Significance
Tuberculosis drug development remains crucial for countering the spread of drug resistance worldwide. New susceptibilities in metabolic pathways must be identified to find novel drugs to eradicate tuberculosis. The electron transport chain (ETC) is the target of recently developed tuberculosis drugs. To assess whether the NADH dehydrogenases of the ETC would be potential drug candidates, we deleted the genes encoding the three Mycobacterium tuberculosis NADH dehydrogenases Nuo, Ndh, and NdhA. We found that although the NADH dehydrogenases were not essential for growth individually, deletion of both nuo and ndh had the most profound effect on Mycobacterium tuberculosis viability and virulence. We propose that screening compound libraries against both Ndh and Nuo will lead to promising drug candidates to fight tuberculosis.
Keywords: NADH, virulence, essentiality, tuberculosis, dehydrogenase
Abstract
Worldwide control of the tuberculosis (TB) epidemic has not been achieved, and the latest statistics show that the TB problem might be more endemic than previously thought. Although drugs and a TB vaccine are available, TB eradication faces the challenges of increasing occurrences of multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis (Mtb) strains. To forestall this trend, the development of drugs targeting novel pathways is actively pursued. Recently, enzymes of the electron transport chain (ETC) have been determined to be the targets of potent antimycobacterial drugs such as bedaquiline. We focused on the three NADH dehydrogenases (Ndh, NdhA, and Nuo) of the Mtb ETC with the purpose of defining their role and essentiality in Mtb. Each NADH dehydrogenase was deleted in both virulent and BSL2-approved Mtb strains, from which the double knockouts ΔndhΔnuoAN and ΔndhAΔnuoAN were constructed. The ΔndhΔndhA double knockout could not be obtained, suggesting that at least one type II NADH dehydrogenase is required for Mtb growth. Δndh and ΔndhΔnuoAN showed growth defects in vitro and in vivo, susceptibility to oxidative stress, and redox alterations, while the phenotypes of ΔndhA, ΔnuoAN, and ΔndhAΔnuoAN were similar to the parental strain. Interestingly, although ΔnuoAN had no phenotype in vivo, ΔndhΔnuoAN was the most severely attenuated strain in mice, suggesting a key role for Nuo in vivo when Ndh is absent. We conclude that Ndh is the main NADH dehydrogenase of Mtb and that compounds that could target both Ndh and Nuo would be good candidates for TB drug development.
Tuberculosis (TB), a disease caused by the bacillus Mycobacterium tuberculosis (Mtb), remains one of the leading causes of mortality due to a single infectious agent. Despite chemotherapy and the bacillus Calmette–Guérin vaccine, worldwide incidences of this disease persist, while multidrug-resistant (MDR) and extensively drug-resistant (XDR) Mtb strains have emerged, rendering TB control even more challenging. The current TB pharmacopeia is divided into three categories: first-line, second-line, and third-line TB drugs. Drug-susceptible TB cases are treated with the first-line TB drugs isoniazid (INH), rifampicin (RIF), ethambutol (EMB), and pyrazinamide (PZA). These drugs target the mycobacterial cell wall (INH and EMB) and transcription (RIF), while the target of PZA is still under investigation. The second-line TB drugs used to treat drug-resistant cases include drugs targeting the DNA gyrase (fluoroquinolones), protein synthesis (aminoglycosides and cyclo-peptides), or the cell wall (thioamides and cycloserine) (1). To fight the TB drug resistance pandemic, novel pathways for drug development need to be explored. One of the most promising new TB drugs is bedaquiline, which targets the oligomeric c ring of the F1Fo-ATP synthase complex. ATP synthesis catalyzed by the F1Fo-ATP synthase is driven by the protonmotive force (pmf) generated by the electron transport chain (ETC). Other components of the oxidative phosphorylation machinery are also showing promise for drug development. The proton-pumping cytochrome bc1 complex is targeted by a novel drug in development, Q203 (Qurient, Infectex) (2). Q203 was shown to be cidal against Mtb when combined with inhibition of the cytochrome bd oxidase activity (3). SQ109 (Sequella) is thought to disrupt the biosynthesis of the electron carrier menaquinone and the pmf through uncoupling activity (4). The pmf generated by the ETC is an essential element for the survival of any organism under both aerobic and hypoxic growth conditions, which makes this system attractive for drug development (5).
