Significance
The role of astrocytes on brain function is controversial in many aspects. It remains challenging to specify the in vivo functional impact of astrocytic calcium signal when mediating vasodilation/constriction at varied physiological or pathophysiological conditions. Here, we applied simultaneous fMRI and GCaMP-mediated Ca2+ optical fiber recording to detect distinct astrocytic Ca2+ signals (evoked vs. intrinsic) coupled to positive and negative blood-oxygen-level-dependent signals, respectively and concurrently, with unique spatial and temporal patterns. Not only did we demonstrate the distinct neurovascular coupling events coupled to the evoked and intrinsic astrocytic calcium signals, but also revealed the thalamic regulation mechanism underlying the astrocytic calcium-mediated brain state switch. This astrocytic-relevant regulatory mechanism could underlie numerous brain disorder and injury models relevant to gliovascular disruption.
Keywords: BOLD-fMRI, astrocyte, calcium, brain states, glia-vascular coupling
Abstract
Astrocytic Ca2+-mediated gliovascular interactions regulate the neurovascular network in situ and in vivo. However, it is difficult to measure directly both the astrocytic activity and fMRI to relate the various forms of blood-oxygen-level-dependent (BOLD) signaling to brain states under normal and pathological conditions. In this study, fMRI and GCaMP-mediated Ca2+ optical fiber recordings revealed distinct evoked astrocytic Ca2+ signals that were coupled with positive BOLD signals and intrinsic astrocytic Ca2+ signals that were coupled with negative BOLD signals. Both evoked and intrinsic astrocytic calcium signal could occur concurrently or respectively during stimulation. The intrinsic astrocytic calcium signal can be detected globally in multiple cortical sites in contrast to the evoked astrocytic calcium signal only detected at the activated cortical region. Unlike propagating Ca2+ waves in spreading depolarization/depression, the intrinsic Ca2+ spikes occurred simultaneously in both hemispheres and were initiated upon the activation of the central thalamus and midbrain reticular formation. The occurrence of the intrinsic astrocytic calcium signal is strongly coincident with an increased EEG power level of the brain resting-state fluctuation. These results demonstrate highly correlated astrocytic Ca2+ spikes with bidirectional fMRI signals based on the thalamic regulation of cortical states, depicting a brain-state dependency of both astrocytic Ca2+ and BOLD fMRI signals.
The blood-oxygen-level-dependent (BOLD) signal is used in many fMRI studies as a surrogate for brain activity, but the link between the two signals is indirect, which makes the interpretation of BOLD signals problematic. The neurovascular coupling that underpins functional MRI (fMRI) brain mapping (1) has been characterized by simultaneous intracortical recordings and fMRI (2). Among the neurovascular signaling events, it remains ambiguous how gliovascular interactions, especially that mediated by the astrocytic Ca2+ signal, are involved in the neurovascular network (3–6). Astrocytic Ca2+ signals in both in situ and in vivo environments can occur in coordination with, or independently of, each other, suggesting specific and variable coupling mechanisms (5, 7–13). It is difficult to directly measure astrocytic activity, which may vary according to different normal brain states and pathological conditions (14–17), while simultaneously utilizing fMRI.
It is possible to use genetically encoded Ca2+ indictors (GECIs, e.g., GCaMP) to acquire cell type-specific Ca2+ signals via optical fiber, which can be measured during fMRI imaging without radio-frequency and magnetic gradient-switch induced interference (2, 18–21). In particular, GCaMP6f shows fast Ca2+ binding kinetics and high sensitivity comparable to the Ca2+-sensitive dyes, such as Oregon Green BAPTA-1 (OGB-1) (18, 20). Besides two-photon microscopy (22) or wide-field calcium imaging (23), the Ca2+-sensitive dye and GCaMP-mediated Ca2+ signal can be directly recorded using optical fiber implanted into animal brains (19, 24, 25). The fMRI signal may be simultaneously acquired with the Ca2+ signal from neurons or astrocytes specifically loaded with Ca2+-sensitive dyes (19). These studies show that it is feasible to monitor the many possible ways that astrocytes mediate BOLD signals through specific neurovascular coupling events.
We have observed the expected positive correlation between the evoked neuronal/astrocytic Ca2+ signals and BOLD fMRI signal in activated cortical areas; however, an unexpected intrinsic astrocytic Ca2+ signal showing negative correlation with both neuronal and fMRI signals can occur concurrently with the evoked neurovascular coupling events. In contrast to the spreading depolarization/depression-based traveling Ca2+ waves in the brain (26, 27), this intrinsic astrocytic Ca2+ signal represents distinct spatiotemporal features that occur simultaneously in both hemispheres without propagation delays (28–32). The instantaneously altered brain activity that is associated with the intrinsic astrocytic Ca2+ signal is a unique astrocyte-mediated change in brain state (9, 33, 34).
We provide evidence that subcortical nuclei projecting to the entire cortex are responsible for regulating the astrocytic calcium-coupled bidirectional fMRI signal. By using astrocytic Ca2+ signal-based event-related fMRI analysis, positive BOLD signals were detected at the central and mediodorsal thalamic nuclei extending to the midbrain reticular formation, which is associated with the intrinsic astrocytic Ca2+ signal followed by the negative BOLD signals in the cortex. This result is consistent with a resting-state fMRI study of unanesthetized macaques, showing increased thalamic fMRI signals with widespread signal decreases in the neocortex during elevated arousal states (15). This work demonstrates that astrocytes might be involved in mediating the bidirectional fMRI signal arising from the thalamic regulation of brain states, demonstrating a way to bridge the study of brain function among the cellular, network, and systems levels.
Results
Identifying the Neurovascular Coupling Events with Simultaneous fMRI and Neuronal/Astrocytic Ca2+ Recordings.
