Significance
E-cigarette smoke (ECS) delivers nicotine through aerosols without burning tobacco. ECS is promoted as noncarcinogenic. We found that ECS induces DNA damage in mouse lung, bladder, and heart and reduces DNA-repair functions and proteins in lung. Nicotine and its nitrosation product 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone can cause the same effects as ECS and enhance mutations and tumorigenic cell transformation in cultured human lung and bladder cells. These results indicate that nicotine nitrosation occurs in the lung, bladder, and heart, and that its products are further metabolized into DNA damaging agents. We propose that ECS, through damaging DNA and inhibiting DNA repair, might contribute to human lung and bladder cancer as well as to heart disease, although further studies are required to substantiate this proposal.
Keywords: E-cigarettes, DNA damage, DNA repair, lung–bladder–heart, cancer
Abstract
E-cigarette smoke delivers stimulant nicotine as aerosol without tobacco or the burning process. It contains neither carcinogenic incomplete combustion byproducts nor tobacco nitrosamines, the nicotine nitrosation products. E-cigarettes are promoted as safe and have gained significant popularity. In this study, instead of detecting nitrosamines, we directly measured DNA damage induced by nitrosamines in different organs of E-cigarette smoke-exposed mice. We found mutagenic O6-methyldeoxyguanosines and γ-hydroxy-1,N2-propano-deoxyguanosines in the lung, bladder, and heart. DNA-repair activity and repair proteins XPC and OGG1/2 are significantly reduced in the lung. We found that nicotine and its metabolite, nicotine-derived nitrosamine ketone, can induce the same effects and enhance mutational susceptibility and tumorigenic transformation of cultured human bronchial epithelial and urothelial cells. These results indicate that nicotine nitrosation occurs in vivo in mice and that E-cigarette smoke is carcinogenic to the murine lung and bladder and harmful to the murine heart. It is therefore possible that E-cigarette smoke may contribute to lung and bladder cancer, as well as heart disease, in humans.
E-cigarettes (E-cigs) are designed to deliver the stimulant nicotine, similar to conventional cigarettes, through an aerosol state. In E-cigs, nicotine is dissolved in relatively harmless organic solvents, such as glycerol and propylene glycol, then aerosolized with the solvents by controlled electric heating. Hence, E-cig smoke (ECS) contains mostly nicotine and the gas phase of the solvents (1–4). In contrast, conventional tobacco smoke (TS), in addition to nicotine and its nitrosamine derivatives, contains numerous (>7,000) incomplete combustion byproducts, such as polycyclic aromatic hydrocarbons (PAHs), aromatic amines, aldehydes, and benzene, many of which are human carcinogens, irritants, and allergens (5, 6). TS also has a strong scent. Therefore, TS is both harmful and carcinogenic to smokers, as well as being unpleasant and harmful to bystanders (7). Because of these effects, TS has become an unwelcome social habit and is no longer acceptable in many social settings and public domains (8). E-cigs have been promoted as an alternative to cigarettes that can deliver a TS “high” without TS’s ill and unpleasant effects. Since it appears that ECS contains neither carcinogens, allergens, nor odors that result from incomplete combustion, as a result of these claims, E-cigs have become increasingly popular, particularly with young people (9). However, the question as to whether ECS is as harmful as TS, particularly with regard to carcinogenicity, remains a serious public health issue that deserves careful examination.
It is well established that most chemical carcinogens, either directly or via metabolic activation, can induce damage in genomic DNA, that unrepaired DNA damage can induce mutations, and that multiple mutations can lead to cancer (10). Many chemical carcinogens can also impair DNA-repair activity (11–13). Therefore, in this study, as a step to understanding the carcinogenicity of ECS, we determined whether ECS can induce DNA damage in different organs of a mouse model and whether ECS can affect DNA-repair activity. We then characterized the chemical nature of ECS-induced DNA damage and how ECS affects DNA repair. Last, we determined the effect of ECS metabolites on the susceptibility to mutations and tumorigenic transformation of cultured human cells.
Results
ECS Induces O6-Methyl-Deoxuguanosine in the Lung, Bladder, and Heart.
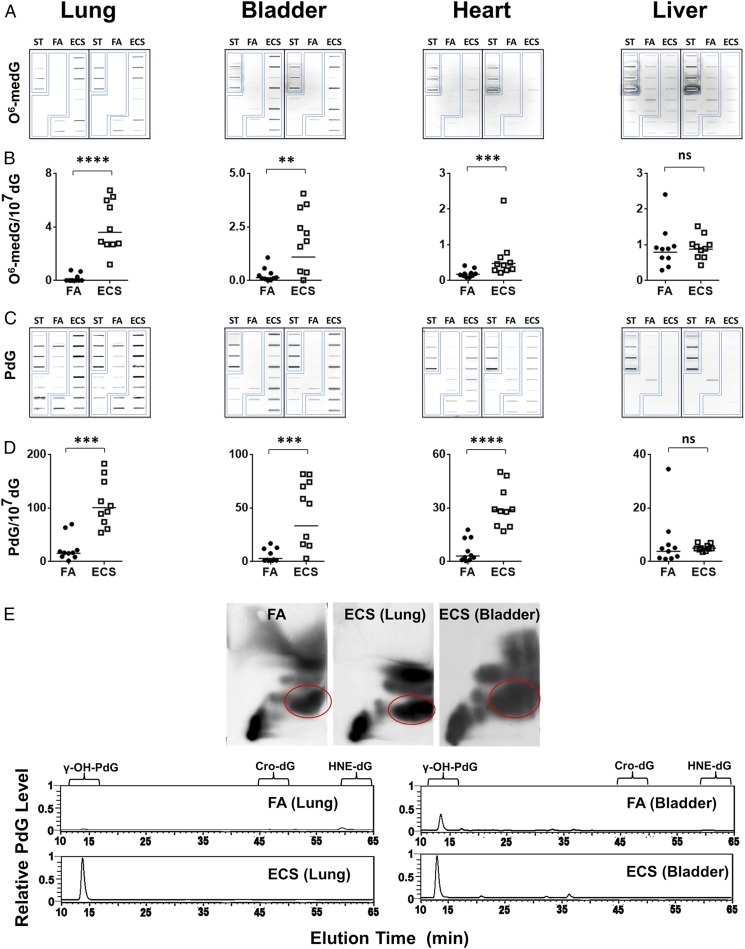
