Significance
As obligate parasites, viruses have evolved strategies to hijack cellular pathways and persist in their host, sometimes without causing overt diseases. As such, they represent unique tools to decipher cellular functions and their consequences on host physiology. Here, we exploited the natural property of a virus-encoded protein known to act as a decoy substrate for protein kinase C (PKC), a pathway thought to play key roles in learning and memory processes. When selectively expressed in the hippocampal dentate gyrus of mice, this protein caused behavioral abnormalities, notably increased anxiety and impaired memory, mostly by interfering with PKC-dependent phosphorylation. Our findings provide further insight into the role of the PKC pathway in controlling cognitive functions.
Keywords: dentate gyrus, hippocampus, virus, memory, protein kinase C
Abstract
The analysis of the biology of neurotropic viruses, notably of their interference with cellular signaling, provides a useful tool to get further insight into the role of specific pathways in the control of behavioral functions. Here, we exploited the natural property of a viral protein identified as a major effector of behavioral disorders during infection. We used the phosphoprotein (P) of Borna disease virus, which acts as a decoy substrate for protein kinase C (PKC) when expressed in neurons and disrupts synaptic plasticity. By a lentiviral-based strategy, we directed the singled-out expression of P in the dentate gyrus of the hippocampus and we examined its impact on mouse behavior. Mice expressing the P protein displayed increased anxiety and impaired long-term memory in contextual and spatial memory tasks. Interestingly, these effects were dependent on P protein phosphorylation by PKC, as expression of a mutant form of P devoid of its PKC phosphorylation sites had no effect on these behaviors. We also revealed features of behavioral impairment induced by P protein expression but that were independent of its phosphorylation by PKC. Altogether, our findings provide insight into the behavioral correlates of viral infection, as well as into the impact of virus-mediated alterations of the PKC pathway on behavioral functions.
The increasing prevalence of mental and behavioral disorders urges the identification and implementation of new therapeutic strategies (1). Unfortunately, effective treatments for these diseases rely on drugs generated decades ago and the development of new medications has not yielded significant improvement (2). Recently, efforts have switched from the short-term improvement of preexisting medications to the design of new approaches focusing on neural circuitry dysfunctions (3). Interestingly, a better knowledge of the biology of viruses can provide new understandings of brain dysfunction. Indeed, as obligate parasites, viruses have evolved highly specific means to hijack cellular pathways to optimize their replication and survival in their host. Hence, studies of viral interference with cell functions have yielded many discoveries on the cell transcription machinery (4), the IFN response (5), the role of mTOR pathways during tumorigenesis (6), or more recently, mitochondrial-driven neuroprotection (7, 8). Thus, away from modeling the core aspects of a given mental illness, the study of neurotropic viruses, whose persistence leads to neurological symptoms, could reveal important insights into neural circuits and their alterations during neuropsychiatric disorders.
In that regard, the noncytolytic and neurotropic Borna disease virus (BDV) is an ideally suited experimental model. Indeed, BDV hijacks the neuronal molecular machinery for its replication and lifelong persistence in the brain, without causing cellular death. In addition, BDV displays a preferential tropism for the neurons of the limbic system and infects a remarkably wide range of warm-blooded animals, from mammalian to avian species (9, 10). Clinical manifestations after both natural and experimental infections are highly heterogeneous but remarkably, they are always accompanied by behavioral alterations (11). In rodents, behavioral features of BDV disease include symptoms such as hyperactivity, movement and posture disorders, stereotypic or perseverative behaviors, chronic emotional abnormalities, abnormal social interactions, and impaired cognitive functions (11).
Among the six proteins encoded by BDV, the viral phosphoprotein (P) represents a major candidate to explain the occurrence of behavioral disorders during infection (12). Besides acting as a cofactor for the viral polymerase, this multifunctional 24-kDa protein interacts with numerous cellular pathways (12–14). In particular, P is preferentially phosphorylated at serine residues 26 and 28 by protein kinase C (PKC) and, to a lesser extent, at serine residues 70 and 86 by casein kinase II (CKII) (15). Our previous work has established that P selectively interferes with PKC-dependent phosphorylation in neurons. By acting as a decoy substrate for neuronal PKC, P diverts part of the enzyme activity toward phosphorylation of its own S26/S28 residues. Consequently, neuronal infection with a virus bearing wild-type P decreases the phosphorylation levels of major PKC neuronal substrates, such as SNAP25 or MARCKS, and selectively impairs neuronal activity and plasticity. In contrast, neurons infected with a virus bearing a P protein mutated in its PKC phosphorylation site exhibit a normal pattern of PKC-dependent phosphorylation, with restored neuronal plasticity (12, 16).
Interestingly, PKC-dependent phosphorylation regulates numerous key brain functions such as neuronal excitability, neurotransmitter release, ion channel activity, and synaptic plasticity (17–19). Therefore, it is likely that any impairment of PKC-dependent phosphorylation may lead to learning and memory disabilities that are observed in many neurobehavioral diseases (20, 21). In addition, PKC plays a major role in neuronal communication and interacts with transduction pathways of a wide variety of neurotransmitters and growth factors implicated in the pathogenesis of mental disorders (22). Indeed, dysregulations of PKC signaling have been associated with neuropsychiatric (schizophrenia) (23) or mood diseases (bipolar disorders, depression) (20, 24), as well as with autism spectrum disorders (25). To date, however, the study of PKC physiological functions supported by the hippocampus has mostly been based on the use of pharmacological tools, such as PKC inhibitors or activators that may lack selectivity (22, 23).
