Significance
The origin of mitochondria is a challenging and intensely debated issue. Mitochondria are ancestrally present in eukaryotes, and their endosymbiotic inclusion was an extremely important step during the transition from prokaryotes to eukaryotes. However, because of the unknown order of eukaryotic inventions (e.g., cytoskeleton, phagocytosis, and endomembranes), it is unknown whether they led to or followed the acquisition of mitochondria. According to the farming hypothesis, the mitochondrial ancestor was captured by a phagocytotic host, but the advantage was not direct metabolic help provided by the symbiont; rather, it was provisioning captured prey to farmers in poor times, like humans farm pigs. Our analytical and computational models prove that farming could lead to stable endosymbiosis without any further benefit assumed between partners.
Keywords: mitochondrial origin, eukaryotes, endosymbiosis, evolution, ecology
Abstract
The origin of mitochondria was a major evolutionary transition leading to eukaryotes, and is a hotly debated issue. It is unknown whether mitochondria were acquired early or late, and whether it was captured via phagocytosis or syntrophic integration. We present dynamical models to directly simulate the emergence of mitochondria in an ecoevolutionary context. Our results show that regulated farming of prey bacteria and delayed digestion can facilitate the establishment of stable endosymbiosis if prey-rich and prey-poor periods alternate. Stable endosymbiosis emerges without assuming any initial metabolic benefit provided by the engulfed partner, in a wide range of parameters, despite that during good periods farming is costly. Our approach lends support to the appearance of mitochondria before any metabolic coupling has emerged, but after the evolution of primitive phagocytosis by the urkaryote.
Mitochondria provide eukaryotic cells with energy through efficient oxygen respiration. They are endosymbiotic descendants of once free-living alphaproteobacteria (1, 2). The origin of this endosymbiosis remains unclear because initial advantages of the association are debatable (for an extensive review, see ref. 3) and we do not know whether mitochondria are early or late partners of the eukaryotic host cell (4–6). Arguably, the origin of the eukaryotic cell is the biggest major transition in the history of life, and also a major transition in individuality (7, 8). Besides the acquisition of mitochondria, which proved to be extremely effective partners in the long term, radical changes happened in many aspects of the host (e.g., cell structures, inheritance mechanisms, and their coordination) relative to what we see in the prokaryotic ancestors. Such a major transition cannot be selectively neutral: thousands of positive mutations must have been selected for, leading to the complex eukaryotic cell that the last eukaryotic common ancestor (LECA) was (9, 10). Out of the many steps of the transition, we focus only on the merger (the integration of mitochondria in the archaeal host), without discussing much of the preceding or succeeding steps. The question naturally arises: What are the benefits of the eukaryotic condition and the merger? Essentially, there are two answers, and both assume markedly different initial conditions and timings for mitochondria to join the party.
The traditional answer for the benefit of eukaryotes is predation by phagocytosis that would have considerably increased the efficiency relative to any bacterial predation (with external digestion) (11, 12). The benefit in this case is the energy saved for processes other than the synthesis of many organic compounds. Although the phagocytotic machinery and its functioning demand energy, (secondarily) amitochondriate eukaryotes, once termed Archezoa (13), are phagocytosing without mitochondria. Note, however, that lack of evidence is not the evidence of lack; the recently discovered Asgard archaea (14) show signs of a cytoskeleton. Mitochondria, in this scenario, were acquired phagocytotically, and provided photosynthates for the host to help survive hard times.
The hypothesis rival to ancestral predation is the energetic boost provided by ancient mitochondria to the evolving host cell (15–17). Because the Archezoan lifestyle exists, the claim has been made that not this condition as such but the evolutionary process leading to it would have required the energetic boost by mitochondria (18). The ancestral eukaryotic lineage is supposed to have experimented with a large combinatorial library of new and costly proteins that might have required up to 100,000 genes per cell (16). No model or any comparative evidence has been offered to support this claim (3). Mitochondria undoubtedly provided an energetic boost to the evolving host cell, but this benefit could only be harvested after the establishment of the adenine nucleotide translocator (ANT), a eukaryotic invention (19) that taps into the symbionts ATP pool. What may have been the benefit to partners before the translocator was in place?
Here we entertain the possibility that phagocytosis evolved in the host archaeon. Several facts support this, at least weakly, in modern Archaea: actin and tubulin homologs (20, 21), primitive cytoskeletal features (20, 22), membrane remodeling (23, 24), signs of possible earlier endosymbiosis (25), and even possibly phagocytosis in the archaeal host (14). For symbiosis, or at least a mutually beneficial relationship to develop, the bacterial partner had to evade digestion.
But this raises a problem: Why would indigestion have been beneficial without any direct profit for the host? This is further complicated by the fact that eating less (relative to well-fed competitors) would have meant an immediate disadvantage. More than 20 y ago it was suggested that perhaps the ancient hosts kept prey bacteria as humans keep pigs (8): they feed them during easy times and eat them during hard times (a bet-hedging strategy) (26). Similar exploitative behavior was observed in the Paramecium–Chlorella endosymbiosis (27). The critical feasibility condition of the proposed scenario is phagocytosis before endosymbiosis, rather than the other way around. Given the fact that members of the recently established but ultrastructurally still-unexplored Asgard archaeal superphylum has genes for cytoskeletal processes and vesicle transport (14), we do not consider early phagocytosis, before mitochondria, unrealistic. In this case, because the host was already predatory, the only benefit for the host cell compared with nonprovisioning phagocytotic predators was the prey stock available during hard times.