Nicotine is the major component of ECS (3). The majority (80%) of inhaled nicotine in smoke is quickly metabolized into cotinine, which is excreted into the bloodstream and subsequently into urine (14). Cotinine is generally believed to be nontoxic and noncarcinogenic (15); however, a small portion (<10%) of inhaled nicotine is believed to be metabolized into nitrosamines in vivo (16–18). Nitrosamines induce tumors in different organs in animal models (6, 19). Inhaled nitrosamines are metabolized into N-nitrosonornicotine (NNN) and nicotine-derived nitrosamine ketone (NNK). It has been proposed that NNK can be further metabolized and spontaneously degraded into methyldiazohydroxide (MDOH), pyridyl-butyl derivatives (PBDs), and formaldehyde, and that NNN degrade into hydroxyl or keto PBDs (20). While nicotine cannot bind to DNA directly, MDOH can methylate deoxyguanosines and thymidines in DNA (21). Although the fate of nitrosamine-induced formaldehyde and PBDs in vivo is less clear, both are capable of inducing DNA damage in vitro (22–25). Therefore, if ECS in fact is a carcinogen, it is likely that its carcinogenicity is derived from nitrosamines that are derived from the nitrosation of nicotine (5, 19, 21). Nitrosamines are potent carcinogens and it is generally believed that their carcinogenicity is via induction of methylation DNA damage (26, 27). As a step in examining the carcinogenicity of ECS, we determined whether ECS can induce O6-methyl-deoxuguanosine (O6-medG) adducts in lung, heart, liver, and bladder tissues of mice. Mice were exposed to ECS (10 mg/mL, 3 h/d, 5 d/wk) for 12 wk; the dose and duration equivalent in human terms to light E-cig smoking for 10 y. The results in Fig. 1 A and B, Fig. S1, and Table S1 show that ECS induced significant amounts of O6-medG adducts in the lung, bladder, and heart and that the level of O6-medG adducts in lung was three- to eightfold higher than in the bladder and heart. These results are consistent with the explanation that nicotine is metabolized into MDOH, which can methylate DNA (16, 20).
Fig. 1.
ECS induces γ-OH-PdG and O6-medG adducts in the lung, bladder and heart. Genomic DNA were isolated from different organs of mice exposed to FA or ECS as described in text. (A–D) O6-medG and PdG formed in the genomic DNA were detected by immunochemical methods (28). (A and C) Slot blot. (B and D) Quantification results. The bar represents the mean value. (E) Identification of γ-OH-PdG adducts formed in the genomic DNA of lung and bladder by the 2D-TLC (Upper) and then HPLC (Lower) (28). ST, PdG, or O6-medG standard DNA. ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05.
ECS Induces γ-OH-PdG in the Lung, Bladder, and Heart.
Recently, we found that aldehyde-derived cyclic 1,N2-propano-dG (PdG), including γ-OH-1,N2-PdG (γ-OH-PdG) and α-methyl-γ-OH-1,N2-PdG adducts, are the major DNA adducts in mouse models (28) induced by TS, which contains abundant nitrosamines and aldehydes (20). We therefore determined the extent of PdG formation in different organs of ECS-exposed mice using a PdG-specific antibody (28–30).
The results in Fig. 1 C and D show that ECS induced PdG adducts in the lung, bladder, and heart, and that the level of PdG in the lung is two- to threefold higher than in the bladder and heart. Moreover, the level of PdG is 25- to 60-fold higher than the level of O6-medG in lung, bladder, and heart tissues, indicating that induction of PdG is more efficient than induction of O6-medG by nicotine metabolic products and/or that O6-medG is more efficiently repaired in these organs. ECS, however, did not induce either O6-medG or PdG in liver DNA.
Due to the relatively minute amount of genomic DNA that is possible to isolate from mouse organs, in this case, specifically from bladder mucosa, which is only able to yield up to 2 μg of genomic DNA from each mouse, we used the sensitive 32P-postlabeling thin layer chromatography (TLC)/HPLC method to identify the species of the PdG formed in lung and bladder tissues (13, 28, 31). The results in Fig. 1E show that the majority of PdG (>95%) formed in these tissues coelute with γ-OH-PdG adduct standards with a minor portion that coelute with α-OH-PdG standards.
Relationship of ECS-Induced PdG and O6-medG Formation in Different Organs of Each Animal.
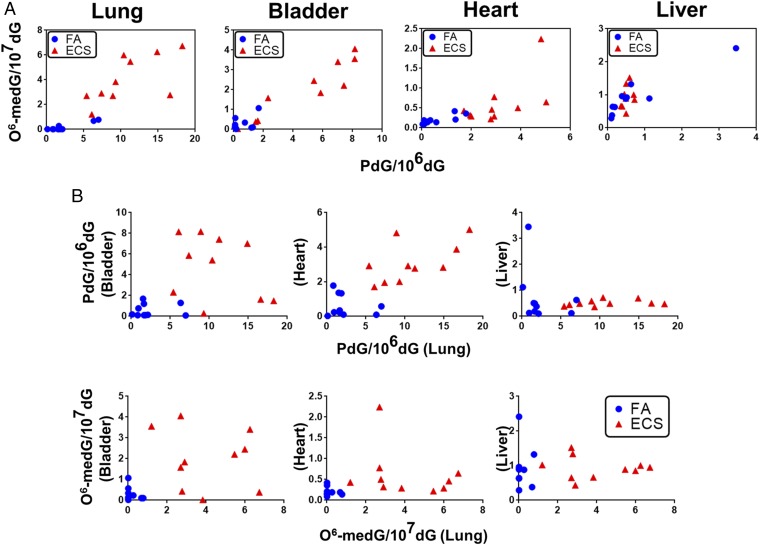
We then determined the relationship of PdG and O6-medG formation in different organs of each animal. The results in Fig. 2A show that the levels of PdG and O6-medG in the same organs are positively related to each other. Thus, a lung tissue sample that had a high level of PdG also had a high level of O6-medG. The same relationship between PdG and O6-medG formation was found in the bladder and heart (Fig. 2A and Table S1). The results in Fig. 2B show that in the same mouse, the levels of PdG and O6-medG formation in different organs also have a positive correlation: Mice with a high level of PdG and O6-medG formation in the lung also had a high level of these DNA adducts in the bladder and heart (Fig. 2B and Table S1). Together, these results indicate that the formation of PdG and O6-medG DNA adducts in the lung, bladder, and heart tissue are the result of DNA damaging agents derived from ECS exposure, and raising the possibility that the ability for nicotine absorption and metabolism and DNA-repair activity of different organs determine their susceptibility to ECS-induced DNA adduct formation.