We recorded the cell type-specific Ca2+ signal simultaneously with the local field potential (LFP). GCaMP6f was expressed by viral injection into the forepaw somatosensory cortex (FP-S1) of the rat brain, specifically into neurons or astrocytes (Fig. 1A). The sensory-evoked Ca2+ signal from neurons matched well with the evoked LFP for each electrical stimulus, and the amplitude of the Ca2+ signal was positively correlated with that of the LFP, as shown in Fig. 1B and SI Appendix, Fig. S1. Spontaneous Ca2+ spikes were also detected from neurons whose amplitudes were positively correlated with those of the spontaneous LFPs (SI Appendix, Fig. S1 D and F). The latency of the neuronal Ca2+ signal reported by GCaMP6f was ∼15 ms with a full width of half maximum (FWHM) of 150–200 ms (SI Appendix, Fig. S2A), similar to the kinetics of GCaMP6f-mediated Ca2+ signals reported previously (20, 24).
Fig. 1.
Sensory-evoked neuronal/astrocytic Ca2+ recordings with simultaneous LFP or BOLD fMRI. (A) The colocalized GCaMP (green) with neurons (NeuN, red), or with astrocytes (GFAP, red). (Lower) Immunostaining image (Left) and a schematic drawing (Right) for simultaneous LFP and fiber-optic Ca2+ recording. (B) Simultaneous LFP (red) and Ca2+ signal traces (blue) from neurons or astrocytes in FP-S1 with forepaw electrical stimulation (3 Hz, 4 s, 1 mA). (Center) Enlarged figures of the dashed box in Left. (Right) The averaged trace of evoked Ca2+ signal (gray lines are individual traces from six rats). (C) The schematic drawing of the two-channel fiber-optic recording system with fMRI (CL, coupling lens; DM, dichroic mirror; EF, emission filter; PM, photomultiplier). (D) The time courses of evoked fMRI signal from bilateral FP-S1 and simultaneous neuronal (Left)/astrocytic (Right) Ca2+ signal (Inset, a representative color-coded BOLD-fMRI map at 2.0 mA). (E) The stimulation intensity-dependent Ca2+ signal from neurons or astrocytes. (F) The scatter plot of the evoked Ca2+ signal amplitude vs. simultaneous fMRI peak amplitude at different stimulation intensities (red dashed circle, outlier). (G) The traces of BOLD-fMRI and astrocytic Ca2+ signals show the outlier event (red dashed box) with increased astrocytic Ca2+ signal, but reduced BOLD-fMRI signal (SI Appendix, Table S1 shows the occurrence rate of the unexpected astrocytic event).
In contrast to the neuronal Ca2+ signal, the sensory-evoked astrocytic Ca2+ signal was a unitary event following a train of electrical stimuli to the forepaw (Fig. 1B). The latency of the astrocytic Ca2+ signal was 1.0–1.7 s, and the FWHM was proportional to the stimulus duration, matching the simultaneously acquired BOLD signal (SI Appendix, Figs. S2 B and C and S3). The latency detected by optical fiber could reflect fast astrocytic Ca2+ signals within astrocyte processes (11, 35). The spread function of the astrocytic Ca2+ signal is derived from the sum of individual astrocytes exposed under the tip of optical fiber, which have varied response kinetics as observed in vivo with two-photon microscopy (5, 10). These results clearly demonstrated that the GCaMP6f-mediated Ca2+ signal from either neurons or astrocytes is specifically detectable in vivo via optical fiber, showing distinct temporal features to sensory stimulation. It is noteworthy that the evoked fluorescent Ca2+ signal is independent of the hemoglobin-based intrinsic optical signal, similar to the previous fiber optic Ca2+ studies (details in Materials and Methods) (21, 25).
A two-channel fluorescent signal recording system was developed to simultaneously detect the BOLD signal and the Ca2+ signal from neurons and astrocytes from the forepaw somatosensory cortex of both hemispheres, respectively (Fig. 1 C and D). The neuronal and astrocytic Ca2+ signals were correlated with the BOLD-fMRI evoked signal across electrical stimulation intensities (Fig. 1 E and F and SI Appendix, Fig. S4). However, a novel event was detected in which a high-amplitude astrocytic Ca2+ signal was coupled with a reduced amplitude BOLD fMRI signal in a single trial-on/off block design paradigm. This outlier indicates that astrocytic Ca2+ signaling might play another role in neuroglial and gliovascular interaction.
We investigated the properties of the intrinsic astrocyte Ca2+ spikes and its relationship to the simultaneously acquired fMRI signal. In rats anesthetized with α-chloralose, intrinsic astrocytic Ca2+ spikes occurred simultaneously with a transient frequency shift in hypersynchronized LFP bursts (Fig. 2A), previously described as intermittent or continuous hypersynchrony during different EEG stages (36). During the spontaneous LFP frequency shifts, there was a transient suppression of the power spectral density [Fig. 2 B and C, nonoverlapping confidence intervals (CIs); SI Appendix, Table S2], as well as a decrease in the fMRI signal throughout the cortex (Fig. 2 D and E). There was a negative correlation between the resting state fMRI signal and the intrinsic astrocytic Ca2+ spikes (Fig. 2D and SI Appendix, Fig. S5). The astrocytic Ca2+ spike-triggered average of the simultaneously acquired LFP power spectral profile and fMRI signal were computed. The negative BOLD signal was much delayed (−2.64 ± 0.25 s) (Fig. 2F) compared with the mean onset of the astrocyte Ca2+ spike (−4.29 ± 1.04 s) (Fig. 2F and SI Appendix, Fig. S6, the negative value is set from the zero time at the peak of astrocytic Ca2+ spikes, nonoverlapping Cis; SI Appendix, Table S2). The mean estimated onset of LFP frequency shift (−4.77 ± 1.44 s) preceded the estimated onset of the astrocyte Ca2+ spike, although the difference was not statistically significant. In addition, previous studies showed that the high frequency LFP power reduction occurred earlier than the astrocytic Ca2+ spikes in both the cortex and hippocampus of mice anesthetized with urethane (9, 30). Thus, the intrinsic astrocytic Ca2+ signal elevation is possibly involved in mediating this particular neurovascular event. It is noteworthy that two-channel bilateral Ca2+ recording (left hemisphere, neurons; right hemisphere, astrocytes) during the resting state also shows the negative correlation of the intrinsic astrocytic Ca2+ spike to the neuronal Ca2+ transients. Power spectral analysis of the neuronal Ca2+ transients shows similar frequency suppression shift to that of the spontaneous LFP signal (SI Appendix, Fig. S7).