In this paper we show by explicit modeling that the farming scenario is a viable route to protomitochondrial establishment: farming by regulated internal digestion could have led to stable endosymbiosis without any other benefit (such as ATP or other metabolic currency from the symbiont) in an environment alternating between rich and poor in prey bacteria. We present an analytical minimum model of farming and a more comprehensive, but consonant, individual-based model. Each model investigates how two types of phagocytotic predatory archaea, one conventional (nonfarmer) and one capable of storing prey for delayed digestion (farmer), compete in an ecological-evolutionary setup. This is a unique and ecologically explicit dynamical model of the establishment phase of the prokaryote-to-eukaryote major transition.
Results
To help the reader, we briefly introduce both models before results are discussed. Detailed explanations are in Materials and Methods and in SI Appendix; a list of all figures and experiments are provided in SI Appendix, Table S2 with parameter values in SI Appendix, Table S3.
Analytical Minimum Model of Farming.
The ecological model simulates the dynamical behavior of farmer and nonfarmer species, assuming a fluctuating resource, causing rich and poor environments. In rich periods, both species grow exponentially, but farmers have a fitness disadvantage (reduced growth rate constant) as farm maintenance inflicts a cost. In poor environments, both species decline, but the farmer has a delayed decline due to its provisions provided by the farm. The longer the poor period is, the closer the farmer’s death rate gets to that of the nonfarmer.
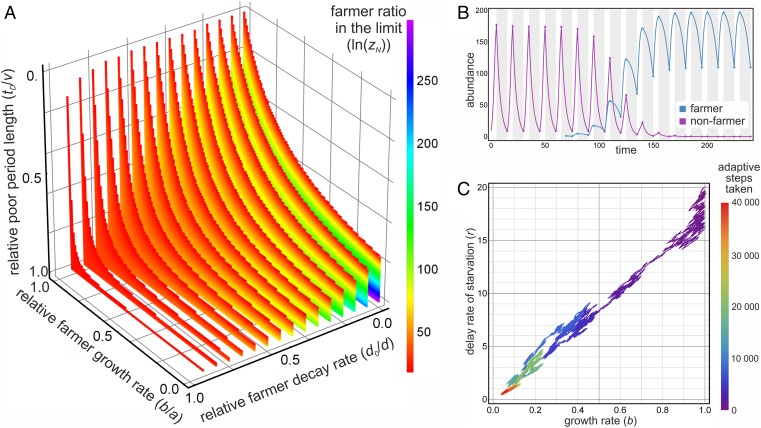
Farmers are introduced in small amounts to a nonfarmer population; the time evolution of dynamics are presented in Fig. 1B. The ratio of farmers to nonfarmers () after resource-rich and resource-poor periods indicates that the farmer population can successfully invade a nonfarmer population if the resource-poor period is longer than the rich period (D and G represent decline and growth; Fig. 1A). The longer the poor period is, the more fitness disadvantages farmers can tolerate in good periods. These disadvantages stem from the diminished per-capita reproduction rate b (compared with nonfarmer’s reproduction rate a).
Fig. 1.
(A) Equilibrium distribution of invading farmers. Colored points indicate cases where farming cells (y) dominate over nonfarmers (x) after cycles of rich–poor periods. Color indicates the logarithm of the ratio ; points are not shown where (farmer is practically extinct). Parameters: . (B) Time evolution of farmers invading nonfarmer population. Mutant (farmer) is introduced at with amount . Calculated by numerical integration of Eq. 1 for rich and Eq. 3 for poor periods; the successive and values can also be calculated by the analytical solutions for the corresponding differential equations (these are used to calculate ; SI Appendix). Parameters: , 16 cycles of rich–poor period pairs; shading indicates poor periods. Results are similar with random period lengths (SI Appendix, Figs. S2 and S3). (C) Adaptive dynamics of invading farmers. Successive farmer mutants can invade a nonfarmer population only if parameters (intrinsic growth rate) and (delayed decline by farming) are in trade-off: if decreases (better delay of death in poor periods), must also decrease (worse growth in good periods). There exist viable evolutionary trajectories toward establishing farming. Parameters: ; mutant is introduced after two rich–poor cycles. For further details, see SI Appendix.
To investigate evolution, we have explored the adaptive dynamics of the model (Fig. 1C). Results show that invading populations of more evolved farming ability (delayed starvation due to farming) successively replace residents, ultimately eliminating nonfarmer behavior (assuming the intrinsic growth rate b is in tradeoff with the delay rate of starvation r; otherwise, the result would be trivial). If r decreases (better delayed starvation in poor times), b must also decrease (worse growth in rich times), which is a realistic biological condition. If this holds, most evolutionary trajectories lead to the strong attractor of small instead of high r,b values (SI Appendix, Fig. S4). In other words, the longer starvation is delayed in poor periods (small r), the smaller intrinsic growth rate farmers can tolerate in good periods (small b), indicating that farming is sustainable.
Farming, Fusion, and Fission.