Fig. 2.
Relationship of ECS-induced PdG versus O6-medG formation in different organs of mice. The levels of PdG and O6-medG detected in different organs from mice exposed to FA and ECS were determined in Fig. 1. In A, O6-medG formation is plotted against PdG formation in each organ in mice exposed to ECS (red triangles) and FA (blue dots). In B, formation of PdG and O6-medG in the bladder, heart, and liver is plotted against PdG and O6-medG formation, respectively, in the lung of mice exposed to ECS and FA. Each symbol represents each individual mouse.
ECS Reduces DNA-Repair Activity in the Lung.
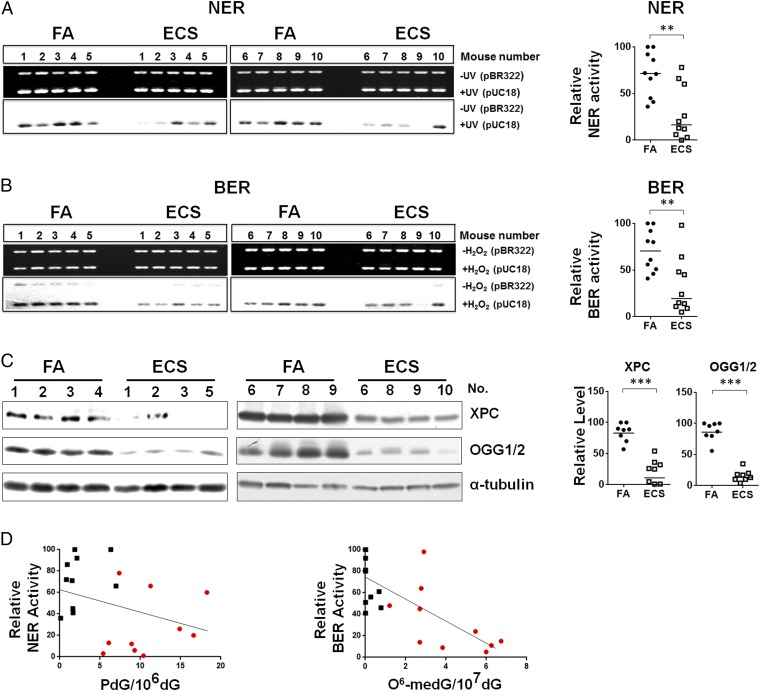
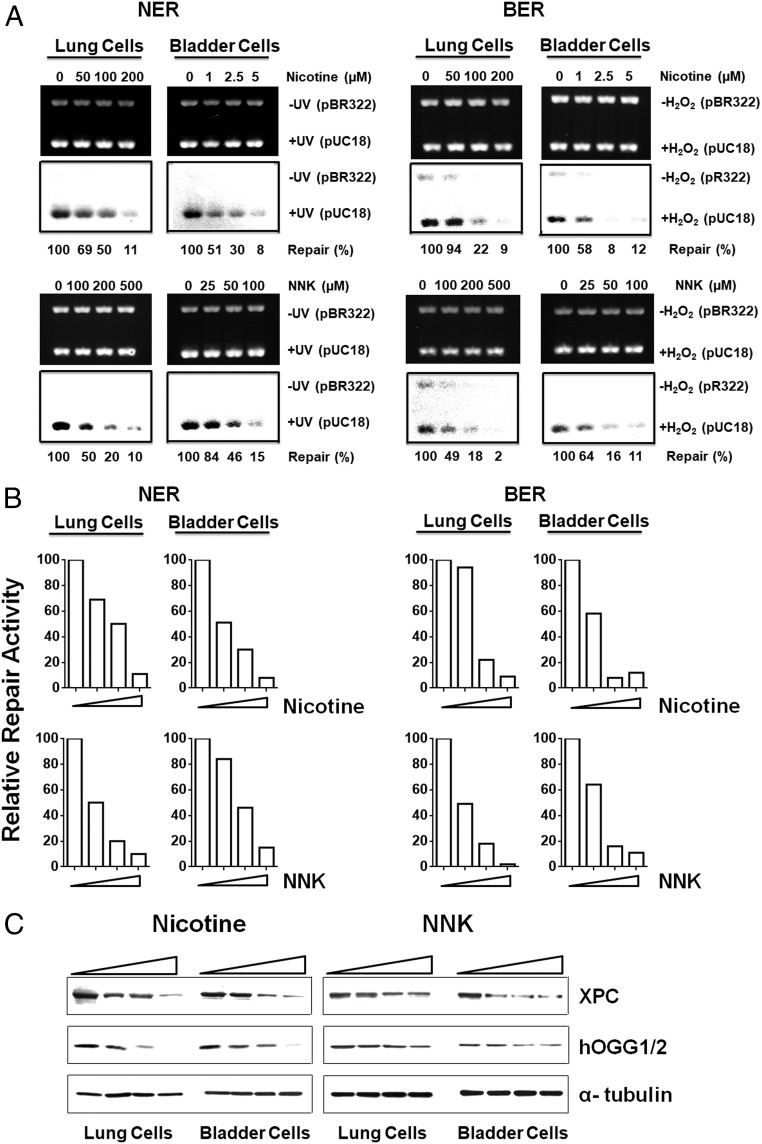
Recently, we have found that lung tissues of mice exposed to TS have lower DNA-repair activity and lower levels of DNA-repair proteins XPC and OGG1/2 and that aldehydes, such as acrolein, acetaldehyde, crotonaldehyde, and 4-hydroxy-2-nonenal, can modify DNA-repair proteins, causing the degradation of these repair proteins and impairing DNA-repair function (11, 12, 28). These findings raise the possibility that, via induction of aldehydes, ECS can impair DNA-repair functions. To test this possibility, we determined the effect of ECS on the activity of the two major DNA-repair mechanisms in mouse lung tissues: nucleotide excision repair (NER) and base excision repair (BER) (32). We adopted a well-established in vitro DNA damage-dependent repair synthesis assay, which requires only 10 μg of freshly prepared cell lysates (11, 13, 28). Since the amount of bladder mucosa collected from individual mice was minute, we were only able to determine DNA-repair activity in lung tissues (28). We used UV-irradiated DNA, which contains cyclobutane pyrimidine dimers as well as <6-4> photoproducts; Acr-modified DNA, which contains γ-OH-PdG; and H2O2-modified DNA, which contains 8-oxo-dG, as substrates (13, 28). It is well established that NER is the major mechanism that repairs cyclobutane pyrimidine dimers, <6-4> photoproducts, and γ-OH-PdG, and that BER is the major mechanism that repairs 8-oxo-dG (32, 33). Therefore, these two types of substrates allow us to determine the NER and BER activity in the cell lysates (11, 13). The results in Fig. 3 A and B and Fig. S2 show that both NER and BER activity in lung tissue of ECS-exposed mice are significantly lower than in lung tissue of filtered air (FA)-exposed mice.