Fig. 2.
Intrinsic astrocytic Ca2+ spikes negatively correlate with neuronal and BOLD signals. (A) The representative traces of the LFP (Top), the power spectra of LFP (Middle), and the intrinsic astrocytic Ca2+ signal (Bottom) in FP-S1 (1-s sliding window with 0.1-s steps, 9 tapers). (B) The average spectrogram of the LFP is aligned based on the simultaneously acquired peak time of the intrinsic astrocytic Ca2+ spikes (red dashed line as time 0). (C) The integrated LFP power spectral density (2 ∼ 50 Hz) at different phases according to the intrinsic astrocytic Ca2+ spikes (*P = 9.5e-22; #P = 3.6e-11, paired t test; 54 traces of 6 rats). (D) The representative traces of the intrinsic astrocytic Ca2+ spikes and simultaneous fMRI signal acquired from the entire cortex (Upper Inset: color-coded negative correlation map; Lower Inset: ROI in red contour; details in SI Appendix, Fig. S5). (E) The averaged time course of the fMRI signal is aligned based on the simultaneous acquired peak time of the intrinsic astrocytic Ca2+ spikes (red dashed line as time 0, 52 traces of 6 rats). (F) Estimated onset times of the intrinsic astrocytic Ca2+ spikes, the LFP spectral power shift, and the fMRI signal reduction (time 0 at the peak time of the astrocytic Ca2+ spikes; one-way ANOVA followed by Tukey’s multiple comparison test F = 31.94; *P = 5.1e-12, nLFP_Ca = 6; nfMRI_Ca = 6 rats).
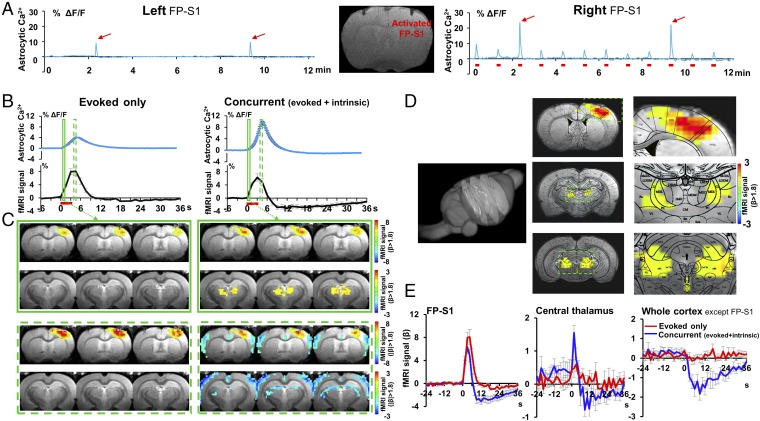
Elevated astrocytic Ca2+ signals can affect vasoconstriction or vasodilation, depending on conditions (7, 8, 12, 37), but these seldom coexist during neurovascular coupling events. Interestingly, intrinsic astrocytic Ca2+ spikes can occur in the FP-S1 and barrel cortex concurrently with the astrocytic Ca2+ signal evoked by forepaw electrical stimulation (Fig. 3 A and B). As with the spontaneous astrocytic Ca2+ recording during the resting state, the intrinsic astrocytic Ca2+ spikes during stimulation were correlated with the negative BOLD signal throughout the cortex, with the exception of FP-S1 (Fig. 3 B and C, nonoverlapping CIs; SI Appendix, Table S2), where the FP-S1 BOLD signal with the concurrent (intrinsic and evoked) astrocytic Ca2+ signals was also lower than that with the evoked-only astrocytic Ca2+signal (P = 0.026, Student t test two-tailed), although it was not showing nonoverlapping CIs (SI Appendix, Table S2). In contrast, the amplitude of the intrinsic astrocytic Ca2+ spike was significantly higher than the normally evoked astrocytic Ca2+ spike (Fig. 3C, nonoverlapping CIs; SI Appendix, Table S2). The concurrent astrocytic Ca2+ spiking events were better characterized in the 30-s forepaw stimulation block design experiment, which showed the intrinsic astrocytic Ca2+ spike superimposed on the early phase of the evoked astrocytic Ca2+ signal (Fig. 3D). The averaged time courses and time-lapsed functional maps revealed the reduced fMRI signal in the FP-S1 and the negative fMRI signal from the whole cortex upon the intrinsic astrocytic Ca2+ spiking events (Fig. 3 E and F). Furthermore, the intrinsic astrocytic Ca2+ spikes were also detected at different phases of the 30-s stimulation and the stimulation-off period (SI Appendix, Fig. S8 A and B). These results clearly demonstrated that two independent astrocytic Ca2+ signals (evoked vs. intrinsic) could concurrently occur with a unique combination of coexisting positive and negative BOLD signals in the brain.
Fig. 3.