We have investigated whether sex (iterated fusion–fission) of the archaeal cells have any effect on the population. We show both via a continuous time and a game theoretical model, that farmers always have an advantage. We assume that, during fusion–fission of host cells, farmed bacteria are exchanged via passive cytoplasmic diffusion, which, if infinite time is allotted for fusion, results in equalized stock sizes where each party donates half of its stock to the other. Nonfarmers cannot pursue an active “stealing” strategy (anything that would bias passive diffusion toward receiving more than half of the partner’s stock) because that would require highly evolved cytoskeletal gymnastics. In this case, because nonfarmer and farmer stock levels would converge only in infinite time, nonfarmers could never win. There is a critical time when farmers have more stock on average than nonfarmers in the poor period, assuming nonfarmers can obtain a fraction of farmer stock:
where is the rate of stock elimination (culling), is the probability that farmers and nonfarmers fuse, is the stock level of farmers at the start of the poor period, and is an arbitrary small number specifying the threshold of convergence of stock levels of farmers and nonfarmers). It ensures that the evolutionarily stable strategy is farming and not stealing.
Individual-Based Computational Model.
The above minimum model deliberately neglects several component processes (Fig. 2 and SI Appendix, Table S1). To provide a more realistic case, we have constructed an individual-based, stochastic, model in discrete time where internal cell states (cell and farm sizes), interactions (eating, farming, and division), and costs are explicitly modeled. Farmer and nonfarmer cells compete in a closed Moran process for prey species. The prey population regenerates at every time step according to the fluctuation of the abiotic resource causing poor and rich periods. Predators capture prey and, according to their evolutionary traits of culling and farming, eat them or store them for later use. Culling the farm happens in a piecemeal fashion. When fed, host cells grow and divide when they reach a certain size. The explicit cost of farm using up host’s resources is realized in negative host growth (dependent on farm size). Farmers also suffer an implicit cost of farming because they cannot store and cull in the same time step, modeling increased handling time. The farm itself can also grow autonomously, depending on the availability of the abiotic resource.
Fig. 2.
Predator–prey interactions in the farming archaeon and interactions in the individual-based model. Free living prey bacteria (blue cells) have density A that is explicitly defined by the abiotic resource density R (1). Predatory archaeon capable of farming (red cell) captures prey (2). The predator either consumes the captured prey to fuel its growth (3) or store prey in the farm (farming, 4) that has density B. Stored living cells can also be digested by the host to grow (culling, 5) which eventually leads to the reproduction of the host (6). Stored bacteria can also reproduce within the host depending on resource R (7). Bacteria multiply in separate phagosomes (red wrappers). Farmed cells could escape the host (8) to reseed environments where prey species have been extinct (omitted in the model). Predators unable to maintain a farm (nonfarmers) lack processes 4, 5, 7, and 8; predators unable to cull their farm lack 5. Any explicit benefit the farm provides for the host (metabolites, energy) is not displayed; if there is no explicit benefit, hosted bacteria are parasites.
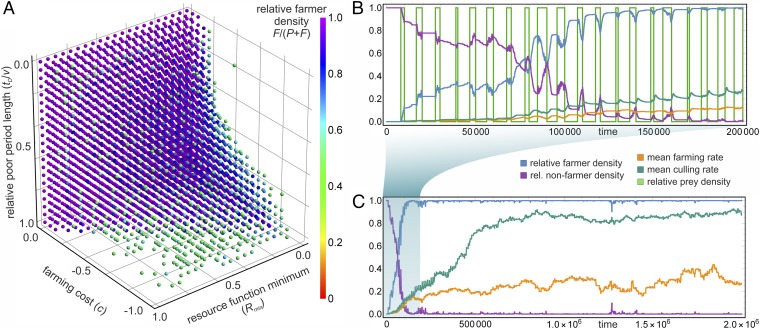
Results are consistent with those of the minimal model: farmers can invade without extra benefit of their symbionts if environment fluctuates (Fig. 3 B and C). Farmer invasion is possible (and likely) whenever there is reduced mortality (or increased growth rate) for the farmers in poor periods. Moreover, farmers can invade in a wide range of the parameters (Fig. 3A).
Fig. 3.
Invasion of farmers in the evolutionary individual-based model. (A) Equilibrium populations where farmers win. Colored points represent equilibrium populations where the relative farmer ratio is above 1/2 (after 107 time steps, 1,000 rich–poor cycles with period length , each point is an average of 10 independent simulations). Each simulation started from a pure nonfarmer population with farming mutation rate and culling mutation rate . To comply with the assumption that no explicit benefit can be expected from the symbiont ab initio, farm cost was not allowed to evolve and turn to benefit. Farmers can set aside prey (with probability rate ), but it is costly to do so (). is the ratio of the poor period in a rich–poor period cycle; c is the cost of farming; is equivalent to the minimum of prey density in poor periods. (B) Characteristic run where farmer population wins over nonfarmer {. (C) Simulation of B in longer time span. Prey density is omitted; blue shading indicates position of B.
Farming ability first appears by mutation, and farmers initiate their farm by storing prey. After this, the farm is increased mostly by its own growth. According to our results, this has the following consequences. First, the host evolves toward culling more and more of its farm, even in good times, maximizing the culling rate, to keep up with nonfarmers (SI Appendix, Figs. S9C and S10C). Second, because further storing to the farm is marginal to its own growth, the allocation ability can be entirely lost (SI Appendix, Figs. S9B and S10B). It only makes sense to maintain the mechanism if the farm can be lost due to culling, and it must be regained after a long resource-poor period (as farming eukaryotes do) (28, 29). Results prove that this is indeed the case (SI Appendix, Figs. S9B and S10B). At any point, nonfarmers cannot invade, due to the still-existent environmental fluctuations that favor farmers with provisions in poor periods (SI Appendix, Fig. S9A).