Primary NADH dehydrogenases play a pivotal role in energization of the mycobacterial respiratory chain. Mtb has three membrane-bound NADH dehydrogenase complexes that are capable of oxidizing the cofactor NADH into NAD+ using menaquinone as an electron acceptor. These include the proton-translocating (type I–NDH-1) Nuo complex and two nonproton-pumping (type II) Ndh and NdhA complexes (NDH-2). To assess which NADH dehydrogenase enzyme was the most relevant target for drug design, we deleted each Mtb gene or operon encoding these enzymes individually and in tandem and tested the resulting knockout strains in vitro and in vivo for viability. NdhI, which is composed of 14 subunits (NuoA-NuoN, Rv3145-Rv3158), had already been shown dispensable, as transposon insertions had been identified in most of the subunits (6), and the full operon has been deleted from the Mtb genome (7). Transposon insertions had also been previously isolated in ndhA (Rv0392c) (8), but ndh (Rv1854c) was considered an essential gene, as specific mutations in ndh had temperature-sensitive lethal phenotypes in Mycobacterium smegmatis (9, 10), transposon insertions in Mtb ndh were rare (11), and attempts at deleting Mtb ndh had been unsuccessful (12). This report describes the construction of single- and double-NADH dehydrogenase deletion mutants in Mtb strains and their phenotypes in vitro and in vivo.
Results
NADH Dehydrogenase Genes Are Individually Dispensable in Mtb.
The NADH dehydrogenase type I operon, encoded by nuoAN, and the two NADH dehydrogenases type II encoded by ndh and ndhA, were deleted from the Mtb strains CDC1551 and mc26230 (Mtb H37Rv ΔRD1ΔpanCD) using the specialized transduction system (13, 14) and replaced by a γδ(sacB-hyg) γδ cassette (Table S1). The hygromycin cassette was excised in each knockout strain to obtain unmarked deletion strains (14). These strains were then confirmed by Southern analysis and by whole-genome sequencing (Fig. S1). The unmarked deletion strains Δndh, ΔndhA, and ΔnuoAN were then used to generate deletions of a second NADH dehydrogenase in each background using the same specialized transduction phages used to generate Δndh (phAE237), ΔndhA (phAE804), and ΔnuoAN (phAE805). The double-knockout ΔndhΔnuoAN and ΔndhAΔnuoAN strains were obtained; however, six independent attempts failed to produce a ΔndhΔndhA mutant. The ΔndhΔnuoAN and ΔndhAΔnuoAN constructions were confirmed by Southern analysis (Fig. S1). ΔndhΔnuoAN was further confirmed by whole-genome sequencing (Fig. S1). This set of deletion strains demonstrates that the NADH dehydrogenases are not essential individually, but that most likely one type II NADH dehydrogenase is required for the viability of Mtb in vitro.
Deletion of NADH Dehydrogenase Genes Affects NADH Dehydrogenase Expression Levels and NADH/NAD+ Ratio.
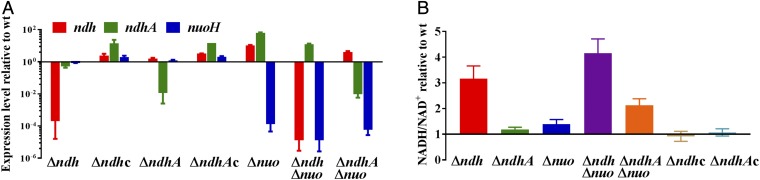
To examine the impact of the deletion mutants on the expression levels of the three NADH dehydrogenase genes in Mtb, qPCR was performed using primers to amplify the ndh, ndhA, and nuoH genes (Fig. 1A and Table S2). The levels of nuo and ndhA expression in Δndh decreased by 15% and 50%, respectively, compared with WT. Complementation of Δndh with Mtb ndh cloned downstream of the hsp60 promoter (Table S3) restored nuo and ndhA expression levels to or above WT expression levels. Deletion of the NADH dehydrogenase type I operon (ΔnuoAN) led to overexpression of both type II NADH dehydrogenases, while the double knockouts, ΔndhΔnuoAN and ΔndhAΔnuoAN, overexpressed ndhA and ndh, respectively. ΔndhA was the only Mtb strain with a similar level of ndh and nuo transcripts compared with the WT strain.
Fig. 1.
