Significance
The NLRP3 inflammasome is an intracellular oligomer regulating the activation of caspase-1 for the processing and secretion of IL-1β and IL-18. Although there is growing evidence to substantiate inflammasome inhibition as a therapeutic option for the treatment of inflammatory diseases, to date, there are no approved humans agents. OLT1177, a β-sulfonyl nitrile molecule, shown to be safe in humans, is a selective inhibitor of the NLRP3 inflammasome, with unique properties to reverse the metabolic costs of inflammation and to treat IL-1β– and IL-18–mediated diseases.
Keywords: interleukin-1, NLRP3, caspase-1
Abstract
Activation of the NLRP3 inflammasome induces maturation of IL-1β and IL-18, both validated targets for treating acute and chronic inflammatory diseases. Here, we demonstrate that OLT1177, an orally active β-sulfonyl nitrile molecule, inhibits activation of the NLRP3 inflammasome. In vitro, nanomolar concentrations of OLT1177 reduced IL-1β and IL-18 release following canonical and noncanonical NLRP3 inflammasome activation. The molecule showed no effect on the NLRC4 and AIM2 inflammasomes, suggesting specificity for NLRP3. In LPS-stimulated human blood-derived macrophages, OLT1177 decreased IL-1β levels by 60% and IL-18 by 70% at concentrations 100-fold lower in vitro than plasma concentrations safely reached in humans. OLT1177 also reduced IL-1β release and caspase-1 activity in freshly obtained human blood neutrophils. In monocytes isolated from patients with cryopyrin-associated periodic syndrome (CAPS), OLT1177 inhibited LPS-induced IL-1β release by 84% and 36%. Immunoprecipitation and FRET analysis demonstrated that OLT1177 prevented NLRP3-ASC, as well as NLRP3-caspase-1 interaction, thus inhibiting NLRP3 inflammasome oligomerization. In a cell-free assay, OLT1177 reduced ATPase activity of recombinant NLRP3, suggesting direct targeting of NLRP3. Mechanistically, OLT1177 did not affect potassium efflux, gene expression, or synthesis of the IL-1β precursor. Steady-state levels of phosphorylated NF-κB and IkB kinase were significantly lowered in spleen cells from OLT1177-treated mice. We observed reduced IL-1β content in tissue homogenates, limited oxidative stress, and increased muscle oxidative metabolism in OLT1177-treated mice challenged with LPS. Healthy humans receiving 1,000 mg of OLT1177 daily for 8 d exhibited neither adverse effects nor biochemical or hematological changes.
Interleukin-1β (IL–1β) is the prototypic inflammatory cytokine that promotes acute and chronic inflammation in a broad spectrum of diseases (1). Lacking a leader sequence, the IL-1β precursor requires maturation into an active cytokine by caspase-1. Activation of caspase-1 takes place by the oligomerization of intracellular proteins, termed inflammasomes, which induce the autocatalytic activation of caspase-1 (2, 3). Whereas several inflammasomes have been described, the NOD-like receptor (NLR) protein 3 (NLRP3) (also known as cryopyrin) has been extensively characterized for its role in models of disease (4, 5). Processing and secretion of active IL-1β and IL-18 via caspase-1 follows activation of the NLRP3 inflammasome. Pharmacologic inhibition or genetic deficiency of NLRP3 results in markedly reduced inflammation in animal models of human disease (6–8). Microbial products via Toll-like receptors (TLRs), host products generated during damaging inflammation, as well as IL-1 itself, activate NLRP3, which leads to release of active IL-1β and IL-18. Although neutralization of IL-1β has proven efficacious in the treatment of inflammation, to date, there is no approved therapeutic that inhibits the activation of the NLRP3 inflammasome in humans.
OLT1177 is an active moiety discovered during the investigation of synthetic reactions containing chlorinating agents and methionine. Structural elucidation established that OLT1177 is a β-sulfonyl nitrile (formula weight 133.17). We report here that OLT1177 is a selective inhibitor of the NLRP3 inflammasome. We also report that, in healthy humans, OLT1177 is safe at oral doses up to 1,000 mg each day for 8 d, with no clinical, hematological, or organ toxicities observed. Thus, OLT1177 has the potential for the treatment of IL-1β– and IL-18–mediated diseases.
Results
OLT1177 Suppresses IL-1β and IL-18 Release with No Effect on Priming or TNFα.
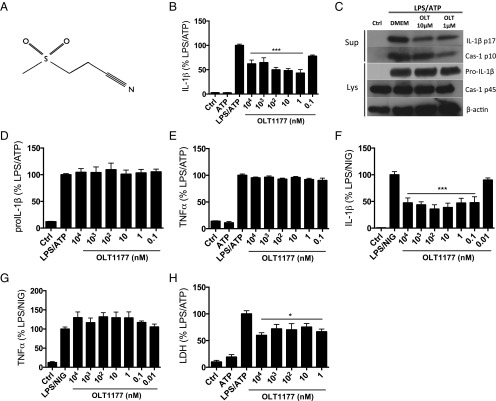
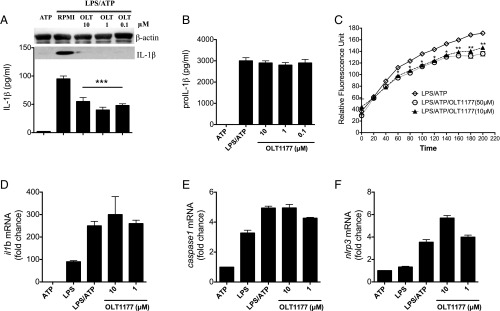
The structure of OLT1177 is depicted in Fig. 1A and is verified as described in SI Appendix, Figs. S1–S4. We evaluated the effect of OLT1177 on cytokine production in murine and human macrophages following NLRP3 inflammasome activation. In the murine macrophages cell line J774A.1, nanomolar concentrations of OLT1177 reduced IL-1β secretion by 50% following LPS/ATP stimulation (Fig. 1B). In human monocyte-derived macrophages (HMDMs), OLT1177 showed maximal inhibition (60%) of IL-1β and (70%) of IL-18 secretion at 1 µM (SI Appendix, Fig. S5 A and B). By Western blotting, mature IL-1β and the generation of the p10 subunit of active caspase-1 were reduced in the supernatant at 1 and 10 µM OLT1177 (Fig. 1C). In cell lysates, activated caspase-1 was reduced by 35% (P = 0.003) (SI Appendix, Fig. S5C) whereas there were no changes in the intracellular level of the IL-1β and caspase-1 precursors (Fig. 1 C and D). In the same supernatants, OLT1177 showed no effect in TNFα levels (Fig. 1E). Similar to cultures stimulated with LPS/ATP, OLT1177 inhibited IL-1β release in murine macrophages stimulated with LPS and nigericin (Fig. 1F), without affecting TNFα levels (Fig. 1G). Although LPS/ATP stimulation can result in lactate dehydrogenase (LDH) release as a measure of cell death, in the presence of OLT1177, the release of LDH was significantly reduced (P < 0.05) (Fig. 1H). To further investigate potential effects of OLT1177 on gene expression, mRNA levels of nlrp3, asc, caspase1, il1b, and il18 were measured. As shown in SI Appendix, Fig. S5 D–H, OLT1177 had no effect on mRNA levels of these genes, suggesting no effect on the priming phase of the NLRP3 inflammasome formation.
