Significance
Cells require specific molecular entities to regulate biological processes, which are often out of balance in diseases. Once identified, their activities may be modulated by specific ligands. DPP4 protein is an example of a target to successfully treat type II diabetes by small molecule ligands. Besides DPP4, other members of this protein family, DPP8 and DPP9 are similarly interesting and relevant in immune response and cancer. It is crucial to understand their structures and enzymatic mechanism to enable structure-based drug development. Here we unveil the crystallographic structures of DPP8 and DPP9, whereby we observe a different active site architecture and substrate binding mechanism in this family. These discoveries open new options for drug development targeting DPP8 and DPP9.
Keywords: DPP4, DPP8, DPP9, SUMO1
Abstract
Dipeptidyl peptidases 8 and 9 are intracellular N-terminal dipeptidyl peptidases (preferentially postproline) associated with pathophysiological roles in immune response and cancer biology. While the DPP family member DPP4 is extensively characterized in molecular terms as a validated therapeutic target of type II diabetes, experimental 3D structures and ligand-/substrate-binding modes of DPP8 and DPP9 have not been reported. In this study we describe crystal and molecular structures of human DPP8 (2.5 Å) and DPP9 (3.0 Å) unliganded and complexed with a noncanonical substrate and a small molecule inhibitor, respectively. Similar to DPP4, DPP8 and DPP9 molecules consist of one β-propeller and α/β hydrolase domain, forming a functional homodimer. However, they differ extensively in the ligand binding site structure. In intriguing contrast to DPP4, where liganded and unliganded forms are closely similar, ligand binding to DPP8/9 induces an extensive rearrangement at the active site through a disorder-order transition of a 26-residue loop segment, which partially folds into an α-helix (R-helix), including R160/133, a key residue for substrate binding. As vestiges of this helix are also seen in one of the copies of the unliganded form, conformational selection may contributes to ligand binding. Molecular dynamics simulations support increased flexibility of the R-helix in the unliganded state. Consistently, enzyme kinetics assays reveal a cooperative allosteric mechanism. DPP8 and DPP9 are closely similar and display few opportunities for targeted ligand design. However, extensive differences from DPP4 provide multiple cues for specific inhibitor design and development of the DPP family members as therapeutic targets or antitargets.
Members of the dipeptidyl peptidase (DPP) family are N-terminal dipeptide postproline-cleaving serine proteases. DPP4, DPP8, and DPP9 have been extensively studied in view of their roles in physiological processes and pathologies of the immune system and inflammation (1–3). DPP4 is extracellular, either as a soluble protein in the body fluids or anchored to the plasma membrane. By controlling the activity of the gastrointestinal incretin hormones, DPP4 plays an important role in glucose homeostasis, is a drug target in clinical use for type II diabetes, and is explored for other disease areas (4).
The two homologs DPP8 and DPP9 are intracellular, localized to the cytosol and nucleus, but also associate with the plasma membrane (5–8). Although DPP8 and DPP9 may be partially redundant due to their cellular localization and similar enzymatic specificities, accumulating evidence suggests that they also have separate physiological roles. Recent findings based on inhibitor studies show a role for DPP9 and DPP8 in the immune system (9–11) and in preadipocyte differentiation (12). Other publications show that DPP9 is essential for neonatal survival (13, 14) and plays a role in antigen maturation (15), cell migration, and cell adhesion (8). Several screens for DPP8/9 natural substrates were performed (16, 17). Recently, the spleen tyrosine kinase (Syk) was shown to be processed by DPP9, thereby regulating B cell signaling (18). DPP9 activity is also connected to pathophysiological conditions, as promoting tumoregenicity and metastasis in nonsmall cell lung cancer (19). Recently, DPP9 fusion genes were identified in high-grade serous ovarian carcinoma, suggesting that DPP9 rearrangements might play a role in tumorigenesis or tumor progression (20).
DPP4 has been broadly characterized structurally by X-ray crystallographic methods in unliganded and liganded forms (21, 22). DPP4 has two characteristic domains: one α/β catalytic and a β-propeller domain. Two channels lead from the surface to the active site, which is located between these two structures. The first one traverses the lumen of the β-propeller domain spanned by its eight blades. The second, perpendicular to the first one, opens sidewise. The latter provides access for substrates in DPP4 (23). The N terminus of substrates binds to two conserved glutamic acid residues in DPP4 located to the EE-helix (E205, E206) and an arginine (R125) at the R-loop. R125 undergoes a side-chain rearrangement in the presence of substrate (24, 25). Besides this small difference upon ligand binding, the structure remains invariant otherwise.
Similar to DPP4, DPP8 and DPP9 are also active dimers with small fractions of tetrameric and monomeric species (26). Mutational studies have been carried out to understand the role of different parts of the proteins and their relation to enzymatic activity (15, 27, 28). Unlike for DPP4, few inhibitors had been developed to modulate DPP8 or DPP9 activities. 1G244, a small molecule, inhibits DPP8 and DPP9 specifically, with a small preference for DPP8, being inactive against DPP4 (29). Since 1G244 bears an isoindoline moiety at P1 and a spacious 1-(4-4′-difluor-benzhydryl)-piperazine group at the P2 position, it has been proposed that DPP8 and DPP9 may have a larger active site cavity compared with DPP4 (30). Despite efforts in the synthesis of variants of this molecular entity, the problem to generate significant specificity toward either DPP8 or DPP9 remained unsolved (31).
With regard to potential interacting partners, DPP4 has been extensively studied and adenosine deaminase was identified early (32). SUMO1 was identified as an interacting partner of DPP8 and DPP9 in SUMO-pulldown assays (33). The region of interaction on SUMO1 was mapped and termed the E67 interacting loop (EIL), since a mutation in SUMO1E67 leads to loss of binding. As expected, an EIL peptide was able to compete with the interaction of SUMO1 with DPP8 or DPP9. Surprisingly however, EIL also exhibited an inhibitory effect on DPP8/9. In further evaluation, a systematic variation in the EIL amino acid sequence rendered a strong inhibitor, SLRFLYEG. Finally, the region of interaction with DPP9 was attributed to a predicted extended arm close to the active site, named SUMO1 binding arm (SUBA). SLRFLYEG was proposed as an allosteric inhibitor with specificity against DPP8 and DPP9 (34).
To understand in detail the nature of interaction between DPP8/9 and the 1G244 and SLRFLYEG inhibitors, we performed crystallographic structural studies of both proteins and their complexes with those ligands. Furthermore, we determined kinetics of complex formation to explore the substrate binding mode. DPP8 and DPP9 sequence comparisons against DPP4 suggest the existence of new structural features, possible candidates to generate specific inhibition of these important pharmacological drug targets.
Experimental Procedures
Protein Expression and Purification.