Deletion of NADH dehydrogenase genes impacts NADH dehydrogenase expression levels and NADH/NAD+ ratio. (A) Expression levels of ndh, ndhA, and nuoH in the NADH dehydrogenase mutants relative to their parental strain CDC1551. (B) NADH/NAD+ ratio in NADH dehydrogenase mutants relative to their parental strain CDC1551. In these experiments, the strains were grown in Middlebrook 7H9, supplemented with OADC, glycerol, and tyloxapol to OD600 nm ≈ 1. The complemented strains Δndh pMV361::ndh and ΔndhA pMV361::ndhA are shown as Δndhc and ΔndhAc, respectively. Δnuo stands for ΔnuoAN. Average of three independent experiments is shown with SD.
The function of the NADH dehydrogenases is to oxidize NADH into NAD+, the ratio of which reflects the redox state of a cell. Therefore, the NADH/NAD+ ratio was determined for each of the NADH dehydrogenase mutants and found to be increased in the Δndh and double-knockout strains while remaining similar to WT level in the ΔndhA and ΔnuoAN strains (Fig. 1B). While the deletion of ndhA or nuoAN may not induce any major redox perturbation in Mtb, the NADH/NAD+ ratio was the most altered when Mtb was lacking ndh, suggesting that this enzyme has an important function in maintaining the redox status of the cell.
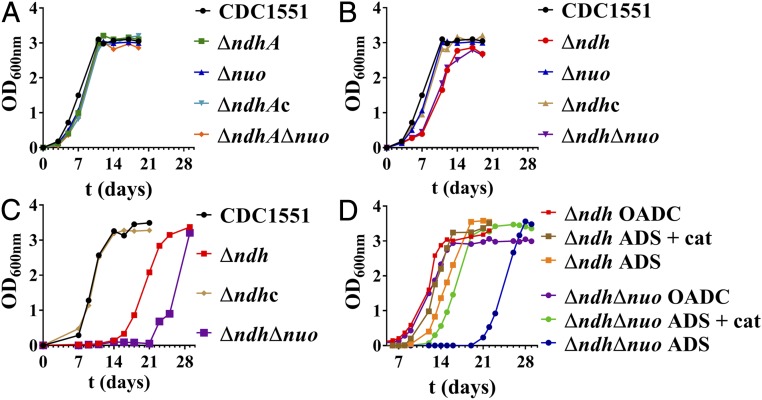
Only Δndh and ΔndhΔnuoAN Have Growth Defects in Vitro.
The five NADH dehydrogenase knockout strains Δndh, ΔndhA, ΔnuoAN, ΔndhΔnuoAN, and ΔndhAΔnuoAN were tested for growth in Middlebrook 7H9-glycerol–OADC (oleic acid, albumin, dextrose, catalase). All of the strains containing ndh grew similarly to the WT strain (Fig. 2A). In contrast, Δndh and ΔndhΔnuoAN showed a longer lag phase during growth than WT, although once the strains had reached log phase, the kinetics of growth and the maximum growth rate achieved were similar to WT (Fig. 2B). This increased lag phase was resolved in the Δndh-complemented strain (Fig. 2B). This growth delay was reproducible and unlikely due to the inoculation of large volume of nonviable bacteria, as cultures were typically reinoculated while in log phase. In Salmonella typhimurium (15) and in Escherichia coli (16), the switch to lag phase was shown to generate a transient oxidative stress when cultures were inoculated into freshly oxygenated medium. To test whether deletion of ndh or ndh and nuoAN could increase Mtb sensitivity to oxidative stress, we grew the NADH dehydrogenase deletion mutants in Middlebrook 7H9-glycerol–ADS, a medium without catalase, an enzyme that converts hydrogen peroxide to oxygen and water and protects the bacteria against oxidative stress (Fig. 2C and Fig. S2). Only the Δndh and ΔndhΔnuoAN mutants were substantially affected by this growth condition, exhibiting an extended lag phase increased by 9 and 15 d, respectively, compared with growth in medium containing catalase (Fig. 2B). The susceptibility of Δndh and ΔndhΔnuoAN to oxidative stress was confirmed when catalase was added to Δndh and ΔndhΔnuoAN grown in Middlebrook 7H9-glycerol–ADS, and a reduction in the lag phase was observed for these two mutants (Fig. 2D). The defects observed in Δndh and ΔndhΔnuoAN during these in vitro growth experiments suggest that in addition to its role in the ETC, Ndh might also protect Mtb from oxidative stress.