Two-channel recording of the evoked and intrinsic astrocytic Ca2+ signal in FP-S1 and barrel cortex (BC). (A) The time courses of the evoked astrocytic Ca2+ signal and the fMRI signal from FP-S1 (Left) upon forepaw stimulation (3 Hz, 4 s, 1.5 mA), and the concurrent intrinsic astrocytic Ca2+ signal from BC and the fMRI signal from the entire cortex except FP-S1 (Right). (B) The correlation map of the fMRI signal with the intrinsic astrocytic Ca2+ spikes detected in BC shows negative correlation across the entire cortex except FP-S1. (C) The amplitude of the astrocytic Ca2+ signal (evoked-only) is significantly lower than that of the concurrent events in FP-S1 (Left, *P = 2.0e-15), but the fMRI signal change is significant lower in the concurrent events (Center, &P = 0.026). The fMRI signal in the entire cortex except FP-S1 shows negative amplitude of the concurrent events, which is significantly lower than the evoked-only events (Right, #P = 2.8e-07; evoked-only, trial # = 81; concurrent, trial # = 50, rats, n = 7). (D) The representative traces of astrocytic Ca2+ and corresponding fMRI signals with 30-s forepaw stimulation (evoked-only vs. concurrent: epoch 2, 5 vs. 1, 3, 4). (E) The mean astrocytic Ca2+ traces of the two events (Left) and the simultaneously acquired fMRI time course (Right; gray line, difference; evoked-only, trial # = 84 vs. concurrent, trial # = 23, mean ± SEM, rats, n = 5). (F) The representative time-lapsed BOLD-fMRI map shows FP-S1 activation during the 30-s stimulation of evoked-only and concurrent events.
Specifying the Spatiotemporal Features of the Intrinsic Astrocytic Ca2+ Spikes.
Given that brain trauma may accompany the surgical procedure, we examined the possibility that the astrocytic Ca2+ signal is associated with spreading depolarization/depression (27). Astrocytic Ca2+ signals from two hemispheres in the 30-s stimulation block design were measured to determine whether the intrinsic astrocytic Ca2+ spike elicited by the stimulation traveled through the cortex. If the astrocytic Ca2+ spike was triggered from the activated FP-S1 due to the stimulation-induced spreading depolarization/depression, it should appear as a signal propagating through the cortex following stimulation (26, 28, 31). Fig. 4A shows that the intrinsic astrocytic Ca2+ spikes not only occurred in the activated FP-S1, but also were detected in FP-S1 of the opposite hemisphere despite the fact that no evoked astrocytic and fMRI signals were detected together with the negative BOLD signal through the cortex. The onset of the intrinsic astrocytic Ca2+ spike in activated FP-S1 during stimulation was measured by subtracting the normally evoked astrocytic Ca2+ spike to clearly display the overlapping onsets of the intrinsic astrocytic Ca2+ spikes detected from both hemispheres (Fig. 4B). The latencies of the intrinsic astrocytic Ca2+ spiking events in the two hemispheres varied from 2 to 4 s after the stimulus, but with little difference between them (Fig. 4C, Left, 2.29 ± 0.31 s vs. Right, 2.42 ± 0.12 s, paired t test, P = 0.26; CI values in SI Appendix, Table S2). Periodically, the intrinsic astrocytic Ca2+ spike appeared at a later phase of the 30-s stimulation period and was detected in the other hemisphere with the same onset time (SI Appendix, Fig. S8 C and D).
Fig. 4.
Bilateral astrocytic Ca2+ recordings with simultaneous fMRI. (A) The representative traces of astrocytic Ca2+ and simultaneous BOLD signal in FP-S1 of both hemispheres with 30-s right forepaw stimulation (Inset: BOLD-fMRI map with left FP-S1 activation; concurrent events: epoch 2, 3, 5). (B) The mean time course of the astrocytic Ca2+ signal from evoked-only events vs. the concurrent (evoked + intrinsic) events (Left). The intrinsic astrocytic Ca2+ spike in the activated FP-S1 can be represented by subtracting the two events, which shows similar onset time to the intrinsic astrocytic Ca2+ spike detected in the opposite hemisphere (Right; mean ± SEM, rats, n = 6). Inset is a magnified figure of the dashed box. (C) The scatter plot of the latency estimated from the intrinsic astrocytic Ca2+ spikes of two hemispheres (Trial # = 32; rats, n = 6).
This simultaneous appearance of the intrinsic astrocytic Ca2+ spikes in both hemispheres is inconsistent with previously reported astrocytic Ca2+ waves of spreading depression with propagation speed at ∼20–40 µm/s (31, 32, 38), as well as the astrocytic Ca2+ wave propagation detected in the hippocampus (60 µm/s) (30). The spreading depression events elicited Ca2+ signals at a higher intensity of electrical stimulation. Despite the high Ca2+ signal detected in both neurons and astrocytes upon the occurrence of spreading depression in the stimulated hemisphere, no Ca2+ signal was detected in the other hemisphere (SI Appendix, Fig. S9), consistent with a previous study showing that spreading depression is confined to the ipsilateral hemisphere with focal ischemia (29). This shows that the intrinsic astrocytic Ca2+ signal that mediates global neurovascular coupling in the cortex is independent of spreading depolarization/depression induced by brain lesions.
Using fMRI to Identify the Subcortical Functional Nuclei Correlated with the Intrinsic Astrocytic Ca2+ Signal.
Subcortical nuclei that project throughout the entire neocortex could be responsible for the intrinsic astrocytic Ca2+-mediated neurovascular coupling events. Based on the on/off stimulation block design, the neurovascular coupling events were separated into two groups: those in which only the evoked astrocytic Ca2+ signal was present in the activated cortex, i.e., evoked-only events, and those with concurrent evoked and intrinsic astrocytic Ca2+ signals, i.e., concurrent (evoked + intrinsic) bilateral events (Fig. 5A). The amplitudes of the astrocytic Ca2+ spike of the concurrent events were significantly higher than those of the evoked-only events (Fig. 3C) and were therefore also used to specify the two categories (Fig. 5B). The event-related fMRI analysis showed focal FP-S1 activation for the evoked events, but for the concurrent events, the amplitude of the BOLD signal in the FP-S1 was significantly reduced and a prolonged negative BOLD signal was detected throughout the cortex as well as in the subcortical regions (Fig. 5C and Movie S1). Interestingly, a positive BOLD signal was also detected in the central and mediodorsal thalamic nuclei at an early phase of the stimulation for concurrent events (Fig. 5 B and C). In addition, the subcortical activity pattern extended beyond the thalamus; particularly, from the central lateral and mediodorsal lateral thalamus to the midbrain reticular formation (Fig. 5D, brain atlas overlapped; SI Appendix, Fig. S10, multislice functional map; Movie S2, 3D rendering video). Simultaneous recording of fMRI and Ca2+ signals can therefore be used to locate specific functional nuclei underlying unique neurovascular coupling on a whole-brain scale.