Human cDNAs of DPP8 isoform 1 (UniProtKB Q6V1X1) was obtained from GeneArt and DPP9 isoform 2 from OriGene (UniProtKB Q86TI2-2). The genes were cloned by standard methods in pFastBacHTb (Invitrogen) and virus generated following the Baculovirus Expression Vector System protocol (Life Technologies). The DPP8_aa1-898–6His protein was expressed in 5L scale in wavebags by infecting Spodoptera frugiperda cells (Sf9), harvesting the cells 64 h after infection. The pellet was thawed in 20 mM phosphate buffer pH 7.4, 0.5 mM NaCl, 40 mM imidazole, 5 mM β-mercaptoethanol, and 1 mM NiSO4. DPP8 protein was purified by a three-step procedure: Ni-nitrilotriacetic acid (NTA) from GE affinity purification, tobacco etch virus protease cleavage, negative affinity on an Ni-NTA column and size-exclusion chromatography on Superdex 200 in 20 mM Tris pH 8.0, 150 mM NaCl, and 2 mM DTT buffer. DPP9_1-892-6-His protein was expressed identically. DPP9-His protein required two purification steps. First, Ni-NTA affinity chromatography purification; and second, size-exclusion chromatography on Superdex 200 in 20 mM Tris pH 8.0, 150 mM NaCl, and 2 mM DTT buffer. Both preparations yield ∼50 mg protein, with a negligible amount of contaminant proteins or aggregates. Analysis of the DPP9-His protein sample used for crystallization by LC-(electrospray ionization-TOF)-MS identified the short and long DPP9 isoforms in about equal amounts. For kinetic and pull-down assays, His-DPP9 isoform 1 (UniProtKB Q86TI2) was purified as described in Pilla et al. (33).
Enzyme Kinetic Assay.
Purified recombinant DPP8 or His-DPP9 (12 nM, assuming monomer) were analyzed for hydrolysis of G-P-7-amido-4-methylcoumarin hydrobromide (GP-AMC) in transport buffer (20 mM Hepes/KOH pH 7.3, 110 mM potassium acetate, 2 mM Mg acetate, 0.5 mM EGTA) supplemented with 0.02% Tween 20. Both inhibitors (SLRFLYEG or 1G244) were stored as 5-mM stocks in DMSO. Different concentrations of 1G244 (0, 5, 10, 20, 40, or 80 nM; or 0, 31.25, 62.5, 125, or 250 nM) or SLRFLYEG (0, 31.25, 62.5, 125, 250, 500, or 1,000 nM) were added to the enzyme, followed by 30 min incubation at 4 °C. Reactions were started by addition of the synthetic fluorogenic substrate GP-AMC (2-mM stock in DMSO, diluted into transport buffer) and were carried out at 24 °C. The following GP-AMC concentrations were tested: 0 µM, 31.25 µM, 62.5 µM, 125 µM, 250 μM, and 500 µM. Fluorescence release was measured at a time interval of 30 s, using the Appliskan microplate fluorimeter (Thermo Scientific) with 380-nm (excitation) and 480-nm (emission) filters and SkanIt software. Both His-tagged DPP9 isoforms were active; His-DPP9 was slightly more active than DPP9-His. Each experiment was performed at least three times, in triplicates. Results were analyzed with the Prism 5.0 (GraphPad) software. The datasets were analyzed using the following equations:
Competitive inhibition model:
Noncompetitive inhibition model:
Allosteric sigmoidal model:
Pull-Down Assays.
Two hundred nanograms of recombinant DPP8 or His-DPP9 in transport buffer supplemented with 0.05% Tween 20 and 0.2 mg/mL ovalbumin was incubated with SLRFLYEG (110 µM) or with 1G244 (40 µM) to allow interaction. Control reactions included His-DPP9 or DPP8 alone. The pull-down assays were performed as previously described (33). In short, following a 1-h incubation at 4 °C, bead-immobilized SUMO1 was added to the reactions. Reactions were incubated for 2 h at 4 °C. Next, beads were washed in transport buffer containing 0.05% Tween 20, and proteins were eluted with sample buffer.
Protein Cocrystallization, Soaking Experiments, and Diffraction Improvement.
Crystallization was performed by the hanging drop method. DPP8 crystals grow at 4 °C, setting drops in a 1:1 ratio of 10 mg/mL protein and 0.46 M Na-citrate pH 6.75 precipitant solution. Crystals appear after 1–2 d, mostly in a P212121 space group. After seeding, crystals with the space group C2221 prevailed, offering the best diffracting crystal form. Soaks with SLRFLYEG peptide powder were done from 1-h to overnight incubations. This method produced the first set of structures at 3.0 Å resolution. In a further method development, we treated C2221 crystals with 1 M TMAO as a cryoprotectant and lattice stabilizer (35) using a free mounting system soaking-based method (36). We found a diffraction improvement of these crystals up to 2.5 Å. DPP9-His crystals appear at 20 °C, setting drops in a 1:1 ratio of 20 mg/mL protein and 10% PEG 8000, 25% glycerol, 0.16 M calcium acetate, and 0.08 M cacodilate pH 6.25 as precipitant solution. Crystals are fully grown after 1 wk in the presence of 0–2 mM 1G244. DPP9-His with 1G244 crystals occur as small 5-μm needles and larger stacks with multiple splits.
Data Collection, Structure Solution, and Model Refinement.
Datasets were collected at SLS-X06SA beamline. Data were processed in two space groups for DPP8 (P212121 and C2221) and one for DPP9-His (P1211) (Table S1) using XDS (37). Molecular replacement was performed based on a DPP4 model [Protein Data Bank (PDB): 1ORV (22)] using Phaser (38). The model was generated guided by amino acid sequence homology and elimination of divergent segments. Multiple sequence alignment between DPP4, DPP8, and DPP9 had shown several insertions, both in DPP4 and DPP8/9, making the choice of an appropriate model for phasing by molecular replacement not a trivial task. We systematically deleted unconserved blades. This approach yielded one truncated version of the model, which produced a correct solution. After phasing, the model was manually inspected searching for conspicuous differences, validating the phased model using Coot (39). The model was subjected to several cycles of restrained refinement, keeping 5% of free reflections to calculate Rfree factor using Refmac5 (40). DPP9 was analyzed by use of the refined DPP8 model. Structure visualization and figure preparation was made using PyMOL (The PyMOL Molecular Graphics System, Version 1.7; Schrödinger LLC) and MOLE 2.5 (41). Each structure has been deposited in the PDB with the following codes: DPP8-SLRFLYEG C2221 (PDB: 6EOP), DPP8 unliganded C2221 (PDB: 6EOO), DPP8-SLRFLYEG P212121 (PDB: 6EOT), DPP8 unliganded P212121 (PDB: 6EOS), DPP9-1G244 P1211 (PDB: 6EOR), and DPP9 unliganded P1211 (PDB: 6EOQ).
Results
DPP8 and DPP9 Structure Solution, Homodimer, and Monomer.