Fig. 1.
OLT1177 reduces IL-1β but not TNFα following NLRP3 inflammasome activation. (A) OLT1177 structure. (B) Mean ± SEM percent change (range 270 to 860 pg/mL) of secreted mature IL-1β from J774A.1 cells stimulated with LPS and ATP in the presence of increasing concentration of OLT1177. (C) Western blots for IL-1β (p17 and IL-1β precursor) and caspase-1 (p10 and p45) of supernatant (Sup) and cell lysates (Lys) from J774A.1 cells stimulated with LPS and ATP in the presence of OLT1177. (D) Mean ± SEM percent change (range 375 to 720 pg/mL) of total IL-1β protein level from J774A.1 cell lysates stimulated with LPS and ATP in the presence of OLT1177. (E) Mean ± SEM percent change (range 4,060 to 18,800 pg/mL) of secreted mature TNFα from J774A.1 cells stimulated with LPS and ATP in the presence of increasing concentration of OLT1177. (F and G) Mean ± SEM percent change of secreted mature IL-1β (range 100 to 3,024 pg/mL) (F) and TNFα (range 10,900 to 14,300 pg/mL) (G) from J774A.1 cells stimulated with LPS and nigericin (NIG) in the presence of OLT1177. (H) Mean ± SEM of LDH from J774A.1 cells stimulated with LPS and ATP in the presence of increasing concentration of OLT1177. Percent changes are calculated as described in Methods. The black bars represent the percent of IL-1β, TNFα, and LDH that is still expressed after treatment. ***P < 0.001, *P < 0.05. Data are representative of three independent experiments.
Noncanonical activation of the NLRP3 inflammasome by intracellular LPS leads to an alternative inflammasome pathway, mediated by caspase-4 in humans and caspase-11 in mice (9). We examined IL-1β release in human THP-1 cells stimulated with the TLR2/4 agonist Pam3CSK4, followed by transfection with LPS in WT and nlrp3-deficient cells. As shown in SI Appendix, Fig. S6, treatment with OLT1177 reduced IL-1β release in THP-1 cells, with maximal inhibition (70%) at 10 µM (P < 0.001). Cells deficient in nlrp3 showed low or no production of IL-1β, confirming the requirement of NLRP3 for IL-1β release in this model (SI Appendix, Fig. S6).
We next examined the effect of OLT1177 on downstream kinase phosphorylation activity in HMDM stimulated with LPS and nigericin. As depicted in SI Appendix, Fig. S7A, OLT1177 reduced several phosphorylated kinases. For example, there was a 26% reduction in Src activation, which, among other functions, activates the NF-κB pathway (10, 11); a 35% reduction in Fyn, which is involved in T-cell activation and motility (12, 13); a 43% reduction in the neutrophil activating kinase Hck (14); and a 33% reduction in STAT3, which affects autoimmune diseases and cancer (15). To demonstrate that OLT1177 does not influence LPS-mediated NF-κB activation, we used the specific IκB kinase inhibitor (IKKi) (ACHP). As shown in SI Appendix, Fig. S7B, ACHP reduced LPS/nigericin induction of IL-1β by 60%; however, in combination with OLT1177, the release of IL-1β was to the levels of unstimulated cells.
OLT1177 Prevents NLRP3 Inflammasome Formation.
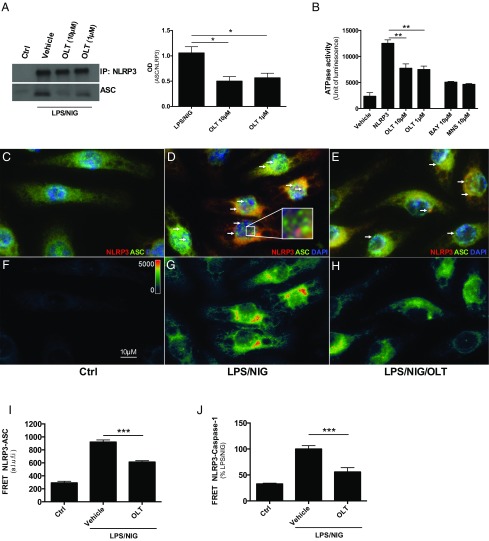
In J774A.1 cells, ASC immunoprecipitated with NLRP3 following LPS and nigericin, confirming the formation of the oligomer upon stimulation of the inflammasome (Fig. 2A). OLT1177 treatment resulted in a reduction in the NLRP3-mediated ASC recruitment (Fig. 2A, Left and Right). Using full-length recombinant human NLRP3 protein, we next measured the effect of OLT1177 on the nucleotide-binding domain of NLRP3. Recombinant NLRP3 exhibited ATPase activity (Fig. 2B), which was inhibited by OLT1177 at 1 and 10 µM (Fig. 2B). In the same assay, known inhibitors of the NLRP3 inflammasome, BAY and MNS, were used as positive controls (16, 17).
