Significance
The compositional basis for the biconcave shape of the human red blood cell is unknown. The present work used red blood cell ghosts, which retain the ability to become biconcave discs, which were centrifuged as spheres onto coated coverslips. It was found that during their centrifugation, they became oriented with a dense band located in an equatorial plane of the sphered ghost. When the ghosts were transformed into biconcave discs, they collapsed in place with the dense band in the rim. These shape changes were recorded microscopically. These results indicate that the biconcave shape depends on differences in the density between their rim and the dimple regions and specify that the underlying cytoskeletal complex is asymmetrically distributed.
Keywords: red blood cell ghosts, biconcave shape, membrane/cytoskeletal complex
Abstract
The main conclusion of the results reported in this article is that during centrifugation, sphered red blood cell ghosts become oriented in their attachment to a coverslip such that a dense band within the ghosts lies parallel to the centrifugal field. The result of the orientation of this dense band is that when the attached spherical ghosts are shrunken to become biconcave discs, they do so by directly collapsing on themselves without any lateral motion. This result is interpreted to suggest that a dense band, relative to the dimple, resides in the rim of the ghost and is responsible for its biconcave shape. These results confirm the conclusions reached in a previous publication in which there was the uncertainty that the shape change of the spherical ghosts to discs could not be directly imaged. The present work corrects this limitation by use of a chamber in which the tonicity of the solutions in the ghosts’ surround could be altered by perfusion coupled with constant microscopic imaging. The identity of the components that are responsible for the differences in the density (mass) between the rim and the dimple regions of the cytoskeletal/membrane complex in the biconcave disk are unknown. It is also unknown what forces apply or what the explanation is for the unique orientation of the dense band during the ghosts’ centrifugation, as described in this article. Nevertheless, the results reported in this article indicate the membrane’s underlying cytoskeletal complex is asymmetrically distributed.
In a beguiling review, Bessis and Delpech (1) emphasize the unlikelihood that no single individual can be regarded to have been the first to have seen or to describe red blood cells. There is no question that Malpighi, Swammerdam, and Leeuwenhoek all observed red globules/corpuscles/particles in the blood of various species of vertebrates in the latter part of the 17th century. At the time, and up until the work of Hodgkin and Lister (2), there were various interpretations of cell shapes not only for human red blood cells but also in other types of cells as well. This is discussed in the treatise of Hewson (3), together with the inclusion of the invaluable footnote annotations provided by Gulliver [ref. 4, as well as in the monograph by Gerber (5)]. From the time of their discovery, cells were variously described, for instance, as being spherical, oval, lenticular, or oat-shaped, with or without a hole or nucleus, but only rarely discoidal. The biochemical/structural basis responsible for the biconcave shape of human red blood cells has eluded resolution since its definitive shape was first discerned. In contrast, various theoretical analyses of the physical forces involved that underlie and characterize the red cell biconcave shape have been remarkably successful and extensively described, as referred to before (6). It was also suggested in the previous paper that the density [i.e., mass differences in the membrane/cytoskeletal (M/CS) complex between the dimple and the rim] was critical in providing insight into the forces/molecular scaffolding responsible for the cell’s biconcave shape.