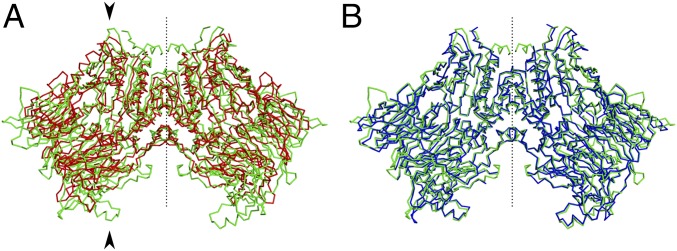
The crystal structure of DPP8 was determined in space group C2221, refined to 2.5 Å and 2.4 Å for the unliganded and liganded forms. The structure was solved using molecular replacement with a DPP4 model (PDB: 1ORV) (22). The R-factors are 22.9% (Rfree 25.4%) and 21.4% (Rfree 23.7%), respectively. There are three polypeptide chains in the asymmetric unit. Two form a noncrystallographic dimer and the remaining molecule forms a crystallographic dimer with a twofold rotation at the “a” axis (0.17 Å average α-carbon rmsd for the three molecules). DPP8 also crystallized in space group P212121, both in unliganded and liganded forms with six polypeptides (three noncrystallographic dimers) in the asymmetric unit (on average 0.24 Å α-carbon rmsd). All liganded forms show full occupation of the ligand sites, indicating that ligand binding is not influenced by crystal packing. While DPP8 produced well-ordered single crystals, DPP9 tended to form clusters of crystals whose diffraction images could be reliably processed but gave high-symmetry R-factors. The unliganded and liganded structures were solved using molecular replacement with the DPP8 model. The space group is P1211. The structures were refined to 3.0 Å and 2.9 Å with R-factor 27.3% (Rfree 33.4%) and 26.5% (Rfree 33.2%), respectively. There are four polypeptides in the asymmetric unit forming two noncrystallographic dimers with an average α-carbon rmsd of 0.18 Å. A summary of all structure statistics is presented in Table S1. DPP4, DPP8, and DPP9 are active dimers in solution. Using a PISA server (42), we determined similar interface areas for each dimer of ∼2,200 Å2 with a complexation significance score of 1. The DPP4, DPP8, and DPP9 dimers are compared in Fig. 1.
Fig. 1.
Homodimer alignment and comparison of DPP4/8 and DPP8/9. (A) DPP4 (red) and DPP8 (green). The catalytic and propeller domains of one monomer are marked with arrows at the top and bottom, respectively. (B) DPP8 (green) and DPP9 (blue). The α-carbon rmsd for DPP4/8 is 2.9 Å and 1.0 Å for DPP8/9. The dotted lines represent the homodimer diad axis.
As the best-defined structure in the series of DPP8 and DPP9 crystals, the DPP8 liganded form was used for comparison with DPP4. The DPP8 unliganded form has interpretable electron density from residues 48–70, 77–105, 109–137, and 165–897, while in DPP8 in complex with SLRFLYEG, residues 48–105, 109–139, and 148–897 are well ordered. DPP9 is less well defined than DPP8 with several additional loops missing electron density in the β-propeller domain. The residues with interpretable electron density are 20–43, 48–79, 82–93, 101–229, 232–266, 270–581, 583–599, and 604–836. Two residues of the His-tag are visible in the DPP9 unliganded structure. The overall DPP-family structure is conserved. The monomer consists of two domains: the C-terminal α/β globular domain, harboring the catalytic triad in DPP4/8/9 (S630/755/730, H740/864/840, and D708/833/708), and the N-terminal β-propeller domain, providing most of the elements required for ligand binding. Using the structure information, we mapped and compared the secondary structures of DPP4, DPP8, and DPP9, suggesting the sequence alignment presented in Fig. S1.
DPP8 and DPP9 β-Propeller Domain.
The β-propeller domain, similar to DPP4, consists of eight blades, which enlace a central round pore. The blades are arranged in two subdomains (blades 3–6 and 1, 2, 7–9) with a β-strand average length of six and eight residues for DPP4 and DPP8/9, respectively. Blades 4 and 5 are longer in DPP8/9 and span up to 13 residues. In DPP8/9, the EE-helix is inserted in blade 4, which arches with a sharp turn at G267/240 toward the active site, forming a helical turn harboring both E275/248 and E276/249, corresponding to the primary binding sites for the substrate N termini.
While blades in DPP4 are invariably four-stranded, the number of strands varies in DPP8 and DPP9. Blade 4 and 5 are tightly packed and intertwined. The fifth β-strand of blade 4 is formed by residues from a loop of blade 5. In DPP8/9, immediately after blade 4, a three-turn helix is observed, not present in DPP4. This helix blocks a surface, which, in DPP4, is the consensus binding region of adenosine deaminase (ADA), offering an explanation for the lack of ADA binding to DPP8/9 (32). Blade 4 shares with DPP4 a characteristic conserved arm of similar size and position of ∼34 residues protruding toward the side opening, named SUBA. This arm structure remains fixed upon substrate binding in DPP4 (33).
R125 in DPP4 is fundamental for substrate fixation, located in a loop of the second blade linking β-strands 2 and 3, named the R-loop (Fig. 2A). Due to a general low homology in the propeller domain, this residue aligns with K190 and R163 in DPP8/9 (Fig. S1). Instead, the molecular structure shows R160 and R133, respectively, adopting the same structural and functional role. Interestingly, they are provided by a different region of the propeller domain located in the R-segment, at the interconnecting loop between blades 1 and 2 (DPP8: 137–165; DPP9: 110–138) (Fig. 2 C and F). Part of this segment folds into the R-helix, which harbors the arginine residues at its C termini. The R-helix becomes ordered upon substrate binding but is mostly disordered in the unliganded forms (Fig. 2 B, D, and E). Disconnected electron density for this helix in unliganded DPP8/9 is visible in some subunits in the asymmetric units, where it adopts a wide range of conformations, suggesting partial order in the unliganded form (Fig. S2). In sharp contrast, in DPP4 such structural change upon ligand/substrate binding has not been observed (22).
Fig. 2.
R-segment order/disorder transition in DPP8 and DPP9. The R-loop and R-segment (including R-helix) are highlighted in magenta. (A) Structure of YPSKPD-liganded DPP4 [PDB: 1R9N (23)]. (B and C) Unliganded and 1G244-liganded DPP9, respectively. Dotted lines indicate undefined segments. (D and E) Unliganded DPP8 in two orientations favoring the visualization of the opened R-segment conformation. Unliganded DPP8 displays two hypothetical conformations partially adopted by the R-segment as observed in different molecules of different unliganded DPP8 structures. (F) SLRFLYEG-liganded DPP8. The black arrowheads indicate the position of the relevant R125/160/133. All panels, except for D, have the same orientation. The monomeric structure is presented for simplification.
DPP8 and DPP9 α/β Hydrolase Domain.