Here, we sought to use the P protein, expressed out of the viral context, to examine its impact on behavior and memory, as well as to gain further insight into the consequences of impaired PKC-dependent phosphorylation on behavior. To discriminate between effects of this protein that would be dependent or not on its phosphorylation by PKC, we expressed either wild-type or mutated P through lentiviral administration in the hippocampus of wild-type adult mice and studied the effects using a battery of behavioral and memory tasks.
Results
Isolated Expression of the P Protein Interferes with PKC-Dependent Phosphorylation in Hippocampal Neurons.
Our previous work using rat neurons demonstrated that BDV interferes with PKC phosphorylation of synaptic proteins, with detrimental effects on synaptic plasticity. Importantly, normal PKC-dependent phosphorylation was restored upon infection with a recombinant BDV bearing a mutated P protein (PAASS), in which the two serine residues in position 26 and 28 of BDV P had been replaced by alanine (A) residues, thereby abrogating its PKC phosphorylation sites (Fig. 1A), whereas the two alanine residues that are phosphorylated by casein kinase II (CKII) were spared (12, 16). To study the impact of the singled-out expression of BDV P on mouse behavior, we constructed lentiviral vectors expressing wild-type P (PWT), PAASS, or GFP as a control (Fig. 1A). We observed that these vectors allowed expression of the P protein at levels comparable to what can be observed upon infection (Fig. S1). Before performing in vivo experiments, we confirmed that interference with PKC-dependent phosphorylation due to expression of P was indeed observed in cultured mouse neurons. To this aim, we used primary cultures of hippocampal neurons prepared from C57BL/6J mice. Nine days after lentiviral transduction with vectors expressing GFP (as a control), PWT, or PAASS, we directly stimulated neuronal PKC using phorbol 12-myristate 13-acetate (PMA) and analyzed phosphorylation levels of the synaptosomal-associated protein of 25 kDa (SNAP25, on Ser187), a major PKC neuronal substrate. Consistent with our previous reports (12, 16), Western blot analysis confirmed that phosphorylated SNAP25 (pSNAP25) levels were decreased upon PKC stimulation in hippocampal neurons transduced with PWT (Fig. 1B). In contrast, levels of pSNAP25 upon PKC stimulation were similar between neurons transduced with PAASS and those transduced with a GFP-expressing vector (or nontransduced neurons), showing that PAASS does not interfere with PKC-dependent phosphorylation. Thus, we confirmed that the PWT protein interferes with PKC-dependent phosphorylation in cultured neurons.
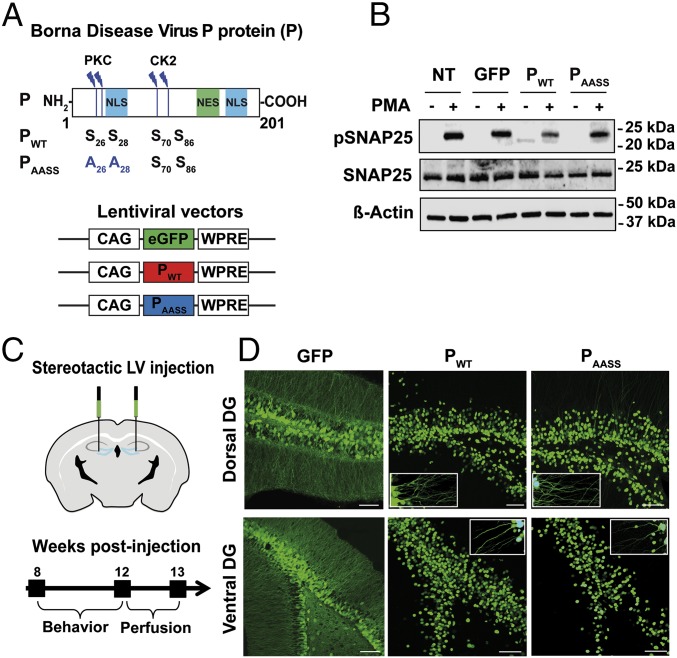
Fig. 1.
Lentiviral expression of the P protein interferes with PKC-dependent phosphorylation in mouse hippocampal neurons. (A) Schematic representation of the P protein, displaying the protein kinase C (PKC) and casein kinase II (CK2) phosphorylation sites. NES, nuclear export sequence; NLS, nuclear localization sequence. Map of the lentiviral vectors (LVs) expressing GFP, wild-type P (PWT), or mutant P (PAASS). Also shown are: positions of the cytomegalovirus enhancer/chicken β-actin (CAG) promoter and woodchuck hepatitis virus posttranscriptional regulatory element (WPRE). (B) Western blot analysis of phospho-SNAP25 levels upon PKC stimulation. Protein extracts were prepared from neurons transduced with LVs expressing GFP (as a control), PWT, or PAASS, after stimulation or not with PMA. Nontransduced (NT) neurons were also processed in parallel. Levels for β-actin and total SNAP25 were used to normalize phosphorylation levels. Data shown are those of a representative experiment out of four that gave similar results. (C) Stereotaxic procedure for in vivo LV delivery and experimental timeline. (D) Expression of GFP, PWT, or PAASS in the DG of mice, 4 mo after surgery. Representative pictures of brain sections expressing GFP, PWT, or PAASS in the dorsal and ventral DG. (Scale bar, 100 μm.) Insets show enlarged view to visualize P expression in the dendrites.
Lentiviral-Mediated Expression of PWT and PAASS in the Hippocampus Is Efficient and Stable.