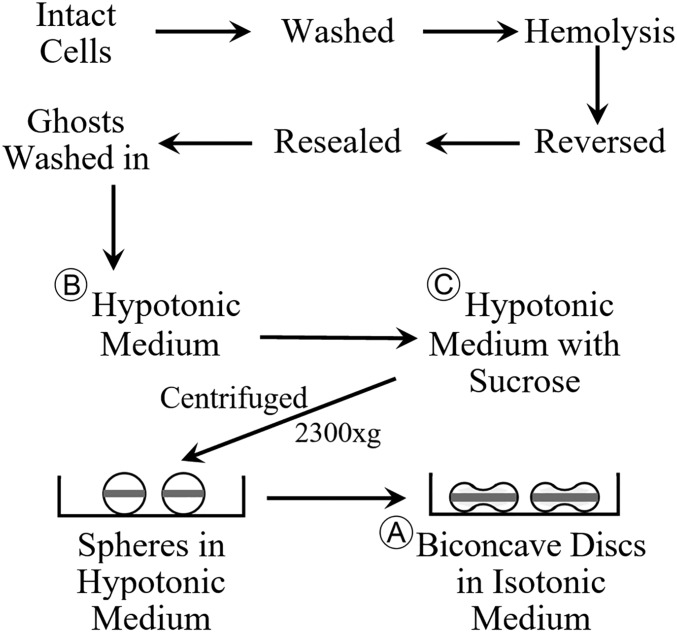
The main purpose of the present article is to provide results that extend those reported before (6). In the previous paper, it was interpreted that spherical human red blood cell ghosts, which were attached to a coverslip after being centrifuged in a hypotonic solution, became directly flattened to biconcave discs when they were subsequently shrunk by exposure to an isotonic solution. Because of microscopic limitations, it was only possible to observe the shape of a different set of disk-shaped ghosts after the spherical ghosts were exposed to an isotonic solution. It was pointed out before (6) that because of the crowded groups of spherically attached ghosts, it was unlikely that there was any rollover when they became discs. The results, using continuous microscopic imaging, as reported in this article, provide clear and direct evidence that the transition of the same group of spherical ghosts to biconcave discs occurs by the ghosts directly shrinking/collapsing onto the coverslip without any lateral displacement/movement. This behavior confirms the interpretation, previously suggested (6), that the sphered ghosts during their centrifugation become oriented with a dense band around their equatorial plane parallel to the direction of the centrifugal force. This dense outer band after the ghost’s shrinkage represents the rim in the resulting biconcave disk. The ghost’s dimple is obviously contained within the central portion of the disk. See Fig. 1 for an overview of the protocol with the resultant horizontal band around the equatorial plane of the sphered ghosts (modified from ref. 6). At the present time, there is no theoretical basis available to understand what force or forces act to promote this unique orientation during the ghost’s centrifugation. It is hoped that the results reported in this article will stimulate the search for an explanation.
Fig. 1.
This figure represents an overview of the experimental protocol, as used in this article. See Materials and Methods for details. Modified with permission from ref. 6.
It is important to again note, as pointed out before (6), that in the instance that reversed ghosts, as prepared in this work, when sphered in hypotonic solution, can become biconcave discs in isotonic solutions, this justifies their use to study the basis for the discoid shape of intact red blood cells. This to say, these ghosts retain within their M/CS complex the structural components necessary to form its discoid shape.
Mention should be made that there is, of course, a possible interaction between the negatively charged surface of the ghost with the positively charged poly-d-lysine (PDL) on the coverslip. It must be assumed that the ghosts are fixed to the coverslip/PDL substrate during its centrifugation. If electrostatic interaction between the ghost and the substrate were to occur, say, during the time before or after centrifugation and before viewing, it would presumably affect the orientation of the ghosts in a random fashion. This would also mean that, on shrinking the ghosts, their resultant orientation as discs would be random, which conflicts with what was observed, as reported before. It should be noted that the presence of 0.3% BSA in all solutions (e.g., ∼1017 molecules in 10 mL solution C) is positively charged at pH 7.4, which would act, as shown by Seaman et al. (7), to neutralize/or make positive the negative charge on the surface of the ghost (because of the presence of N-acetylneuraminic acid. This would presumably act to inhibit any lateral movement of the ghosts on the PDL-coated coverslip. It is known that albumin is absorbed by the red cell surface (8, 9) and, when bound, can act to reduce the cell’s electrophoretic mobility (10).
Results and Discussion
As mentioned earlier, the primary thrust of this article is to show the results of continuously imaging spherical ghosts in their transition of shape to biconcave discs, when the tonicity of the medium is changed to isotonic. The method used in the present article provides direct evidence that the transition of the spheres to discs occurs by the ghosts simply sinking/collapsing directly in place with no lateral movement or rollover. This was made possible, as described in Materials and Methods, by the use of a chamber, and depicted in Fig. 2. Here it was possible to image the transition of the ghosts attached to the coverslip during their changes in shape. Perfusion of the chamber was accomplished by first flushing with a hypotonic solution to clear the field of loosely attached ghosts before switching to an isotonic solution. The perfusion times used were of the order of ∼5 s to allow volume equilibration both of the spheres and then of the discs. Fig. 1 provides an overview of the protocol used in conjunction with the chamber manipulations.
