Significance
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality among allogeneic stem-cell transplantation recipients. An effort to identify selective immune suppression whereby GVHD is reduced and the antitumor activity of the graft is preserved is key to improving the success of blood and marrow transplantation. Here we demonstrate that inhibition of Janus kinase 2 (JAK2) significantly decreases GVHD and maintains tumor killing by the donor T cells. Pharmacologic blockade of JAK1 and JAK2 in myelofibrosis patients is known to impair human T cell subsets broadly. Conversely, we show that JAK2 inhibition impairs alloreactive T cells yet promotes beneficial regulatory T cell and Th2 differentiation. This study emphasizes the relevance of JAK2 in GVHD pathogenesis and prevention.
Keywords: GVHD, GVL, graft rejection, JAK2
Abstract
Janus kinase 2 (JAK2) signal transduction is a critical mediator of the immune response. JAK2 is implicated in the onset of graft-versus-host disease (GVHD), which is a significant cause of transplant-related mortality after allogeneic hematopoietic cell transplantation (allo-HCT). Transfer of JAK2−/− donor T cells to allogeneic recipients leads to attenuated GVHD yet maintains graft-versus-leukemia. Th1 differentiation among JAK2−/− T cells is significantly decreased compared with wild-type controls. Conversely, iTreg and Th2 polarization is significantly increased among JAK2−/− T cells. Pacritinib is a multikinase inhibitor with potent activity against JAK2. Pacritinib significantly reduces GVHD and xenogeneic skin graft rejection in distinct rodent models and maintains donor antitumor immunity. Moreover, pacritinib spares iTregs and polarizes Th2 responses as observed among JAK2−/− T cells. Collectively, these data clearly identify JAK2 as a therapeutic target to control donor alloreactivity and promote iTreg responses after allo-HCT or solid organ transplantation. As such, a phase I/II acute GVHD prevention trial combining pacritinib with standard immune suppression after allo-HCT is actively being investigated (https://clinicaltrials.gov/ct2/show/NCT02891603).
Janus kinase 2 (JAK2) signal transduction is implicated in human autoimmune syndromes (1, 2) and graft-versus-host disease (3–5). IL-6, IL-12, and IL-23 mediate inflammation and activate T cells via JAK2 (3, 4, 6). IL-6 receptor blockade has demonstrated efficacy in a phase II graft-versus-host disease (GVHD) prevention trial (7), but does not fully impair pathogenic Th1/Th17 responses (8). IL-12 and IL-23 promote Th1 and Th17 differentiation via JAK2 (2). Neutralizing the shared p40 subunit of these cytokines prevents GVHD in rodents (6) and may ameliorate steroid-refractory GVHD (9).
JAK2 inhibition is an alternative approach to suppress IL-6 and p40 receptor signal transduction and induce durable tolerance to alloantigens (3). JAK2 inhibitors are clinically efficacious in myelofibrosis, a hematologic disease often driven by constitutive JAK2 activation (10). The existing evidence regarding JAK2 as a therapeutic target for acute GVHD is primarily supported by observations using ruxolitinib, an equimolar inhibitor of JAK1 and JAK2 (4, 11–14). Ruxolitinib has demonstrated activity in treating steroid-refractory GVHD and is clearly immune suppressive (4, 11). In part, JAK1 mediates the biologic effects of common gamma chain cytokines, including IL-2 and IL-15 (3). Ruxolitinib suppresses host-reactive T cells in mice (4, 12–14) and humans (4, 11). Although not observed in murine transplant studies (4, 12–14), ruxolitinib reduces the quantity of human Tregs (15) and natural killer (NK) cells (16, 17). Therefore, targeting JAK2 has the potential to prevent GVHD without conceding JAK1-mediated functions provided by donor lymphocytes.
We report that genetic deletion or pharmacologic inhibition of JAK2 significantly reduces GVHD lethality and spares the graft-versus-leukemia (GVL) effect. Moreover, we demonstrate that JAK2 blockade significantly delays skin graft rejection. We have shown that JAK2 blockade abrogates human Th1 and Th17 responses using TG101348 (3, 18). TG101348 is now regarded as a tool compound, as its use has been associated with Wernicke encephalopathy caused by off-target inhibition of thiamine uptake (19). Therefore, we investigated the use of pacritinib, a JAK2 inhibitor that does not impair thiamine metabolism (20). Distinct from ruxolitinib, pacritinib spares JAK1 activity required by antitumor cytotoxic T lymphocytes and Tregs (21). Here, we identify that eliminating JAK2 signal transduction significantly enhances Th2 and Treg differentiation while dramatically reducing Th1 responses. Thus, we prove that JAK2 inhibition significantly suppresses donor T cells across species without untoward effects on Tregs or GVL.
Results
JAK2 Signaling Promotes Th1 Differentiation, but Inhibits Th2 and Treg Responses by Allo-Activation in Vitro.
To examine the effect of JAK2 in regulating T cell activation and function, we used mice in which JAK2 was conditionally deleted in T cells (JAK2flox/flox × CD4 Cre+). The frequency of CD4+ T cells among JAK2−/− mice was decreased compared with naive B6 mice (Fig. S1 A and D), although proportions of CD8+ T cells and CD4+ Tregs were similar (Fig. S1 A, B, D, and E). The composition of T cell subsets within the naive and central memory compartments was also similar between JAK2−/− and WT mice (Fig. S1 C–G) with only a subtle increase in CD8+ effector memory T cells in JAK2−/− mice (Fig. S1 C–G). In response to alloantigen stimulation, proliferating (CFSElow) JAK2−/− CD4+ and CD8+ T cells produced significantly less IFNγ, yet higher levels of IL-4/5 and IL-10 compared with WT T cells (Fig. S2). These data indicate that JAK2 signaling is critical for Th1 differentiation and argue against Th2 and Treg development after alloantigen stimulation in vitro.