Ultimately, we allowed evolution to turn explicit farming cost to benefit, mimicking the invention of ANT or other means by which a symbiont could provide metabolic help (this benefit, dependent on farm size, is above the implicit benefit of providing provisions). The cost of farming is assumed to decrease most slowly during evolution; otherwise the result is trivial, because turning any cost to benefit is an obviously adaptive step, and farmers would easily win. Even with slowest mutation rate (compared with storing and culling), cost will turn to benefit. Although this is trivial, we were interested in whether a large enough explicit benefit can cause the host to abandon culling for good. Results prove that this is possible: when explicit benefit is at least four times the unit gained from eating a single prey, culling rate can be reduced to zero (SI Appendix, Figs. S12D and S13). This proves that there is a viable evolutionary route from predation through farming to metabolic coupling.
Discussion
We have found in both the analytical and the computational models that no explicit benefit is required from the partners for a stable integration to evolve, provided parties receive implicit benefit (food for the host and shelter for the symbiont in poor times). Farming is a form of bet hedging: the host applies different strategies in good and hard times to minimize its overall risk of extinction. In consequence, relative fitness becomes higher in poor environments and overall temporal variance of fitness is reduced in expense of reduced fitness in rich environments (26). Although no examples of bet hedging are known in Archaea, it is prevalent and well documented in Bacteria and Eukarya. Because there is no need to assume any further metabolic interaction, our bet-hedging strategy can explain stable integration of endosymbiont with host without preexisting metabolic coupling. Given that in both of our models, there is a wide range of parameters where farmers can spread and dominate the population, we claim that ours is a general result that could explain many cases of evolution towards stable endosymbiosis.
Whereas neither phagocytotic archaea nor endosymbionts in archaea are known (30) [the association of haloarchaean genes with a hypothetical early bacterial endosymbiont (31) seems to be an artifact (32)], recall that no intermediate of any stage toward eukaryotes is known (neither mitochondriate prokaryotes nor primarily amitochondriate eukaryotes are known to exist). It is obvious, however, that some must have existed. An early appearance of phagocytosis in Archaea is increasingly, albeit as yet inconclusively, supported by finding the necessary components (14, 20–24). Assumption of phagocytosis implies that the farming strategy can be applied to the establishment of mitochondria. What makes our models specific to mitochondrial origins are (i) the complete lack of any preexisting metabolic interaction or preadaptation (that could provide any explicit benefit) that are certainly there in any modern eukaryote harboring endosymbionts; (ii) the lack of synchronized cell cycles for host and symbiont; (iii) phagocytosis; and (iv) farming and delayed digestion of the farm. Consequently, the lack of relevant examples makes it very hard to estimate parameter values for an association that occurred ∼1–2 Ga ago (33). If the host was increased in size (required for effective phagocytosis), it is not unreasonable to assume an internal endosymbiotic population size in the order of 102–103 [e.g., in ciliates (34); modern amoeba might contain as many as 106 (35)]. Some Korarchaea and Thaumarchaea (in fact in symbiosis with epibiotic bacteria) can reach sizes of 10–100 μm (36). For a detailed explanation on our parameter choices, see SI Appendix, Parameterization.
An analogy of our proposed mechanism lies in the farming behavior of Dictyostelium discoidum. Some clones of this slime mold can establish a symbiosis with members of the Burkholderia bacterial genus. By incorporating the bacteria in the fruiting body instead of eating them all (prudent predation), the amoebae can ensure that the germinating spores find themselves equipped with edible food in a nutrient-poor medium (28). The bacteria can confer the farming behavior on the amoebae (29). Remarkably, another bacterium, Pseudomonas fluorescens is also associated with this phenomenon with two different strains: one is inedible but produces useful small molecules to the farmers (and possibly harmful to nonfarmers) and the other one is edible. The difference between the two strains is caused by a mutation in a regulatory gene (37). A recent experimental paper (27) also shows that full exploitation is a feasible route to the establishment of endosymbiosis (among eukaryotic partners). In the Paramecium–Chlorella symbiosis, the algal partner never gains net benefit from endosymbiosis, whereas the ciliate host benefits from engulfed algae under high light irradiance, and suffers from a cost in dim light. This seems to be the closest analogy to the interim evolutionary phase considered here. Taken together, these farming phenomena support our scenario for mitochondrial origins, with the caveat that living examples always involve highly evolved eukaryotic hosts. They also prompt us to hypothesize that some extra benefits (not considered in our model, such as resistance against other bacteria) may have provided advantage to host cells even when eating of ingested prey would have been still inefficient.
A serious problem of farming is divisional dilution: even in good times (when the farm is not supposed to be culled), the actual farm size will reduce in successive divisions, unless something counters it. (This is even more pronounced in case of nonsynchronous host and symbiont cell cycles.) Storing more prey does not help; it reduces the relative reproduction rate of farmers compared with nonfarmers because farmers can eat less in unit time (see above). The only factor that can counter divisional dilution at the start of the partnership is autonomous growth of the farm (SI Appendix, Fig. S11). Accordingly, the farm’s ability to grow inside the host must have been paramount in countering occasional culling and halving at every division (in the minimal model, this is implicitly assumed). Furthermore, farmed bacteria directly depend on the external resource (i.e., the environment), so in poor periods they can only grow very slowly (or not at all). Therefore, the farm will not last indefinitely in poor times because the host will ultimately consume faster than it can grow. If the poor period is any longer than the provisions, it means a death sentence for farmers (even if nonfarmers have already been starved to death). Thus, farmers must balance between building up a farm, paying costs, and competing with nonfarmers in good periods, and rationing their farms in poor periods such that in the long run they outperform nonfarmers.