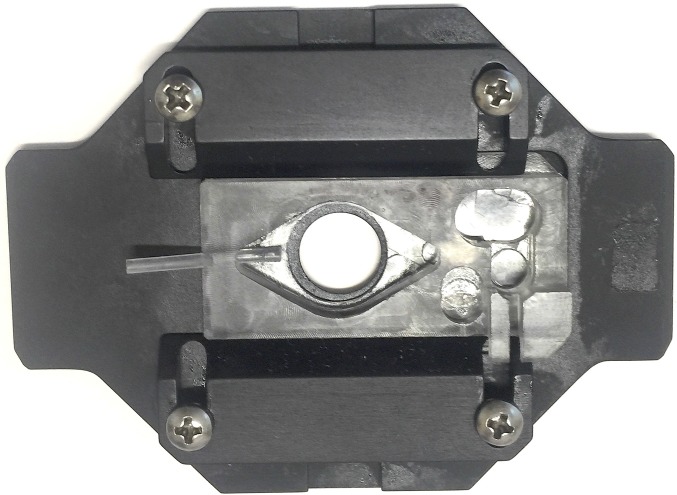
Fig. 2.
This figure is a picture of the chamber used in the various experiments to image the changes in the shape (volume) of individual ghosts on changes in the tonicity of the medium, as detailed in the Materials and Methods.
The time course of net water movement driven by an osmotic gradient in shifting the shape from spheres to discs, as in the experiments reported here, is less than 0.5 s (11). This means that the changes in the ghost’s volume from a sphere to a disk was at equilibrium before and after the perfusion of an isotonic solution. It should be remembered that the types of ghosts that result from the preparatory method used results in a heterogeneous population of shapes and sizes, as discussed previously (6). Nevertheless, as seen before (6), and illustrated in the current figures, there are many spherical ghosts that become discs with the shift (change) in osmotic pressure.
The results of three different experiments are presented in Figs. 3–5. Each of these pairs of figures shows that the transition of the shape of the ghosts from spheres to discs takes place by flattening in place without any lateral motion. The images in Figs. 3–5 were the result of the use of a differential interference contrast (DIC) microscope. It needs to be emphasized that the DIC images indicate differences in refractive indices (mass, density). Thus, in the spherical ghosts, the darkened edges indicate the summed density of the vertical sides of the spheres and are not to be taken as biconcave components. An attempt to indicate these differences is given by the cartoons at the bottom of Fig. 1. Importantly, these results support the conclusion, as stated before (6), that the biconcave shape of the ghost, and presumably of the intact cell, depends on differences (i.e., composition) in the density (mass) of the rim versus the dimple.
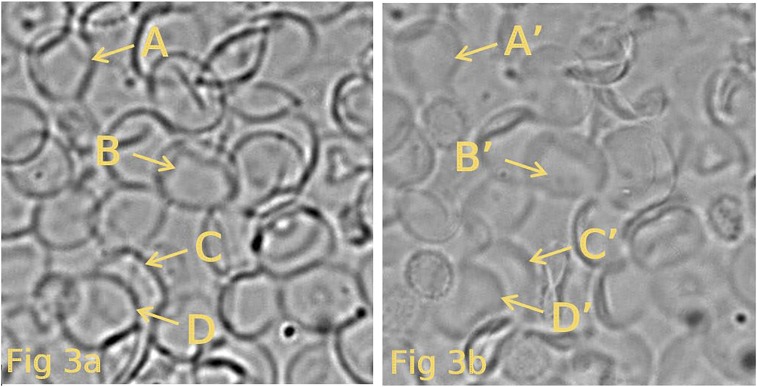
Fig. 3.
This figure depicts the results of one experiment in which resealed ghosts have been centrifuged onto a PDL-coated coverslip, as described in the text. The ghosts in A represent images of spherical ghosts (in hypotonic solution B) attached to the coverslip. (B) Shape of the same ghosts seen in A after they have shrunken by exposure to an isotonic solution (solution B). The shapes of the resultant ghosts are now seen to be mainly biconcave discs. The yellow letters (A–D and A′–D′) highlight the same ghosts before and after the transition of shape. Comparison of other pairs of ghosts in the paired illustrations show similar collapse of the spheres to discs.