This domain is the most conserved region in DPP4, DPP8, and DPP9. In DPP8, it encompasses the C-terminal residues 629–897 with the contribution of an α-helix from the N terminus of residues 48–70. It is composed of eight parallel twisted β-strands flanked by five close α-helices and three additional more distant helices. These appear to stabilize and link the hydrolase and propeller modules. An interesting observation in DPP8 and DPP9 is the different orientation of the first α-helix in the hydrolase domain. This change causes a shift in the side opening relative to DPP4, thus explaining the different paths followed by the peptides bound in the active site of DPP8 and DPP4 (Fig. S3).
We observed a strong elongated electron density in a hydrophobic cavity of the α/β catalytic domain, accessible via the side entry. A pentadecanoic acid was modeled with its acidic group fixing the side chain of -R- at P1′ in the DPP8 liganded structure. The unliganded DPP8 also has visible, albeit lower, electron density in this cavity.
SLRFLYEG and 1G244 Binding Expose the Active Site Architecture of DPP8 and DPP9.
Next, we analyzed the interaction of DPP8 and DPP9 with two well-characterized inhibitors. 1G244 was developed as a competitive inhibitor of DPP8 and DPP9 (30). The peptide SLRFLYEG was designed as an allosteric inhibitor of these peptidases. This inhibitory peptide was previously described and developed based on amino acids 61–67 of SUMO1, corresponding to a fraction of the SUMO1 E67 interacting loop (EIL). Furthermore, a synthetic peptide corresponding to the EIL (SLRFLFEGQRIADNH) competed with SUMO1 for binding to DPP9 and acted as a DPP9 inhibitor, with a Ki of 5.4 µM (5.6 µM for DPP8) when analyzed with a noncompetitive fit. SLRFLYEG shows Ki values in the nanomolar range for DPP8 and DPP9 when analyzed with a noncompetitive fit (34). Surprisingly, the crystal structure of DPP8 in complex with SLRFLYEG disclosed the peptide bound in a substrate-like manner. Crystals soaked overnight with SLRFLYEG displayed a clear difference electron density with well-defined amino acid side chains (Fig. 3A).
Fig. 3.
SLRFLYEG and 1G244 active site binding and induced fit. (A) Overlay of liganded DPP4 (red) and liganded DPP8 (green). The omit map difference electron density (Fo-Fc) for SLRFLYEG is displayed at 3σ. (B) Overlay of liganded DPP8 (green) and DPP9 (blue). The omit map difference electron density (Fo-Fc) for 1G244 is displayed at 3σ. (C and D) Surface representations of residues forming the S1, S1′, S2′, and S3′ subsites of liganded DPP8 (green) and unliganded DPP8 (gray), respectively. The side chains of P1 and P2′ are represented as sticks; the rest are omitted for simplification. Dashed rectangles correspond to the peptide binding region. (E) Parallel β-strand arrangement of SLRFLYEG with the residues H865 and I867 in DPP8. The arrowed circle highlights the psi angle change of H865 upon peptide binding.
Further refinements including a link between Oγ of S755 in DPP8 and the carbonyl carbon of the scissile -L-R-peptide bond resulted in negative electron density between these two atoms, suggesting a tight noncovalent interaction rather than a tetrahedral intermediate, as had been observed in a peptide complex of serine protease trypsin (43). The presence of an oxyanion hole and the polarization of the carbonyl oxygen of the scissile bond by a hydrogen bond with the side-chain hydroxyl group of Y669 is a precondition for enzymatic activity. This residue is embedded in a fully conserved segment in all DPPs.
The S1 subsite, which accommodates the side chain of the scissile peptide, is the most conserved region among all three proteins. It possesses a conserved particular arrangement of four residues perpendicular to each other (T-shaped), starting with W353/446/420 to Y662/787/762, endowing it with a hydrophobic character (Fig. 3C). Regardless of the high homology of S1, the comparison of unliganded and liganded structures of DPP8 highlights significant differences induced by peptide binding in other subsites with respect to DPP4. One major change is the reorganization of the amino acid sequence H865-S866-I867, not observed in DPP4 (Fig. 3 C–E). The H865 psi torsion angle changes from −54° to +49° upon peptide binding. This remodeling generates a parallel β-sheet interaction with the incoming peptide formed between H865/I867 and the P3′ residue -L-. It allows the formation of a hydrophobic pocket of S2′, where -F- fits (Fig. 3 C–E). It is noticeable that the SLRFLYEG peptide is also involved in a β-sheet in native SUMO1 (Fig. S4) (44).
1G244 bound to DPP9 provides further information regarding the active site. Strong electron density for the R-helix is observed in all four monomers in the asymmetric unit, similar to liganded DPP8; this feature is attributed to the ligand bound state. The isoindoline group in 1G244 with a clear difference electron density fills the S1 subsite and hydrophobic pocket. Its amino substituent binds the E248, E249, and R133 side chains. The 1-(4-4′-difluor-benzhydryl)-piperazine substituent is not defined in electron density (Fig. 3B). A modeling study based on DPP4 had predicted that the S2 subsite is more voluminous in DPP8/9 (45). Our results confirm these findings. Three loops in DPP8 and DPP9 present significant differences compared with DPP4 forming the S2 subsite (Fig. 4). First, enforced by the sequence change of G355 (DPP4) to N448/422 (DPP8/9), the main and side chain of residue H450/424 is displaced by 7 Å, in an opposite orientation with respect to F357 in DPP4, generating a more spacious S2 subsite (Fig. 4A). Second, an additional new feature of this subsite is an extra loop, which buds from the first β-strand of the seventh blade of the β-propeller domain. This loop is absent in DPP4, with the residue H525/500 in DPP8/9 lining the pocket (Fig. 4B). A further difference is provoked by the sequence exchange of C551 (DPP4) to Q673/648 (DPP8/9), offering additional contact fixing the SLRFYLEG peptide, here interacting with serine at P2 (Fig. 4C).
Fig. 4.
S2 subsite loop comparison between DPP4, DPP8, and DPP9. The monomer α-carbon alignment of DPP4 (red), DPP8 (green), and DPP9 (blue) is shown. In the background the liganded DPP8 secondary structure serves as a loop position reference. The main loop differences contributing to the S2 subsite are highlighted and zoomed in at the discontinuous line boxes. (A) F357 in DPP4 is exchanged for the equivalent residues H450/424 (loop segment; DPP8: 447–453, DPP9: 421–427). (B) The most different loop of all has an H525/500 (loop segment; DPP8: 522–258, DPP9: 497–503), whereas this loop does not exist in DPP4. (C) Loop bearing a C551 in DPP4 exchanged for Q673/648 (loop segment; DPP8: 670–676, DPP9: 645–651).
DPP8 and 9 β-Propeller Tunnel, Active Site Cavity, and Side Opening.