JAK2 Contributes to T Cell-Mediated GVHD, but Is Dispensable for the GVL Effect.
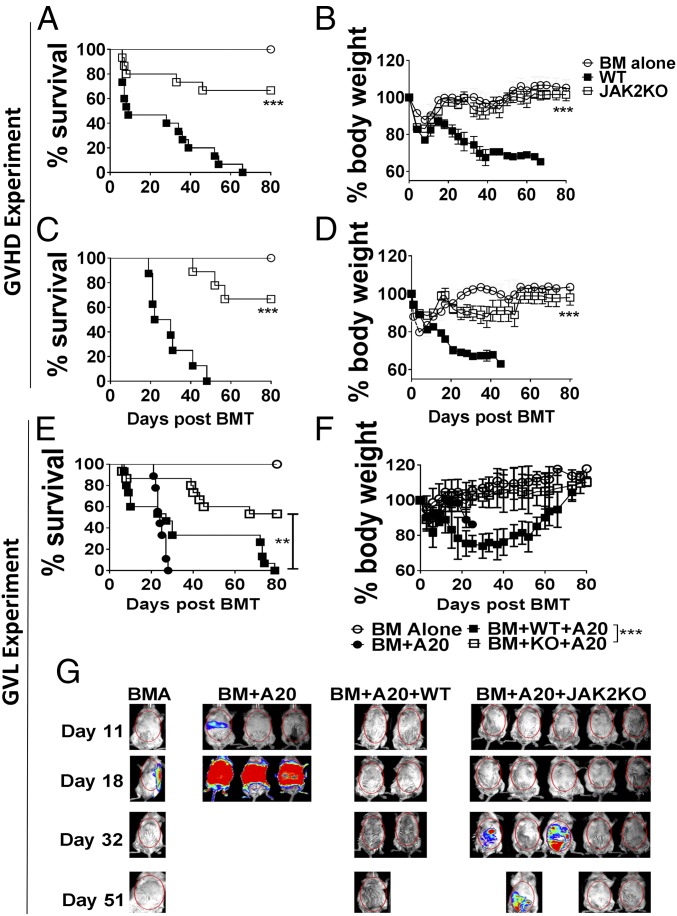
To study how JAK2 affects T cell responses to alloantigen in vivo, we first evaluated GVHD severity after transfer of JAK2−/− or WT T cells to major MHC-mismatched recipients. JAK2−/− T cells had a significantly reduced ability to induce lethal GVHD compared with WT T cells (Fig. 1 A and B). Furthermore, the reduced alloresponse observed in cohorts that received JAK2−/− T cells was replicated in a minor MHC-mismatch model of bone marrow transplantation (BMT) (Fig. 1 C and D). Impaired immune reconstitution is associated with GVHD (22). We therefore examined donor-derived T- and B-cell reconstitution in surviving BALB/c recipients 80 d post BMT. In the thymus, the percentage and absolute number of double-positive thymocytes (CD4+CD8+) were comparable to cohorts receiving T cell-depleted bone marrow (TCD-BM) only (Fig. S3). In the spleen, the absolute numbers of CD4+ T cells and B cells in recipients of JAK2−/− T cells were reduced compared with those of TCD-BM alone (Fig. S3B); the frequencies of donor-derived CD4+ and CD8+ T cells and of B cells were comparable (Fig. S3A). These data indicate that JAK2 signaling in T cells facilitates recipient thymic damage typically associated with GVHD (22), yet may enhance peripheral T- and B-cell numbers after allogeneic hematopoietic cell transplantation (allo-HCT).
Fig. 1.
JAK2 contributes to T cell-mediated GVHD, but is dispensable for GVL. Lethally irradiated BALB/c (A and B) or BALB/b (C and D) mice were transplanted with 5 × 106 TCD-BM alone or plus 1 × 106 (BALB/c) or 3 × 106 (BALB/b) purified T cells from WT B6 or JAK2 KO mice. Survival and body weight loss of BALB/c (A and B) and BALB/b recipients (C and D) are shown. Data shown are pooled from two to three replicate experiments with a total of 6–15 mice per group. Lethally irradiated BALB/c mice were transplanted with 5 × 106 TCD-BM alone or plus 2 × 103 luc-A20 cells and either 1 × 106 purified T cells from WT or JAK2 KO mice plus 2 × 103 luc-A20 cells. Recipient survival (E), body weight loss (F), and tumor burden (G) are shown. Percentage survival and tumor mortality data shown were pooled from three replicate experiments with a total of 6–15 mice per group. Representative BLI images were taken from one of three replicate experiments. **P < 0.01; ***P < 0.001.
Based on in vivo bioluminescence measurements of tumor growth, recipients of JAK2−/− or WT donor T cells exhibited significantly less tumor mortality compared with those that did not receive T cells, indicating that JAK2−/− T cells mediate GVL (Fig. 1 E and G). The tumor mortality among recipients of JAK2 KO T cells was not significantly different (P = 0.15, log-rank test) compared with that of WT T cells (Fig. 1 E and G). These experiments demonstrate that JAK2 signaling in donor T cells contributes to GVHD but is partially dispensable for the GVL effect.
JAK2 Inhibits Th2 and Treg Polarization in Vivo.