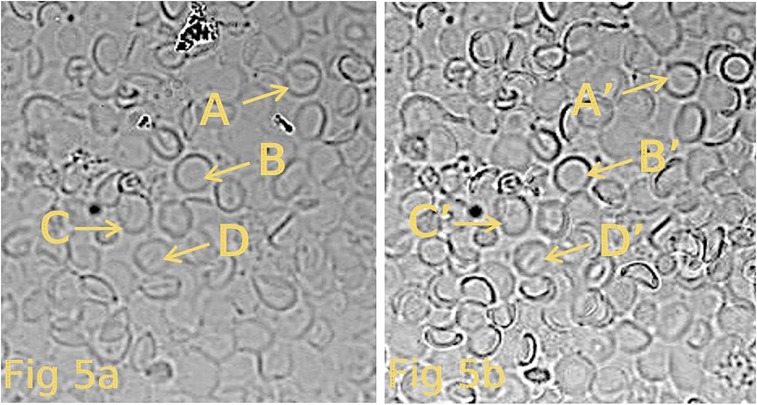
Fig. 5.
This figure depicts the results of a third experiment carried out in the same way as described in the legend to Fig. 3. The ghosts in A represent images of spherical ghosts (in hypotonic solution B) attached to the coverslip. (B) Shape of the same ghosts seen in A after they have shrunken by exposure to an isotonic solution (solution B). The shapes of the resultant ghosts are now seen to be mainly biconcave discs. Again, the yellow letters A–D and A′–D′ highlight the same ghosts before and after the transition of ghost shape. Careful comparison of other pairs of ghosts also show similar results.
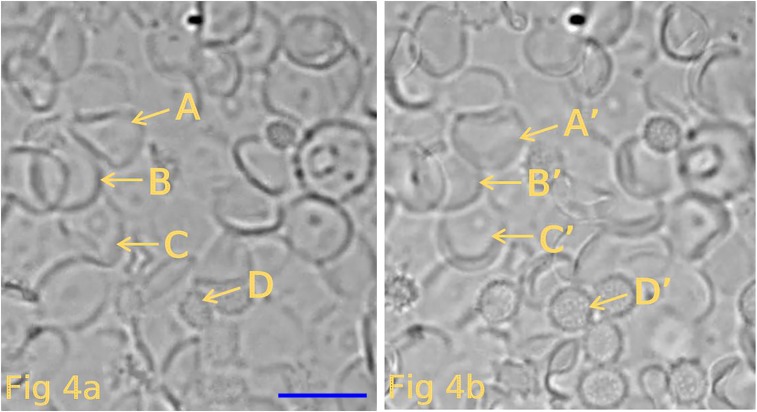
Fig. 4.
This figure depicts the results of different experiment carried out as described in the legend to Fig. 3. The ghosts in A represent images of spherical ghosts (in hypotonic solution B) attached to the coverslip. (Scale bar, 10 μm.) (B) Shape of the same ghosts seen in A after they have shrunken by exposure to an isotonic solution (solution B). The shapes of the resultant ghosts are now seen to be mainly biconcave discs. Again, the yellow letters A–D and A′–D′ highlight the same ghosts before and after the transition of ghost shape. Close scrutiny of other pairs of ghosts show similar results.
The M/CS complex is, of course, thought to cover the entire inside surface to the ghost/cell. What is attempted to be depicted in Fig. 1 is the notion, compatible with the results reported here, that there is a denser region of the M/CS complex around the equatorial circumference/perimeter on the inside of the sphere that functions to form the biconcave shape when it shrinks in an isotonic solution. This denser band of the M/CS complex present in the rim becomes responsible for the biconcave shape of the resultant ghost/cell. This implies that the M/CS is heterogeneous, with at least some of its structural/architectural components being asymmetrically distributed or arranged. At the current time, there is no evidence on whether or not some type of asymmetry exists in the M/CS complex. The recent report that MIIA (nonmyosin IIA) (12), in association with actin is attached to the M/CS, provides a possible link for the stabilization of the biconcave shape of the human red blood cells. In contrast, because its distribution appears uniform within the M/CS, it is unlikely that it contributes to the density differences between the rim and the dimple. Possible memory properties of the M/CS associated with shape and cell tank-treading have been referred to in the previous paper (6).