In DPP4, the active site cavity is connected to the exterior via two pores: a tunnel of ∼6 Å along the center of the β-propeller domain and a wide side opening of 8 Å (Fig. 5A). Based on homology models and sequence comparisons, the existence of a side opening in DPP8/9 was not clear (45). Interestingly, we see both structures: a tunnel of similar proportions as in DPP4 and a side opening. The latter has variable dimensions, depending on whether a substrate is bound or not. In the unliganded form, the R-segment is not ordered, leaving a wide side opening of ∼7 Å, close to the values observed for DPP4. In turn, after binding of a substrate, the side opening tightens to a narrow tunnel (Fig. 5B). Our data show the 8-residue polypeptide SLRFLYEG bound in the active site of DPP8, pointing to the side opening as the primary access of unprocessed substrates, similar to a DPP4 bound decapeptide (Fig. 2 A and F) (23).
Fig. 5.
DPP4 and DPP8 pore size comparison. (A) DPP4 in complex with YPSKPD [PDB: 1R9N (23)]. The peptide has been omitted to calculate the pore size void volume represented by red spheres. (B) Void volume of DPP8 in complex with SLRFLYEG (omitted for calculation) is shown with green spheres. In both cases, the size of the side exit is indicated above it. The R-loop (left) and R-helix (right) are marked with an arrowhead. The structures are displayed in the same orientation.
SLRFLYEG and 1G244 Reveal Allosteric and Cooperative Inhibition.
The binding of SLRFLYEG to the active site of DPP8 suggests that it acts as a competitive inhibitor. This finding was unexpected since SLRFLYEG is a variant of the EIL SUMO1 peptide, which acted as an inhibitor of DPP8 and DPP9, and competed with SUMO1 for binding to DPP9, suggesting a noncompetitive inhibition (33, 34). Indeed, we find that, similar to the EIL, incubation of DPP8 or DPP9 with SLRFLYEG reduces their interaction with SUMO1, suggesting that SLRFLYEG also competes with the interaction of DPP8 and DPP9 with SUMO1. Strikingly however, incubation of DPP8 or DPP9 with 1G244 leads to a similar effect (Fig. 6A).
Fig. 6.
DPP8 and DPP9 enzyme kinetics with SLRFLYEG and 1G244 reveal allosteric behavior due to cooperative substrate binding. (A) Pull-down assays with immobilized SUMO1 showing that interaction of DPP8 and DPP9 with SUMO1 is strongly reduced in the presence of 1G244 or SLRFLYEG. (B) Inhibition of DPP8 by SLRFLYEG showing an allosteric fit. The experiment was performed three times in triplicates; shown are the results from one experiment, with error bars within one experiment. (C) Table summarizing the results of B, showing calculated values of Vmax, Hill coefficient K0.5, and R2 showing the results for substrate conversion in the presence of inhibitor, analyzed with an equation for an allosteric sigmoidal model [Y = Vmax × Xh/(K0.5h + Xh)]. Also, the table summarizes the data for DPP9 inhibition by SLRFLYEG, analyzed with the same equation. (D and E) Data analysis as above but for inhibition of DPP8 by 1G244.
To further study the inhibitory effect of 1G244 and SLRFLYEG, we performed enzyme kinetic assays in the presence of these inhibitors and analyzed the data using nonlinear regression. We assumed a noncompetitive inhibition [null hypothesis: Vmaxinh = Vmax/(1 + I/Ki), Y = Vmaxinh × X/(Km + X)] and compared the fitting with a competitive model [KmObserved = Km × (1 + [I]/Ki), Y = Vmax × X/(KmObserved + X)], and vice versa. The extra sum of squares F test was used to compare two equations at a time. This analysis revealed no preference to either model. On the other hand, the interaction of the substrate with the enzyme in the presence of the inhibitor fits with an allosteric model [Y = Vmax × Xh/(K0.5h + Xh)], showing a sigmoid behavior (Fig. 6B). Furthermore, the average value of the Hill coefficient for DPP8 in the presence of SLRFLYEG was 1.49, suggesting cooperative substrate binding (Fig. 6C and Tables S2–S5). Similar observations were made for DPP9 inhibition by SLRFLYEG. The Hill coefficient in the presence of SLRFLYEG also points to a cooperative interaction of DPP9 with its substrate, with calculated average Hill values of 1.28, and an R2 average value of 0.98 (Fig. 6B). Consistently, cooperative binding of DPP8 to its substrate was also revealed in the presence of 1G244 (Fig. 6D), with a maximal Hill coefficient value of 3.55 (Fig. 6E).
Discussion
DPP8 and DPP9 Molecular Structure.
DPP8 and DPP9 are intracellular serine dipeptidyl peptidases that modify in a nonreversible manner the N terminus of their substrates. The outcome of this processing and formation of a neo N terminus may alter the life span or activity of a variety of proteins (15, 18). They are a focus of attention because of their relevance in immune response and cancer (9, 11, 18–20, 46). Therefore, molecular structures of both targets are a valuable basis for development of specific inhibitors. The DPP4 Activity Structure Homolog (DASH) family of proteases with its members DPP10, DPP6, DPP4, FAP, DPP8, and DPP9 share a common modular structure, consisting of the N-terminal β-propeller domain and the C-terminal α/β hydrolase domain, despite a very low sequence homology in the former module. The last four members commonly occur as active functional homodimers, whereby the association is mediated by the α/β hydrolase domain.
The first crystal structure of a member of the protein family was published in 2003 for DPP4 (22). Here we report the structures of DPP8 and DPP9 and extend earlier studies of in vitro functional investigations.
Only using a DPP4 modified molecular model allowed phasing by molecular replacement of DPP8 and DPP9 crystal forms. All crystal forms contain multiple copies of the polypeptide chain in the asymmetric unit. The comparison of DPP8 and DPP9 with DPP4 disclosed extensive variations in the β-propeller domain by additional secondary structures, strand exchanges, and loop alterations (Fig. 1 and Fig. S1).
The binding mechanism unveiled by the structures of unliganded and liganded DPP8/9 and enzyme kinetic assays deserves particular attention and will be the focus of the discussion.
In contrast to DPP4, where ligand binding does not significantly alter the protein structure, the binding of the inhibitory SUMO1-derived peptide SLRFLYEG to DPP8 induces ordering of the R-helix, which is part of the R-segment, shaping the substrate binding site. The unliganded structure of DPP8 shows no or disconnected electron density, which may be traced as pieces of the R-helix, albeit differently positioned. These observations hint at induced fit and/or conformational selection for ligand binding.
The unexpected discovery of SLRFLYEG binding in the active site was instrumental in revealing the essential structural features of substrate binding.
Although it has the canonical proline residue replaced by leucine at P1, its ϕ angle is compatible with proline. Discontinuous electron density between Oγ of S755 and the carbonyl carbon of the scissile peptide bond -L-R- indicates a tight noncovalent binding. The peptide displays tight interactions at P1′, P2′, and P3′. In particular, the phenylalanine’s role might be underestimated in defining enzyme specificity, fitting in an additional hydrophobic pocket not existent in DPP4. The octa-peptide extends toward the surface occupying the side entry/exit tunnel similar to DPP4 but following a somewhat different path (Fig. S3) (23).