Fig. 2.
OLT1177 inhibits NLRP3 inflammasome formation. (A) Immunoprecipitation (IP, Left) and analysis (Right, mean of 3 ± SEM) of the NLRP3-ASC association in J774A.1 cells stimulated with LPS/nigericin (NIG). (B) ATPase activity of recombinant NLRP3 in the presence of OLT1177 (n = 3), Bay11-7082 (BAY) (n = 2), and 3,4-methylenedioxy-β-nitrostyrene (MNS) (n = 2). (C–E) Immunofluorescent staining and (F–H) FRET from NLRP3 (in red) and ASC (in green) in J774A.1 cells stimulated with LPS and NIG in presence of OLT1177 (10 µM). Representative ASC speck-like structures are indicated (white arrows) and highlighted in the Inset (D). Images were acquired using Slide Book 6 (Intelligent Imaging Innovation). (I and J) Mean ± SEM of FRET intensity from NLRP3-ASC (I) and percent change of FRET intensity from NLRP3-caspase-1 (J). ***P < 0.001, **P < 0.01, *P < 0.05. The data are mean of three independent experiments. a.l.u.f.i., arbitrary linear units of fluorescence intensity.
We next investigated the effect of OLT1177 on NLRP3 inflammasome formation using immunofluorescence and fluorescent resonance energy transfer (FRET) analysis. In unstimulated J774A.1 cells, NLRP3, ASC, and caspase-1 were diffuse in the cell (Fig. 2C and SI Appendix, Fig. S9A). However, following LPS and nigericin, ASC oligomerization was observed in the characteristic speck-like structure in the cytosol of J774A.1 cells (Fig. 2D). Cells treated with OLT1177 showed a reduction in speck structures, suggesting reduced inflammasome formation (Fig. 2E). ASC-NLRP3 association was further confirmed using FRET. FRET positivity requires the distance between two molecules, in this case NLRP3 and ASC, to be less than 30 nm. As shown in Fig. 2G, LPS and nigericin stimulation of J774A.1 cells showed higher FRET intensity for NLRP3 and ASC, indicating inflammasome oligomerization. Following treatment with OLT1177, the association between these two proteins was significant lower (Fig. 2 H and I). FRET analysis was also conducted to measure the proximity of NLRP3 and caspase-1 (SI Appendix, Fig. S9A). OLT1177 reduced NLRP3 and caspase-1 association following LPS and nigericin (Fig. 2J and SI Appendix, Fig. S9). Taken together, these data demonstrate that OLT1177 reduces the release of mature IL-1β by preventing NLRP3 inflammasome oligomerization.
OLT1177 Has No Effect on ATP-Induced Ion Current and Has No Effect on AIM2 and NLRC4 Inflammasomes.
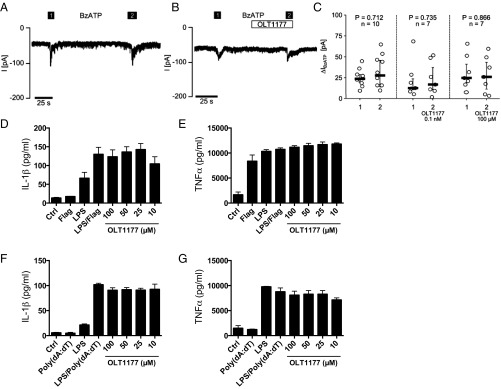
We next investigated the effect of OLT1177 on K+ efflux, a common activator of inflammasome function via activation of the P2X7 receptor (P2X7R) by extracellular ATP (18, 19). Ion current was measured in human LPS-primed monocytic U937 cells by whole-cell patch-clamp measurements. Two successive applications of 100 µM 2′(3′)-O-(4-benzoylbenzoyl)adenosine-5′-triphosphate tri(triethylammonium) salt (BzATP) resulted in the expected current change due to P2X7R activation (Fig. 3A); however, this was not affected by OLT1177 (Fig. 3 B and C). The lack of effect of OLT1177 on ion flux was confirmed using Xenopus laevis oocytes, which heterologously express human P2X7R. OLT1177 had no effect on ion flux in oocytes when treated with BzATP (SI Appendix, Fig. S10). We conclude that OLT1177 inhibits NLRP3 inflammasome activation by a mechanism downstream of P2X7R.
Fig. 3.
OLT1177 has no effect on ATP-induced ion currents and on NLRC4 and AIM2 inflammasomes. (A) Representative current change of whole-cell patch-clamp measurements performed on human U937 cells primed with LPS (1 µg/mL, 5 h) and treated with BzATP (100 µM) to induce P2X7R activation. (B) Treatment with 100 µM OLT1177 in parallel cultures following BzATP. (C) Analysis of multiple measurements of BzATP-induced current changes (∆IBzATP) in the absence and presence of OLT1177 (0.1 nM and 100 µM, Wilcoxon signed-rank test). ∆IBzATP values are shown as individual data points, bars represent median, and whiskers percentiles 25 and 75. (D and E) Mean ± SEM of secreted mature IL-1β and TNFα from J774A.1 cells stimulated with LPS and flagellin (Flag) in the presence of OLT1177. (F and G) Mean ± SEM of secreted mature IL-1β and TNFα from J774A.1 cells stimulated with LPS and Poly (dA:dT) in the presence of OLT1177 as measured in the supernatants. Data are derived from three independent experiments.
To investigate the potential effect of OLT1177 on other inflammasomes, murine macrophages were primed with LPS and stimulated with either flagellin to activate the NLRC4 inflammasome or the dsDNA analog Poly(dA:dT) to activate the AIM2 inflammasome. The release of IL-1β and TNFα was determined. Compared with the levels induced by the agonists, OLT1177 showed no effect on the release of these cytokines following NLRC4 (Fig. 3 D and E) or AIM2 (Fig. 3 F and G) inflammasome activation. These data indicate that OLT1177 is specific for the NLRP3 inflammasome.