To study the impact of P expression on mouse cognition, lentiviral vectors expressing GFP, PWT, or PAASS were bilaterally injected into the hippocampal dentate gyrus (DG), the gateway to hippocampal circuits (26) (Fig. 1C). Correct targeting of the DG and long-term expression of GFP, PWT, or PAASS were assessed histologically after completion of behavioral testing, i.e., 12 wk after lentiviral injection (Fig. 1D). Lentiviral delivery into two sites (dorsal and ventral) of each DG allowed robust neuronal expression of the different proteins (Fig. 1D). Positive signals were observed throughout the rostrocaudal axis of the DG and covered a length of ∼2,000 µm. PWT and PAASS expression was particularly strong in the nuclei, as expected for this protein with two nuclear localization sequences (Fig. 1A and Fig. S2) (27). Lentiviral transduction also enabled a strong expression of PWT and PAASS in the cytoplasm, both in the cell bodies and dendrites (Fig. 1D, Insets). Proteins were also detected by Western blot analysis on dissected hippocampi (Fig. S3).
To gain insight into the efficiency of viral transduction before behavioral experiments, we stereologically estimated the percentage of cells expressing the two forms of the P protein in the DG (Fig. S4). Transduction rates were similar between PWT and PAASS, with an average rate of 31.6 ± 0.45% cells expressing PWT and 32.4 ± 1.1% cells expressing PAASS. Analysis of the colocalization with the neuronal marker NeuN showed that the vast majority of transduced cells (>90%) were neurons (Fig. S5), the rest being composed of astrocytes (positive for the marker GFAP, Fig. S6). Importantly, expression of the transgenes did not elicit any marked activation of astrocytes (Fig. S7). Likewise, it did not trigger infiltration of T cells or microgliosis (Fig. S8). Thus, our lentiviral vectors-based strategy enabled a stable and long-lasting expression of the P protein, with a similar pattern for its wild-type and mutated variants, without inducing any overt intraparenchymal host responses.
Long-Term Expression of the P Protein in the Hippocampus Increases Anxiety-Related Traits.
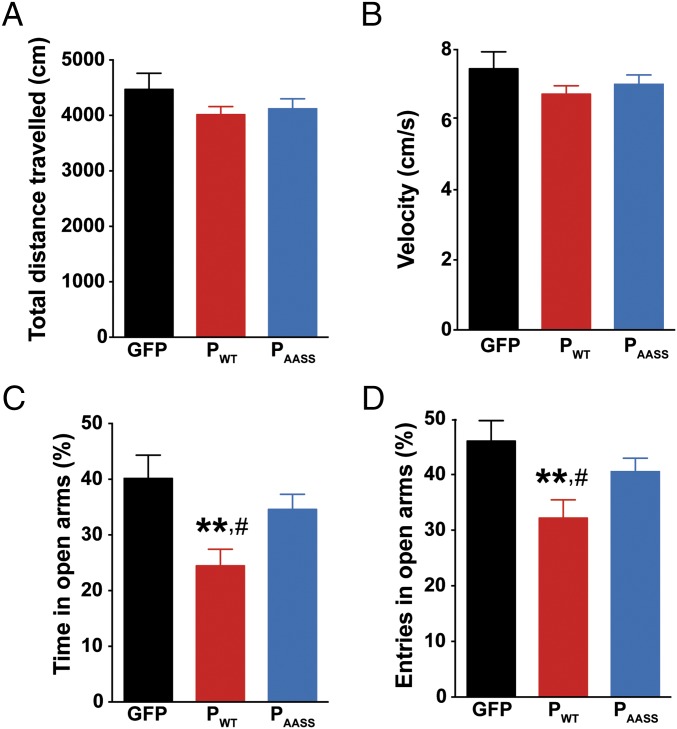
We first investigated whether hippocampal expression of P could affect mouse activity and exploratory behavior. Two months after lentiviral administration, cohorts of animals expressing GFP, PWT, or PAASS in the DG were analyzed for spontaneous locomotor activity in the open field. Mice from all groups traveled the same distance (Fig. 2A), with a similar velocity (Fig. 2B), with no difference in the number of rearings on the walls and in the center of the arena. Thus, hippocampal expression of the P protein had no impact on mouse locomotor activity or exploratory behavior. Next, another cohort of animals was screened for anxiety-related behavior in the elevated plus maze (Fig. 2 C and D). Analysis of the time spent in the open arms revealed a significant group effect. Strikingly, mice expressing PWT spent less time in the open arms compared with the other groups (Fig. 2C). Similarly, the relative number of entries in the open arms was also significantly decreased for animals expressing PWT (Fig. 2D). Altogether, these data suggest that hippocampal expression of PWT, the PKC-phosphorylatable form of the protein, favors anxiety-related traits in mice.
Fig. 2.
Impact of hippocampal P expression on locomotor activity and basal anxiety. (A) Distance traveled and (B) mean velocity during exploration in the open field. (C) Analysis of anxiety-like behavior in the elevated plus maze. PWT decreased the percent of time spent in the open arms (one-way ANOVA, P < 0.01). (D) PWT also decreased the number of visits in the open arms of the elevated plus maze (one-way ANOVA, P < 0.05). Data are expressed as means ± SEM (GFP n = 8; PWT n = 11; PAASS n = 10). **P < 0.01, #P < 0.05 by post hoc Fisher’s least significant difference test for, respectively, PWT vs. GFP and PWT vs. PAASS.
Hippocampal Expression of the P Protein Impairs Long-Term Contextual Fear Memory.