Fig. 3.
ECS reduces DNA-repair activity and XPC and OGG1/2 in the lung. Cell lysates were isolated from lung tissues of mice exposed to FA (n = 10) or to ECS (n = 10) the same as in Fig. 1. The NER and the BER activity in the cell lysates were determined by the in vitro DNA damage-dependent repair synthesis assay as described (13, 28). (A and B) Ethidium bromide-stained gels (Upper) and autoradiograms (Lower) are shown in Left. In Right, the radioactive counts in the autoradiograms were normalized to input DNA. The relative repair activity was calculated using the highest band as 100%. (C) Detection of XPC and OGG1/2 protein in lung tissues (n = 8) by Western blot (Left). Right graphs are quantifications of ECS effect on the abundance of XPC and OGG1/2. The bar represents the mean value. (D) The relationship between the level of PdG and O6-medG adduct and the NER and BER activity in lung tissues of FA- (black square) and ECS (red dot)-exposed mice.
ECS Causes a Reduction of Repair Protein XPC and OGG1/2.
We then determined the level of XPC and OGG1/2, the two crucial proteins, respectively, for NER and BER (34, 35). The results in Fig. 3C show that the level of XPC and OGG1/2 in lung tissues of ECS-exposed mice was significantly lower than in control mice. We further determined the relationship between DNA adduct formation and DNA-repair activity in lung tissues of FA- and ECS-exposed mice. Since NER is the major repair mechanism for bulky DNA damage such as γ-OH-PdG and photodimers (11, 33) and BER is a major repair mechanism for base damage (32), we compared BER activity with the level of O6-medG adducts and NER activity with the level of γ-OH-PdG adducts. The results in Fig. 3D show that NER and BER activity in lung tissue of different mice is inversely related to the level of γ-OH-PdG and O6-medG adducts, respectively. These results indicate that in lung tissue, NER and BER activities are crucial factors in determining the level of ECS-induced γ-OH-PdG and O6-medG DNA damage; mice that are more sensitive to ECS-induced DNA-repair inhibition accumulate more ECS-induced DNA damage in their lung and, perhaps, bladder and heart. It should be noted that in human cells, repair of O6-medG adducts is mainly carried out by O6-methylguanine DNA methyltransferase (MGMT) (36, 37). The positive relationship between BER activity and the O6-medG level in lung tissues of mice implies that ECS impairs BER enzymes as well as MGMT, and/or O6-medG is repaired by a BER mechanism in mice.
Nicotine Induces DNA Damage in Human Cells.
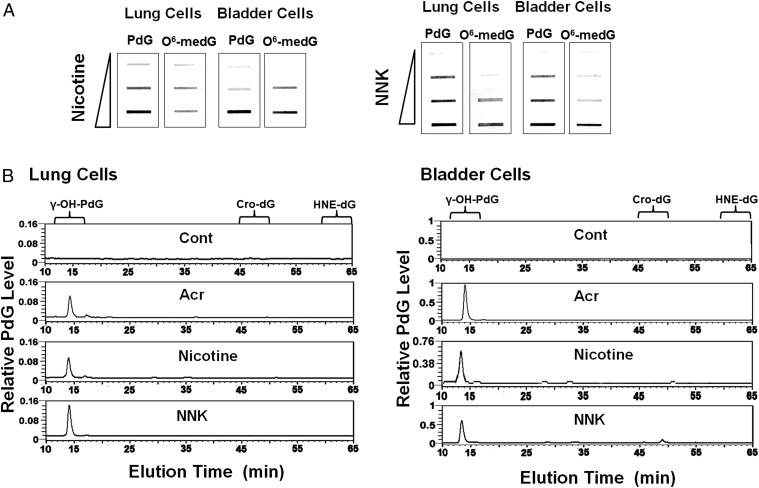
Many tobacco-specific nitrosamines that result from the nitrosation of nicotine, such as NNN and NNK, are potent carcinogens and can induce cancer in different organs, including the lung (20, 21, 27). While NNK and NNN cannot covalently bind with DNA directly, it has been proposed that one of NNK’s metabolic products, MDOH, can interact with DNA to induce mutagenic O6-medG adducts (20, 21, 27). These results raise the possibility that ECS-induced O6-medG is due to the nitrosation of nicotine, and that NNK resulting from nicotine nitrosation then further transforms into MDOH in lung and bladder tissue (20). To test this possibility, we determined the DNA adducts induced by nicotine and NNK in cultured human bronchial epithelial and urothelial cells, and the effect of nicotine and NNK treatments on DNA repair, using the same methods indicated in Fig. 1. The results in Fig. 4 show that both nicotine and NNK can induce the same type of γ-OH-PdG adducts, and O6-medG adducts. Since it is well established that many aldehydes can induce cyclic PdG in cells (38–40), these results suggest that aldehydes as well as MDOH are NNK metabolites, which induce γ-OH-PdG and O6-medG.
Fig. 4.
Nicotine and NNK induce γ-OH-PdG and O6-medG in cultured human lung and bladder epithelial cells. Human lung epithelial (BEAS-2B) cells and urothelial (UROtsa) cells were treated with different concentrations of nicotine and NNK as described in text. O6-medG and PdG formed in the genomic DNA were determined as described in Fig. 1. (A) The DNA adducts were detected by immunochemical methods (13, 28). (B) The PdG adducts formed in the genomic DNA were further identified as γ-OH-PdG adducts by the 32P postlabeling followed by 2D-TLC/HPLC method (13, 28).