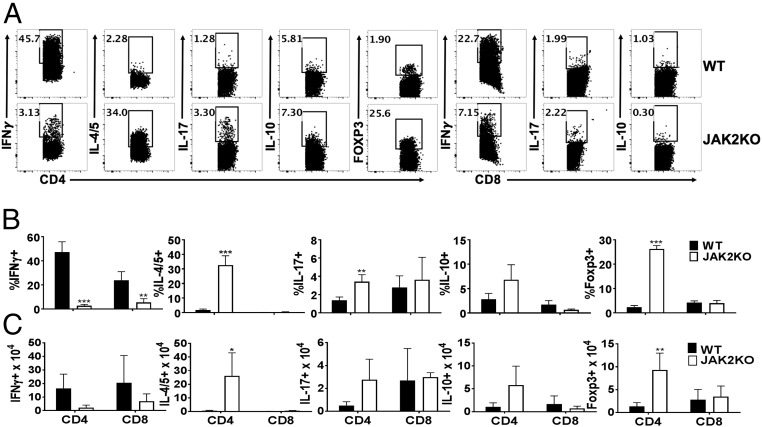
We analyzed the cytokine production profile of WT and JAK2−/− T cells in allogeneic recipients. JAK2−/− T cells had significantly less IFNγ+ Th1 differentiation in the spleen (Fig. 2). Conversely, JAK2−/− T cells had significantly enhanced IL-4/5+ Th2 and Foxp3+ Treg polarization (Fig. 2). JAK2−/− CD8+ T cells also produced less IFNγ compared with WT controls (Fig. 2). Although limited to a small positive population of cells, JAK2−/− T cells exhibited increased Th17 differentiation (Fig. 2). Similar results were observed in recipient livers (Fig. S4).
Fig. 2.
Donor T cells deficient for JAK2 are prone to Th2 and Treg polarization in vivo. Lethally irradiated BALB/c mice were transplanted with 5 × 106 Ly5.1+ TCD-BM alone or plus 1 × 106 purified T cells (Ly5.2+) from WT B6 or JAK2 KO mice. Recipient splenic mononuclear cells were isolated for immunophenotyping on day +14 post-BMT. Data depict one representative mouse per group for IFNγ+, IL-4/5+, IL-17+, Foxp3+ (Tregs), or IL-10+ among gated H2Kb+Ly5.1-CD4+ or CD8+ cells (A). Average percentages +SD (B) or absolute numbers (C) of splenic T cell subsets are shown from one of three replicate experiments. Splenic cells from 9 to 11 mice per group were analyzed in total. *P < 0.05; **P < 0.01; ***P < 0.001.
JAK2 Contributes to the Migratory Capacity of Donor T Cells.
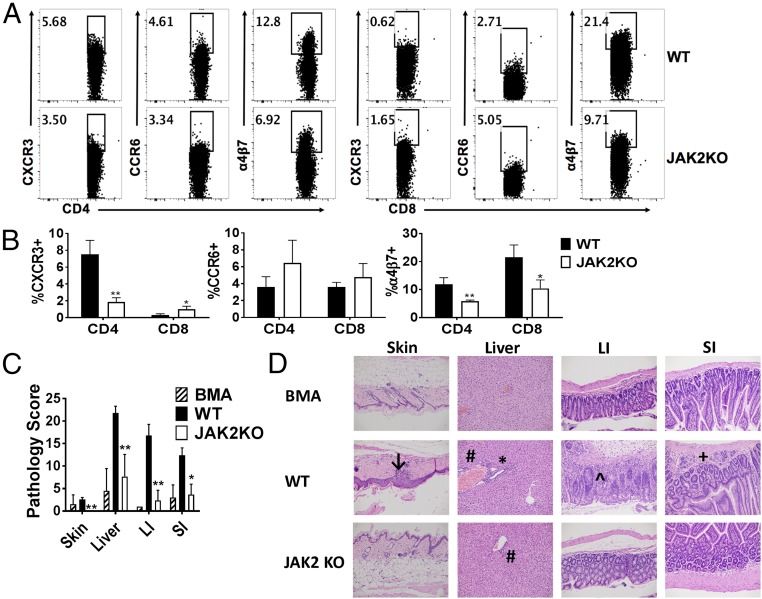
We then investigated the impact of JAK2 signaling on donor T cell migration. Expression of the chemokine receptor CXCR3 and the integrin α4β7 was significantly decreased among CD4+ JAK2−/− T cells in the spleen of recipients (Fig. 3 A and B). This reduction in CXCR3 expression is indicative of reduced trafficking among Th1 cells (23), while decreased α4β7 demonstrates impaired migration to intestinal tissues (24, 25). However, the expression of CCR6l, a key Th17 chemokine receptor, was similar among cohorts (Fig. 3 A and B). These data show that JAK2 activation promotes T cell homing potential to GVHD target organs. Consistent with the observed decrease in α4β7 expression, recipients of JAK2−/− T cells had significantly less tissue damage in the small and large intestine (Fig. 3 C and D). GVHD was also significantly reduced in the skin and liver of JAK2−/− T cell recipients (Fig. 3 C and D). Although these results do not directly assess T cell trafficking, the data suggest that JAK2 contributes to the expression of necessary chemokines and integrins required for lymphocyte migration.
Fig. 3.
JAK2 contributes to the migratory capacity of donor T cells. Lethally irradiated BALB/c mice were transplanted with 5 × 106 Ly5.1+ TCD-BM alone or plus 1 × 106 purified T cells (Ly5.2+) from WT B6 or JAK2 KO mice. Recipient splenic mononuclear cells were isolated for T cell migration surface markers on day +14 post-BMT. Flow plots show expression of CXCR3, CCR6, or α4β7 (A) among gated H2Kb+Ly5.1-CD4+ or CD8+ cells. (B) The mean frequency of T cell CXCR3, CCR6, or α4β7 expression ±SD for one of three replicate experiments is shown. A total of 9–11 mice per group were analyzed. (C) Quantified GVHD tissue damage scores ±SD from one representative experiment are shown (BMA: 6 mice; WT: 14 mice; JAK2 KO: 15 mice). (D) Representative H&E sections of GVHD target organs from each cohort showing vacuolar changes in the skin (↓); endothelialitis (#) and mononuclear infiltrates in the liver (*); crypt regeneration in the large intestine (LI, ^); and lamina propria inflammation in the small bowel (SI, +). *P < 0.05; **P < 0.01. (Magnification: 200×.)