To model a worst-case scenario, we deliberately implied costs on everything the farmer does to prevent any trivial advantage over nonfarmers, so as not to beg the question. Farming has an explicit cost, dependent on farm size, that the host must pay in the form of reduced growth. Furthermore, we also added an implicit cost of farming: farmers cannot store and cull in the same time step (modeling increased handling time). Because any food stored is not consumed right away, it means that farming equals giving up eating. This ensures that farming is not a zero-sum process and has a disadvantage in good periods: farmers grow less in unit time compared with nonfarmers (even if farming has zero explicit cost). Consequently, farmers must have superior reproductive rates compared with nonfarmers in poor environments, otherwise they will go extinct or cannot invade. This is achieved by delaying death in poor periods (or even being able to reproduce in the individual-based model) when nonfarmers simply starve. Thus, according to our models, hosts do not receive free lunch; nevertheless, protomitochondria are able to stably integrate.
In our model, captured bacteria end up enclosed in the host’s phagosome, just like in real and laboratory conditions (35) (though this is not always the case; see ref. 34). Wrapping symbionts in a phagosomal membrane has consequences. It might limit the symbiont metabolism if it relies on extensive transport of large molecules. However, this does not seem to be a problem if the symbiont is phototrophic (as we assume). Many modern examples attest to this, most prominently the various plastids, which sometimes retain extra membranes. Although losing the phagosomal membrane could have been a late invention (followed by the integration of ANT), the reproduction of symbionts within a phagosome provides a natural solution as to how they could have ended up in the host’s cytoplasm: the phagosome simply burst due to overpopulation. No phagosomal remnants can be found around modern mitochondria, whose outer membrane is partly of bacterial origin.
The most important consequence of the phagosomal membrane is, however, that the symbiont could only reproduce clonally. Unless the host was sexual (discussed later), the symbiont is also exclusive to the host’s lineage. As a result, the symbiont genome becomes closely linked with the host’s genome, even before nuclear transfer of any genes. In the individual-based model, we assume asexual hosts. We also associate the farming ability with the host’s genome (instead of the symbionts), as is the case with Dictyostelium, where carrying a farm is a clone-specific trait (28). Consequently, all evolutionary traits presently associated with the asexual host could equally be associated with its clonal symbiont, i.e., the farm allocation rate of the host could in fact be the digestion-evasion rate of the symbiont. The evolved trait of culling can thus be interpreted as the ability to slowly overcome this evasive strategy. If, however, bacteria can evade host’s digestion, nonfarmers lacking the culling ability can end up with internal bacteria that only imply costs and do not provide any benefit. This would be a parasitic scenario.
Although the above argument holds, ancient Archaea might have practiced sex and fused to share genes and farms. Eury- and Crenarchaeota are known to undergo fusion and fission (38, 39). We presented a simple game theoretical model that nevertheless captures the essence of the situation. Assuming that fusion is triggered by starvation, we find that farmer–farmer interactions are less critical because nonfarmers can practically “steal” part of the stock when fusing (and splitting) with farmers. We show that nonfarmers can never build up stock larger than farmers if diffusion is responsible for exchange. In other words, farmers in the poor period always have more stock than nonfarmers, which maintains their selective advantage (in terms of survival) in poor times. There are other important considerations that favor farmers in the long-term. Repeated fusion–fission is costly (draining stocks), and leads to selective death of those running out of their reserves. Remorseless decline in population density entails an Allee effect that favors farmers: as densities drop (there is no reproduction in the poor period), mating probability (also of farmers and nonfarmers) decreases hyperbolically. Ultimately, internal stock levels will decide who survives the poor period and in what density. That is, farmers must maintain a farm large enough to survive the poor period with an end period density that prohibits nonfarmers to outgrow them in the following good period.
Furthermore, if members of the farm can occasionally escape the host (not modeled), farmers can (re)colonize habitats where prey is missing (like Dictyostelium does) (28). Consequently, farm escape, or other means that facilitate farm-sharing, renders the prey as a public good in poor times (and farming/seeding ability a useful asset in the population), which minimizes risk for all population members, thus stabilizing the overall population. We have not modeled this, but the effect was proven in a eukaryotic example (40). Because rare escape of farmed bacteria is enough to reseed habitats and has only a marginal cost, we believe that this beneficial mechanism could have been additional to provisioning.
There also seems to be a natural way to lose the farm-allocation ability—something we indeed cannot see in established mitochondrial dynamics. If the farm autonomously grows within the host, allocation becomes a neutral trait (SI Appendix, Fig. S9). Our results specifically show that there is no real need for an allocation mechanism at all from the host’s side: any process that serves to delay digestion up to the point where the symbiont can reproduce is sufficient. Furthermore, we found that when symbionts provide more benefit than what they inflict as cost on their host, and the host receives more benefit than what it pays to feed its farm, then maintaining the farm becomes more profitable than eating it (SI Appendix, Fig. S12). Consequently, culling is abandoned when the farm can provide more explicit benefit [e.g., photosynthate (41), metabolite (42, 43), or ATP (11, 44)] than what the host would receive by eating it. The route to synchronized cell cycles opens up.