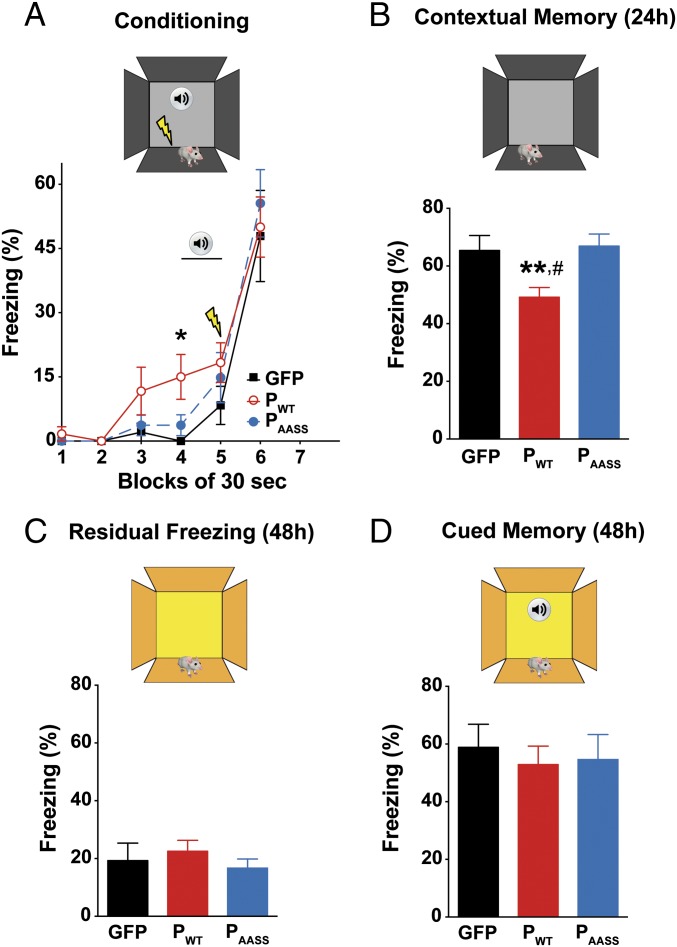
We next assessed the effects of PWT and PAASS expression in the DG on learning and memory, using hippocampal-dependent tasks. First, mice were subjected to contextual followed by cued fear conditioning. We assessed a possible effect of P on fear expression and learning performances by monitoring freezing behavior during the conditioning session (Fig. 3A). Consistent with increased anxiety-related traits observed in the elevated plus maze (Fig. 2), expression of PWT led to a significant increase in baseline freezing measured at tone delivery (Fig. 3A). As expected, all groups displayed a similar increase in their freezing response after delivery of the electric shock. However, when contextual fear memory was evaluated 24 h later (Fig. 3B), PWT mice displayed significantly reduced freezing levels compared with other groups, reflecting impaired acquisition and/or consolidation of contextual fear memory. Two days (48 h) after conditioning, cued memory was assessed in a new context. Residual freezing to the new context before tone emission was similar across groups (Fig. 3C). Moreover, all groups of mice displayed the same freezing response to the tone (Fig. 3D). Overall, these results demonstrate that hippocampal expression of PWT has a specific impact on contextual memory, while sparing amygdala-driven association between the tone and the shock. Our findings also reveal that the P protein needs to be phosphorylated by PKC to exert its effect on contextual memory.
Fig. 3.
Effects of P expression on contextual memory. (A) Fear expression in GFP, PWT, and PAASS mice during conditioning. Repeated ANOVA ran on 30-s blocks during the whole session (P < 0.05), and independent one-way ANOVA ran on block 4 (*P < 0.05). All groups displayed a similar increase in their freezing response after delivery of the electric shock (P > 0.05, independent one-way ANOVA ran on block 6). The lightning bolt icon indicates the time of shock delivery; the speaker indicates the tone delivery block. (B) Contextual memory assessed 24 h after conditioning and expressed as normalized data (SI Materials and Methods), showing the selective impairment of contextual memory due to PWT expression in the DG. One-way ANOVA, P < 0.01; #P < 0.05 for PWT vs. GFP and **P < 0.01 for PWT vs. PAASS by post hoc Fisher’s least significant difference test. (C) Analysis of residual freezing to the modified context before tone emission, 48 h after training. (D) Cued memory, assessed in a modified context, 48 h after training. Data are expressed as means ± SEM (GFP n = 8; PWT n = 10; PAASS n = 9).
Hippocampal Expression of the P Protein Impairs Long-Term Spatial Memory.
Given the critical involvement of the dentate gyrus in spatial learning and memory processes (28), we next evaluated the effects of P expression in the object location task and the Morris water maze (MWM), two tests that assess spatial memory.
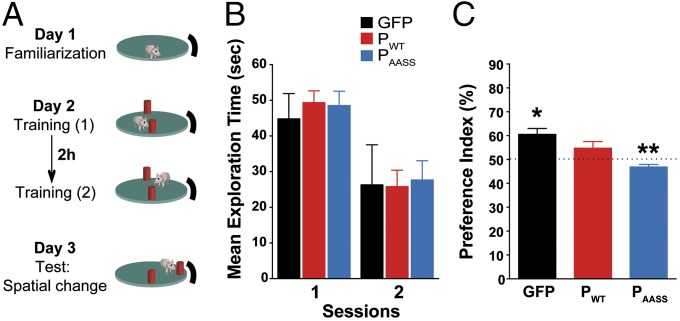
The object location task addresses the ability of rodents to evaluate spatial relations between objects, a cognitive operation that relies on the hippocampus (Fig. 4A) (29). After familiarization to the setup during which all groups performed similarly (Fig. S9), mice were allowed to explore two identical objects. Importantly, both objects were similarly explored and elicited a similar interest from all groups of animals, as indicated by the same cumulated time of exploration between objects and at each session (Fig. 4B). All groups also displayed an equivalent decrease in time spent exploring the objects during the second training session, indicating habituation to the presence and position of the objects in the arena (Fig. 4B). The next day, GFP-expressing controls preferentially explored the object that had been moved to a new location (Fig. 4C). Likewise, mice expressing PAASS also detected the new spatial configuration of the objects, while showing a significant preference for the nondisplaced object. In contrast, PWT expressing mice showed no exploratory preference for the displaced object compared with chance level (50%) (Fig. 4C), indicating that they did not detect the spatial change.