Fig. 2.
Δndh and ΔndhΔnuoAN have growth defect in vitro. The NADH dehydrogenase mutants and their parental strain CDC1551 were grown to midlog (OD600 nm ≈ 0.8) and diluted 1/50. Growth was followed by measuring OD600 nm over time. (A and B) Growth in Middlebrook 7H9, supplemented with OADC, glycerol, and tyloxapol. (C) Growth in Middlebrook 7H9, supplemented with ADS, glycerol, and tyloxapol. (D) Growth of Δndh and ΔndhΔnuoAN in Middlebrook 7H9, supplemented with, glycerol, tyloxapol, and OADC, ADS, or ADS-containing catalase (3 mg/L, same concentration as in OADC). The complemented strains Δndh pMV361::ndh and ΔndhA pMV361::ndhA are shown as Δndhc and ΔndhAc, respectively. Δnuo stands for ΔnuoAN. The graphs show single replicates, which are representative of at least two independent experiments.
Δndh and ΔndhΔnuoAN Are More Susceptible to Oxidative Stress Reagents, but Not to Potential NADH Dehydrogenase Inhibitors.
The data generated by the growth condition studies led us to investigate the susceptibility of the NADH dehydrogenase mutants to agents generating oxidative stress. Minimum inhibitory concentrations (MIC) were determined for the NADH dehydrogenase mutants against hydrogen peroxide and ascorbic acid, which can generate an oxidative environment in Mtb (17). The NADH dehydrogenase mutants had similar levels of susceptibility to hydrogen peroxide as their parental strain, while Δndh and ΔndhΔnuoAN were slightly more susceptible to ascorbic acid (two- to fourfold) than CDC1551 (Table 1).
Table 1.
Susceptibility of NADH dehydrogenase mutants to drugs and oxidative stress agents
| Strain/MIC | Ascorbic acid (mM) | H2O2 (mM) | CPZ (mg/L) | TPZ (mg/L) | CFZ (mg/L) | INH (mg/L) |
| CDC1551 | 1.0 | 0.5 | 12.5 | 25 | 0.6–1.25 | 0.03 |
| Δndh | 0.25–0.5 | 0.25 | 12.5 | 25 | 2.5 | 0.06–0.12 |
| Δndh pMV361::ndh | 1.0–2.0 | 0.5 | Not done | Not done | 1.25 | 0.03 |
| ΔndhA | 1.0 | 0.25–0.5 | 12.5 | 12.5 | 0.6–1.25 | 0.03 |
| ΔnuoAN | 1.0 | 0.5 | 25 | 25 | 0.6–1.25 | 0.03 |
| ΔndhΔnuoAN | 0.5 | 0.25 | 25 | 12.5–25 | 2.5 | 0.12 |
| ΔndhAΔnuoAN | 1.0 | 0.5 | 12.5–25 | 25 | 0.6–1.25 | 0.03–0.06 |
We next measured the MICs of compounds that target the NADH dehydrogenase type II, such as trifluoperazine (TPZ; 18), chlorpromazine (CPZ; 18), clofazimine (CFZ; 19), and INH (9, 10), against the NADH dehydrogenase mutants. The two neuroleptic drugs TPZ and CPZ, used in the treatment of psychiatric patients infected with TB in the 1950s, inhibit purified recombinant Ndh and NdhA (18) and had similar MICs across all of the strains tested (Table 1). A low-level (up to fourfold) resistance was observed for Δndh and ΔndhΔnuoAN against CFZ, a prodrug that requires Ndh for its activation (19), and INH (Table 1).
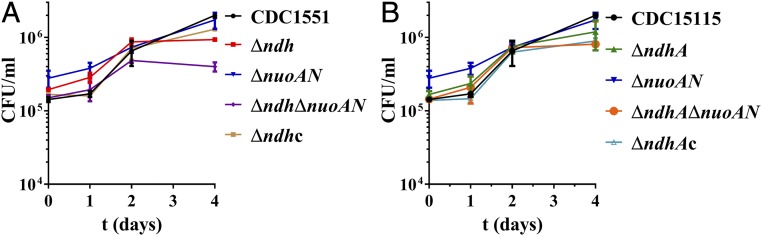
Δndh and ΔndhΔnuoAN Have a Late-Growth Defect in Murine Macrophages.