The farming hypothesis of Maynard Smith and Szathmáry (7, 8) is a plausible scenario for the origin of mitochondria. Our models provide strong support for the farming hypothesis and, consequently, for the origin of mitochondria right after phagocytosis and before any metabolic coupling, especially before the invention of ANT. Our work explicitly tests a mitochondrial origin hypothesis in a dynamical model, and is intended to bridge the gap between telltale evolutionary scenarios and ecological assumptions within the origin of eukaryotes. Our theory of mitochondrial origin, as any other, has to explain the same questions raised previously (3). Although our scenario does not explain all of the issues of eukaryogenesis (neither of the hypotheses do) (3), it provides a plausible explanation for the early endosymbiotic relationship between partners (not just in the mitochondrial context) and the emergence of relevant evolutionary innovations (farming, prudent predation).
Materials and Methods
Analytical Minimal Model of Farming.
We consider two predatory cell types: nonfarmer and farmer. Farmers do not digest a fraction of ingested prey during good times, and instead digest them when no prey is available in the environment. Relative to the nonfarmer, the farmer enjoys a benefit during prey scarcity by feeding on its prey stock, but suffers from a cost of slower growth during prey richness. The dynamics during the growth period G is
| [1] |
where and are nonfarmer and farmer population densities at time t, and a and b are respective Malthusian growth rates. Because of farming, . is the total production in the system and is the carrying capacity to which the total density relaxes. Prey density is not explicitly modeled. The ratio of farmer to nonfarmer after a period of can be solved
| [2] |
During prey scarcity there is only death (period D) no predation. Hence,
| [3] |
The initial death rate of farmers is much less than d of nonfarmers. With time, death rate of farmers converges to that of nonfarmers. We assume that the increase in the death rate of farmers from to proceeds along a time-dependent sigmoid curve, r affecting the shape of the sigmoid (SI Appendix, Fig. S1). Both and have analytic solutions expressing population densities at the end of a -long period (SI Appendix). Their ratio is
| [4] |
After N cycles of G–D pair of periods, the ratio is
| [5] |
where and are the lengths of the growth and decline periods, respectively, and is the ratio at the start of the period. It is now straightforward to see when the farmer wins over its competitor: Fig. 1 shows , the farmer/nonfarmer ratio after N G–D cycles. A concise condition for is not known.
Farming, Fusion, and Fission.
We present a game theoretical model here, and an extension of the above minimum model in SI Appendix, to model archaeal sex. We follow the assumptions of the ecological minimal model above. There are two predatory types: farmer and nonfarmer. In good periods, both types grow exponentially, but only farmers can store living cells in phagosomes. In poor times, there is only decay, and cells without farms starve to death. Fusion is triggered by starvation: both types are of the same archaeal stock and can fuse with each other. During fusion, they exchange cytoplasmic components, e.g., farmed cells, and then split. Because in the poor period there is no growth or reproduction, we can ignore replicator dynamics for the game theoretical model and assume iterated pairwise interactions. For the simplest case we assume that when cells fuse, empty nonfarmers steal a fraction s of the stock of their farming partners, who retain a fraction (1 − s) of their stock. The below payoff matrix provides the average internal food levels (and average payoffs) after first round of fusion–fission, where intact farmers can accumulate a farm of size . It is easy to see that farmer is the evolutionarily stable strategy in case of .
Individual-Based Computational Model.
The resource input to the system is provided by an abiotic resource function that directly defines the actual amount of external prey. In every time step, a fixed amount of prey enters the system. Until the population is fully updated, no new prey cells enter. Hence, there is competition for them and they can be depleted in the given time step. The next time step will introduce again a new batch of prey. With this simplification (i.e., assuming regulated growth of prey species) we can directly focus on the competitive dynamics of the various predator types. Predators find external prey according to their density. When they acquire prey, they either eat it and grow or (if able to farm) store the prey for later processing. A predator can only ever consume a single prey per turn. This means that the farmer only eats a farmed prey item if it cannot find a free-living prey in that turn. In contrast, if a farmer captures an external prey, it will not cull its farm, regardless of whether it has consumed or farmed the captured prey. Farmed bacteria are not essential for the farmers: if there is no farm, they can live the same life as the nonfarmer predators (with a small decrease in fitness due to the maintenance cost of the farming apparatus). Any eaten prey increases the internal growth state of the predator. When the growth state reaches split size, the cell inevitably splits to two. A farm’s inherent growth is implicitly modeled to avoid complicated individual updating of prey populations within host populations.
The population of predators is limited and closed, to keep dynamics at bay. Competition is modeled as a Moran process where reproducing individuals produce a single new offspring that replaces another individual in the population. This way, types with faster growth rates will overpopulate those with lower growth rates. Predators able to farm must pay costs. The baseline cost comes from maintaining the apparatus of farming and does not depend on the number of farmed cells. The farm-dependent cost explicitly depends on the size of the farm: the bigger the farm is, the more the host must pay. Costs are deducted from the hosts’ internal growth state to slow down its reproduction rate (explicit benefit, if present, is added to the growth state). When a predator splits, a daughter cell is generated, which overwrites another existing cell in the population. Both the parent’s and the daughter’s growth states are reset to zero and the farm is randomly distributed among them according to binomial distribution. The daughter cell inherits the parent’s evolutionary traits with mutations.