The R-helix plays a major role in ligand binding by providing R160/133 at its C terminus. The arginine side chain anchors the peptide through hydrogen bonds to the carbonyl oxygens of the P2 and P1′ residues, thereby stabilizing the proline turn conformation at P1. R125 plays this role in DPP4, but emanates from a different structural segment of the protein, the R-loop (Fig. 3 A and B and Fig. S3) (22, 23).
The active sites of DPP4, DPP8, and DPP9 exhibit a conserved characteristic S1 subsite, with similar dimensions in the three species. The site is almost fully occupied by 1G244 in DPP9, offering little room for expansion. On the other hand, the S2 subsite in DPP8 and DPP9 diverges significantly from DPP4, presenting different features, most remarkably the positions of two loops. First, the H450/424 loop in DPP8/9, with the side chain pointing away from the active site, increasing the size of S2 subsite compared with DPP4. Second, the loop H525/500 contributing to the S2 subsite in DPP8 and DPP9 does not exist in DPP4. The H525/500 loop is a possible candidate to interact with large P2 side chains as in 1G244 the 1-(4-4′-difluor-benzhydryl)-piperazine. Furthermore, the significant size expansion of the P2 subsite in DPP8 and DPP9 causes an overlap of S2 and S1′. This feature provides options for specific ligand generation. The S2 subsite can accept a variety of residues, with a preference for voluminous hydrophobic groups (15).
A significant difference between DPP8 and DPP9 is a region contained within the R-segment. This solvent-exposed loop possesses two consecutive histidines, H117 and H118 in DPP9, ordered in the liganded form. DPP8 has D134 and Y135 in the same positions, which are disordered and not visible either in the unliganded crystal structure or in the peptide-complex structure. This segment offers itself as an epitope for antibodies with specific inhibitory properties in a similar approach as for DPP4 (47).
DPP8 and DPP9 Display Allosteric and Cooperative Binding.
1G244 was designed for specificity against DPP8 and DPP9 and discriminating against DPP4. The analysis of the enzymatic binding mode of 1G244 had indicated a small difference between both proteins, with competitive and slow-tight competitive inhibition for DPP9 and DPP8, respectively (29). However, the kinetic data presented here are consistent with an allosteric interaction between the two subunits of DPP8 and DPP9, resulting in a cooperativity in their substrate binding. The allosteric effect of both 1G244 and SLRFLYEG on substrate turnover is supported by the observation that although both inhibitors bind in the active site, they have little effect on K0.5 (Fig. 6). The structural features described, specifically the ligand-induced rearrangements and formation of the substrate binding site and the strap of contacts between the active sites in the dimer formed by “ligand-[R-helix]-SUBA-SUBA-[R-helix]-ligand,” suggest a tentative molecular interpretation of these data, whereby ligands first bind to the partially disordered unliganded conformation or, alternatively, select competent conformers, ensued by active site stabilization, which is signaled to the other subunit (Fig. 7). Fast kinetic measurements would need to be performed to further study the substrate binding mode and conformational selection mechanism associated with partially defined R-helices in unliganded structures. The discovery of communication between the subunits in the dimer and the putative transduction signal pathway offers opportunities for specific functional interference.
Fig. 7.
Active sites contact pathway viewed along the diad axis. A relay connecting the two active sites in a DPP8 homodimer is depicted. The SLRFLYEG peptide (yellow) bound in the the left subunit interacts with the R-helix in red. The R-helix is in contact with SUBA (green), which, in turn, is interfaced with its diad related counterpart in the dimer to allow signal transmission. The two arrows point to the side entries. The important residues interacting in each subdomain are indicated and hydrogen bonds are marked with discontinuous lines.
Molecular Dynamics Simulation.
To assess the stabilization that the bound ligand provides to the overall structure and the R-helix, respectively, molecular dynamics simulations were carried out. Hence, experimental DPP8 and DPP9 structures, crystallized with their respective ligands, were modeled under two different conditions: (i) the ligand bound protein structure with the ligand being present and (ii) the ligand bound protein structure with the ligand removed. Four independent simulations, on each of the four modeled systems, were carried out at 310 K for 200 ns to provide statistical robustness to the observations. The rmsd showed deviations of 1.8 Å and 1.9 Å of the overall structures for DPP8 and DPP9, respectively, independent of the presence of the ligand, while the R-helix deviates by 1.2 Å and 2.5 Å for DPP8 and 0.4 Å and 2.3 Å for DPP9 under the same conditions. Interestingly, while the global protein fold is preserved during the dynamics runs, the R-helix structure is highly sensitive to the presence of the ligand, in agreement with the crystallographic structure observations (Fig. S5).
Bacterial DPP4 Structural Diversity.
Screening the PDB database comparing hDPP4/8/9 with bacterial DPP4 reveals several features. Some bacterial DPP4s display an R-loop and are structurally related to human DPP4 (48) (Fig. S6 A and F), whereas DPP4 from other species harbors an R-segment, lacks the R-loop, and is closer to DPP8/9 (Fig. S6 B–E). The R-segment in DPP4 of Stenotrophomonas maltophilia is disordered as in human DPP8/9 (49), but data of a liganded structure are not available. Furthermore, a third case is compared in Fig. S6E. The R-loop does not exist, and the R-segment is ordered in an open conformation, having a lysine instead of an arginine. The diversity observed in bacteria is quite complex, reflecting a broad function and flexibility of these proteins across species.
DPP8 and DPP9 Interaction with SUMO1.
A specific interaction between DPP8/9 and SUMO1 has been described based on pull-down experiments with bead-immobilized SUMO1. We therefore set up cocrystallization experiments with DPP8/9 and SUMO1, which, however, were not successful. Also, we did not observe the complex in solution using size exclusion chromatography. These observations denote a transient and low-affinity interaction. SUBA has been characterized as the interaction region of SUMO1, and a single mutation, V285A, in this subdomain abolishes binding (33, 34).
SLRFLYEG, a peptide derived from SUMO1, where it is a terminal strand of the central β-sheet in the molecular structure, was found to displace SUMO1 from its complex. It was tempting to assume that the peptide mirrors SUMO1 binding. However, the structural data, described here, present SLRFLYEG at the active site of DPP8, which is a narrow crevice and unfit to receive SUMO1. Additionally, E67 was described to be fundamental for SUMO1 binding, whereas an -E- mutation on the peptide did not affect binding (34). Extensive unfolding of either ligand or receptor is unlikely and not supported by experimental data.
Modeling by docking of SUMO1 to the DPP8 dimer was pursued and demonstrated that there is sufficient space to allow the approach of the EIL strand to SUBAs, such that the specific contacts defined earlier (L321 and F329) by mutations in SUBA and sequence variations of the peptide can be satisfied (Fig. S7).