Pharmacologic Inhibition of JAK2 with Pacritinib Reduces GVHD and Spares GVL.
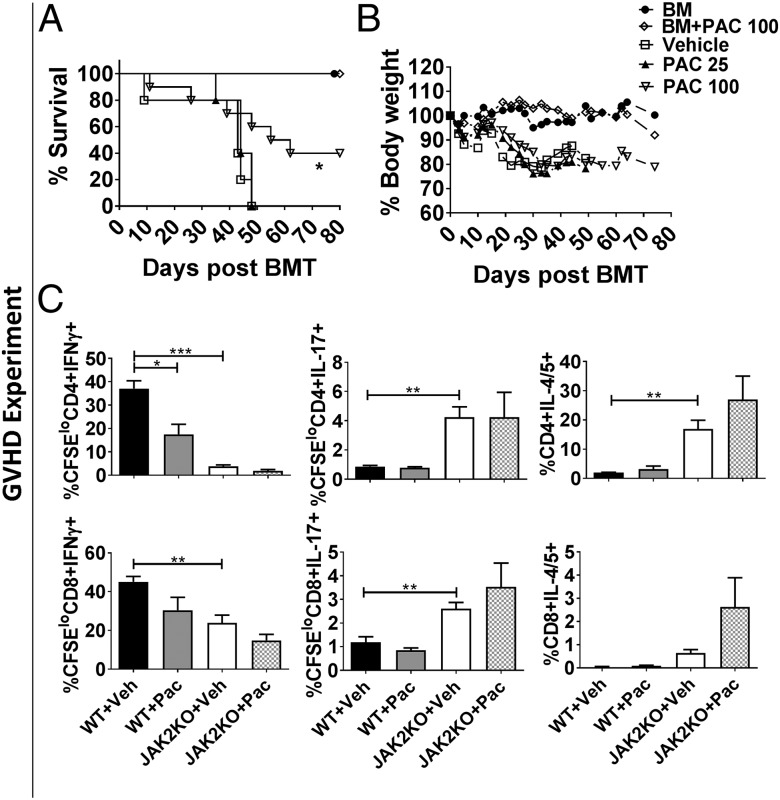
To translate the observations made using the JAK2−/− T cells, host WT BALB/c mice received B6 allografts and were treated with pacritinib, a JAK2 inhibitor, or vehicle for 3 wk. Pacritinib significantly reduced acute GVHD mortality among the recipient BALB/c mice (Fig. 4 A and B). Similar to the JAK2 KO T cells, pacritinib significantly reduced CD4+ T cell production of IFNγ and the proliferation of Th1 cells (Fig. 4C). Notably, pacritinib treatment did not significantly impact JAK2−/− T cells in these studies, indicating that the potential off-target effects of pacritinib in this context were minimal (Fig. 4C). While ruxolitinib (JAK1/2 inhibitor) impaired murine cytotoxic T-lymphocyte (CTL) activity against tumor in vitro (Fig. S5), neither pacritinib nor ruxolitinib interfered with the GVL effect in vivo (Fig. S6 A–C). Additionally, ruxolitinib demonstrated greater immune suppression against GVHD (P < 0.05, Fig. S6 A–C), indicating that dual JAK1/2 inhibition induces broad donor T cell inactivation compared with pacritinib. Pacritinib had no direct effect on P815 cells (Fig. S6 D–F).
Fig. 4.
Pharmacological inhibition of JAK2 with pacritinib reduces GVHD and spares GVL. Lethally irradiated BALB/c mice were transplanted with 5 × 106 TCD-BM alone or plus 1 × 106 T cells/mouse from WT B6 donors. Pacritinib 100 mg/kg or methylcellulose vehicle was given by oral gavage daily for 3 wk starting on day 0 of BMT. Recipient survival (A) and body weight loss (B) are shown. In separate experiments, lethally irradiated BALB/c mice were transplanted with carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells from either WT or JAK2 KO donors and treated with pacritinib or vehicle. Average percentages +SD of CFSE-diluted CD4+ and CD8+ T cells positive for IFNγ, IL-4/5, and IL-17 for are shown (C). A total of 12 mice/group were used across the three experiments (A–C). *P < 0.05; **P < 0.01; ***P < 0.001. Pac, pacritinib; Veh, vehicle.
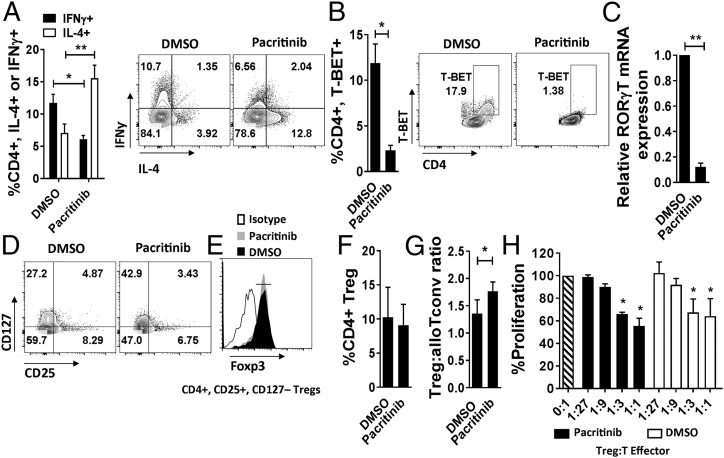
Pacritinib Polarizes a Th2 Response Among Allostimulated Human T Cells.