Fig. 5.
Subcortical fMRI activation patterns underlying the intrinsic astrocytic Ca2+ spikes. (A) The representative traces of the FP-S1 astrocytic Ca2+ signals in both hemispheres (Right FP-S1 activation, 12 epochs, 3 Hz, 4 s, 1.5 mA; Inset, fiber traces in the anatomical MRI image; concurrent events, epoch 3 and 10, red arrows). (B) The averaged astrocytic Ca2+ and simultaneous fMRI signal of the evoked-only and concurrent events. (C) The time-lapsed function maps at 1.5 s (solid green box) and 4.5 s (dashed green box) after onset of stimulation (Left: evoked-only FP-S1 activation; Right: concurrent events, thalamic activation at 1.5 s, followed by the negative fMRI signal in the cortex and ventricle areas at 4.5 s). (D) The concurrent event functional maps at 1.5 s were overlapped on three anatomic slices characterized from a 3D whole brain and the corresponding brain atlas (Right: the enlarged images with activity patterns on the FP-S1, central thalamus, and the midbrain reticular formation region). (E) The time course of the BOLD fMRI signal from FP-S1, central thalamic region, and the whole cortex except FP-S1 (trial # = 72; rats: n = 6, mean ± SEM).
To confirm the thalamic BOLD activation during concurrent events, the LFP in the central thalamic region was recorded simultaneously with the astrocytic Ca2+ recording in the cortex. The central lateral (CL) thalamic regions were specifically targeted based on the BOLD functional map with the concurrent events, showing bilateral activation pattern covering a region of the brain atlas 400–500 µm in extant (Fig. 5D). For precise localization, the position of the electrode tip for each experiment was imaged by MRI (SI Appendix, Fig. S11). Fig. 6A shows the power spectral density of the LFP signal recorded from both the central thalamic region and the FP-S1, as well as the astrocytic Ca2+ signal detected in the FP-S1 in response to electrical stimulation of the forepaw. Interestingly, an elevated spectral power level was detected in both the thalamus and cortex before the induction of the intrinsic astrocytic Ca2+ spike from the on/off stimulation trial, followed by reduced power levels. A similar phenomenon was shown by the two-channel bilateral Ca2+ recording setup (left FP-S1, neuronal Ca2+ vs. right FP-S1, astrocytic Ca2+). An increased EEG-like power level of neuronal Ca2+ transients was detected before the intrinsic astrocytic Ca2+ events upon electrical stimulation, followed by reduced power levels as well (SI Appendix, Fig. S12). These results indicate that the intrinsic astrocytic Ca2+ events may rely on the brain state fluctuation.
Fig. 6.
Simultaneous astrocytic Ca2+ recording and the LFP recordings in both central thalamus and FP-S1. (A) The representative trace of the FP-S1 astrocytic Ca2+ signal (Top Right), the LFP power spectrogram in FP-S1 (Middle Right) and the contralateral central thalamus (Bottom Right) with forepaw electrical stimulation (3 Hz, 4 s, 1.5 mA; Inset, 2D slices with electrode insertion in the central thalamic region). (B) Evoked-only and concurrent events were separated to show the averaged astrocytic Ca2+ signal and LFP power spectrogram (1-s sliding window with 0.1-s steps, 9 tapers). (C) The normalized mean spectral power of different EEG bands (evoked-only, red, trial # = 98; concurrent, blue, trial # = 57, rats, n = 4) at different phases of the stimulation on/off trials (before: −40 to −20 s; during: 0–1.5 s; and after: 60–80 s; FP-S1: theta, *P = 1.5e-05, #P = 0.012; alpha, *P = 1.3e-07, #P = 0.019; beta, *P = 1.6e-07; central thalamic region: theta, *P = 0.00018, #, P = 0.0025; alpha, *P = 3.3e-05, #, P = 0.0068; beta, *P = 0.00010, &P = 0.0022, #P = 0.0025; mean ± SEM; delta power was calculated from 4-s windows in 0.1-s steps).
The event-related analysis scheme used previously was applied to acquire the mean power spectral density of the LFP signal based on the evoked-only events and concurrent events of the simultaneously recorded astrocytic Ca2+ signal (Fig. 6 B and C). The power levels in different EEG frequency bands before and after the stimulation in the evoked-only events indicated no difference between recordings from the thalamus and cortex (Fig. 6 B and C). In contrast, for the concurrent events, a higher EEG power level occurred before the onset of stimulation and a short transient in the EEG power spectrum was detected at frequencies up to 40 Hz upon stimulation (Fig. 6B). Quantitative analysis of the different EEG frequency bands in both the thalamus and cortex showed no detectable difference between both events in the delta band (1–4 Hz). For concurrent events, both the theta (4–8 Hz) and alpha band (8–13 Hz) power levels were significantly higher before stimulation than in evoked-only events (Fig. 6C, nonoverlapping CIs; SI Appendix, Table S2). Similar patterns were also shown in the EEG-like power spectral analysis for the simultaneously acquired neuronal Ca2+ signal (SI Appendix, Fig. S12). Also noteworthy is that the beta band (13–30 Hz) power level increased upon stimulation and was significantly higher than during the evoked events, which could contribute to the positive BOLD signal detected only in the concurrent events (Fig. 6B). The increased power in the beta frequency band in the thalamus may be attributable to a desynchronized arousal fluctuation that may shift the cortex to more alert brain states and underlie the intrinsic astrocytic Ca2+-mediated neurovascular coupling events.