Evolution allows the fine-tuning of three traits. The farm allocation rate specifies the probability that an engulfed prey is allocated to the internal farm instead of being consumed. The farm culling rate specifies the probability that the host digests its farm (instead of capturing free prey), based on prey density. The explicit farming cost specifies the direct cost or benefit of the farm. For more details, parameter values, and pseudocode of the model, see SI Appendix. To compare the individual based and analytical models, see SI Appendix, Table S1.
Supplementary material containing extended materials and methods, tables, and figures is available in the online version of the paper (SI Appendix).
Supplementary Material
Acknowledgments
We are grateful for the insightful suggestions provided by two anonymous reviewers, and for discussions and ideas from the late John Maynard Smith, István Scheuring, Balázs Könnyű, Viktor Müller, Gergely Boza, Harold P. de Vladar, and Ádám Kun. We acknowledge the National Information Infrastructure Development Institute (NIIF Institute) for granting access to their computational resources based in Hungary at Budapest, Debrecen, and Szeged. The research was funded by National Research, Development, and Innovation Office Grants NKFI-112788 (to I.Z.), NKFI-K119347 (to E.S. and A.S.), NKFI-K124438 (to A.S.), and GINOP-2.3.2-15-2016-00057 (to E.S.); European Research Council Project 294332 (E.S.); the Volkswagen Stiftung initiative “Leben? – Ein neuer Blick der Naturwissenschaften auf die grundlegenden Prinzipien des Lebens” under project “A unified model of recombination in life” (E.S.); and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme Grant 648693 (to S.S.)
Footnotes
The authors declare no conflict of interest.
Data deposition: The code for the individual-based model reported in this paper has been deposited in GitHub, https://github.com/IstvanZachar/FarmingMitochondria/releases/latest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718707115/-/DCSupplemental.
References
- 1.Gray MW. Mosaic nature of the mitochondrial proteome: Implications for the origin and evolution of mitochondria. Proc Natl Acad Sci USA. 2015;112:10133–10138. doi: 10.1073/pnas.1421379112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Ezpeleta N, Embley TM. The SAR11 group of alpha-proteobacteria is not related to the origin of mitochondria. PLoS One. 2012;7:e30520. doi: 10.1371/journal.pone.0030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachar I, Szathmáry E. Breath-giving cooperation: Critical review of origin of mitochondria hypotheses: Major unanswered questions point to the importance of early ecology. Biol Direct. 2017;12:19. doi: 10.1186/s13062-017-0190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-García P, Moreira D. Open questions on the origin of eukaryotes. Trends Ecol Evol. 2015;30:697–708. doi: 10.1016/j.tree.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin WF, Neukirchen S, Zimorski V, Gould SB, Sousa FL. Energy for two: New archaeal lineages and the origin of mitochondria. BioEssays. 2016;38:850–856. doi: 10.1002/bies.201600089. [DOI] [PubMed] [Google Scholar]
- 6.Pittis AA, Gabaldón T. Late acquisition of mitochondria by a host with chimaeric prokaryotic ancestry. Nature. 2016;531:101–104. doi: 10.1038/nature16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szathmáry E. Toward major evolutionary transitions theory 2.0. Proc Natl Acad Sci USA. 2015;112:10104–10111. doi: 10.1073/pnas.1421398112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Freeman; New York: 1995. [Google Scholar]
- 9.Koumandou VL, et al. Molecular paleontology and complexity in the last eukaryotic common ancestor. Crit Rev Biochem Mol Biol. 2013;48:373–396. doi: 10.3109/10409238.2013.821444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koonin EV. The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol. 2010;11:209. doi: 10.1186/gb-2010-11-5-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martijn J, Ettema TJG. From archaeon to eukaryote: The evolutionary dark ages of the eukaryotic cell. Biochem Soc Trans. 2013;41:451–457. doi: 10.1042/BST20120292. [DOI] [PubMed] [Google Scholar]
- 12.Cavalier-Smith T. The phagotrophic origin of eukaryotes and phylogenetic classification of protozoa. Int J Syst Evol Microbiol. 2002;52:297–354. doi: 10.1099/00207713-52-2-297. [DOI] [PubMed] [Google Scholar]
- 13.Cavalier-Smith T. The origin of eukaryotic and archaebacterial cells. Ann N Y Acad Sci. 1987;503:17–54. doi: 10.1111/j.1749-6632.1987.tb40596.x. [DOI] [PubMed] [Google Scholar]
- 14.Zaremba-Niedzwiedzka K, et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature. 2017;541:353–358. doi: 10.1038/nature21031. [DOI] [PubMed] [Google Scholar]
- 15.Vellai T, Takács K, Vida G. A new aspect to the origin and evolution of eukaryotes. J Mol Evol. 1998;46:499–507. doi: 10.1007/pl00006331. [DOI] [PubMed] [Google Scholar]
- 16.Lane N. Energetics and genetics across the prokaryote-eukaryote divide. Biol Direct. 2011;6:35. doi: 10.1186/1745-6150-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467:929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 18.Lane N, Martin WF. Mitochondria, complexity, and evolutionary deficit spending. Proc Natl Acad Sci USA. 2016;113:E666. doi: 10.1073/pnas.1522213113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurland CG, Andersson SGE. Origin and evolution of the mitochondrial proteome. Microbiol Mol Biol Rev. 2000;64:786–820. doi: 10.1128/mmbr.64.4.786-820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ettema TJG, Lindås AC, Bernander R. An actin-based cytoskeleton in archaea. Mol Microbiol. 2011;80:1052–1061. doi: 10.1111/j.1365-2958.2011.07635.x. [DOI] [PubMed] [Google Scholar]