Fig. 4.
Effects of P expression on long-term spatial memory in the object location task. (A) Schematic representation of the object location task and experimental timeline. (B) Cumulated time spent exploring the objects during training sessions (seconds). (C) Analysis of the preference index (as detailed in SI Materials and Methods). The horizontal dotted line represents equal exploration of both objects (50%). Comparison with 50%: *P < 0.05, **P < 0.01 for index vs. chance level, Wilcoxon signed-rank test. Data are expressed as means ± SEM (GFP n = 8; PWT n = 11; PAASS n = 10).
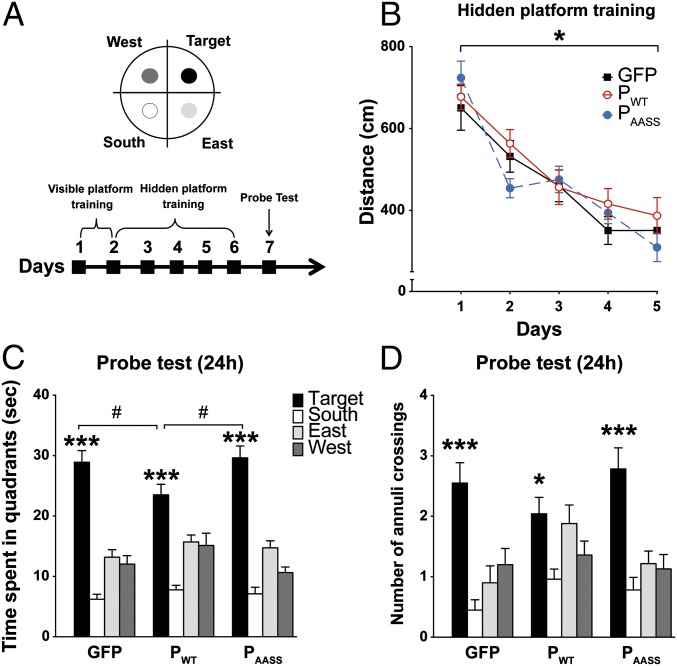
In the MWM, mice have to locate a hidden platform using distal visual cues (30) (Fig. 5A). First, we found that P expression did not influence performance during spatial training (Fig. 5B). Indeed, a repeated ANOVA revealed no significant group effect but a session effect with no time × group interaction. Thus, all groups learned to locate the hidden platform across the 5 d of training and performed equally well at the end of training. All three groups also showed similar swim speed and thigmotactism (Fig. S10). We then assessed long-term spatial memory in a probe test conducted 24 h after the last training session (Fig. 5 A and C). All groups spent significantly more time in the target quadrant where the platform was located during training than in the three other quadrants (Fig. 5C), indicating that mice remembered its original location. However, animals expressing PWT spent significantly less time in the target quadrant compared with mice from the other groups. To evaluate the precision of spatial memory, we measured the number of annuli crossings in the four quadrants during the probe test for each group of mice (Fig. 5D). Intragroup analyses revealed that mice expressing GFP or PAASS crossed significantly more the target annulus than the three other annuli (Fig. 5D). In contrast, PWT-expressing animals showed a less precise search strategy and crossed equally the target and its adjacent annuli (east and west). Hence, while mice expressing PAASS or GFP remembered precisely where the platform was located during training, mice expressing PWT displayed a less accurate spatial search, indicative of impaired long-term spatial memory. Altogether, our results demonstrate significant spatial memory impairment due to hippocampal expression of PWT and confirm that the deleterious effects of the P protein on long-term memory in the MWM depend on its phosphorylation by PKC.
Fig. 5.
Effects of P expression on long-term spatial memory in the Morris water maze. (A) Schematic representation of the setup and experimental timeline. (B) Mean distance traveled to find the hidden platform during training (one-way ANOVA with repeated measures *P < 0.001). (C and D) Effects of P expression on spatial memory during the 24 h posttraining probe test. (C) Time (seconds) spent in each quadrant of the pool, showing that all mice spent more time in the target quadrant compared with the three others (***P < 0.001 by one-way ANOVA intragroup analyses). Comparison of time spent in the target quadrant between PWT mice and GFP and PAASS animals. One-way ANOVA, #P < 0.05. (D) Spatial search precision: analysis of the number of annuli crossings between groups of mice. *P < 0.05 for PWT and ***P < 0.001 for GFP and PAASS, by one-way ANOVA intragroup analyses. (P > 0.65 and P > 0.07 for, respectively, target vs. east and target vs. west, by post hoc Fisher’s least significant difference test). Data are expressed as means ± SEM (GFP n = 20; PWT n = 25; PAASS n = 23).
Discussion
The goal of our study was to provide further insight into the mechanisms whereby a viral protein may lead to behavioral disorders in mammals, as well as to unravel the role of PKC-dependent phosphorylation in cognitive functions. We singled out the P protein, clearly established by our team as a selective blocker of neuronal plasticity (12, 16, 31) and expressed this protein in a restricted brain region, out of the viral context. We focused on the effects of P in the dentate gyrus, the gateway to hippocampal circuitry, to address its behavioral and cognitive impacts. Importantly, using both PWT and its mutant counterpart PAASS allowed us to discriminate between effects of the P protein that would be dependent or independent of its phosphorylation by PKC (12). Our results clearly demonstrate that a single viral protein is able to induce a wide range of behavioral abnormalities, mostly resulting from its ability to interfere with PKC-dependent phosphorylation in the CNS, herewith confirming the fascinating features of the interplay between Borna disease virus and the brain (32).