Changes in the composition by insertion or depletion of molecules in the outer or inner lipid layer can induce shape changes (13–15). This type of rearrangement of the bilayer is also related to disk/sphere transformations of constant volume (16). However, these kinds of alteration in the membrane structure are unlikely to occur in the present experiments, and therefore can be excluded as having an input in the rim/dimple density differences. It remains for future work to determine the molecular/structural basis accounting for the asymmetry of the M/CS relative to the membrane density differences, as described in this article. It should be emphasized again that a rationale for the orientation of the presumed rim region of the ghost’s sphere parallel to the centrifugal field during its centrifugation is in need of explanation. That said, it should be appreciated that, from the results reported in this article, the cytoskeletal complex that resides within the membrane is necessarily asymmetrical in its construction/distribution.
Materials and Methods
Normal human blood was taken as approved by Yale's Human Investigation Committee after informed consent was obtained. The solutions used were the same as before (6), in which isotonic solution A contained 150 mM NaCl, 20 mM Tris⋅Cl, and 3% BSA; hypotonic solution B contained 75 mM NaCl, 20 mM Tris⋅Cl, and 3% BSA; and hypotonic solution C was the same as solution B, but with sucrose substituted for NaCl to keep the same osmolality while making the density of the solution equal to 1.003. The pH of all solutions was 7.2 at 23 °C.
The protocol used in this study to produce resealed red blood cell ghosts is identical to that described before (6). In brief, intact red blood cells (50% Hct) were hemolyzed in 20 mL of a hypotonic solution containing Mg and ATP at pH 6.0 at −1.0 °C, then brought to pH 7.4, reversed, and incubated for 40 min at 37 °C. The resultant resealed ghosts, which contained less than 2% Hb and were subsequently washed and ultimately added to and mixed in the very top of solution C contained in a polycarbonate tube (#355631; Beckman). The centrifuge tubes used in the present work were different from those used before. These centrifuge tubes (1″ × 3.5″) contained 4 mL clear epoxy cement (Home #31345; Devcon) to make a soft flat surface in the bottom of the tube. A PDL-coated coverslip (#354086, #1, 12 mm diameter; Corning) was put on top of the epoxy support before 10 mL solution C and 4λ (∼2 × 104 ghosts) of the ghost suspension was added. The tubes were brought to temperature equilibrium (1 h at 4 °C) before being centrifuged in a LE-80K Beckman ultracentrifuge for 1 h at 4 °C at 3,300 × g in a Beckman horizontal rotor (SW28). The hypotonic solution C contained, among other constituents, sucrose and BSA, as described earlier. It may be of interest to note that the number of N-acetylneuraminic acid molecules/ghost is ∼2 × 107 (7, 17), whereas the number of BSA molecules in 10 mL solution C is ∼1017, which is far in excess of the number of N-acetylneuraminic acid charges on the ghost population being centrifuged. Although the concentration of PDL is known, the amount added per coverslip was considered proprietary, and is therefore unknown. Even so, it is clear, as discussed earlier, as well as from the results reported here, that electrostatic interaction between the ghosts and the PDL substrate was not an issue (i.e., the ghosts collapsed in place without lateral movement or rollover).
Before microscopic observation, the coverslip with attached ghosts was mounted in a chamber (RC-25, with a P-3 base; Warner Instruments), which is pictured in Fig. 2. The coverslip, with some solution C (or B) on top, was positioned on top of the hole (∼9 mm diameter) in the bottom of the perfusion chamber. The polycarbonate perfusion chamber, containing a thin layer of silicone grease around the bottom of its opening, was then pressed over the coverslip. After the chamber was sealed, the bottom of the coverslip was dried. The attached ghosts were then imaged with an inverted DIC microscope, which was a Zeiss Axio Observer.Z1, 40× oil immersion, with its high-speed CMOS camera (pco.edge 4.2) attached. As before, the photographs were modified in brightness and contrast to make the results clearer. Perfusion of the chamber was carried out by use of a syringe attached via a capillary tube to the insert shown on the left of the plastic chamber. The procedure used was to first flush (slowly, <10 s) the chamber with about 1 mL hypotonic solution B to remove any unattached or floating ghosts. The accumulated flush solution that filled the small reservoir in the upper right was removed with a plastic pipette. With continuous recorded imaging of the spherical attached ghosts, the chamber was then perfused with isotonic solution A, again with about 1 mL, during which the ghosts were seen to become flattened biconcave discs. The time of perfusing the chamber with solutions B and A was 3–5 s. Images of the resultant shape transitions are presented in the body of this article. All microscopic observations were carried out at room temperature.