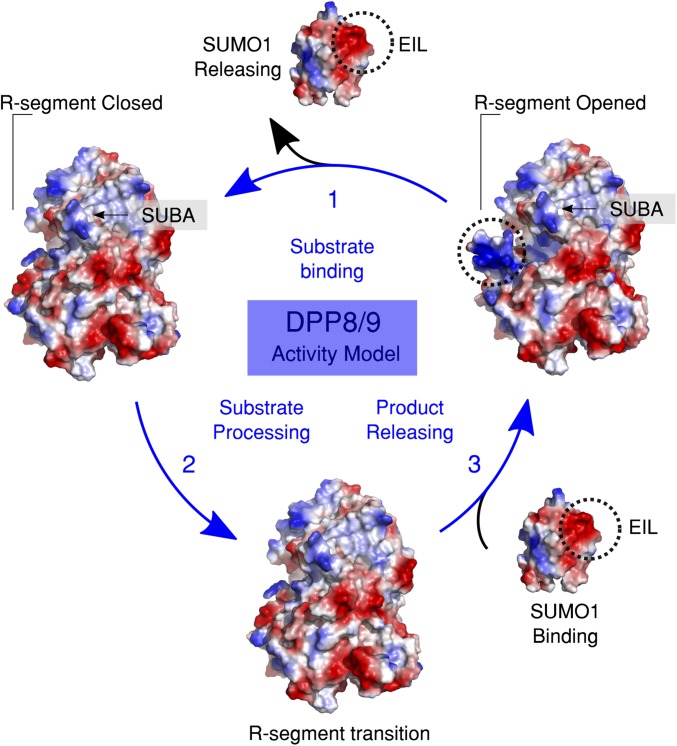
To explain the competition experiments (34), we propose an essential role of the R-segment and the R-helix, which undergo a profound change and structural fixation upon ligand binding at the active site and suggest SUMO1 binding to the unliganded enzymes. The model (Fig. 8) also proposes contacts of complementary charged patches. When ligands bind, the ensuing rearrangement of the R-helix disrupts these interactions, leading to the dissociation of the complex (Figs. 6A and 7).
Fig. 8.
Model of DPP8 and DPP9 activity cycle regulated by SUMO1. Substrate transitions are indicated with blue arrows and SUMO1 with black. In step 1, a substrate binds to the monomer of DPP8/9, releasing SUMO1, generating a closed DPP8/9 conformation. Step 2, the substrate is processed. In step 3, the product is released and SUMO1 binds, favoring an opened conformation. The discontinuous circles highlight the putative interacting negative and positive electrostatic surfaces in SUMO1 and DPP8/9 respectively. Only one member of DPP8/9 dimer is presented for simplification.
Supplementary Material
Acknowledgments
R.G.-F. thanks Blanche Schwappach for her generous support. M. Arciniega acknowledges Dirección General de Cómputo y de Tecnologías de información y Comunicación–Universidad Nacional Autónoma de México for granting the use of the supercomputer Miztli. The authors thank Ulrike Möller for technical assistance, Michael Blaesse for structure modeling advice, the Paul Scherrer Institut for providing synchrotron radiation beam time at beamline X06SA of the Swiss Light Source, and Dr. C. Huang and Dr. V. Olieric for assistance. Part of the research leading to these results received funding from the European Union’s Horizon 2020 Research and Innovation Program under Grant 730872, Project CALIPSOplus. R.G.-F. is supported by Deutsche Forschungsgemeinschaft Grant 2234/1-2.
Footnotes
The authors declare no conflict of interest.
Data deposition: The structure factors have been deposited in the Protein Data Bank, www.wwpdb.org [PDB ID codes 6EOP (DPP8-SLRFLYEG C2221), 6EOO (DPP8 unliganded C2221), 6EOT (DPP8-SLRFLYEG P212121), 6EOS (DPP8 unliganded P212121), 6EOR (DPP9-1G244 P1211), and 6EOQ (DPP9 unliganded P1211)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717565115/-/DCSupplemental.
References
- 1.Zhang H, Chen Y, Keane FM, Gorrell MD. Advances in understanding the expression and function of dipeptidyl peptidase 8 and 9. Mol Cancer Res. 2013;11:1487–1496. doi: 10.1158/1541-7786.MCR-13-0272. [DOI] [PubMed] [Google Scholar]
- 2.Waumans Y, Baerts L, Kehoe K, Lambeir AM, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol. 2015;6:387. doi: 10.3389/fimmu.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner L, Klemann C, Stephan M, von Hörsten S. Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins. Clin Exp Immunol. 2016;184:265–283. doi: 10.1111/cei.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambeir AM, Scharpé S, De Meester I. DPP4 inhibitors for diabetes:–What next? Biochem Pharmacol. 2008;76:1637–1643. doi: 10.1016/j.bcp.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Ajami K, Abbott CA, McCaughan GW, Gorrell MD. Dipeptidyl peptidase 9 has two forms, a broad tissue distribution, cytoplasmic localization and DPIV-like peptidase activity. Biochim Biophys Acta. 2004;1679:18–28. doi: 10.1016/j.bbaexp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Abbott CA, et al. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur J Biochem. 2000;267:6140–6150. doi: 10.1046/j.1432-1327.2000.01617.x. [DOI] [PubMed] [Google Scholar]
- 7.Justa-Schuch D, Möller U, Geiss-Friedlander R. The amino terminus extension in the long dipeptidyl peptidase 9 isoform contains a nuclear localization signal targeting the active peptidase to the nucleus. Cell Mol Life Sci. 2014;71:3611–3626. doi: 10.1007/s00018-014-1591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, et al. Dipeptidyl peptidase 9 subcellular localization and a role in cell adhesion involving focal adhesion kinase and paxillin. Biochim Biophys Acta. 2015;1853:470–480. doi: 10.1016/j.bbamcr.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Okondo MC, et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13:46–53. doi: 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514.e4. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnuolo PA, et al. Inhibition of intracellular dipeptidyl peptidases 8 and 9 enhances parthenolide’s anti-leukemic activity. Leukemia. 2013;27:1236–1244. doi: 10.1038/leu.2013.9. [DOI] [PubMed] [Google Scholar]
- 12.Han R, Wang X, Bachovchin W, Zukowska Z, Osborn JW. Inhibition of dipeptidyl peptidase 8/9 impairs preadipocyte differentiation. Sci Rep. 2015;5:12348. doi: 10.1038/srep12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gall MG, et al. Targeted inactivation of dipeptidyl peptidase 9 enzymatic activity causes mouse neonate lethality. PLoS One. 2013;8:e78378. doi: 10.1371/journal.pone.0078378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, et al. Dipeptidyl peptidase 9 enzymatic activity influences the expression of neonatal metabolic genes. Exp Cell Res. 2016;342:72–82. doi: 10.1016/j.yexcr.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Geiss-Friedlander R, et al. The cytoplasmic peptidase DPP9 is rate-limiting for degradation of proline-containing peptides. J Biol Chem. 2009;284:27211–27219. doi: 10.1074/jbc.M109.041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson CH, Zhang HE, Gorrell MD, Abbott CA. Dipeptidyl peptidase 9 substrates and their discovery: Current progress and the application of mass spectrometry-based approaches. Biol Chem. 2016;397:837–856. doi: 10.1515/hsz-2016-0174. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, et al. Identification of novel dipeptidyl peptidase 9 substrates by two-dimensional differential in-gel electrophoresis. FEBS J. 2015;282:3737–3757. doi: 10.1111/febs.13371. [DOI] [PubMed] [Google Scholar]