Nicotine Reduces DNA Repair in Human Cells.
We next determined the effects of nicotine and NNK treatment on DNA-repair activity and repair protein levels in human lung and bladder epithelial cells using the method described in Fig. 3. The results in Fig. 5 show that nicotine and NNK treatments not only inhibit NER and BER activities, they also reduce the protein levels of XPC and hOGG1/2. We found that these reductions of XPC and hOGG1/2 induced by nicotine and NNK can be prevented or attenuated by the proteasome and autophagosome inhibitors MG132, 3-methyladenine (3-MA), and lactacystin (Fig. S3) (13, 41–43). These results indicate that metabolites of nicotine and NNK can modify DNA-repair proteins and cause proteosomal and autophagosomal degradation of these proteins and that ECS’s effect on the inhibition of DNA-repair activity is via modifications and degradation of DNA-repair proteins by its metabolites.
Fig. 5.
Nicotine and NNK reduce DNA-repair activity and the level of repair proteins XPC and hOGG1/2 in cultured human lung and bladder epithelial cells. Cell-free cell lysates were isolated from human lung (BEAS-2B) and bladder epithelial (UROtsa) cells treated with different concentrations of nicotine and NNK 1 h at 37 °C. The NER and the BER activity in the cell lysates were determined by the in vitro DNA damage-dependent repair synthesis assay as described in Fig. 3. (A) Ethidium bromide-stained gels (Upper) and autoradiograms (Lower) are shown. (B) Quantifications results. The radioactive counts in the autoradiograms were normalized to input DNA. The relative repair activity was calculated using the control band as 100%. (C) The effect of nicotine and NNK treatment on abundance of XPC and hOGG1/2 in human lung and bladder urothelial cells were determined as described in Fig. 3.
Together, these results indicate that human bronchial epithelial and urothelial cells as well as lung, heart, and bladder tissues in the mouse are able to nitrosate nicotine and metabolize nitrosated nicotine into NNK and then MDOH and aldehydes. Furthermore, whereas MDOH induces O6-medG adducts, aldehydes not only can induce γ-OH-PdG, they also can inhibit DNA repair and cause repair protein degradation.
Nicotine Enhances Mutations and Cell Transformation.
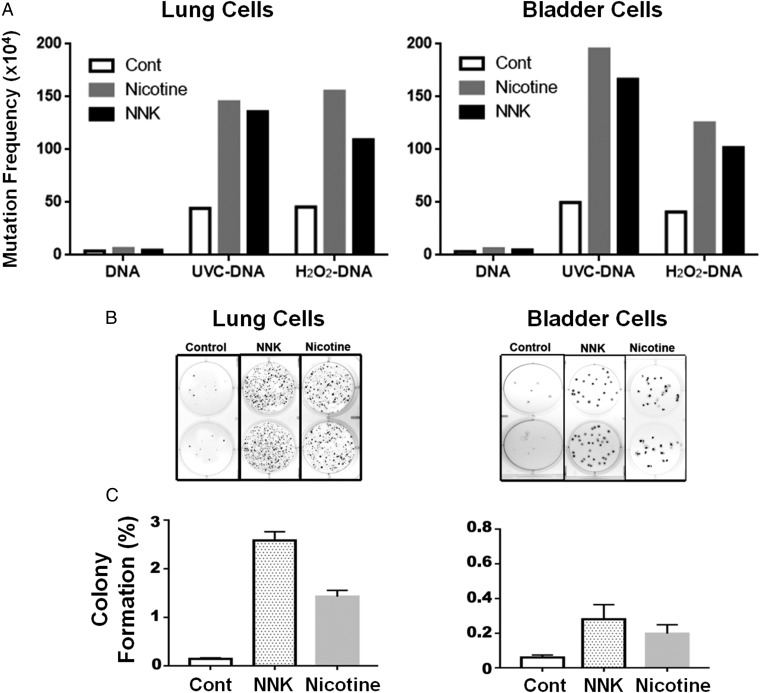
The aforementioned results demonstrate that ECS’s major component nicotine, via its metabolites, MDOH, and aldehydes, not only can induce mutagenic DNA adducts, but that they also can inhibit DNA repair in human lung and bladder epithelial cells. These results raise the possibility that ECS and its metabolites can function not only as mutagens but also as comutagens to enhance DNA damage-induced mutagenesis. To test this possibility, we determined the effect of these agents on cell mutation susceptibility on UV- and H2O2-induced DNA damage using the well-established supF mutation system (13). The results in Fig. 6A show that nicotine and NNK treatment in both human lung and bladder epithelial cells enhances the spontaneous mutation frequency as well as UV- and H2O2-induced mutation frequency by two- to fourfold. These results indicate that nicotine and NNK treatment sensitize these human cells to the extent that they are more susceptible to mutagenesis. We further tested the effect of these agents on induction of tumorigenic transformation using the anchorage-independent soft-agar growth assay (44, 45). The results in Fig. 6 B and C show that nicotine and NNK greatly induce soft-agar anchorage-independent growth of human lung and bladder cells, a necessary ability for tumorigenic cells (46–49).
Fig. 6.
Nicotine and NNK treatments enhance mutational susceptibility and cell transformation. Human lung and bladder epithelial cells (BEAS-2B and UROtsa) were treated with NNK (0.5 mM) and nicotine (25 mM for BEAS-2B cells, and 5 mM for UROtsa cells) for 1 h at 37 °C; these treatments render 50% cell killing. (A) UVC-irradiated (1,500 J/m2) or H2O2 modified (100 mM, 1 h at 37 °C) plasmid DNAs containing the supF gene were transfected into these cells, and the mutations in control, and nicotine- and NNK- treated cells were detected and quantified as previously described (13, 28). (B) Detection of anchorage-independent soft-agar growth. A total of 5,000 treated cells were seeded in a soft-agar plate. The method for anchorage-independent soft-agar growth is the same as previously described (28). Typical soft-agar growth plates stained with crystal violet were shown. (C) Quantifications of percent of control, nicotine, and NNK-treated cells formed colonies in soft-agar plates.