It may be of interest to mention that in an early experiment in which intact red blood cells were centrifuged in a manner similar to the overall protocol used here, it was found that when the sphered cells, stuck on a collodion-coated coverslip, were made into biconcave discs, their shape distribution was in many different/random types of orientation. The interpretation at the time was that if there was possible preferential orientation of the cells during centrifugation, because of density differences between the rim and dimple, it was prevented not only by the masking of the differences by the high concentration of hemoglobin but also by convection currents disorienting the cells as a result of the lack of temperature control during centrifugation. This experience led to the design of the protocol used before (6) and in the current work.
Acknowledgments
I thank Mr. Kenneth Allen for his critical help and skilled performance in all aspects of these experiments. I also thank Mr. Duncan Wong for his invaluable help with the illustrations and the manuscript. I also appreciate the expertise of Dr. Jean-Ju Chung and Dr. Jae Yeon Hwang regarding their use of their DIC microscope, as well as the help of Dr. David Zenisek during the course of this work.
Footnotes
The author declares no conflict of interest.
References
- 1.Bessis M, Delpech G. Discovery of the red blood cell with notes on priorities and credits of discoveries, past, present and future. Blood Cells. 1981;7:447–480. [PubMed] [Google Scholar]
- 2.Hodgkin T, Lister J. Notice of some microscopic observations of the blood and animal tissues. Philos Mag. 1827;2:130–138. [Google Scholar]
- 3.Hewson W. On the figure and composition of the red particles of the blood, commonly called the red globules. By Mr. William Hewson, F. R. S. and Teacher of Anatomy. Philos Trans. 1773;63:303–323. [Google Scholar]
- 4.Gulliver G. 1846. The Works of William Hewson, F.R.S. London (London, Sydeham Society), p 360.
- 5.Gerber F. 1842. Elements of the General and Minute Anatomy of Man and the Mammals, Added Notes and Appendix Comprising Researches on the Anatomy of the Blood, Chyle, etc., ed Gulliver G (Hippolyte Bailliere, London)
- 6.Hoffman JF. Biconcave shape of human red-blood-cell ghosts relies on density differences between the rim and dimple of the ghost’s plasma membrane. Proc Natl Acad Sci USA. 2016;113:14847–14851. doi: 10.1073/pnas.1615452113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seaman GVF, Knox RJ, Nordt FJ, Regan DH. Red cell aging. I. Surface charge density and sialic acid content of density-fractionated human erythrocytes. Blood. 1977;50:1001–1011. [PubMed] [Google Scholar]
- 8.Ponder E. Hemolysis and Related Phenomena. Grune and Stratton; New York: 1948. [Google Scholar]
- 9.Jay AWL. Geometry of the human erythrocyte. I. Effect of albumin on cell geometry. Biophys J. 1975;15:205–222. doi: 10.1016/S0006-3495(75)85812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson HA. Electrokinetic Phenomena and Their Application to Biology and Medicine. Chemical Catalog Company; New York: 1934. [Google Scholar]
- 11.Sidel VW, Solomon AK. Entrance of water into human red cells under an osmotic pressure gradient. J Gen Physiol. 1957;41:243–257. doi: 10.1085/jgp.41.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AS, et al. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. bioRxiv. October 19, 2017 doi: 10.1101/202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svetina S, Zeks B. Bilayer couple as a possible mechanism of biological shape formation. Biomed Biochim Acta. 1985;44:979–986. [PubMed] [Google Scholar]
- 14.Leonard C, et al. Contribution of plasma membrane lipid domains to red blood cell (re)shaping. Sci Rep. 2017;7:4264. doi: 10.1038/s41598-017-04388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Käs J, Sackmann E. Shape transitions and shape stability of giant phospholipid vesicles in pure water induced by area-to-volume changes. Biophys J. 1991;60:825–844. doi: 10.1016/S0006-3495(91)82117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman JF. On the mechanism and measurement of shape transformations of constant volume of human red blood cells. Blood Cells. 1987;12:565–586. [PubMed] [Google Scholar]
- 17.Eylar EH, Madoff MA, Brody OV, Oncley JL. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962;237:1992–2000. [PubMed] [Google Scholar]