We went on to verify the immune suppressive effects of pacritinib in a human system. Using cytokine- or dendritic cell (DC)-stimulated human T cells, pacritinib inhibited JAK2-dependent phosphorylation of STAT3 and significantly suppressed alloreactive T cell proliferation (Fig. S7 A and B). IL-2–mediated STAT5 signal transduction, required by Treg and CTL alike, was largely preserved among T cells exposed to pacritinib (Fig. S7A). JAK2 inhibition of human T cells directed robust Th2 polarization and significantly decreased Th1 and Th17 differentiation (Fig. 5 A–C). Pacritinib also suppressed lymphocyte production of IL-6, IL-17A, IL-17F, and TNF-alpha (26) (Fig. S7C). As demonstrated in rodents (14), both pacritinib and ruxolitinib reduced IFNγ-mediated signaling in human T cells (Fig. S8).
Fig. 5.
Pacritinib polarizes a Th2 response by human T cells after allogeneic stimulation in vitro and permits the differentiation of suppressive iTreg. Data show the effect of pacritinib (2.5 μM) on (A) Th1 (IFNγ) versus Th2 (IL-4), (B) T-BET expression (replicate means ± SEM, n = 4 experiments), and (C) RORγT expression ±SD among CD4+ T cells after DC allostimulation (one of two experiments is shown, performed in triplicate). (D and E) iTregs were generated from Treg-depleted, DC-allostimulated CD4+ T cells in the presence of pacritinib (2.5 μM) or vehicle control. Contour plots show iTregs and corresponding Foxp3 expression. (F and G) Graphs show the mean frequency of iTreg and ratio of iTreg to alloreactive Tconv (CD4+, CD25+, CD127+) ±SD after DC allostimulation. n = 6 experiments. (H) iTreg-suppressive potency is demonstrated against self-T cell responders stimulated by allogeneic DCs without additional drugs. One of two experiments are shown, each performed in triplicate. *P < 0.05; **P < 0.01.
Pacritinib Permits the Differentiation of Suppressive Human-Induced Treg.
CD4+ T cells were purified, depleted of natural Tregs as previously described (>99% non-Treg), and stimulated with allogeneic, cytokine-matured DCs for 5 d with pacritinib or DMSO added once on day 0. Induced Treg (iTreg) differentiation was similar following DC allostimulation, regardless of pacritinib treatment (Fig. 5 D–F). Conversely, pacritinib significantly increased the ratio of iTreg to activated conventional T cells (Tconv) compared with DMSO (Fig. 5G). The suppressive potency of pacritinib- or DMSO-pretreated iTregs was similar, suggesting that JAK2 is not required for human iTreg function (Fig. 5H).
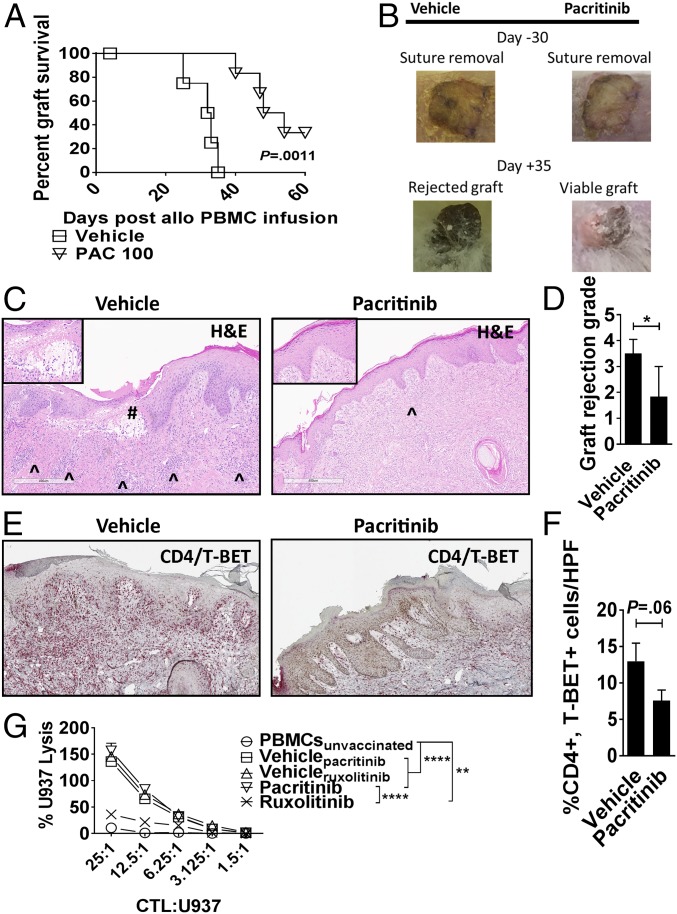
Pacritinib Reduces Xenograft Rejection but Maintains CD8+ CTL Activity Against Tumor.
The immune-suppressive effect of pacritinib on human T cells was tested in vivo. Immunodeficient nonobese diabetic-scid gamma-deficient (NSG) mice received a dorsally positioned, 1-cm2, split-thickness human skin graft. After a 30-d rest period, 5 × 106 human peripheral blood mononuclear cells (PBMCs), allogeneic to the skin donor, were injected into the mouse (18, 27). Mice received pacritinib (100 mg/kg) or vehicle twice a day by oral gavage from day 0 until day +14. Pacritinib significantly delayed skin graft rejection by allogeneic T cells compared with vehicle (Fig. 6 A and B). Skin xenografts from pacritinib-treated mice therefore exhibited significantly less pathologic rejection at day +21 (Fig. 6 C and D), compared with the vehicle-treated controls. Immunohistochemistry analysis suggested a modest reduction in Th1 cells (CD4+, T-BET+) in the skin of pacritinib-treated mice (Fig. 6 E and F). We used an established method to generate human antitumor CTL in vivo and then tested specific killing by the T cells in vitro (18). NSG mice received human PBMCs (30 × 106) and were inoculated with irradiated U937 cells (10 × 106) on days 0 and +7. Mice were treated with pacritinib, ruxolitinib [30 mg/kg twice a day (4)], or vehicle as described. Human CD8+ T cells were isolated from the spleens of euthanized recipients during days +10–12 and cultured against fresh U937 target cells without further drug exposure in vitro. Ruxolitinib significantly impaired antitumor activity, while CTL function was preserved among mice treated with pacritinib or vehicle controls (Fig. 6G).