OLT1177 Reduces IL-1β Release in Freshly Obtained Human Blood Neutrophils.
Neutrophil recruitment and tissue infiltration are hallmarks of the inflammatory response to injury and infection. Therefore, we investigated the effect of OLT1177 on freshly obtained human blood neutrophils following LPS and ATP stimulation. Processing and release of IL-1β precursor in primary human neutrophils was observed in cells stimulated with LPS and ATP (Fig. 4A). OLT1177 prevented the release of IL-1β (Fig. 4A) without affecting the total intracellular pool of the IL-1β precursor (Fig. 4B). Treatment of neutrophils with OLT1177 also reduced caspase-1 activity at 50 and 10 µM (Fig. 4C) without affecting il1b, caspase1, and nlrp3 mRNA levels (Fig. 4 D–F).
Fig. 4.
OLT1177 reduces IL-1β release, caspase-1 activity in primary human blood neutrophils. (A) IL-1β levels in the supernatants from primary human neutrophils stimulated with LPS and ATP in the presence of concentrations of OLT1177 (0.1, 1, and 10 µM) by ELISA (Below) and Western blot (Above) (mature 17 kDa IL-1β). (B) IL-1β precursor levels in the lysates of the cells shown in A. (C) Caspase-1 activity in human neutrophils following LPS/ATP in the presence of OLT1177. Data are expressed as mean ± SEM of neutrophils from three healthy donors. (D–F) Fold change of mRNA levels from neutrophils stimulated with LPS and ATP in the presence of OLT1177. Data are expressed as mean ± SEM of cells from four healthy donors. ***P < 0.001, **P < 0.01, *P < 0.05.
OLT1177 Reduces the Severity of LPS-Induced Systemic Inflammation in Vivo.
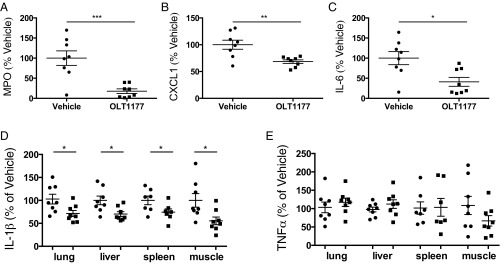
We next examined the efficacy of OLT1177 treatment in mice challenged with LPS-induced systemic inflammation. As shown in Fig. 5 A–C, OLT1177 significantly reduced peritoneal fluid myeloperoxidase (MPO) by 80%, the neutrophil chemokine (C-X-C motif) ligand 1 (CXCL1) by 30%, and IL-6 by 44%. The total IL-1β content in lung, liver, spleen, and skeletal muscle was also significantly decreased (Fig. 5D); no significant changes were detected for TNFα content in these tissues (Fig. 5E).
Fig. 5.
OLT1177 reduces LPS-induced inflammation in vivo. (A–C) Mean ± SEM percent change of (A) myeloperoxidase (MPO) (range 1,755 to 1,870 pg/mL), (B) CXCL1 (range 971 to 10,200 pg/mL), and (C) IL-6 (range 807 to 1,580 pg/mL) in vehicle (round symbol) and OLT1177-treated group (square symbol) in the peritoneal fluid 2 h after i.p. LPS (5 mg/kg). Data are expressed as percent change in vehicle-treated mice. (D and E) Mean ± SEM percent change of IL-1β (range 70 to 1,242 pg/mL) (D) and TNFα (range 70 to 301 pg/mL) (E) level in organ homogenates of mice treated with OLT1177 (square symbol) relative to vehicle (round symbol). Percent changes are calculated as described in Methods. n = 7 to 8 mice per group. ***P < 0.001, **P < 0.01, *P < 0.05.
OLT1177 Increases Oxidative Metabolism in Vivo.
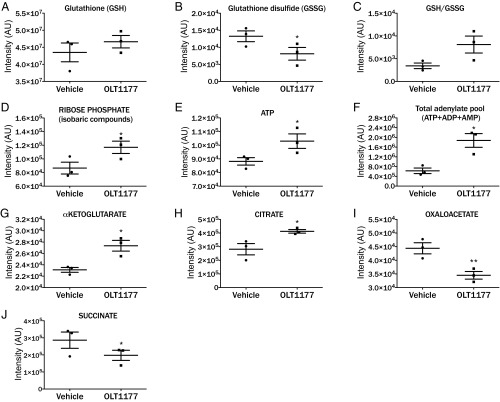
Marked alterations in muscle metabolism are observed during acute and chronic inflammation (20, 21). We investigated the effect of OLT1177 on metabolic changes in muscle following LPS-induced systemic inflammation. Muscle tissue was excised 2 h after LPS administration and examined for metabolic changes (Fig. 6). Treatment of mice with OLT1177 decreased metabolites of oxidative stress with increased reduced glutathione (GSH) and decreased oxidized glutathione (GSSG) levels, leading to an increased GSH/GSSG ratio (Fig. 6 A–C). Improved glutathione homeostasis in OLT1177-treated mice could be explained, in part, by an increase in the NADPH-generating pentose phosphate pathway, for antioxidant effects or to sustain nucleoside anabolism, which is reflected by increased ribose phosphate levels (Fig. 6D). Since pentose phosphate moieties can also fuel the nucleoside biosynthetic pathways, we determined the relative levels of high energy purine nucleosides (ATP) and the total levels of adenylate metabolites (ATP, ADP, and AMP). As shown in Fig. 6 E and F, higher levels of ATP were measured in muscle tissue from mice treated with OLT1177. These higher levels of adenylate metabolites can be due to increased adenylate synthesis or decreased consumption of high energy phosphate compounds, both hallmarks of cell activation following LPS stimulation (22). Consistent with a reduction in oxidative stress, steady-state levels of TCA cycle intermediate α-ketoglutarate were increased in OLT1177-treated animals (Fig. 6G). Treatment with OLT1177 also showed accumulation of citrate and decreased levels of oxaloacetate (Fig. 6 H and I). These data are indicative of activation of early oxidative reactions of mitochondrial metabolism. Consistent with increased oxidative metabolism, tissue levels of the proinflammatory metabolic marker succinate were decreased in response to OLT1177 administration (Fig. 6J).