Considering that Mtb is an intracellular pathogen, we asked whether the in vitro growth defect we had observed with the Δndh and ΔndhΔnuoAN mutants could be reproduced in murine macrophages. Murine J774 macrophages were infected at a multiplicity of infection of 1 with the NADH dehydrogenase mutants, and growth of the mutants was followed for 4 d (Fig. 3). None of the mutants had any growth defect compared with the parental strain early on, but a significant (P < 0.05) growth defect was observed at the last day of infection (day 4) for Δndh and ΔndhΔnuoAN (Fig. 3A). This suggested that these two mutant strains might have an in vivo growth defect phenotype.
Fig. 3.
Δndh and ΔndhΔnuoAN have a late growth defect in macrophages. J774 macrophages were infected at an MOI (multiplicity of infection) of 1 with CDC1551 or the NADH dehydrogenase mutants. At 1, 2, and 4 d postinfection, macrophages were lysed and plated to determine bacterial cfu. The complemented strains Δndh pMV361::ndh and ΔndhA pMV361::ndhA are shown as Δndhc and ΔndhAc, respectively. The average of two independent experiments done in duplicate is shown with SD.
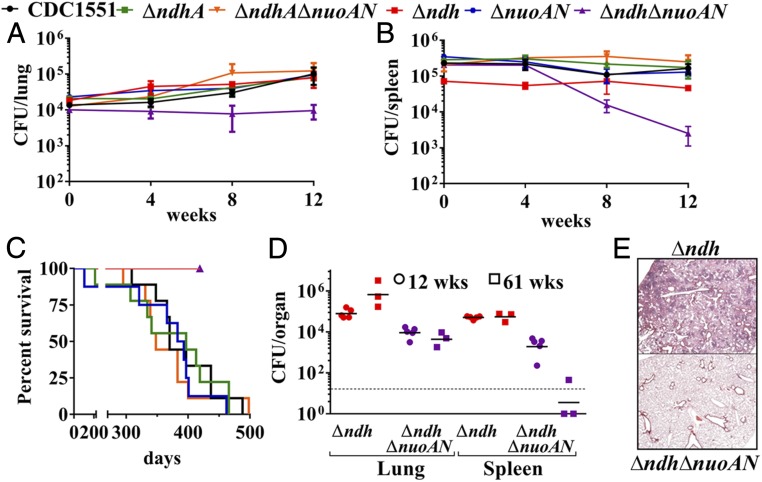
Δndh and ΔndhΔnuoAN Are Attenuated in Vivo.
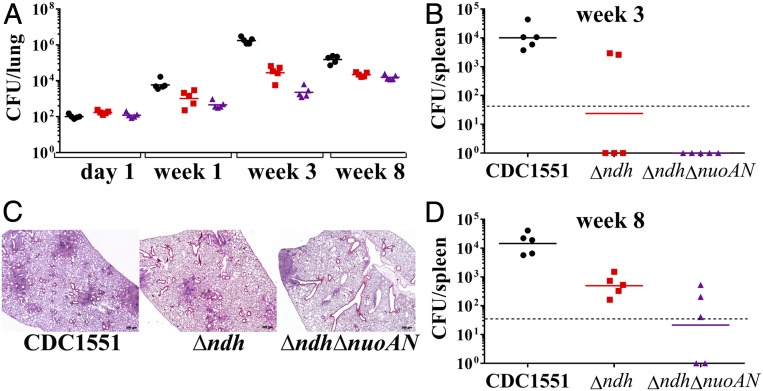
Immunocompetent mice were infected i.v. with the NADH dehydrogenase mutants at a dose of ∼106 bacteria to assess both the in vivo growth and the virulence of the NADH dehydrogenase mutants. Δndh, ΔndhA, ΔnuoAN, and ΔndhAΔnuoAN grew comparably to the WT strain in the lungs (Fig. 4A) and spleens (Fig. 4B) of infected mice. Burden of ΔndhΔnuoAN failed to increase in the lungs of infected mice, and, in the spleen, the ΔndhΔnuoAN titer dropped drastically after the first 4 wk of infection. In parallel, the survival study (nine mice per group; Fig. 4C) showed that the mice infected with the parental strain, ΔndhA, ΔnuoAN, and ΔndhAΔnuoAN, died in the same time range, while the mice infected with Δndh or ΔndhΔnuoAN all survived. To further evaluate the virulence defect of Δndh and ΔndhΔnuoAN observed during the survival experiment, three mice infected with Δndh or ΔndhΔnuoAN from the survival experiment were euthanized to determine lung and spleen bacterial burdens at 61 wk postinfection. The mice infected with Δndh had similar spleen burden at 61 wk compared with week 12 and a higher burden in the lungs at week 61 (Fig. 4D). The lung burden of the mice infected with ΔndhΔnuoAN had not changed at 61 wk compared with week 12, but the spleen burden was near undetectable levels at 61 wk. Pathology revealed that the lungs of mice infected with Δndh had widespread chronic scattered granulomatous inflammation with high numbers of lymphocytes both in the inflammation and around vessels (Fig. 4E). In contrast, the ΔndhΔnuoAN-infected lung samples had very little evidence of inflammation, which represented less than 5% of the lung area and was histiocytic and lymphocytic. The lack of virulence of Δndh and ΔndhΔnuoAN led us next to examine the possibility of protection against virulent Mtb. The six remaining mice initially infected with Δndh or ΔndhΔnuoAN from the survival experiment