Discussion
Simultaneously recorded fMRI and GCaMP-mediated Ca2+ signals were used to characterize the cell-specific neurovascular coupling events underlying two types of fMRI signals in anesthetized rats. In addition to the conventionally evoked BOLD fMRI signal positively correlated to the neuronal and astrocytic Ca2+ signal, an intrinsic astrocytic Ca2+ spike was observed that showed unique spatiotemporal features in the cortex and was accompanied by reduced power levels in the EEG across a broad range of frequencies and a negative BOLD signal throughout the cortex. Periodically, the intrinsic and evoked astrocytic Ca2+ signals occurred concurrently with neurovascular coupling events at opposite signs, demonstrating that two distinct gliovascular effects on vessel constriction and dilation can occur in vivo under the same conditions. Furthermore, both fMRI brain mapping and LFP recording revealed that increased activity in the thalamic nuclei preceded the intrinsic astrocytic Ca2+ signal followed by the negative BOLD signal throughout the cortex. This suggests that the astrocyte-coupled bidirectional fMRI signal originates from thalamic regulation of brain state changes, which may be related to the anticorrelated thalamic and cortical fMRI signals observed during fluctuations in the state of arousal (15).
Previous studies of simultaneous fMRI and electrophysiology have demonstrated that the positive BOLD signal is correlated with increased neuronal activity, and that the negative BOLD signal from the surrounding cortical area is associated with decreased neuronal activity (2, 39). It has also been established that the BOLD fMRI signal is directly linked to the hemodynamic signal coupled to the blood flow changes mediated by vascular activity, but the detailed neurovascular coupling events contributing to the bidirectional fMRI signal changes remain unclear (1). Earlier studies have shown that the astrocytic Ca2+ signal plays a crucial role in controlling blood flow through gliovascular interaction (3, 4) and especially vasoconstriction and vasodilation under a variety of conditions (7, 8, 12, 40). Upon sensory stimulation, increased neuronal activity (both LFP and neuronal Ca2+ signals) is correlated with the evoked astrocytic Ca2+ in vivo signal as well as the BOLD fMRI signal, enabling the direct measurement of the fMRI signal with the underlying neuron–glia–vascular interaction (Fig. 1). Optical fiber was used to record the Ca2+ signal from astrocytes in deep cortical layers, in contrast to the previous in vivo optical measurement from superficial layers of the cortex (5, 10, 19, 41), enabling the detection of the Ca2+ signal from a population of astrocytes located in layer 5 of the cortex. The optical fiber integrated the Ca2+ signal from both astrocytic soma and processes directly coupled to pyramidal neurons located at layer 5, which could account for the 1- to 1.7-s latency of the evoked astrocytic Ca2+ signal, which is shorter than reported in previous studies (5, 10). Several other optical imaging studies have also reported the rapid onset of evoked Ca2+ signal in astrocytes in vivo (11, 35), as well as fast miniature Ca2+ transients in the hippocampal slice (42), indicating that the evoked astrocytic signal may be involved in neurovascular coupled vessel dilation underlying the positive BOLD fMRI signal. It is noteworthy that the activity-coupled vasodilation persists in mice with IP3-2 receptor knock-out (IP3R2−/−) to inhibit the astrocytic Ca2+ intracellular release, therefore indicating that there may be a parallel pathway to mediate the astrocytic Ca2+-independent vasodilation (5, 43). Interestingly, a recent study reported the astrocytic calcium fluctuation in IP3R2−/− mice upon startle responses (44). In contrast, the capillary dilation could be controlled by glial Ca2+ signal (43). In IP3R2−/− mice, the capillary dilation is abolished where the glial Ca2+ signal is reduced (6). This study suggests that the astrocytic Ca2+ signal may mediate the neurovascular coupling through capillary control. In addition, the causal relationship of the astrocytic Ca2+ signal with BOLD fMRI can be further specified in transgenic mice with inhibited astrocytic Ca2+ intracellular release and pericyte-deficient mice (5, 45).
Astrocytic Ca2+-mediated vessel constriction has been observed in vivo under spreading suppression (31). Similarly, the death of pericytes in the ischemic brain also causes capillary constriction in vivo (46). It remains to be determined whether the elevated astrocytic Ca2+ causes negative BOLD signal changes through vasoconstriction during normal functioning. Here, we showed that the intrinsic astrocytic Ca2+ spike was the crucial linkage of neurovascular coupling to mediate the negative BOLD signal in the whole cortex. The data show that the intrinsic astrocytic Ca2+ spike is accompanied by decreased high frequency (3–40 Hz) power of the spontaneous LFP signal with a slight lag, consistent with two-photon imaging studies in mice anesthetized with urethane (9). The absence of slow-frequency oscillations in the present study could be due to anesthetics and differences with α-chloralose (36, 47). Interestingly, in the hippocampus of mice anesthetized with urethane, astrocytic Ca2+ waves have been reported to correlate with the decreased power level in both infraslow and high-frequency ranges, as well as with reduced blood flow (30). Besides the spontaneous neuronal oscillation, the astrocytic Ca2+ transients have also been reported to be coupled with carbachol-induced oscillations in the hippocampal brain slices (34). Therefore, the intrinsic astrocytic Ca2+-coupled negative BOLD signal may represent one of the multiple ways that astrocytes affect neurovascular coupling and modulate brain states (7).