Fig. 6.
OLT1177 reduces muscle oxidative stress and increases oxidative metabolism in vivo. Metabolomic analyses were performed on muscle 2 h following LPS challenge from mice treated with OLT1177 or vehicle. Mean ± SEM in arbitrary units (AU) for reduced glutathione (GSH) (A); oxidized glutathione (GSSG) (B); GSH/GSSG ratios (C); ribose phosphate (D); ATP (E); and total adenylate pool (F); α-ketoglutarate (G); citrate (H); oxaloacetate (I); and succinate (J). n = 3 mice per group. **P < 0.01, *P < 0.05.
Effects of Treatment with OLT1177 on Constitutive Kinase Phosphorylation in Vivo.
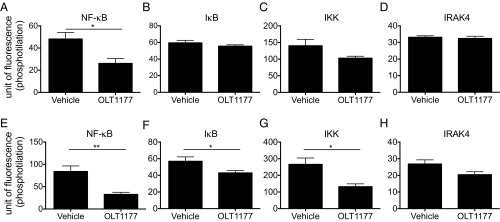
Mice were treated with OLT1177, and spleen cells were isolated and studied ex vivo for changes in phosphorylation of NF-κB, IκB, IKK, and interleukin-1 receptor-associated kinase 4 (IRAK4). As shown in Fig. 7A, compared with vehicle-treated mice, steady-state constitutive phosphorylated NFκB was significantly reduced in mice treated with OLT1177 at the time of spleen cell isolation. There were no statistically significant changes in the other kinases (Fig. 7 B–D). However, when spleen cells from OLT1177-treated mice were activated in vitro with LPS, there was a significant reduction in phosphorylated NF-κB, IκB, and IKK after 30 min, with no change in IRAK4 (Fig. 7 E–H). Thus, OLT1177 primes these kinases in vivo to be less responsive to an inflammatory stimulus.
Fig. 7.
OLT1177 reduces phosphorylation of NF-κB. (A–D) Mean ± SEM of NF-κB, IκB, IKK, and IRAK4 phosphorylation measured in fresh CD11b+CD3− spleen-derived cells from mice treated with saline (vehicle) or OLT1177 under steady-state conditions. (E–H) Mean ± SEM of NF-κB, IκB, IKK, and IRAK4 phosphorylation measured in CD11b+CD3− spleen-derived cells from mice treated with saline (vehicle) or OLT1177 stimulated for 30 min with LPS. n = 3 mice per group. **P < 0.01, *P < 0.05.
OLT1177 Reduces IL-1β Release in Cells from Subjects with NLRP3 Mutations.
OLT1177 was tested in adherent monocytes from patients affected by cryopyrin-associated periodic syndrome (CAPS), a rare autoinflammatory disease characterized by gain-of-function mutations in NLRP3 (23). Patients with CAPS suffer from recurrent episodes of systemic inflammation, fever, and debilitation (24, 25). Blood monocytes isolated from two CAPS patients were stimulated with LPS in the presence and absence of OLT1177, which was added 30 min following LPS. As shown in Fig. 8, OLT1177 reduced IL-1β levels in the supernatants by 84% and 36%, respectively.
Fig. 8.

OLT1177 reduces IL-1β release in peripheral blood mononuclear cells (PBMCs) from human subjects with NLRP3 mutations. Mean levels of IL-1β in the supernatants of PBMCs from two patients affected by CAPS. Patient A (A) represents a severe case of neonatal onset multisystem inflammatory disease (NOMID) with mutation E567K, and patient B (B) represents a case of Muckle–Wells syndrome (MWS) with mutation E525K. Cells were stimulated with LPS (100 ng/mL) for 18 h in the presence of vehicle (PBS) or OLT1177 (1 µM) (OLT). Vehicle and OLT1177 were added 30 min after LPS.
Human Pharmacokinetic Profile.
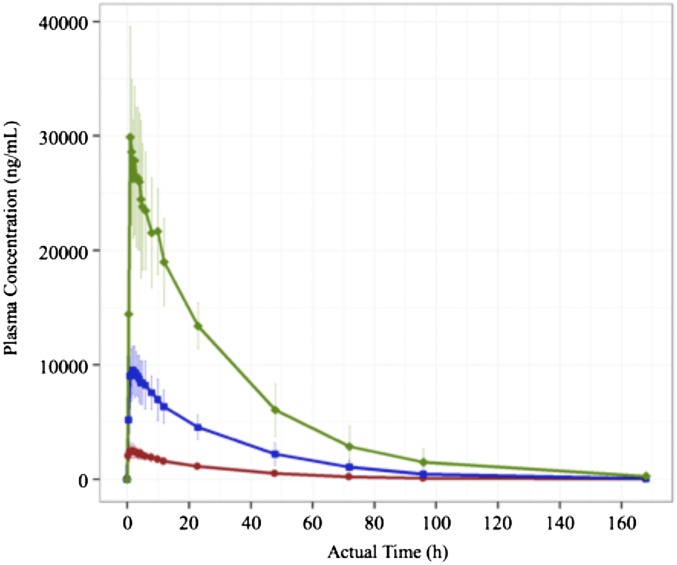
Following a single oral dose, the group mean plasma maximum concentrations (Cmax) were 2,700 ng/mL for the 100-mg dose, 9,800 ng/mL for the 300-mg dose, and 32,000 ng/mL for the 1,000-mg dose (Fig. 9). The mean plasma elimination half-life for each group was 23.0, 22.8, and 24.2 h for the 100-mg, 300-mg, and 1,000-mg dose groups, respectively. Mean pharmacokinetic parameters for OLT1177 after a single dose in fasted subjects are summarized in SI Appendix, Table S1. Plasma OLT1177 exposure was also measured after repeated daily oral dosing. Mean plasma concentrations of OLT1177 (±SD) on the first (day 1) and last (day 8) of 8 d of oral dosing are presented in SI Appendix, Fig. S11. The mean group maximum concentrations on day 8 were 4,800 ng/mL for the 100-mg dose, 15,800 ng/mL for the 300-mg dose, and 41,400 ng/mL for the 1,000-mg dose. The plasma elimination half-life for each multidose group on day 8 and other pharmacokinetic parameters for OLT1177 after multiple doses in fasted subjects are summarized in SI Appendix, Table S2.