Fig. 6.
Pacritinib reduces xenograft rejection. NSG mice received a 1-cm2 split thickness human skin graft. After 30 d of rest to ensure engraftment, an inoculum of 5 × 106 human PBMCs (allogeneic to the skin) were administered by i.p. injection. Unique pairs of donor skin and allogeneic PBMCs were used for each set of experiments. Pacritinib 100 mg/kg or vehicle was given twice a day from days 0 to +14. (A) Graph shows human skin graft survival among pacritinib- or vehicle-treated NSG hosts (log-rank test). (B) Representative images show skin at time of suture removal (day −30) and at day +35. (C) Histologic representations of the skin grafts uniformly harvested on day +21 demonstrate that pacritinib reduces lymphocytic infiltration (^) and severe basal vacuolar changes of the graft, such as the subepidermal blister (#). Low power at 6×, high-power Inset at 20×. (Scale bar, 400 µm.) (D) Bar graph shows pathologic skin graft rejection scores at day +21 among vehicle- and pacritinib-treated mice. (E and F) Immunohistochemistry and accompanying bar graph shows skin-resident Th1 cells at day +21 by dual staining of CD4 (red) and T-BET (brown). n = 2 experiments, 5–6 mice per arm. (G) Graph depicts mean specific lysis ±SEM by human CD8+ CTL generated in vivo using NSG mice transplanted with human PBMCs (30 × 106) and vaccinated with irradiated U937cells (10 × 106) on days 0 and +7. Mice were treated with pacritinib (100 mg/kg twice a day), ruxolitinib (30 mg/kg twice a day), or vehicle from day 0 up to day +12. Human CD8+ T cells were harvested from euthanized mice between days +10 to +12. Results shown are from one of two independent experiments. U937 lysis was measured by colorimetric assay after 4 h. *P < 0.05; **P < 0.01; ****P < 0.0001. (Magnification: C and E, slides were scanned using a 20×/0.8 N.A. objective and viewed using a 6.5× digital zoom in ImageScope software.)
Discussion
While it is known that JAK2 inhibition spares Tregs and reduces Th1 responses (3, 18), we demonstrate that genetic ablation of JAK2 on donor T cells or treatment with pacritinib significantly enhances Th2 differentiation after allo-HCT. Strategies to modulate Th2 over Th1 have proven effective in reducing GVHD lethality in rodents. IL-18 promotes STAT6-dependent Th2 polarization, suppresses Th1 cells, and reduces GVHD yet spares GVL (28). Th2 cells are also required for myeloid-derived suppressor-cell–mediated immune suppression and GVHD prevention (29). Additionally, the GVHD biomarker, soluble suppression of tumorigenicity 2 (sST2), orchestrates alloreactivity in part by binding IL-33 and preventing Th2 differentiation (30). Therefore, we surmise that Th2 polarization contributes to the immune suppressive activity of JAK2 inhibition.
The recipients of JAK2−/− T cells had longer survival, higher body weights, and less GVHD pathology compared with WT controls. We also observed significantly less Th1 differentiation and CXCR3 expression among JAK2−/− T cells. Furthermore, JAK2−/− donor T cells had reduced expression of the α4β7 integrin that is required for gut homing by Th1 cells. Our data suggest that targeting JAK2 may reduce GVHD in part by limiting Th1 differentiation and the migratory capacity of alloreactive T cells (23, 24).
Species-specific immune effects were observed among the murine GVHD experiments compared with the human in vitro and xenogeneic tissue rejection experiments. Pacritinib significantly reduced human Th17 differentiation, which is similar to the published results using the JAK2 inhibitor TG101348 (3, 18). Conversely, recipients of JAK2−/− T cells and those treated with pacritinib exhibited moderately increased Th17 cells after allo-HCT. This is distinct from STAT3 KO donor T cells, which result in reduced Th17 differentiation in transplanted mice (31). TGFβ can promiscuously activate STAT3 in T cells to facilitate murine Th17 development (32, 33). The loss of JAK2 countered by STAT3 activity via alternative pathways may enhance Th17 differentiation in mice but not humans due to the species-specific effects of TGFβ (34). This is further supported by the observation that JAK2−/− T cells exhibited significant Treg differentiation compared with WT controls, which may serve as a source of TGFβ in vivo (35). Our in vitro human data demonstrated that JAK2 inhibition with pacritinib spared Treg differentiation, but did not increase the amount of Tregs compared with controls (Fig. 5F). However, the ratio of Tregs to activated Tconv was increased with pacritinib (Fig. 5G).
An important limitation of these data is that pacritinib is a multikinase inhibitor with effects on pathways potentially relevant to GVHD (21). Pacritinib potently inhibits JAK2, FLT3, IRAK1, TNK1, ROS, and HIPK (IC50 < 50 nM) and also has activity against JAK3 and TYK2 (IC50 50–100 nM) in the JAK-STAT system (21). Such activity by pacritinib may be important to differences seen between JAK2−/− T cells and the studies using pacritinib as a JAK2 inhibitor in GVHD prevention. However, the anti-JAK3 activity of pacritinib did not prevent IL-2–mediated phosphorylation of STAT5, which is required by Tregs and CTL. Tyk2 and JAK2 regulate p40 cytokine receptor signal transduction (36), and Tyk2 inhibition by pacritinib might also impact Th1 and Th17 differentiation beyond the effects of the JAK2 blockade. Additionally, suppression of IRAK1 and IL-1β receptor activity may modulate donor alloresponses (37). In comparison, ruxolitinib inhibits JAK1 and JAK2 equally (4). Ruxolitinib is known to suppress STAT5 phosphorylation in human T cells and NK cells via JAK1 inhibition (15, 17). Others and we show that ruxolitinib, as opposed to pacritinib, significantly impairs human CTL function (15), although this is not observed among murine T cells in vivo (Fig. S6 A–C) (13). These data suggest that human and mouse T cells have a somewhat different sensitivity to Jak inhibition. Similarly to ruxolitinib, we show that pacritinib also limits human NK-cell proliferation and function in vitro (Fig. S9). While pacritinib spares JAK1, we surmise that its effect on NK cells is due to suppression of Tyk2 and impaired IL-12 activity (38). Given that pacritinib permits common gamma-chain cytokine signal transduction, such as IL-2, its effect on JAK3 does not explain the drug’s inhibition of NK cells. Our data also support that ruxolitinib induces profound immune suppression compared with pacritinib, yet potentially at the cost of CTL function required for antitumor activity.