- 21.Yutin N, Koonin EV. Archaeal origin of tubulin. Biol Direct. 2012;7:10. doi: 10.1186/1745-6150-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindås AC, Bernander R. The cell cycle of archaea. Nat Rev Microbiol. 2013;11:627–638. doi: 10.1038/nrmicro3077. [DOI] [PubMed] [Google Scholar]
- 23.Spang A, et al. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature. 2015;521:173–179. doi: 10.1038/nature14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godde JS. Breaking through a phylogenetic impasse: A pair of associated archaea might have played host in the endosymbiotic origin of eukaryotes. Cell Biosci. 2012;2:29. doi: 10.1186/2045-3701-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson-Sathi S, et al. Origins of major archaeal clades correspond to gene acquisitions from bacteria. Nature. 2015;517:77–80. doi: 10.1038/nature13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripa J, Olofsson H, Jonzén N. What is bet-hedging, really? Proc Biol Sci. 2010;277:1153–1154. doi: 10.1098/rspb.2009.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe CD, Minter EJ, Cameron DD, Brockhurst MA. Shining a light on exploitative host control in a photosynthetic endosymbiosis. Curr Biol. 2016;26:207–211. doi: 10.1016/j.cub.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 28.Brock DA, Douglas TE, Queller DC, Strassmann JE. Primitive agriculture in a social amoeba. Nature. 2011;469:393–396. doi: 10.1038/nature09668. [DOI] [PubMed] [Google Scholar]
- 29.DiSalvo S, et al. Burkholderia bacteria infectiously induce the proto-farming symbiosis of Dictyostelium amoebae and food bacteria. Proc Natl Acad Sci USA. 2015;112:E5029–E5037. doi: 10.1073/pnas.1511878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin WF, Tielens AGM, Mentel M, Garg SG, Gould SB. The physiology of phagocytosis in the context of mitochondrial origin. Microbiol Mol Biol Rev. 2017;81:e00008-17. doi: 10.1128/MMBR.00008-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson-Sathi S, et al. Acquisition of 1,000 eubacterial genes physiologically transformed a methanogen at the origin of haloarchaea. Proc Natl Acad Sci USA. 2012;109:20537–20542. doi: 10.1073/pnas.1209119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groussin M, et al. Gene acquisitions from bacteria at the origins of major archaeal clades are vastly overestimated. Mol Biol Evol. 2016;33:305–310. doi: 10.1093/molbev/msv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eme L, Sharpe SC, Brown MW, Roger AJ. On the age of eukaryotes: Evaluating evidence from fossils and molecular clocks. Cold Spring Harb Perspect Biol. 2014;6:165–180. doi: 10.1101/cshperspect.a016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenchel T, Bernard C. A purple protist. Nature. 1993;362:300. doi: 10.1038/362300a0. [DOI] [PubMed] [Google Scholar]
- 35.Jeon KW. The large, free-living amoebae: Wonderful cells for biological studies. J Eukaryot Microbiol. 1995;42:1–7. doi: 10.1111/j.1550-7408.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 36.Muller F, Brissac T, Le Bris N, Felbeck H, Gros O. First description of giant Archaea (Thaumarchaeota) associated with putative bacterial ectosymbionts in a sulfidic marine habitat. Environ Microbiol. 2010;12:2371–2383. doi: 10.1111/j.1462-2920.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 37.Stallforth P, et al. A bacterial symbiont is converted from an inedible producer of beneficial molecules into food by a single mutation in the gacA gene. Proc Natl Acad Sci USA. 2013;110:14528–14533. doi: 10.1073/pnas.1308199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenshine I, Tchelet R, Mevarech M. The mechanism of DNA transfer in the mating system of an archaebacterium. Science. 1989;245:1387–1389. doi: 10.1126/science.2818746. [DOI] [PubMed] [Google Scholar]
- 39.Naor A, Gophna U. Cell fusion and hybrids in archaea: Prospects for genome shuffling and accelerated strain development for biotechnology. Bioengineered. 2013;4:126–129. doi: 10.4161/bioe.22649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thutupalli S, et al. Farming and public goods production in Caenorhabditis elegans populations. Proc Natl Acad Sci USA. 2017;114:2289–2294. doi: 10.1073/pnas.1608961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavalier-Smith T. Origin of mitochondria by intracellular enslavement of a photosynthetic purple bacterium. Proc Biol Sci. 2006;273:1943–1952. doi: 10.1098/rspb.2006.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin W, Müller M. The hydrogen hypothesis for the first eukaryote. Nature. 1998;392:37–41. doi: 10.1038/32096. [DOI] [PubMed] [Google Scholar]
- 43.López-García P, Moreira D. Selective forces for the origin of the eukaryotic nucleus. BioEssays. 2006;28:525–533. doi: 10.1002/bies.20413. [DOI] [PubMed] [Google Scholar]
- 44.Blackstone NW. Why did eukaryotes evolve only once? Genetic and energetic aspects of conflict and conflict mediation. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120266. doi: 10.1098/rstb.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.