Fig. 9.
OLT1177 is rapidly absorbed after oral administration. Plasma OLT1177 concentration versus time after a single oral dose of 100 mg (red), 300 mg (blue), and 1,000 mg (green). n = 5 for each dose group.
OLT1177 Safety Profile.
Seven of 35 subjects reported a total of seven treatment-emergent adverse events (SI Appendix, Table S3), and one subject reported a non–treatment-emergent adverse event (AE) during the course of the study. Five of the seven events occurred after a single dose of OLT1177, and two occurred during the multidose regimen (both in the 100-mg dose group). The reported AEs were considered to be unrelated to OLT1177 by the investigator, and each resolved spontaneously. Physical examination in the subjects exposed to OLT1177 revealed no change from the predrug examination. Systolic and diastolic blood pressure, heart rate, and body temperature showed minor fluctuations from baseline to the end of the study. Overall, there was no correlation between changes in vital signs and OLT1177 dose or timing of treatment. No significant changes were observed in hematologic or coagulation values at any time during the study, compared with baseline values. In addition, there were no significant changes from baseline in serum lipids, urinalysis, liver function enzymes, or acute phase proteins in any cohort after eight consecutive days of up to 1,000-mg dosing.
Discussion
Although inhibition of the NLRP3 inflammasome reduces inflammation in murine models of acute and chronic inflammatory diseases (6, 17, 26), there is currently no approved inflammasome inhibitor for human use. Here, we characterize the sulfonyl nitrile compound OLT1177 as a specific inhibitor of the NLRP3 inflammasome, with no effect on the NLRC4 and AIM2 inflammasomes. Unlike other NLRP3 inhibitors (27), we report here the findings of OLT1177 administered orally to healthy subjects in a phase 1 study. As presented, OLT1177 was safe and well-tolerated and achieved meaningful exposure and a long half-life without any clinical, hematological, or organ toxicities observed at any dose tested.
In addition, adherent monocytes from two patients with CAPS were stimulated with LPS and released less IL-1β in vitro in the presence of 1 µM OLT1177.
We investigated the properties OLT1177 in human blood monocyte-derived macrophages, human and murine cell lines, and primary human blood neutrophils. Neutrophils play a pathological role in many acute diseases, and elevated blood neutrophil counts are characteristic of autoinflammatory diseases mediated by IL-1β: for example, acute gout flares (28–30) and familial Mediterranean fever (31–33). We observed that OLT1177 reduces the processing of the IL-1β precursor in freshly obtained human neutrophils, with no change in caspase1, nlrp3, and il1b mRNA.
OLT1177 treatment consistently reduced IL-1β release from human blood macrophages, differentiated THP-1 cells, and the murine macrophage cell line J774A.1. We observed that the in vitro concentration of OLT1177 necessary to reduce IL-1β differed among cell types and experimental conditions. We also compared OLT1177 with MCC950, a reported NLRP3 inflammasome inhibitor (6) in J774A.1 cells. OLT1177 reduced IL-1β secretion in a comparable manner compared with MCC950 following activation of the NLRP3 inflammasome with LPS and ATP (SI Appendix, Fig. S8). Nevertheless, OLT1177 significantly reduced IL-1β release at 1 µM or lower concentrations in vitro. By comparison, mean peak plasma levels after a single oral dose in humans reached concentrations of 20.3, 73.6, and 240.3 µM, respectively, for doses of 100, 300, and 1,000 mg per subject. Subjects also received 100, 300, or 1,000 mg/d for eight consecutive days, which resulted in mean maximum plasma concentrations at steady state of 36.0, 118.6, and 310.9 µM, respectively. Thus, based on the observed in vitro potency levels, OLT1177 reaches effective plasma levels in vivo at molar concentrations greater than 100-fold those needed to inhibit activation of the NLRP3 inflammasome in vitro.
NLRP3 inflammasome formation also induces maturation and release of IL-18. Consistent with inhibition of the NLRP3 inflammasome activation, OLT1177 significantly reduced IL-18 release in human monocyte-derived macrophages (70% inhibition at 1 µM). Preclinical studies reveal that IL-18 is a target in several models of inflammation (34), including heart failure (35) and macrophage activation syndrome (MAS) (36), each treated with IL-1β blockade by the IL-1β antagonist anakinra. These and other data (35) support the concept that IL-1 drives the activation of caspase-1, resulting in increased IL-18 processing and release. In patients with CAPS, a classic NLRP3 gain-of-function disease, blocking IL-1 with anakinra reduced caspase-1 blood monocyte caspase-1 mRNA levels (37).
FRET analysis confirmed that LPS/nigericin treatment results in the formation of NLRP3-ASC and NLRP3-(pro)caspase-1 complexes at a distance of less than 30 nm, which was significantly reduced in the presence of OLT1177. Inhibition of the NLRP3-ASC oligomerization by OLT1177 was confirmed by immunoprecipitation. The central domain of NLRP3 exhibits nucleotide-binding properties associated with ATPase activity, which is required for the NLRP3 self-oligomerization and ASC recruitment (38). In a cell-free system, OLT1177 inhibited ATPase activity, suggesting a direct interaction with the NLRP3 protein.
The P2X7 receptor plays a crucial role in inflammasome activation and ion currents (19). Here, we show that OLT1177 reduces NLRP3 inflammasome activation and IL-1β release subsequent to ATP and nigericin stimulation, which induce K+ efflux. However, OLT1177 does not affect K+ efflux, and we conclude that the properties of OLT1177 are independent of ion currents.