were then infected i.v. with a high dose of WT Mtb H37Rv (5 × 106 bacteria). Following the H37Rv challenge, the six mice initially infected with Δndh had a median survival of 37 d. Three of the six mice initially infected with ΔndhΔnuoAN had a median survival of 98 d following H37Rv challenge, and at 135 d post H37Rv challenge, the other three mice had to be euthanized due to dermatitis. These data establish ΔndhΔnuoAN as the most attenuated NADH dehydrogenase strain in vivo.
Fig. 4.
Δndh and ΔndhΔnuoAN are avirulent in immunocompetent mice infected intravenously. (A and B) Lung and spleen burdens of infected C57BL/6 mice at day 1, weeks 4, 8, and 12 (five mice per group and per time point). (C) Survival of infected C57BL/6 mice (nine mice per group). (D) Three C57BL/6 mice infected with Δndh and ΔndhΔnuoAN from the survival experiment shown in C were euthanized at 61 wk (squares) postinfection to determine their lung and spleen burdens and are compared with the 12-wk organ burdens (circles). cfu counts in lungs and spleen are shown for each individual mouse, and geometric mean is indicated for each group. The limit of detection is indicated by a dotted line. (E) Lung tissue sections of C57BL/6 mice infected with Δndh (Top) or ΔndhΔnuoAN (Bottom) at 61 wk postinfection were stained with hematoxylin/eosin and observed at a magnification of 2.5×.
To further assess the in vivo virulence defect of the Δndh and ΔndhΔnuoAN mutants, a low-dose aerosol infection of immunocompetent mice was performed. Mice were euthanized at 1, 3, and 8 wk to determine lung (Fig. 5A) and spleen (Fig. 5 B and D) bacterial burdens. ΔndhΔnuoAN mutant was the most attenuated strain, although it did grow in both organs. The Δndh mutant grew better than the ΔndhΔnuoAN mutant in both organs but less than the WT strain. Pathology examination of the lung tissues at 8 wk postinfection (Fig. 5C) showed that the mice infected with CDC1551 or Δndh had small to large nodular to diffuse aggregates of large macrophages admixed with lymphocytes and, occasionally, a small amount of necrotic debris multifocally. These histologic findings were typical of Mtb infection and filled alveolar spaces and obscured normal pulmonary architecture. The lungs of the mice infected with ΔndhΔnuoAN exhibited the fewest lesions, with small to moderate numbers of lymphocytes admixed with reduced numbers of macrophages, rare plasma cells, and neutrophils multifocally surrounding bronchioles.
Fig. 5.
The growth of Δndh and ΔndhΔnuoAN is attenuated in immunocompetent mice infected via the aerosol route. C57BL/6 mice were infected with CDC1551 (black circles), Δndh (red squares), and ΔndhΔnuoAN (purple triangles) via a low-dose (∼100 bacilli) aerosol. Lungs (A) and spleens (B and D) were collected, homogenized, and plated to determine organ bacterial burden at 1, 3, and 8 wk postinfection. cfu counts in lungs and spleen are shown for each individual mouse, and geometric mean is indicated for each group. The limit of detection is indicated by a dotted line. (C) Lung tissue sections at 8 wk postinfection were stained with hematoxylin/eosin and observed at a magnification of 2.5×.