What is the relationship between spreading depression of the lesioned brain and the intrinsic astrocytic Ca2+ signal with decreased BOLD signal? Spreading depolarization/depression can be elicited by numerous noxious triggers, such as chemical treatment with potassium and glutamate, hypoxia, and focal ischemia, as well as in the healthy intact cortex (26, 27). The persistent depression of activity and reduction of blood flow usually follows spreading depolarization in the cortex, which is also accompanied with Ca2+ waves propagating through the local neuronal and astrocytic network from the triggered source (28, 48, 49). However, the novel intrinsic astrocytic Ca2+-mediated neurovascular coupling events showed spatiotemporal features distinct from spreading depression (SI Appendix, Fig. S8). First, intrinsic astrocytic Ca2+ signals can occur concurrently with the evoked astrocytic Ca2+ signal, mediating, respectively, both positive and negative BOLD fMRI signals in the brain. Although the elevated astrocytic Ca2+ leads to the secretion of different vasoactive agents, such as prostaglandin E2, epoxyeicosatrienoic acids, and 20-HETE, under different conditions (7, 8, 12, 37), the large-scale astrocytic Ca2+-mediated vasoconstriction that is usually detected in the pathological conditions of the cortex is not accompanied by simultaneous positive events in vivo (31). Second, there was no propagation delay of the intrinsic astrocytic Ca2+ spikes in the two hemispheres (Fig. 4), which is different from Ca2+ waves traveling through the astrocytic network from the source of spreading depression (31, 32). We presented evidence that the intrinsic astrocytic Ca2+ signals detected instantaneously throughout the cortex (Figs. 3 and 4) were initiated by subcortical projections activating the intrinsic astrocytic Ca2+-mediated neurovascular coupling events (50, 51).
The intrinsic astrocytic Ca2+ signal mediates the regulation of brain states through the reticular formation ascending pathway, which is known to regulate arousal (52). Although initiated in the anesthetized rat brain, the intrinsic astrocytic Ca2+-mediated neuronal activity changes may shed light on the thalamic regulation of the state switch leading to arousal. The simultaneously acquired astrocytic Ca2+ signal led to the identification of the activated thalamic and midbrain reticular formation regions in fMRI brain mapping based on the occurrence of the intrinsic astrocytic Ca2+ spikes (Figs. 5 and 6, SI Appendix, Fig. S10, and Movie S2). Two striking features were detected: First, positive BOLD signals were detected in the thalamic regions in contrast to negative BOLD signals detected throughout the entire cortex. These results are consistent with the fMRI activity pattern reported in a recent resting-state fMRI study, showing that increased fMRI signals in the thalamus and decreased fMRI signals in the whole cortex are correlated with state fluctuation associated with arousal at eye opening (15). Optogenetic activation of the central lateral thalamus at low frequency also initiates the negative BOLD signal across the whole cortex of the anesthetized rat brain (53). Second, the increased alpha power in both the thalamus and cortex was detected before the occurrence of the intrinsic astrocytic spike, which was then followed by decreased alpha power. This could provide insights into what cortical states predispose toward the induction of these Ca2+ spikes. Alpha power is also reduced following eye opening (54). The regulation of alpha-power oscillation-dependent brain states is less efficient when delivered upon the high alpha-power state (55, 56). It is plausible that the intrinsic astrocytic Ca2+ spikes may be involved in the spontaneous fluctuation of the alpha-band EEG activity to mediate the brain excitability (56).
Two issues remain to be solved to better understand the interaction of astrocytic calcium and BOLD fMRI signal during the brain state fluctuation. The first issue is the brain state dependency and potential anesthetic effects on the correlation of astrocytic calcium signal and BOLD fMRI signal. Besides α-chloralose, the negative BOLD signal coupled to the intrinsic astrocytic Ca2+ spikes was observed in rats anesthetized with urethane (SI Appendix, Fig. S13). This result demonstrates that the negative correlation is not just caused by the α-chloralose anesthetic effect. Interestingly, the negative BOLD correlation pattern varied across different animals, indicating a potential dependency on the anesthetic effect of urethane (SI Appendix, Fig. S14). In a preliminary experiment, the astrocytic Ca2+ signal was recorded simultaneously with LFP in free-moving rats, showing the intrinsic astrocytic Ca2+ spikes in coincidence with the EEG state changes during sleep cycles (SI Appendix, Fig. S15). This preliminary result further implicates the highly correlated intrinsic astrocytic Ca2+ signals with brain state changes, similar to what has been reported in the urethane-anesthetized rats (9).
The second issue is to clarify the causal relationship of the intrinsic astrocytic Ca2+ signal to the negative BOLD signal and their underlying molecular mechanism. In the present study, both intrinsic astrocytic Ca2+ signal and negative BOLD signal were dampened in rats anesthetized with medetomidine, an adrenergic alpha-2 receptor agonist, which can inhibit norepinephrine effect through negative feedback (SI Appendix, Fig. S16). It has been shown that the locomotion- or startle-induced astrocytic Ca2+ signal can be blocked by trazodone or prazosin, the inhibitor or antagonist of adrenergic receptors, and abolished by neurotoxin DSP4 with local norepinephrine depletion (57, 58). These results indicate that the noradrenergic system may mediate both intrinsic astrocytic Ca2+ signal and vasoconstriction, respectively or sequentially (59). It is also possible that the central thalamic nuclei and the midbrain reticular formation are directly mediated by the norepinephrine projections or are coactivated to elicit the intrinsic astrocytic Ca2+ signal and the negative BOLD signal in the whole cortical area (60). Optogenetic activation of the locus coeruleus or central thalamic nulcei offers further prospects for studying the causal relationship and the direct norepinephrine-driven mechanism. The simultaneous astrocytic calcium recording with fMRI could be combined with optogenetic activation to specify the molecular and circuit mechanism underlying astrocyte-mediated gliovascular interaction and correlated brain state changes.
Materials and Methods
Detailed methods are provided in SI Appendix, SI Materials and Methods. The study was performed in accordance with the German Animal Welfare Act (TierSchG) and Animal Welfare Laboratory Animal Ordinance (TierSchVersV). This is in full compliance with the guidelines of the EU Directive on the protection of animals used for scientific purposes (2010/63/EU). The study was reviewed by the ethics commission (§15 TierSchG) and approved by the state authority (Regierungspräsidium, Tuebingen, Baden-Württemberg, Germany). A total of 88 Sprague–Dawley rats were used in this study.
