTC 3′ | 5′ TGAGGCCATTCAGTATGTGG 3′ |
| Gata3 | 5′-CCAAGGCACGATCCAGCACAGA-3 | 5′-TGCCGACAGCCTTCGCTTGG-3′ |
IL-33 Administration and IL-33 Blockade.
IL-33 (eBioscience or R&D Systems) was administered at the indicated doses by intraperitoneal injection daily for 3 consecutive days or every 3 d. The specific activity of IL-33 was >3 × 107 units/mg (eBioscience or R&D Systems), corresponding to an ED50 of 0.012–0.05 ng/mL. Anti-mouse IL-33 blocking antibody (Bondy1-1) or an IgG2b isotype (BPC4) control (0.2 mL, 100 μg) antibody (Adipogen) was given every other day for 8 d starting at day of immunization.
BMMC Derivation, Activation, and Reconstitution.
Bone marrow was harvested from the femurs of 3- to 6-wk-old mice and cultured with 10 ng/mL recombinant murine IL-3 (Miltenyi) and 12.5 ng/mL recombinant murine SCF (Miltenyi) in complete RPMI for 6–8 wk. Purity was determined by flow cytometry (>95% live cells coexpressing c-kit and FcεRIα).
For mast cell activation assays, BMMCs (2 × 106 per well) were stimulated for 6 h. BMMCs were preincubated with anti–DNP-IgE (2 μg/mL) for 16 h before addition of DNP-HSA (10 ng/mL). Mtb was used at 100 μg/mL. Pooled serum was collected from naïve or immunized mice using serum separator tubes and 200 μL serum was added to BMMCs. Testosterone (Sigma) was diluted in ethanol and used at the indicated concentrations. Flutamide (Sigma) was diluted to 0.1 M in ethanol and used at a concentration of 100 nM. For mast cell reconstitution, 1 × 106 BMMCs were injected intracranially into 3- to 5-wk-old SJL-KitW/Wv mice. After 8 wk, reconstitution was confirmed by histological analysis of the peritoneum and meninges.
Meningeal Mast Cell Enumeration.
Following perfusion, the calvarium was dissected and fixed in formalin for 24 h before transfer and storage in PBS. The calvarium was stained with acidic Toluidine blue for 15 s followed by distilled water rinses. The meninges were separated from the calvarium while submerged in distilled water and floated onto glass slides. Tissues were dried, adhered to the slide, and covered with clear nail polish. The average number of mast cells in 18 1-mm × 1-mm squares in six different regions was calculated and expressed as mast cell number/mm2.
Serum Testosterone Analysis.
For testosterone analyses, blood (∼100 μL) was collected via tail snips at the indicated days post immunization from group-housed males. The serum was isolated using Microtainer Serum Separation tubes (BD) and serum testosterone levels were assessed using Parameter testosterone immunoassay (R&D Systems). Five- to 8-wk-old SJL and C57BL/6 mice were used for comparisons of serum testosterone in naïve mice.
Statistical Analysis.
All statistics were performed using Prism 6 software (GraphPad Software). Details of specific statistic analyses are included in the corresponding figure legends.
Study Approval.
Animal studies were approved by the Northwestern University Animal Care and Use committee.
Supplementary Material
Acknowledgments
We thank Kevin Cao and Dr. Guiqing Zhao for assistance with animal experiments and measurement of testosterone levels; and Dr. Jim Miller, University of Rochester, for helpful discussions. This work was supported by the Northwestern University–Flow Cytometry Core Facility supported by Cancer Center Support Grant (NCI CA060553). Flow Cytometry Cell Sorting was performed on a BD FACSAria SORP system, purchased through the support of NIH Grant 1S10OD011996-01. This study was supported by National Multiple Sclerosis Society Grants RG 4684A5/1 and RG 5281-A-3 (to M.A.B.), and NIH Grants R21 NS081598-01 and F31 NS084691 (to A.E.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710401115/-/DCSupplemental.