In summary, the ΔndhA, ΔnuoAN, and ΔndhAΔnuoAN strains are as virulent as the parental strain. Mtb strains lacking ndh are attenuated for growth and virulence in mice.
Discussion
Of the three NADH dehydrogenases present in Mtb, only ndh encoding the type II NADH dehydrogenase Ndh had been previously described as an essential gene (11, 12). In this study, we show that none of the NADH dehydrogenases are essential in vitro or in vivo, highlighting the need to validate high-throughput transposon essentiality screening with detailed gene-deletion and gene-silencing studies. The failure to obtain the double-deletion mutant ΔndhΔndhA suggests that Mtb requires the presence of at least one type II nonproton-translocating NADH dehydrogenase for growth. The nonproton-translocating activity of these enzymes may be an important feature in allowing Mtb to maintain an energized membrane in the absence of growth using lower-efficiency complexes. For example, coupling NDH-2 to cytochrome bd would allow Mtb to run respiration coupled to nonproton-pumping complexes, and therefore electron flow would not be impeded by pmf backpressure in the absence of growth and high rates of ATP synthesis (proton consumption) (20). When NdhA is the only NADH dehydrogenase type II present in Mtb, the strain (Δndh) is impaired for growth in vitro, more susceptible to oxidative stress, and is less virulent in vivo. When NdhA is the only NADH dehydrogenase present in Mtb, the strain (ΔndhΔnuoAN) has the most drastic growth-defect phenotype in vitro and in vivo, suggesting a compensatory role for Nuo in the absence of Ndh. In contrast, when Ndh is the only NADH dehydrogenase present in Mtb, the strain (ΔndhAΔnuoAN) has no growth defect in vitro or in vivo. The data designate Ndh as the relevant NADH dehydrogenase in vitro and in vivo. Although the ΔnuoAN mutant had no phenotype in vitro or in vivo, the severely attenuated phenotype of the ΔndhΔnuoAN strain compared with the Δndh strain in mice reveals that Nuo may play an important role in the virulence of Mtb. Previously, the deletion of a single subunit of the nuo operon, nuoG, in Mtb resulted in a proapoptotic phenotype in human macrophages and increased survival in mice (21). The authors observed no growth defect of the ΔnuoG mutant in vitro but a significant reduction in bacterial load in the lungs (although not in the spleen or liver) of immunocompetent mice infected i.v. compared with mice infected with WT Mtb. Furthermore, a nuoG deletion in the bacillus Calmette–Guérin ΔureC::hly vaccine candidate strain (22) increased vaccine safety (23). Deletion of nuoG in bacillus Calmette–Guérin ΔureC::hly led to enhancement of apoptosis and autophagy, two immune cellular pathways that are intimately linked with Mtb survival and eradication in the host, and downstream enhancement of anti-Mtb immune responses. The molecular basis for the discrepancies between our ΔnuoAN mutant and the phenotypes observed with the deletion of a single subunit of nuo will require additional studies to elucidate.
Although ndh is found in all mycobacteria, ndhA is absent in some mycobacterial species such as M. smegmatis, M. abscessus, and M. leprae, suggesting a nonpivotal role for ndhA in the presence of ndh. Questions remain as to the presence of two NADH dehydrogenases type II in Mtb. Are ndh and ndhA redundant or required for specific growth conditions? The fact that Δndh and ΔndhA grew in vitro and in vivo shows that both enzymes are functional, confirming previous biochemical data (24). Interestingly, when grown in vitro, we noticed that the Δndh strain showed a longer lag time compared with WT or ΔndhA. Lag phase is often considered as the time required by a bacterium to adapt to a new growth condition. Jacques Monod had suggested that the lag time per phase might reflect “an insufficient supply of metabolite(s) or the state of inactivity of an enzyme” (ref. 25, p. 387). It may suggest that, although NdhA is an active NADH dehydrogenase during log phase, its ability to oxidize NADH into NAD+ might be reduced during lag phase. We did not find an in vitro growth condition where ndhA was required, but it is possible that ndhA might be necessary for growth with specific nutrient or oxygen conditions. In mice, ΔndhA had no phenotype, while Δndh displayed a virulence defect. We hypothesize that the oxygen environment in the mouse lung might impair Δndh. We had previously shown that ndh and ndhA modulate oxygen consumption differently and both at a slower rate than the parental strain (26). As the level of oxygen decreased, ΔndhA slowed down its oxygen consumption faster than Δndh, suggesting that ΔndhA controls its respiration and conserves oxygen better than Δndh.