Viral vectors were directly ordered and packaged from the University of Pennsylvania Vector Core: AAV5.Syn.GCaMP6f.WPRE.SV40 and AAV5.GfaABC1D.cyto-GCaMP6f.SV40 (Addgene52925; GfaABC1D.cyto promoter for cytoplasmic expression of GCaMP6f; ref. 61).
Similar procedures of fMRI animal preparation have been previously described (62). The two-channel optical setup for fiber-optic Ca2+ recordings has been developed base on a previous report (Fig. 1C) (19). The astrocytic calcium signal-dependent event-related fMRI analysis was applied to specify the subcortical brain activity patterns from the whole brain 3D-EPI fMRI images.
A paired Student’s t test and one-way ANOVA were performed for statistical analysis. The data with error bars are mean ± SEM in each statistic graph. In addition, the mean and 95% CIs for the distribution of LFP power spectra density, estimated onset times, amplitude of the astrocytic Ca2+, and BOLD signal were calculated using the bootstrap procedure (63). The 95% CIs are equivalent to hypothesis testing with a significance level of 0.05. Both confidence intervals with (nonoverlapping CIs) and P values for all statistical analysis were listed in SI Appendix, Table S2. Sample size was not previously estimated for the animal experiments, and no blinding and randomization design was needed in this work.
Supplementary Material
Acknowledgments
We thank Dr. R. Pohmann, Dr. H. Merkle, Dr. K. Buckenmaier, Dr. Y. Jiang, Ms. P. Pais, and Ms. X. Chen for technical support; Ms. H. Schulz and S. Fischer for animal maintenance support; the Analysis of Functional NeuroImages (AFNI) team for the software support; and Dr. B. S. Khakh, the Genetically-Encoded Neuronal Indicator and Effector (GENIE) Program, and the Janelia Farm Research Campus for providing the astrocyte-specific promoter and GCaMP6 plasmids. This research was supported by German Research Foundation (DFG) SPP-1655, internal funding from Max Planck Society (to X.Y.), and HHMI support (to T.J.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711692115/-/DCSupplemental.
References
- 1.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 2.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 3.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Nizar K, et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biesecker KR, et al. Glial cell calcium signaling mediates capillary regulation of blood flow in the retina. J Neurosci. 2016;36:9435–9445. doi: 10.1523/JNEUROSCI.1782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 9.Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci USA. 2016;113:E2675–E2684. doi: 10.1073/pnas.1520759113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 11.Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA. 2013;110:E4678–E4687. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 13.Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang C, et al. Tracking brain arousal fluctuations with fMRI. Proc Natl Acad Sci USA. 2016;113:4518–4523. doi: 10.1073/pnas.1520613113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logothetis NK, et al. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 17.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 18.Akerboom J, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz K, et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods. 2012;9:597–602. doi: 10.1038/nmeth.2013. [DOI] [PubMed] [Google Scholar]
- 20.Chen TW, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–990. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- 22.Lütcke H, et al. Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice. Front Neural Circuits. 2010;4:9. doi: 10.3389/fncir.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanganahalli BG, et al. Comparison of glomerular activity patterns by fMRI and wide-field calcium imaging: Implications for principles underlying odor mapping. Neuroimage. 2016;126:208–218. doi: 10.1016/j.neuroimage.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adelsberger H, Zainos A, Alvarez M, Romo R, Konnerth A. Local domains of motor cortical activity revealed by fiber-optic calcium recordings in behaving nonhuman primates. Proc Natl Acad Sci USA. 2014;111:463–468. doi: 10.1073/pnas.1321612111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CK, et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods. 2016;13:325–328. doi: 10.1038/nmeth.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 27.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev. 2015;95:953–993. doi: 10.1152/physrev.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nedergaard M, Cooper AJL, Goldman SA. Gap junctions are required for the propagation of spreading depression. J Neurobiol. 1995;28:433–444. doi: 10.1002/neu.480280404. [DOI] [PubMed] [Google Scholar]
- 29.Nedergaard M, Hansen AJ. Characterization of cortical depolarizations evoked in focal cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:568–574. doi: 10.1038/jcbfm.1993.74. [DOI] [PubMed] [Google Scholar]
- 30.Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci. 2007;27:4036–4044. doi: 10.1523/JNEUROSCI.0721-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci. 2003;23:9888–9896. doi: 10.1523/JNEUROSCI.23-30-09888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci USA. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HS, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci USA. 2014;111:E3343–E3352. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winters WD, Spooner CE. A neurophysiological comparison of alpha-chloralose with gamma-hydroxybutyrate in cats. Electroencephalogr Clin Neurophysiol. 1966;20:83–90. doi: 10.1016/0013-4694(66)90144-1. [DOI] [PubMed] [Google Scholar]
- 37.Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GCR, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 40.Devor A, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan R, et al. New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in Vivo. Neuron. 2016;92:1181–1195. doi: 10.1016/j.neuron.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Castro MA, et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 43.Mishra A, et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016;19:1619–1627. doi: 10.1038/nn.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan R, et al. Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat Neurosci. 2015;18:708–717. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 46.Hall CN, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 49.Parpura V, et al. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 50.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 51.Cauli B, et al. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edlow BL, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71:531–546. doi: 10.1097/NEN.0b013e3182588293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, et al. Frequency-selective control of cortical and subcortical networks by central thalamus. eLife. 2015;4:e09215. doi: 10.7554/eLife.09215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- 55.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Romei V, et al. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding F, et al. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–394. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paukert M, et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron. 2014;82:1263–1270. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Donnell J, Ding F, Nedergaard M. Distinct functional states of astrocytes during sleep and wakefulness: Is norepinephrine the master regulator? Curr Sleep Med Rep. 2015;1:1–8. doi: 10.1007/s40675-014-0004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shigetomi E, et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu X, et al. Sensory and optogenetically driven single-vessel fMRI. Nat Methods. 2016;13:337–340. doi: 10.1038/nmeth.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Dort CJ, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci USA. 2015;112:584–589. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.