We first observed that PWT expression triggered increased basal anxiety in the elevated plus maze. This anxiogenic effect was also observed in the fear conditioning test. Animals expressing PWT exhibited increased fear reaction to the context before any shock delivery, in an environment that should have been perceived as nonharmful (33). As a part of the limbic circuit, the hippocampus is a major actor in the control of mood and anxiety (34) and plays a central role in the pathophysiology of anxiety disorders (35). Furthermore, DG granule cells contribute to learning and/or anxiety processes, according to their position along the dorsoventral axis of the hippocampus (35). Interestingly, our stereological counting revealed widespread transduction of the DG, including in the ventral region where granule cells have been shown to play a suppressive action on innate anxiety, while sparing exploratory behaviors (28, 35). We could thus hypothesize that the anxiogenic effects due to the P protein may result from impaired activity of granule cells. Our results are also in agreement with studies demonstrating anxiogenic effects of PKC ablation in various transgenic mouse models (36, 37), as well as of targeted injections of PKC inhibitors in the hippocampus (38, 39).
Consistent with the major role played by the hippocampus in learning and memory processes, expression of the P protein led to a clear PKC-dependent impairment of two types of episodic-like memory, i.e., long-term contextual fear memory and spatial memory in the water maze and object location tasks. These effects are consistent with the deleterious effects on mouse learning and memory that result from PKC inhibition or genetic ablation (40–43), and the converse cognition-enhancing effects of PKC activation or overexpression (44).
Surprisingly, although PAASS mice detected the spatial change in the object location task, they displayed a preference for the nondisplaced object, which may reflect the expression of neophobia. This is indicative of additional effects of P on memory, which appear to be distinct from its PKC decoy activity and are revealed when the PKC phosphorylated site is mutated, thereby sparing the capacity of mice to detect the spatial change. In addition to PKC pathways, the multifunctional P has been reported to interact with the Traf family member-associated NF-κB–binding kinase 1 (TBK-1), the gamma-aminobutyric acid receptor-associated protein (GABARAP), and the neurite outgrowth factor amphoterin/HMGB-1 (12, 45). For instance, it has been reported that P protein modifies the epigenetic environment of the chromatin through its interaction with HMGB1 (46, 47). Thus, expression of P may have induced memory impairments both through its phosphorylation by PKC, including modifications of histone acetylation (48), and by other mechanisms such as effects on chromatin structure dynamics. In addition, the PAASS mutant still retains sites for phosphorylation by CKII (S70/86) and TBK1 (S8/11) (15, 49) that may contribute to impaired neuronal activity. Notably, CKII activity is particularly elevated in the cortex and hippocampus and has been involved in learning and memory processes (48, 50). Altogether, our data confirm the central role of P as a key player for behavioral alterations, including anxiety-related traits and spatial memory defects through its interference with PKC. They also unveil a previously uncharacterized PKC-independent effect of P.
Infection with BDV preferentially targets CNS regions where activity of the epsilon isoform of PKC (PKCε) is high (51). Indeed, P was originally thought of as a selective substrate for this isoform (15). However, our subsequent work on neuronal cultures indicated a broader activity on PKC-dependent phosphorylation (12). However, we cannot exclude that P could have differential effects on various neuronal PKC isoforms, such as nuclear-translocated PKCs. As a matter of fact, we recently demonstrated that the P protein induces a specific and PKC-dependent set of epigenetic dysregulations, including impaired acetylation on selected lysines of several core histones (52).
In most behavioral studies consecutive to CNS infections, the idiosyncratic effects of the virus are often blurred by coinciding immune reactions or developmental damage. In any event, our results fit well with the reported increased anxiety in neonatally BDV-infected rats (53). They also match with increased freezing responses to novel environments, as well as with spatial learning and memory deficits observed in adult infected rats (53–58). In addition, spatial memory deficits were also found in a line of transgenic mice expressing P in astrocytes, which displayed a defined set of molecular dysregulations (59, 60). In our case, we cannot formally exclude the possibility that our results may also be due, at least in part, to the expression of P in other neurons than the DG present in the vicinity of the injection site (e.g., interneurons) or even in glial cells. We, however, think this is unlikely, considering the precise targeting of the lentiviral vectors and the minority of glial cells that were found to express P (Figs. S5 and S6).
The idea that a parasite or pathogen could modify the behavior or cognitive performances of its host is attractive (61). In particular, some parasites like the protozoan Toxoplasma gondii are even thought to facilitate their own transmission through the modification of mouse behavioral responses to their predators (62). In the case of neurotropic viruses, including BDV, studies of peculiar pathogens that have evolved to preserve the neuronal network of their host may thus reveal surprising insights into the neurobiology of rodent behavior.
Materials and Methods
The materials and methods used are detailed at length in SI Materials and Methods. Construction and production of lentiviral vectors, stimulation and Western blot analysis of mouse primary hippocampal cultures, surgery and infusion of lentiviral vectors, histology and immunohistochemistry, behavioral characterization (elevated plus maze, open field, contextual fear conditioning, object location, and Morris water maze), data analysis, and statistics are described therein. Experiments on mice were performed in accordance with the European Union (86/609/EEC) and the French Committee of Ethics (87/848) policies. Our protocol received approval from the local ministry-approved committee on ethics in animal experimentation (Ethics Committee of the US 006 / CREFRE) (permit no. 04-U1043-DG-06).
Supplementary Material
Acknowledgments
We thank M. Takahashi and S. Yamamori (Kitasato University) for their generous gift of pSNAP25 antibody; M. Belloy and N. Blanchard (Centre de Physiopathologie de Toulouse-Purpan) for providing brains from T. gondii-infected mice; H. Halley and S. Pech for their technical support with animals at the ABC facility from ANEXPLO; K. Richetin for his contribution to the design of some figures; and R. Liblau, A. Saoudi, and L. Verret for their critical reading and insightful comments on our manuscript. This work was supported by grants from the Agence Nationale de la Recherche (ANR-10-BLANC-1322), INSERM, CNRS, and University Paul Sabatier. We also acknowledge support from the Aninfimip EquipEx program (Investments for the Future ANR-11-EQPX- 0003). A.B. was supported by fellowships from the Fondation Orange and Région Midi-Pyrénées.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711977115/-/DCSupplemental.