Discussion
The major purpose of E-cig smoking as well as tobacco smoking is to deliver the stimulant nicotine via aerosols, which allow smokers to obtain instant gratification. Unlike TS, which contains nitrosamines and numerous carcinogenic chemicals resulted from burning, ECS contains nicotine and relatively harmless organic solvents. Therefore, E-cig has been promoted as noncarcinogenic and a safer substitute for tobacco. In fact, recent studies show that E-cig smokers, similar to individuals on nicotine replacement therapy, have 97% less 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), an isoform form of NNK, a tobacco nitrosamine and lung carcinogen, in their body fluid than tobacco smokers (50). Based on these results, ECS has been recommended as a substitute for TS (50). However, E-cig smoking is gaining popularity rapidly particularly in young individuals and it is important to note that many of these E-cig smokers have taken up E-cig smoking habit are not necessary doing it for the purpose of quitting TS, rather, it is because they are assuming that E-cig smoking is safe. Currently, there are 18 million E-cig smokers in the United States and 16% of high school students smoke E-cig (51, 52). Understanding the carcinogenicity of ECS is an urgent public health issue. Since it takes decades for carcinogen exposure to induce cancer in humans, for decades to come there will be no meaningful epidemiological study to address the carcinogenicity of ECS. Therefore, animal models and cell culture models are the reasonable alternatives to address this question.
Nicotine has not been shown to be carcinogenic in animal models (7). However, during tobacco curing, substantial amounts of nicotine are transformed into tobacco-specific nitrosamines (TSA) via nitrosation, and many of these TSA, such as NNK and NNN, are carcinogenic in animal models (19, 53–55). Because of these findings, the occurrence and the level of nitrosamines in blood fluid have been used as the gold standard for determination of the potential carcinogenicity of smoking (56). While the NNAL level in E-cig smokers is 97% lower than in tobacco smokers, nonetheless, it is significant higher than in nonsmokers (50). This finding indicates that nitrosation of nicotine occurs in the human body and that ECS is potentially carcinogenic.
It is well established that cytochrome p450 enzymes in human and animal cells can metabolize and transform NNK, NNAL, and NNN into different products, which can modify DNA as well as proteins (20, 57, 58). This finding raises the possibility that the level of these nitrosamines detected in the blood stream of E-cig smokers at any given time may grossly underestimate the level of nicotine nitrosation. We undertake the approach of detecting DNA damage induced by nicotine rather than detecting nitrosamine level to address the potential mutagenic and carcinogenic effect of ECS. It should be noted that in vivo DNA damage can remain in genomic DNA for many hours and even days (13, 59, 60). Therefore, this approach not only is direct but also more sensitive in determining the carcinogenicity of ECS.
The level of γ-OH-PdG adducts induced by E-cig smoke in mice and by nicotine and NNK in cultured human cells is 10-fold higher than O6-medG (Fig. 1). We have shown that γ-OH-PdG adducts are as mutagenic as BPDE-dG and UV photoproducts and induce G to T and G to A mutations similar to the mutations in the p53 gene in tobacco smoker lung cancer patients (11). Together, these results suggest that γ-OH-PdG adducts are the major cause of nitrosamine lung carcinogenicity.
The current understanding of NNK and NNN metabolism indicates that NNK metabolites are further transformed into PBDs, formaldehyde, and MDOH (20, 21, 61), while NNN metabolites are hydroxyl and keto forms of PBD (20, 21, 61). While MDOH can induce O6-medG adducts, it is unclear what metabolites induce γ-OH-PdG adducts. It is well established that acrolein–DNA interaction generates γ-OH-PdG adducts (11, 13, 30) and that formaldehyde induces hydroxymethylated nucleotides, mainly dG, in animal models (62). It has been found that in vitro formaldehyde combined with acetaldehyde can induce γ-OH-PdG (63). Therefore, it possible that ECS, nicotine, and NNK induce γ-OH-PdG via their metabolite formaldehyde, which triggers lipid peroxidation and produces acrolein and acetaldehyde byproducts; consequently, these byproducts induce γ-OH-PdG.
In summary, we found that ECS induces mutagenic γ-OH-PdG and O6-medG adducts in lung, bladder, and heart tissues of exposed mice. ECS also causes reduction of DNA-repair activity and repair proteins XPC and OGG1/2 in lung tissue. Furthermore, nicotine and NNK induce the same effects in human lung and bladder epithelial cells. We propose that nicotine can be nitrosated, metabolized, and further transformed into aldehydes and MDOH in lung, bladder, and heart tissues of humans and mice. Whereas MDOH induced O6-medG, aldehydes not only induce γ-OH-PdG, but also inhibit DNA repair and reduce XPC and OGG1 proteins (Fig. S3). We also found that nicotine and NNK can enhance mutational susceptibility and induced tumorigenic transformation of human lung and bladder epithelial cells. Based on these results, we propose that ECS is carcinogenic and that E-cig smokers have a higher risk than nonsmokers to develop lung and bladder cancer and heart diseases.
Materials and Methods
Materials.
Acr-dG monoclonal antibodies and plasmid pSP189 were prepared, as described (13, 41). Acr-dG antibodies are specific for PdG adducts including Acr-, HNE-, and crotonaldehyde (Cro)-dG (29). Antibodies for XPC, hOGG1/2 (cross reacts with mouse OGG1/2), α-tubulin, and mouse/rabbit IgG; enzymes, T4 kinase, protease K, nuclease P, and RNase A; and chemicals, acrolein, nicotine, and NNK were commercially available. Immortalized human lung (BEAS-2B) and bladder epithelial (UROtsa) cells were obtained from American Type Culture Collection and J.R. Masters, University College London, London. All animal procedures were approved by the Institutional Animal Care and Use Committee, New York University School of Medicine.
ECS Generation and Mice Exposure.
Twenty FVBN (Jackson Laboratory, Charles River) male mice were randomized into two groups, 10 each. Mice were exposed to ECS (10 mg/mL), 3 h/d, 5 d/wk, for 12 wk. ECS was generated by an E-cig machine, as previously described (64). An automated three-port E-cigarette aerosol generator (e∼Aerosols) was used to produce E-cigarette aerosols from NJOY top fill tanks (NJOY, Inc.) filled with 1.6 mL of e-juice with 10 mg/mL nicotine in a propylene glycol/vegetable glycerin mixture (50/50 by volume; MtBakerVapor MESA). Each day the tanks were filled with fresh e-juice from a stock mixture, and the voltage was adjusted to produce a consistent wattage (∼1.96 A at 4.2 V) for each tank. The puff aerosols were generated with charcoal and high-efficiency particulate filtered air using a rotorless and brushless diaphragm pump and a puff regime consisting of 35-mL puff volumes of 4-s duration at 30-s intervals. Each puff was mixed with filtered air before entering the exposure chamber (1 m3). Tanks were refilled with fresh e-juice at 1.5 h into the exposure period during the pause between puffs. Mass concentrations of the exposure atmospheres were monitored in real time using a DataRam4 (Thermo Fisher Scientific) and also determined gravimetrically by collecting particles on Teflon filters (Teflo, 2 mm pore size; Pall) weighed before and after sample collection using an electrobalance (MT-5; Metler).