We assessed the in vivo effect of OLT1177 following systemic administration of LPS in mice. Treatment with OLT1177 limited LPS-induced acute inflammatory response by reducing MPO, CXCL1, and IL-6 levels in the peritoneal fluid and IL-1β in whole tissue homogenates. Secondary effects of OLT1177 when administered in vivo are likely to be downstream from NLRP3 inflammasome activation. For example, without LPS stimulation, constitutive levels of phosphorylated NF-κB were decreased in spleen cells from mice treated with OLT1177. Systemic inflammation affects cell metabolism as it represents the energy costs of inflammation. Metabolic derangements, such as the Warburg effect, have been well-characterized in tumors (39) when malignant cells produce energy via glycolysis (39). Proinflammatory stimuli, i.e., LPS, activate macrophages and dendritic cells, driving systemic and tissue metabolic reprogramming similar to that of the Warburg effect (22, 40, 41). We show here that OLT1177 protects from LPS-induced oxidative stress, by promoting glutathione homeostasis. The steady-state observations suggest increased fluxes through the pentose phosphate pathway in muscle of OLT1177-treated mice, which may explain the improved redox state observed. Additionally, accumulation of pentose phosphate moieties may fuel the biosynthesis of high energy phosphate nucleosides, contributing to the improved energy balance observed in the muscles of OLT1177-treated mice.
OLT1177 treatment also resulted in the accumulation of citrate, with a reduction in oxaloacetate, which limits LPS-induced citrate metabolism (42, 43). Indeed, succinate levels correlate with the stabilization of HIF-1α and the induction of proinflammatory cascades leading to the accumulation and release of IL-1β (44). In the present study, we showed that, parallel to decreased IL-1β levels, OLT1177 also reduced muscle succinate levels. Succinate accumulation is mostly driven by mitochondrial uncoupling at the electron transport chain level, secondary to increased oxidative stress. Here, we show that OLT1177 promotes mitochondrial metabolism in a tissue with high energy demands while preventing the accumulation of proinflammatory succinate.
Methods
OLT1177 Synthesis.
The drug substance was manufactured by alkylation of sodium methanesulfinate with 3-bromopropionitrile to yield methylsulfonylpropionitrile (crude drug substance). The crude drug substance was then purified by dissolution into acetone, filtration of the sodium bromide byproduct, solvent exchange via distillation, and recrystallization from ethanol.
Inflammasome Stimulation.
Details for NLRP3 inflammasome formation in HMDM, human neutrophils, and J774A.1 murine macrophages are presented in SI Appendix.
NLRP3 ATPase Activity.
Details for the NLRP3 ATPase activity measurements can be found in SI Appendix.
Immunoprecipitation.
Details for the immunoprecipitation can be found in SI Appendix.
Immunofluorescent Microscopy and Fluorescent Resonance Energy Transfer Analysis.
J774A.1 cells were stimulated as described above. After washing with PBS, the cells were fixed and permeated with 70–30 acetone–methanol as previously described (45). Briefly, cells were placed in a humidified slide chamber blocked for 1 h in 10% normal donkey serum (Jackson Immunologicals). After removal of the donkey serum, the primary antibodies to NLRP3 (Cryo-2; AdipoGen), caspase-1 (sc-514; Santa Cruz Biotechnology), and ASC (D2W8U; Cell Signaling) or isomolar, species-specific anti-IgG (R&D Systems, Inc.) were added and incubated overnight at 4 °C. The slides were then washed three times with PBS, and cells were incubated for 1 h at room temperature with donkey anti-mouse–Alexa555 (Life Technologies) or donkey anti-rabbit–Alexa488 (Life Technologies) conjugated secondary antibodies. Nuclei were stained with DAPI (Life Technologies). FRET images were acquired using a Marianas Imaging Station (Intelligent Imaging Innovations) using a Zeiss 639 Plan-Apochromat objective (1.4 N.A.), a Sutter Xenon light source, and a Cooke SensiCam (The Cooke Corporation) as previously described (45). The FRET was calculated as FRET = Transfer − (Fd/D) − (Fa/Aa) as reported (46).
Flow Cytometry.
Details on flow cytometry to assess levels of phosphokinases can be found in SI Appendix.
Whole-Cell Patch-Clamp Recordings.
For electrophysiological recordings, U937 cells were placed in poly-l-lysine–coated cell culture dishes (Nunc) as previously described (47). Briefly, after incubation with 1 μg/mL LPS from Escherichia coli (L2654; Sigma-Aldrich) for 5 h, whole-cell patch-clamp recordings were performed at room temperature. Cells were clamped to −60 mV, and transmembrane currents were recorded using an EPC-9 amplifier (HEKA) and acquired via an ITC-16 interface with Pulse software (HEKA). A 100-mM stock solution of 2′(3′)-O-(4-benzoylbenzoyl)adenosine-5′-triphosphate tri(triethylammonium) salt (BzATP) (Jena Bioscience) was prepared and dissolved in the bath solution to a 100-µM working solution and applied via a pressure-driven microperfusion system.
In Vivo LPS Challenge.
Animal protocols were approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. C57BL/6J male mice, 22 to 26 g (The Jackson Laboratory), were treated with 200 mg/kg OLT1177 in 200 µL of saline or the matching volume of vehicle (saline) intraperitoneally (i.p.) every 12 h for five doses. One hour after the last dose of OLT1177 or saline, the animals were injected i.p. with 200 μL of LPS (E. coli, 055:B5; Sigma-Aldrich) at 5 mg/kg and killed after 2 h. Cytokines were measured in the peritoneal fluid and in homogenates of spleen, lung, liver, and skeleton muscle (quadriceps). Tissue homogenates were centrifuged at 13,000 × g for 20 min at 4 °C, and the supernatants were assayed for cytokine levels. The cytokine concentrations were corrected for total protein content. For experiments with peritoneal lavage, 10 mL of cold PBS were injected in the peritoneal cavity before organ collection. The peritoneal lavage fluids were then centrifuged at 1,000 × g for 5 min, and the supernatants were used for cytokine measurement.
Metabolomics Analysis.