References
- 1.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–2196, and erratum (2013) 381:628. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marder SR. Perspective: Retreat from the radical. Nature. 2014;508:S18. doi: 10.1038/508S18a. [DOI] [PubMed] [Google Scholar]
- 3.Abbott A. Novartis reboots brain division. Nature. 2013;502:153–154. doi: 10.1038/502153a. [DOI] [PubMed] [Google Scholar]
- 4.Jang SK, Davies MV, Kaufman RJ, Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5′ nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989;63:1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitajewski J, et al. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986;45:195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- 6.O’Shea CC, Choi S, McCormick F, Stokoe D. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle. 2005;4:883–888. doi: 10.4161/cc.4.7.1791. [DOI] [PubMed] [Google Scholar]
- 7.Ferré CA, et al. Manipulation of the N-terminal sequence of the Borna disease virus X protein improves its mitochondrial targeting and neuroprotective potential. FASEB J. 2016;30:1523–1533. doi: 10.1096/fj.15-279620. [DOI] [PubMed] [Google Scholar]
- 8.Szelechowski M, et al. A viral peptide that targets mitochondria protects against neuronal degeneration in models of Parkinson’s disease. Nat Commun. 2014;5:5181. doi: 10.1038/ncomms6181. [DOI] [PubMed] [Google Scholar]
- 9.Kistler AL, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: Identification of a candidate etiologic agent. Virol J. 2008;5:88. doi: 10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu YJ, et al. Borna disease virus-induced neuronal degeneration dependent on host genetic background and prevented by soluble factors. Proc Natl Acad Sci USA. 2013;110:1899–1904. doi: 10.1073/pnas.1214939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig H, Bode L. Borna disease virus: New aspects on infection, disease, diagnosis and epidemiology. Rev Sci Tech. 2000;19:259–288. doi: 10.20506/rst.19.1.1217. [DOI] [PubMed] [Google Scholar]
- 12.Prat CM, et al. Mutation of the protein kinase C site in borna disease virus phosphoprotein abrogates viral interference with neuronal signaling and restores normal synaptic activity. PLoS Pathog. 2009;5:e1000425. doi: 10.1371/journal.ppat.1000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng G, et al. Borna disease virus P protein affects neural transmission through interactions with gamma-aminobutyric acid receptor-associated protein. J Virol. 2008;82:12487–12497. doi: 10.1128/JVI.00877-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planz O, Pleschka S, Wolff T. Borna disease virus: A unique pathogen and its interaction with intracellular signalling pathways. Cell Microbiol. 2009;11:872–879. doi: 10.1111/j.1462-5822.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwemmle M, De B, Shi L, Banerjee A, Lipkin WI. Borna disease virus P-protein is phosphorylated by protein kinase Cepsilon and casein kinase II. J Biol Chem. 1997;272:21818–21823. doi: 10.1074/jbc.272.35.21818. [DOI] [PubMed] [Google Scholar]
- 16.Volmer R, Monnet C, Gonzalez-Dunia D. Borna disease virus blocks potentiation of presynaptic activity through inhibition of protein kinase C signaling. PLoS Pathog. 2006;2:e19. doi: 10.1371/journal.ppat.0020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citri A, Malenka RC. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 18.Newton AC. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 19.Shapira R, Silberberg SD, Ginsburg S, Rahamimoff R. Activation of protein kinase C augments evoked transmitter release. Nature. 1987;325:58–60. doi: 10.1038/325058a0. [DOI] [PubMed] [Google Scholar]
- 20.Birnbaum SG, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 21.Lei Z, Liu B, Wang JH. Reward memory relieves anxiety-related behavior through synaptic strengthening and protein kinase C in dentate gyrus. Hippocampus. 2016;26:502–516. doi: 10.1002/hipo.22540. [DOI] [PubMed] [Google Scholar]
- 22.Abrial E, Lucas G, Scarna H, Haddjeri N, Lambás-Señas L. A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: Involvement of a functional imbalance? Mol Neurobiol. 2011;44:407–419. doi: 10.1007/s12035-011-8210-4. [DOI] [PubMed] [Google Scholar]
- 23.Hains AB, et al. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara RK, Lenox RH. The myristoylated alanine-rich C kinase substrate: A lithium-regulated protein linking cellular signaling and cytoskeletal plasticity. Clin Neurosci Res. 2004;4:155–169. [Google Scholar]
- 25.Lintas C, et al. Involvement of the PRKCB1 gene in autistic disorder: Significant genetic association and reduced neocortical gene expression. Mol Psychiatry. 2009;14:705–718. doi: 10.1038/mp.2008.21. [DOI] [PubMed] [Google Scholar]
- 26.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoya Y, et al. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J Virol. 1998;72:9755–9762. doi: 10.1128/jvi.72.12.9755-9762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu MV, Hen R. Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus. 2014;24:751–761. doi: 10.1002/hipo.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richetin K, et al. Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer’s disease. Brain. 2015;138:440–455. doi: 10.1093/brain/awu354. [DOI] [PubMed] [Google Scholar]