Cell Cultures and Treatments of Nicotine and NNK.
Exponentially growing BEAS-2B and UROtsa were treated with different concentrations of nicotine (BEAS-2B: 0, 100, 200 µM; UROtsa: 0, 1, 2.5 µM), and NNK (BEAS-2B: 0, 100, 300, 1,000 µM; UROtsa: 0, 50, 100, 200 µM) for determination of DNA adduct and DNA-repair activity. For XPC and hOGG1/2 detection, BEAS-2B were treated with nicotine (0, 50, 100, 200 µM), and NNK (0, 500, 750, 1,000 µM) and UROtsa were treated with nicotine (0, 1, 2.5, 5 µM) and NNK (0, 100, 200, 400 µM) for 1 h at 37 °C. Genomic DNA and cell lysate isolation from these cells was the same as described (28).
PdG and O6-medG Adduct Detection.
Cyclic PdG and O6-medG adducts formed in the genomic DNA were determined by the immunochemical slot blot hybridization method using Acr-dG and O6-medG antibodies and quantum dot labeled second antibody, as described (13, 28). PdG adducts formed in cultured human cells, and mouse lung tissue were further analyzed by the 32P postlabeling-2D-TLC/HPLC method, as previously described (28).
In Vitro DNA-Damage-Dependent Repair Synthesis Assay.
The DNA-repair activity was assessed by an in vitro DNA damage-dependent repair synthesis assay, as previously described (13).
DNA Repairs Proteins Detection.
The levels of XPC and OGG1/2 proteins in lung tissues of mice with and without ECS exposure, and in BEAS-2B and UROtsa cells treated with nicotine and NNK, were determined, as described (13).
Mutation Susceptibility Determination.
Shuttle vector pSP189 plasmids, which contain the tyrosine suppressor tRNA coding gene the supF, were UV (1,500 J/m2) irradiated or modified with H2O2 (100 mM, 1 h at 37 °C), then transfected into cells with and without pretreated with nicotine and NNK for 1 h at 37 °C. Mutations in the supF mutations were detected, as previously described (13).
Anchorage-Independent Soft-Agar Growth.
Lung (BEAS-2B) and bladder (UROtsa) epithelial cells were treated with NNK (0.5 mM) and nicotine (25 and 5 mM) for 1 h at 37 °C; these treatments rendered 50% cell killing. The method for anchorage-independent soft-agar growth is the same as previously described (28).
Statistical Analysis.
Statistical analysis and graphs were performed with Prism 6 (GraphPad) software. Two group comparisons were conducted with the unpaired, two-tailed Mann–Whitney u test or the unpaired, two-tailed t test with Welsh’s correction for unequal variances. A P value <0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank Drs. Frederic Beland and Catherine B. Klein for reviewing this manuscript and Ms. Mona I. Churchwell for technical assistance. This work was supported by National Institutes of Health Grants R01CA190678, 1P01CA165980, ES00260, and P30CA16087.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1406.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718185115/-/DCSupplemental.
References
- 1.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther Adv Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javed F, Kellesarian SV, Sundar IK, Romanos GE, Rahman I. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis. 2017;23:1052–1057. doi: 10.1111/odi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grana R, Benowitz N, Glantz SA. E-cigarettes: A scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23:ii11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 6.NTP (National Toxicology Program) 2016 Report on carcinogens,14th Edition (US Department of Health and Human Services, Public Health Service, Research Triangle Park, NC). Available at http://ntp.niehs.nih.gov/go/roc14. Accessed March 10, 2017.
- 7.US Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2014. [Google Scholar]
- 8.US Department of Health and Human Services . The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2006. [Google Scholar]
- 9.O’Loughlin J, Wellman RJ, Potvin L. Whither the e-cigarette? Int J Public Health. 2016;61:147–148. doi: 10.1007/s00038-016-0800-5. [DOI] [PubMed] [Google Scholar]
- 10.Loeb LA, Loeb KR, Anderson JP. Multiple mutations and cancer. Proc Natl Acad Sci USA. 2003;100:776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: A possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci USA. 2004;101:8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HT, et al. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem. 2012;287:12379–12386. doi: 10.1074/jbc.M111.329623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 16.Stepanov I, et al. Evidence for endogenous formation of N′-nitrosonornicotine in some long-term nicotine patch users. Nicotine Tob Res. 2009;11:99–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N′-nitrosonornicotine in humans. Nicotine Tob Res. 2013;15:591–595. doi: 10.1093/ntr/nts172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IARC . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 89. Intl Agency Res Cancer; Lyon, France: 2007. pp. 457–480. [Google Scholar]
- 20.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 21.Hecht SS, Carmella SG, Foiles PG, Murphy SE, Peterson LA. Tobacco-specific nitrosamine adducts: Studies in laboratory animals and humans. Environ Health Perspect. 1993;99:57–63. doi: 10.1289/ehp.939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swenberg JA, et al. Endogenous versus exogenous DNA adducts: Their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci. 2011;120:S130–S145. doi: 10.1093/toxsci/kfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyaya P, Kalscheuer S, Hochalter JB, Villalta PW, Hecht SS. Quantitation of pyridylhydroxybutyl-DNA adducts in liver and lung of F-344 rats treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2008;21:1468–1476. doi: 10.1021/tx8001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson LA, et al. Role of aldehydes in the toxic and mutagenic effects of nitrosamines. Chem Res Toxicol. 2013;26:1464–1473. doi: 10.1021/tx400196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beland FA, Fullerton NF, Heflich RH. Rapid isolation, hydrolysis and chromatography of formaldehyde-modified DNA. J Chromatogr A. 1984;308:121–131. doi: 10.1016/s0021-9673(01)87539-7. [DOI] [PubMed] [Google Scholar]