Metabolomics analysis was performed, and details can be found in SI Appendix.
CAPS Patients.
Blood samples from two CAPS patients positive for the mutation of the nlrp3 gene were taken after informed consent by patients or their legal guardian and as approved by the “G. Gaslini” Ethical Board.
Human Safety and Pharmacokinetic Study.
Details of the human study can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank Ralph J. A. Mass and Niklas Lonnemann for assistance in cytokine measurements; and Julie R. Haines for assistance in the metabolomics analysis. We also thank G. Schmalzing and R. Hausmann (Rheinisch-Westfälische Technische Hochschule Aachen University) for providing us the human P2X7R cRNA and for their assistance and support. These studies are supported by NIH Grant AI-15614, The Interleukin Foundation, Olatec Therapeutics LLC, and German Research Foundation Grant GR 1094/7-1.
Footnotes
Conflict of interest statement: C.A.D. serves as Chairman of Olatec’s Scientific Advisory Board, is co-Chief Scientific Officer, and receives compensation. L.A.B.J. serves on Olatec’s Scientific Advisory Board and receives compensation. J.P.S.L. has equity in Olatec. D.B.S. serves as Chairman and Chief Executive Officer of Olatec, R.B.B. serves as Chief Operating Officer of Olatec, and C.L.S. and R.D.A. serve on Olatec’s Scientific Advisory Board.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716095115/-/DCSupplemental.
References
- 1.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Callaway JB, Ting JP. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman HM, Wanderer AA. Inflammasome and IL-1beta-mediated disorders. Curr Allergy Asthma Rep. 2010;10:229–235. doi: 10.1007/s11882-010-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coll RC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youm YH, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 10.Lee HS, et al. Src tyrosine kinases mediate activations of NF-kappaB and integrin signal during lipopolysaccharide-induced acute lung injury. J Immunol. 2007;179:7001–7011. doi: 10.4049/jimmunol.179.10.7001. [DOI] [PubMed] [Google Scholar]
- 11.Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: Activation of nuclear factor-kappaB via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 12.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 13.Sugie K, Jeon MS, Grey HM. Activation of naïve CD4 T cells by anti-CD3 reveals an important role for Fyn in Lck-mediated signaling. Proc Natl Acad Sci USA. 2004;101:14859–14864. doi: 10.1073/pnas.0406168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, et al. Src kinase Hck association with the WASp and mDia1 cytoskeletal regulators promotes chemoattractant-induced Hck membrane targeting and activation in neutrophils. Biochem Cell Biol. 2009;87:207–216. doi: 10.1139/O08-130. [DOI] [PubMed] [Google Scholar]
- 15.Milner JD, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, et al. 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juliana C, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Planillo R, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marette A, Liu Y, Sweeney G. Skeletal muscle glucose metabolism and inflammation in the development of the metabolic syndrome. Rev Endocr Metab Disord. 2014;15:299–305. doi: 10.1007/s11154-014-9296-6. [DOI] [PubMed] [Google Scholar]
- 21.Shearer JD, Amaral JF, Caldwell MD. Glucose metabolism of injured skeletal muscle: The contribution of inflammatory cells. Circ Shock. 1988;25:131–138. [PubMed] [Google Scholar]
- 22.Krawczyk CM, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman HM, et al. Efficacy and safety of rilonacept (interleukin-1 trap) in patients with cryopyrin-associated periodic syndromes: Results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 25.Lachmann HJ, et al. Canakinumab in CAPS Study Group Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 26.Cook GP, Savic S, Wittmann M, McDermott MF. The NLRP3 inflammasome, a target for therapy in diverse disease states. Eur J Immunol. 2010;40:631–634. doi: 10.1002/eji.200940162. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin AG, Brough D, Freeman S. Inhibiting the inflammasome: A chemical perspective. J Med Chem. 2016;59:1691–1710. doi: 10.1021/acs.jmedchem.5b01091. [DOI] [PubMed] [Google Scholar]
- 28.Cleophas MC, Crişan TO, Joosten LA. Factors modulating the inflammatory response in acute gouty arthritis. Curr Opin Rheumatol. 2017;29:163–170. doi: 10.1097/BOR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 29.Liu-Bryan R. Intracellular innate immunity in gouty arthritis: Role of NALP3 inflammasome. Immunol Cell Biol. 2010;88:20–23. doi: 10.1038/icb.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1β, and gout: Is there a link? Semin Immunopathol. 2013;35:501–512. doi: 10.1007/s00281-013-0361-0. [DOI] [PubMed] [Google Scholar]
- 31.Chae JJ, Aksentijevich I, Kastner DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol. 2009;146:467–478. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manukyan G, et al. Activated phenotype of circulating neutrophils in familial Mediterranean fever. Immunobiology. 2013;218:892–898. doi: 10.1016/j.imbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Ozen S. Familial mediterranean fever: Revisiting an ancient disease. Eur J Pediatr. 2003;162:449–454. doi: 10.1007/s00431-003-1223-x. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toldo S, et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–H1031. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazodier K, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–3489. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan JA, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everts B, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKε supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15:323–332. doi: 10.1038/ni.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Infantino V, et al. The mitochondrial citrate carrier: A new player in inflammation. Biochem J. 2011;438:433–436. doi: 10.1042/BJ20111275. [DOI] [PubMed] [Google Scholar]
- 43.Infantino V, Iacobazzi V, Palmieri F, Menga A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem Biophys Res Commun. 2013;440:105–111. doi: 10.1016/j.bbrc.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gamboni F, et al. Clathrin complexes with the inhibitor kappa B kinase signalosome: Imaging the interactome. Physiol Rep. 2014;2:e12035. doi: 10.14814/phy2.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berney C, Danuser G. FRET or no FRET: A quantitative comparison. Biophys J. 2003;84:3992–4010. doi: 10.1016/S0006-3495(03)75126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richter K, et al. Phosphocholine–An agonist of metabotropic but not of ionotropic functions of α9-containing nicotinic acetylcholine receptors. Sci Rep. 2016;6:28660. doi: 10.1038/srep28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.