- 30.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Volmer R, Prat CM, Le Masson G, Garenne A, Gonzalez-Dunia D. Borna disease virus infection impairs synaptic plasticity. J Virol. 2007;81:8833–8837. doi: 10.1128/JVI.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horie M, et al. Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature. 2010;463:84–87. doi: 10.1038/nature08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anagnostaras SG, et al. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Front Behav Neurosci. 2010;4:158. doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tannenholz L, Jimenez JC, Kheirbek MA. Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Front Behav Neurosci. 2014;8:147. doi: 10.3389/fnbeh.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kheirbek MA, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kataoka M, et al. A single amino acid mutation in SNAP-25 induces anxiety-related behavior in mouse. PLoS One. 2011;6:e25158. doi: 10.1371/journal.pone.0025158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbier E, Wang JB. Anti-depressant and anxiolytic like behaviors in PKCI/HINT1 knockout mice associated with elevated plasma corticosterone level. BMC Neurosci. 2009;10:132. doi: 10.1186/1471-2202-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vianna MR, et al. Pharmacological demonstration of the differential involvement of protein kinase C isoforms in short- and long-term memory formation and retrieval of one-trial avoidance in rats. Psychopharmacology (Berl) 2000;150:77–84. doi: 10.1007/s002130000396. [DOI] [PubMed] [Google Scholar]
- 39.Abrial E, et al. Protein kinase C regulates mood-related behaviors and adult hippocampal cell proliferation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:40–48. doi: 10.1016/j.pnpbp.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Abeliovich A, et al. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 41.Bonini JS, et al. On the participation of hippocampal PKC in acquisition, consolidation and reconsolidation of spatial memory. Neuroscience. 2007;147:37–45. doi: 10.1016/j.neuroscience.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Li XB, Inoue T, Koyama T. Effect of chronic treatment with the protein kinase C inhibitor staurosporine on the acquisition and expression of contextual fear conditioning. Eur J Pharmacol. 2002;441:151–155. doi: 10.1016/s0014-2999(02)01441-3. [DOI] [PubMed] [Google Scholar]
- 43.Sun MK, Alkon DL. The “memory kinases”: Roles of PKC isoforms in signal processing and memory formation. Prog Mol Biol Transl Sci. 2014;122:31–59. doi: 10.1016/B978-0-12-420170-5.00002-7. [DOI] [PubMed] [Google Scholar]
- 44.Shema R, et al. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- 45.Kamitani W, et al. Borna disease virus phosphoprotein binds a neurite outgrowth factor, amphoterin/HMG-1. J Virol. 2001;75:8742–8751. doi: 10.1128/JVI.75.18.8742-8751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto Y, et al. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe. 2012;11:492–503. doi: 10.1016/j.chom.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Suberbielle E, et al. Proteomic analysis reveals selective impediment of neuronal remodeling upon Borna disease virus infection. J Virol. 2008;82:12265–12279. doi: 10.1128/JVI.01615-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura R, Matsuki N. Protein kinase CK2 modulates synaptic plasticity by modification of synaptic NMDA receptors in the hippocampus. J Physiol. 2008;586:3195–3206. doi: 10.1113/jphysiol.2008.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unterstab G, et al. Viral targeting of the interferon-beta-inducing Traf family member-associated NF-kappaB activator (TANK)-binding kinase-1. Proc Natl Acad Sci USA. 2005;102:13640–13645. doi: 10.1073/pnas.0502883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saito N, et al. Cellular and intracellular localization of epsilon-subspecies of protein kinase C in the rat brain; presynaptic localization of the epsilon-subspecies. Brain Res. 1993;607:241–248. doi: 10.1016/0006-8993(93)91512-q. [DOI] [PubMed] [Google Scholar]
- 52.Bonnaud EM, et al. Borna disease virus phosphoprotein modulates epigenetic signaling in neurons to control viral replication. J Virol. 2015;89:5996–6008. doi: 10.1128/JVI.00454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pletnikov MV, et al. Persistent neonatal Borna disease virus (BDV) infection of the brain causes chronic emotional abnormalities in adult rats. Physiol Behav. 1999;66:823–831. doi: 10.1016/s0031-9384(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 54.Dittrich W, Bode L, Ludwig H, Kao M, Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;26:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 55.Hornig M, Weissenböck H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pletnikov MV, Rubin SA, Vasudevan K, Moran TH, Carbone KM. Developmental brain injury associated with abnormal play behavior in neonatally Borna disease virus-infected Lewis rats: A model of autism. Behav Brain Res. 1999;100:43–50. doi: 10.1016/s0166-4328(98)00111-9. [DOI] [PubMed] [Google Scholar]
- 57.Rubin SA, et al. Borna disease virus-induced hippocampal dentate gyrus damage is associated with spatial learning and memory deficits. Brain Res Bull. 1999;48:23–30. doi: 10.1016/s0361-9230(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 58.Rubin SA, Waltrip RW, 2nd, Bautista JR, Carbone KM. Borna disease virus in mice: Host-specific differences in disease expression. J Virol. 1993;67:548–552. doi: 10.1128/jvi.67.1.548-552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honda T, et al. Upregulation of insulin-like growth factor binding protein 3 in astrocytes of transgenic mice that express Borna disease virus phosphoprotein. J Virol. 2011;85:4567–4571. doi: 10.1128/JVI.01817-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamitani W, et al. Glial expression of Borna disease virus phosphoprotein induces behavioral and neurological abnormalities in transgenic mice. Proc Natl Acad Sci USA. 2003;100:8969–8974. doi: 10.1073/pnas.1531155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worth AR, Andrew Thompson RC, Lymbery AJ. Reevaluating the evidence for Toxoplasma gondii-induced behavioural changes in rodents. Adv Parasitol. 2014;85:109–142. doi: 10.1016/B978-0-12-800182-0.00003-9. [DOI] [PubMed] [Google Scholar]
- 62.Ingram WM, Goodrich LM, Robey EA, Eisen MB. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS One. 2013;8:e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.