References
- 1.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11:A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. 2015;125:2187–2193. doi: 10.1172/JCI78082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 4.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 5.Khalid R. Contributing factors in multiple sclerosis and the female sex bias. Immunol Lett. 2014;162:223–232. doi: 10.1016/j.imlet.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. 2012;8:255–263. doi: 10.1038/nrneurol.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papenfuss TL, et al. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J Neuroimmunol. 2004;150:59–69. doi: 10.1016/j.jneuroim.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Bebo BF, Jr, et al. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J Immunol. 1995;155:4052–4059. [PubMed] [Google Scholar]
- 11.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 12.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Gender differences in experimental autoimmune encephalomyelitis develop during the induction of the immune response to encephalitogenic peptides. J Neurosci Res. 1998;52:420–426. doi: 10.1002/(SICI)1097-4547(19980515)52:4<420::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 14.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. J Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005;174:2387–2395. doi: 10.4049/jimmunol.174.4.2387. [DOI] [PubMed] [Google Scholar]
- 16.Torjesen PA, Sandnes L. Serum testosterone in women as measured by an automated immunoassay and a RIA. Clin Chem. 2004;50:678, author reply 678–679. doi: 10.1373/clinchem.2003.027565. [DOI] [PubMed] [Google Scholar]
- 17.Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol. 1994;36:S6–S11. doi: 10.1002/ana.410360704. [DOI] [PubMed] [Google Scholar]
- 18.Kurth F, et al. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin. 2014;4:454–460. doi: 10.1016/j.nicl.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sicotte NL, et al. Testosterone treatment in multiple sclerosis: A pilot study. Arch Neurol. 2007;64:683–688. doi: 10.1001/archneur.64.5.683. [DOI] [PubMed] [Google Scholar]
- 20.Russi AE, Walker-Caulfield ME, Ebel ME, Brown MA. Cutting edge: c-kit signaling differentially regulates type 2 innate lymphoid cell accumulation and susceptibility to central nervous system demyelination in male and female SJL mice. J Immunol. 2015;194:5609–5613. doi: 10.4049/jimmunol.1500068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Paul WE. Inflammatory group 2 innate lymphoid cells. Int Immunol. 2016;28:23–28. doi: 10.1093/intimm/dxv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halim TY. Group 2 innate lymphoid cells in disease. Int Immunol. 2016;28:13–22. doi: 10.1093/intimm/dxv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Pearson C, et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife. 2016;5:e10066. doi: 10.7554/eLife.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwong B, et al. T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat Immunol. 2017;18:1117–1127. doi: 10.1038/ni.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirchandani AS, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 28.Oliphant CJ, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong SH, et al. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow JL, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CL, Bryce PJ. Inducible IL-33 expression by mast cells is regulated by a calcium-dependent pathway. J Immunol. 2012;189:3421–3429. doi: 10.4049/jimmunol.1201224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno T, et al. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol. 2009;183:7890–7897. doi: 10.4049/jimmunol.0802449. [DOI] [PubMed] [Google Scholar]
- 37.Zhao WH, Hu ZQ. Up-regulation of IL-33 expression in various types of murine cells by IL-3 and IL-4. Cytokine. 2012;58:267–273. doi: 10.1016/j.cyto.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: A role for neutrophil recruitment? J Immunol. 2010;184:6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 40.Russi AE, Walker-Caulfield ME, Guo Y, Lucchinetti CF, Brown MA. Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity. J Autoimmun. 2016;73:100–110. doi: 10.1016/j.jaut.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaitsu M, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1977–1985. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W, et al. Human mast cells express androgen receptors but treatment with testosterone exerts no influence on IgE-independent mast cell degranulation elicited by neuromuscular blocking agents. Exp Dermatol. 2010;19:302–304. doi: 10.1111/j.1600-0625.2009.00969.x. [DOI] [PubMed] [Google Scholar]
- 43.Brouillette J, Rivard K, Lizotte E, Fiset C. Sex and strain differences in adult mouse cardiac repolarization: Importance of androgens. Cardiovasc Res. 2005;65:148–157. doi: 10.1016/j.cardiores.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson D, Yasui D, Seeldrayers P. An analysis of mast cell frequency in the rodent nervous system: Numbers vary between different strains and can be reconstituted in mast cell-deficient mice. J Neuropathol Exp Neurol. 1991;50:227–234. doi: 10.1097/00005072-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Halim TY, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. [DOI] [PubMed] [Google Scholar]
- 49.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 50.Kivisäkk P, et al. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 53.Lefrançais E, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci USA. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beil WJ, et al. Tumor necrosis factor alpha immunoreactivity of rat peritoneal mast cell granules decreases during early secretion induced by compound 48/80: An ultrastructural immunogold morphometric analysis. Int Arch Allergy Immunol. 1996;109:383–389. doi: 10.1159/000237267. [DOI] [PubMed] [Google Scholar]
- 55.Beil WJ, Login GR, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the cytoplasmic granules of rat peritoneal mast cells with rapid microwave fixation. J Allergy Clin Immunol. 1994;94:531–536. doi: 10.1016/0091-6749(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 56.Walker-Caulfield ME, Hatfield JK, Brown MA. Dynamic changes in meningeal inflammation correspond to clinical exacerbations in a murine model of relapsing-remitting multiple sclerosis. J Neuroimmunol. 2015;278:112–122. doi: 10.1016/j.jneuroim.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Schiering C, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aharoni R. Immunomodulation neuroprotection and remyelination–The fundamental therapeutic effects of glatiramer acetate: A critical review. J Autoimmun. 2014;54:81–92. doi: 10.1016/j.jaut.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Gross CC, et al. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;3:e183. doi: 10.1212/NXI.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allan D, et al. Role of IL-33 and ST2 signalling pathway in multiple sclerosis: Expression by oligodendrocytes and inhibition of myelination in central nervous system. Acta Neuropathol Commun. 2016;4:75. doi: 10.1186/s40478-016-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christophi GP, et al. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin Immunol. 2012;142:308–319. doi: 10.1016/j.clim.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natarajan C, Yao SY, Sriram S. TLR3 agonist poly-IC induces IL-33 and promotes myelin repair. PLoS One. 2016;11:e0152163. doi: 10.1371/journal.pone.0152163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H, et al. Interleukin-33 is released in spinal cord and suppresses experimental autoimmune encephalomyelitis in mice. Neuroscience. 2015;308:157–168. doi: 10.1016/j.neuroscience.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Luo Y, et al. Interleukin-33 ameliorates ischemic brain injury in experimental stroke through promoting Th2 response and suppressing Th17 response. Brain Res. 2015;1597:86–94. doi: 10.1016/j.brainres.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Pomeshchik Y, et al. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun. 2015;44:68–81. doi: 10.1016/j.bbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Fu AK, et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc Natl Acad Sci USA. 2016;113:E2705–E2713. doi: 10.1073/pnas.1604032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.