- 26.Bartsch H, Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984;5:1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- 27.Xue J, Yang S, Seng S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers (Basel) 2014;6:1138–1156. doi: 10.3390/cancers6021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HW, et al. Cigarette side-stream smoke lung and bladder carcinogenesis: Inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget. 2015;6:33226–33236. doi: 10.18632/oncotarget.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, et al. Detection of acrolein-derived cyclic DNA adducts in human cells by monoclonal antibodies. Chem Res Toxicol. 2012;25:2788–2795. doi: 10.1021/tx3004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HT, et al. Effect of CpG methylation at different sequence context on acrolein- and BPDE-DNA binding and mutagenesis. Carcinogenesis. 2013;34:220–227. doi: 10.1093/carcin/bgs323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HT, Zhang S, Hu Y, Tang MS. Mutagenicity and sequence specificity of acrolein-DNA adducts. Chem Res Toxicol. 2009;22:511–517. doi: 10.1021/tx800369y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 34.Sugasawa K, et al. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell. 1998;2:223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 35.Radicella JP, Dherin C, Desmaze C, Fox MS, Boiteux S. Cloning and characterization of hOGG1, a human homolog of the OGG1 gene of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:8010–8015. doi: 10.1073/pnas.94.15.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tano K, Shiota S, Collier J, Foote RS, Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc Natl Acad Sci USA. 1990;87:686–690. doi: 10.1073/pnas.87.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Natarajan AT, et al. Chromosomal localization of human O6-methylguanine-DNA methyltransferase (MGMT) gene by in situ hybridization. Mutagenesis. 1992;7:83–85. doi: 10.1093/mutage/7.1.83. [DOI] [PubMed] [Google Scholar]
- 38.Esterbauer H, Zollner H. Methods for determination of aldehydic lipid peroxidation products. Free Radic Biol Med. 1989;7:197–203. doi: 10.1016/0891-5849(89)90015-4. [DOI] [PubMed] [Google Scholar]
- 39.Guéraud F, et al. Chemistry and biochemistry of lipid peroxidation products. Free Radic Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 40.Gentile F, et al. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017;4:103–137. doi: 10.3934/genet.2017.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HW, et al. Acrolein- and 4-Aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget. 2014;5:3526–3540. doi: 10.18632/oncotarget.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft C, Peter M, Hofmann K. Selective autophagy: Ubiquitin-mediated recognition and beyond. Nat Cell Biol. 2010;12:836–841. doi: 10.1038/ncb0910-836. [DOI] [PubMed] [Google Scholar]
- 43.Schrader EK, Harstad KG, Matouschek A. Targeting proteins for degradation. Nat Chem Biol. 2009;5:815–822. doi: 10.1038/nchembio.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freedman VH, Shin SI. Cellular tumorigenicity in nude mice: Correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 45.Borowicz S, et al. The soft agar colony formation assay. J Vis Exp. 2014:e51998. doi: 10.3791/51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cressey D. E-cigarettes affect cells. Nature. 2014;508:159. doi: 10.1038/508159a. [DOI] [PubMed] [Google Scholar]
- 47.Zhou H, Calaf GM, Hei TK. Malignant transformation of human bronchial epithelial cells with the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Cancer. 2003;106:821–826. doi: 10.1002/ijc.11319. [DOI] [PubMed] [Google Scholar]
- 48.Plesner BH, Hansen K. Formaldehyde and hexamethylenetetramine in Styles’ cell transformation assay. Carcinogenesis. 1983;4:457–459. doi: 10.1093/carcin/4.4.457. [DOI] [PubMed] [Google Scholar]
- 49.Heidelberger C, et al. Cell transformation by chemical agents–A review and analysis of the literature. A report of the US environmental protection agency gene-tox program. Mutat Res. 1983;114:283–385. doi: 10.1016/0165-1110(83)90036-2. [DOI] [PubMed] [Google Scholar]
- 50.Shahab L, et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: A cross-sectional study. Ann Intern Med. 2017;166:390–400. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coleman BN, et al. Electronic cigarette use among US adults in the population assessment of tobacco and health (PATH) study, 2013-2014. Tob Control. 2017;26:e117–e126. doi: 10.1136/tobaccocontrol-2016-053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh T, et al. Tobacco use among middle and high school students–United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65:361–367. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 53.Hecht SS, et al. Tobacco-specific nitrosamines: Formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J Natl Cancer Inst. 1978;60:819–824. doi: 10.1093/jnci/60.4.819. [DOI] [PubMed] [Google Scholar]
- 54.Hecht SS, Adams JD, Numoto S, Hoffmann D. Induction of respiratory tract tumors in Syrian golden hamsters by a single dose of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and the effect of smoke inhalation. Carcinogenesis. 1983;4:1287–1290. doi: 10.1093/carcin/4.10.1287. [DOI] [PubMed] [Google Scholar]
- 55.Hecht SS, et al. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986;46:4162–4166. [PubMed] [Google Scholar]
- 56.Carmella SG, et al. Mass spectrometric analysis of tobacco-specific nitrosamine hemoglobin adducts in snuff dippers, smokers, and nonsmokers. Cancer Res. 1990;50:5438–5445. [PubMed] [Google Scholar]
- 57.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 58.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 59.Morris RJ, Fischer SM, Slaga TJ. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res. 1986;46:3061–3066. [PubMed] [Google Scholar]
- 60.Denissenko MF, Pao A, Pfeifer GP, Tang M. Slow repair of bulky DNA adducts along the nontranscribed strand of the human p53 gene may explain the strand bias of transversion mutations in cancers. Oncogene. 1998;16:1241–1247. doi: 10.1038/sj.onc.1201647. [DOI] [PubMed] [Google Scholar]
- 61.Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat Res. 1999;424:127–142. doi: 10.1016/s0027-5107(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 62.Lu K, Gul H, Upton PB, Moeller BC, Swenberg JA. Formation of hydroxymethyl DNA adducts in rats orally exposed to stable isotope labeled methanol. Toxicol Sci. 2012;126:28–38. doi: 10.1093/toxsci/kfr328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng G, et al. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: Formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem Res Toxicol. 2003;16:145–152. doi: 10.1021/tx025614r. [DOI] [PubMed] [Google Scholar]
- 64.Zhao J, Pyrgiotakis G, Demokritou P. Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal Toxicol. 2016;28:658–669. doi: 10.1080/08958378.2016.1246628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.