- 18.Justa-Schuch D, et al. DPP9 is a novel component of the N-end rule pathway targeting the tyrosine kinase Syk. Elife. 2016;5:e16370. doi: 10.7554/eLife.16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Z, et al. Contribution of upregulated dipeptidyl peptidase 9 (DPP9) in promoting tumoregenicity, metastasis and the prediction of poor prognosis in non-small cell lung cancer (NSCLC) Int J Cancer. 2017;140:1620–1632. doi: 10.1002/ijc.30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smebye ML, et al. Involvement of DPP9 in gene fusions in serous ovarian carcinoma. BMC Cancer. 2017;17:642. doi: 10.1186/s12885-017-3625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol. 2003;10:19–25. doi: 10.1038/nsb882. [DOI] [PubMed] [Google Scholar]
- 22.Engel M, et al. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci USA. 2003;100:5063–5068. doi: 10.1073/pnas.0230620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aertgeerts K, et al. Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Sci. 2004;13:412–421. doi: 10.1110/ps.03460604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oefner C, et al. High-resolution structure of human apo dipeptidyl peptidase IV/CD26 and its complex with 1-[([2-[(5-iodopyridin-2-yl)amino]-ethyl]amino)-acetyl]-2-cyano-(S)-pyrrolidine. Acta Crystallogr D Biol Crystallogr. 2003;59:1206–1212. doi: 10.1107/s0907444903010059. [DOI] [PubMed] [Google Scholar]
- 25.Thoma R, et al. Structural basis of proline-specific exopeptidase activity as observed in human dipeptidyl peptidase-IV. Structure. 2003;11:947–959. doi: 10.1016/s0969-2126(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, et al. Investigation of the dimer interface and substrate specificity of prolyl dipeptidase DPP8. J Biol Chem. 2006;281:38653–38662. doi: 10.1074/jbc.M603895200. [DOI] [PubMed] [Google Scholar]
- 27.Ajami K, et al. Structural requirements for catalysis, expression, and dimerization in the CD26/DPIV gene family. Biochemistry. 2003;42:694–701. doi: 10.1021/bi026846s. [DOI] [PubMed] [Google Scholar]
- 28.Tang HK, et al. Role of a propeller loop in the quaternary structure and enzymatic activity of prolyl dipeptidases DPP-IV and DPP9. FEBS Lett. 2011;585:3409–3414. doi: 10.1016/j.febslet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu JJ, et al. Biochemistry, pharmacokinetics, and toxicology of a potent and selective DPP8/9 inhibitor. Biochem Pharmacol. 2009;78:203–210. doi: 10.1016/j.bcp.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Jiaang WT, et al. Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg Med Chem Lett. 2005;15:687–691. doi: 10.1016/j.bmcl.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Van Goethem S, et al. Structure-activity relationship studies on isoindoline inhibitors of dipeptidyl peptidases 8 and 9 (DPP8, DPP9): Is DPP8-selectivity an attainable goal? J Med Chem. 2011;54:5737–5746. doi: 10.1021/jm200383j. [DOI] [PubMed] [Google Scholar]
- 32.Weihofen WA, Liu J, Reutter W, Saenger W, Fan H. Crystal structure of CD26/dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface. J Biol Chem. 2004;279:43330–43335. doi: 10.1074/jbc.M405001200. [DOI] [PubMed] [Google Scholar]
- 33.Pilla E, et al. A novel SUMO1-specific interacting motif in dipeptidyl peptidase 9 (DPP9) that is important for enzymatic regulation. J Biol Chem. 2012;287:44320–44329. doi: 10.1074/jbc.M112.397224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pilla E, Kilisch M, Lenz C, Urlaub H, Geiss-Friedlander R. The SUMO1-E67 interacting loop peptide is an allosteric inhibitor of the dipeptidyl peptidases 8 and 9. J Biol Chem. 2013;288:32787–32796. doi: 10.1074/jbc.M113.489179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall H, Venkat M, Seng NS, Cahn J, Juers DH. The use of trimethylamine N-oxide as a primary precipitating agent and related methylamine osmolytes as cryoprotective agents for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2012;68:69–81. doi: 10.1107/S0907444911050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiefersauer R, et al. A novel free-mounting system for protein crystals: Transformation and improvement of diffraction power by accurately controlled humidity changes. J Appl Crystallogr. 2000;33:1223–1230. [Google Scholar]
- 37.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 41.Sehnal D, et al. MOLE 2.0: Advanced approach for analysis of biomacromolecular channels. J Cheminform. 2013;5:39. doi: 10.1186/1758-2946-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Marquart M, Walter J, Deisenhofer J, Bode W, Huber R. The geometry of the reactive site and of the peptide groups in trypsin, trypsinogen and its complexes with inhibitors. Acta Crystallogr B. 1983;39:480–490. [Google Scholar]
- 44.Capili AD, Lima CD. Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. J Mol Biol. 2007;369:608–618. doi: 10.1016/j.jmb.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rummey C, Metz G. Homology models of dipeptidyl peptidases 8 and 9 with a focus on loop predictions near the active site. Proteins. 2007;66:160–171. doi: 10.1002/prot.21138. [DOI] [PubMed] [Google Scholar]
- 46.Waumans Y, et al. The dipeptidyl peptidases 4, 8, and 9 in mouse monocytes and macrophages: DPP8/9 inhibition attenuates M1 macrophage activation in mice. Inflammation. 2016;39:413–424. doi: 10.1007/s10753-015-0263-5. [DOI] [PubMed] [Google Scholar]
- 47.Tang J, et al. An inhibitory antibody against dipeptidyl peptidase IV improves glucose tolerance in vivo. J Biol Chem. 2013;288:1307–1316. doi: 10.1074/jbc.M112.396317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rea D, et al. Crystal structure of Porphyromonas gingivalis dipeptidyl peptidase 4 and structure-activity relationships based on inhibitor profiling. Eur J Med Chem. 2017;139:482–491. doi: 10.1016/j.ejmech.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 49.Nakajima Y, et al. Dipeptidyl aminopeptidase IV from Stenotrophomonas maltophilia exhibits activity against a substrate containing a 4-hydroxyproline residue. J Bacteriol. 2008;190:7819–7829. doi: 10.1128/JB.02010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.