Our data support that neutralization or pharmacologic blockade of JAK2 with pacritinib preserves donor T cell-mediated GVL activity. We propose that targeting JAK2, but sparing JAK1, with agents such as pacritinib is sufficient to control alloreactivity without impairing normal T-effector and Treg function. A phase I/II acute GVHD prevention trial combining pacritinib with standard immune suppression after allo-HCT is actively being investigated (https://clinicaltrials.gov/ct2/show/NCT02891603). Therefore, such an approach has direct translational implications in GVHD and solid organ rejection prophylaxis.
Materials and Methods
Detailed methods can be found in SI Materials and Methods.
Mice.
C57BL/6 (B6; H-2b), B6.Ly5.1 (H-2b), BALB/b (H2b), BALB/c (H2d), and (B6 × DBA2)F1 (H2b/d) mice were purchased from the National Cancer Institute/National Institutes of Health (NIH). Mice with a conditional KO of JAK2 on the T cell lineage were generated by breeding JAK2fl/fl [provided by K. U. Wagner, University of Nebraska, Omaha, NE (39)] with CD4-Cre (purchased from Taconic). NSG mice were purchased from the Jackson Laboratory. With the exception of the NSG mice that were housed at the Moffitt Cancer Center, all other animals were housed at the American Association for Laboratory Animal Care-accredited Animal Resource Center at the Medical University of South Carolina. All mice were treated in adherence with the NIH Guide for the Care and Use of Laboratory Animals and their respective protocols approved by local institutional animal care and use committees.
Murine GVHD and Bioluminescent Imaging.
Lethally irradiated BALB/c (MHC-mismatched) or BALB/b (MHC-matched, minor histocompatibility antigen-mismatched) mice received 5 × 106 TCD-BM alone or plus 1 × 106 (BALB/c) or 3 × 106 (BALB/b) T cells from WT B6 or JAK2 KO mice. Recipient body weight and survival were assessed twice weekly. GVHD pathology scores were assessed in a blinded fashion by an independent pathologist. Where indicated, B6.Ly5.1+ TCD-BM and B6.Ly5.2+ WT and JAK2 KO T cells were used. For GVL experiments, BALB/c recipients received TCD-BM alone, TCD-BM with 2 × 103 luciferase-transduced A20, or TCD-BM plus WT B6 or JAK2 KO T cells and luciferase-transduced A20. (B6 × DBA2)F1 mice received TCD-BM alone, TCD-BM with 5 × 103 luciferase-transduced P815 tumor cells, or 3 × 106 WT B6 T cells and luciferase-transduced P815 tumor cells. Bioluminescent imaging (BLI) was performed as previously described (6). As indicated, pacritinib or its vehicle control were administered at 25–100 mg/kg by oral gavage daily from day 0 for 3 wk after BMT. Where indicated, ruxolitinib (a JAK1/2 inhibitor control) was given at 30 mg/kg twice a day by oral gavage.
Xenograft Model.
NSG mice (male or female, 6–24 wk old) received a 1-cm2 split thickness human skin graft under anesthesia. Consent was obtained from eligible patients undergoing mastectomy, and their skin was collected in accordance with an IRB-approved protocol at the Moffitt Cancer Center (MCC 17634). The bandage and sutures were removed after 7–10 d. Thirty days later, recipient mice then received 5 × 106 fresh, human PBMCs (OneBlood) i.p. using a random donor allogeneic to the skin graft (18, 27). Each transplant experiment used a unique donor pair of skin and PBMCs. Pacritinib 100 mg/kg or vehicle was given by oral gavage twice a day from day 0 until day +14. The skin grafts were followed closely for signs of rejection, such as ulceration, necrosis, or scabbing. Skin grafts that were >75% nonviable were considered rejected. Pathologic skin rejection grading was performed according to criteria set forth by Bejarano et al. (40). Tissue samples were prepared, stained (Ventana Medical Systems), and imaged (Vista) as previously described (5).
Statistics.
For comparisons of independent murine data, the two-tailed Student’s t test was used. For comparisons of dependent human data, the two-tailed paired t test was used. The Mann–Whitney test was used for comparisons of nonparametric data. ANOVA was used for group comparisons. The log-rank test was used to analyze GVHD and skin graft survival.
Supplementary Material
Acknowledgments
The Flow Cytometry, University of South Florida (USF) Comparative Medicine and Vivarium, Analytic Microscopy, and Tissue Cores at Moffitt/USF were utilized in completing this work. The core facilities are supported partially by the Moffitt Cancer Center Support Grant P30-CA076292. This work was supported by Grants R01 CA143812 and CA169116 (to X.-Z.Y.) and Grants K08 HL11654701A1 and R01 HL133823 (to B.C.B.) from the National Institutes of Health.
Footnotes
Conflict of interest statement: B.C.B. has participated in advisory boards related to pacritinib (CTI BioPharma) and ruxolitinib (Incyte) in GVHD. J.S. is employed by CTI BioPharma with related equity ownership. A.O. is an employee at BioSeek. All other authors have no competing financial interests to declare.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712452115/-/DCSupplemental.
References
- 1.Steward-Tharp SM, et al. A mouse model of HIES reveals pro- and anti-inflammatory functions of STAT3. Blood. 2014;123:2978–2987. doi: 10.1182/blood-2013-09-523167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng MW, et al. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 3.Betts BC, et al. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood. 2011;118:5330–5339. doi: 10.1182/blood-2011-06-363408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spoerl S, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 5.Betts BC, et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol. 2015;97:807–819. doi: 10.1189/jlb.5A1114-532RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, et al. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118:5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy GA, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: A phase 1/2 trial. Lancet Oncol. 2014;15:1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 8.Betts BC, St Angelo ET, Kennedy M, Young JW. Anti-IL6-receptor-alpha (tocilizumab) does not inhibit human monocyte-derived dendritic cell maturation or alloreactive T-cell responses. Blood. 2011;118:5340–5343. doi: 10.1182/blood-2011-06-363390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pidala J, Perez L, Beato F, Anasetti C. Ustekinumab demonstrates activity in glucocorticoid-refractory acute GVHD. Bone Marrow Transplant. 2012;47:747–748. doi: 10.1038/bmt.2011.172. [DOI] [PubMed] [Google Scholar]
- 10.Harrison C, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 11.Zeiser R, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia. 2015;29:2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carniti C, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21:3740–3749. doi: 10.1158/1078-0432.CCR-14-2758. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, et al. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS One. 2014;9:e109799. doi: 10.1371/journal.pone.0109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, et al. IFNγR signaling mediates alloreactive T-cell trafficking and GVHD. Blood. 2012;120:4093–4103. doi: 10.1182/blood-2012-01-403196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parampalli Yajnanarayana S, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol. 2015;169:824–833. doi: 10.1111/bjh.13373. [DOI] [PubMed] [Google Scholar]
- 16.Schönberg K, et al. JAK inhibition impairs NK cell function in myeloproliferative neoplasms. Cancer Res. 2015;75:2187–2199. doi: 10.1158/0008-5472.CAN-14-3198. [DOI] [PubMed] [Google Scholar]
- 17.Curran SA, et al. Human dendritic cells mitigate NK-cell dysfunction mediated by nonselective JAK1/2 blockade. Cancer Immunol Res. 2017;5:52–60. doi: 10.1158/2326-6066.CIR-16-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Betts BC, et al. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Sci Transl Med. 2017;9:eaai8269. doi: 10.1126/scitranslmed.aai8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardanani A, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A randomized clinical trial. JAMA Oncol. 2015;1:643–651. doi: 10.1001/jamaoncol.2015.1590. [DOI] [PubMed] [Google Scholar]
- 20.Mesa RA, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): An international, randomised, phase 3 trial. Lancet Haematol. 2017;4:e225–e236. doi: 10.1016/S2352-3026(17)30027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer JW, et al. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol. 2016;8:11–19. doi: 10.2147/JEP.S110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999;189:1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadwa M, et al. IL-10 downregulates CXCR3 expression on Th1 cells and interferes with their migration to intestinal inflammatory sites. Mucosal Immunol. 2016;9:1263–1277. doi: 10.1038/mi.2015.132. [DOI] [PubMed] [Google Scholar]
- 24.Chen YB, et al. Expression of α4β7 integrin on memory CD8(+) T cells at the presentation of acute intestinal GVHD. Bone Marrow Transplant. 2013;48:598–603. doi: 10.1038/bmt.2012.191. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melton AC, et al. Regulation of IL-17A production is distinct from IL-17F in a primary human cell co-culture model of T cell-mediated B cell activation. PLoS One. 2013;8:e58966. doi: 10.1371/journal.pone.0058966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Issa F, et al. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90:1321–1327. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy P, et al. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood. 2003;101:2877–2885. doi: 10.1182/blood-2002-08-2566. [DOI] [PubMed] [Google Scholar]
- 29.Messmann JJ, et al. In vitro-generated MDSCs prevent murine GVHD by inducing type 2 T cells without disabling antitumor cytotoxicity. Blood. 2015;126:1138–1148. doi: 10.1182/blood-2015-01-624163. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med. 2015;7:308ra160. doi: 10.1126/scitranslmed.aab0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurence A, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Yoon JH, et al. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat Commun. 2015;6:7600. doi: 10.1038/ncomms8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebel K, et al. IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells. J Immunol. 2011;187:5627–5635. doi: 10.4049/jimmunol.1003998. [DOI] [PubMed] [Google Scholar]
- 35.Schmitt N, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Mooij CEM, Netea MG, van der Velden WJFM, Blijlevens NMA. Targeting the interleukin-1 pathway in patients with hematological disorders. Blood. 2017;129:3155–3164. doi: 10.1182/blood-2016-12-754994. [DOI] [PubMed] [Google Scholar]
- 38.Curran SA, Romano E, Kennedy MG, Hsu KC, Young JW. Phenotypic and functional activation of hyporesponsive KIRnegNKG2Aneg human NK-cell precursors requires IL12p70 provided by Poly(I:C)-matured monocyte-derived dendritic cells. Cancer Immunol Res. 2014;2:1000–1010. doi: 10.1158/2326-6066.CIR-14-0054-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krempler A, et al. Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis. 2004;40:52–57. doi: 10.1002/gene.20063. [DOI] [PubMed] [Google Scholar]
- 40.Bejarano PA, et al. The pathology of full-thickness cadaver skin transplant for large abdominal defects: A proposed grading system for skin allograft acute rejection. Am J Surg Pathol. 2004;28:670–675. doi: 10.1097/00000478-200405000-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.