Enzymes of the ETC-mediating oxidative phosphorylation in Mtb are validated drug targets (e.g., ATP synthase, cytochrome bc1). The NADH dehydrogenase Ndh has no homolog in humans, so Mtb Ndh inhibitors could be developed with limited toxicity risk. One consequence of inhibiting Ndh is an increase in the NADH/NAD+ ratio toward a higher reducing potential. Because redox homeostasis is important for the survival of cells in a slowing–replicating state (27, 28), Ndh inhibitors might also be good candidates to target persister or dormant Mtb bacteria. In that context, new Ndh inhibitors have been synthesized and shown to have antimycobacterial activity under aerobic and hypoxic conditions against both drug-susceptible and drug-resistant Mtb strains (29). Furthermore, redox homeostasis is also an important factor in drug activity and resistance, since many drugs against Mtb are prodrugs activated via a reductive process. INH is activated by the catalase peroxidase KatG to form an isonicotinoyl radical that reacts with NAD+ yielding an INH-NAD adduct (30, 31). It had been previously shown by us and others that mutations in ndh led to resistance to INH in mycobacteria (32). M. smegmatis and Mycobacterium bovis bacillus Calmette–Guérin ndh mutants are 20-fold and up to sixfold more resistant to INH, respectively. We had postulated that INH resistance in M. smegmatis and M. bovis bacillus Calmette–Guérin ndh mutants was due to an increase in cellular NADH concentration, which competitively inhibited the binding of the INH-NAD adduct to the NADH-dependent enoyl–ACP reductase InhA (10). The Mtb NADH dehydrogenase deletion strains Δndh and ΔndhΔnuoAN had NADH/NAD+ ratios three to four times higher than their parental strain CDC1551, so we expected them to be INH-resistant, yet they had only very low-level resistance to INH (two- to fourfold). The difference between the phenotypes of the Mtb, M. smegmatis, and M. bovis bacillus Calmette–Guérin Pasteur ndh mutants in regard to INH resistance might reflect the NADH concentration in the different mycobacterial species. In M. smegmatis, the highly INH-resistant (20-fold) ndh mutants had NADH concentrations approaching 2 mM, while the NADH concentrations in the low-level INH-resistant ndh mutants of M. bovis bacillus Calmette–Guérin were between 0.6 and 0.7 mM (10). In Mtb, Δndh, and ΔndhΔnuoAN, NADH concentrations never exceeded 0.3 mM. We hypothesize that the increase in NADH concentration in Mtb, Δndh, and ΔndhΔnuoAN might not be sufficiently high to efficiently prevent InhA inactivation by the INH-NAD adduct in Mtb.
Inhibitors of the NADH dehydrogenases type II such as TPZ and CPZ have good activity against Mtb both in vitro and in vivo (24). When tested against the Δndh and ΔndhA strains, both TPZ and CPZ had similar MIC, confirming that TPZ and CPZ inhibit Ndh and NdhA equally. CFZ, which was shown to be efficient in the treatment of MDR-TB (33, 34) and was recently recommended by WHO to be included in the treatment of MDR-TB, has a complex mode of action. In M. smegmatis, Yano et al. (19) showed that CFZ is a prodrug that is reduced by Ndh, and oxidation of reduced CFZ by oxygen generates reactive oxygen species. Since NdhA is not present in M. smegmatis, both enzymes, Ndh and NdhA, could be involved in the reduction of CFZ in Mtb. The MIC of CFZ was higher for Δndh but not for ΔndhA compared with the WT Mtb strain, supporting the involvement of Ndh and not NdhA in the mechanism of action of CFZ.
This study underlines the critical role of the type I NADH dehydrogenase in Mtb. The loss of both nuoAN and ndh in Mtb resulted in the most pronounced phenotypes in terms of growth and virulence. Although this is not a true synthetic lethality, this work clearly demonstrates that both the type I and type II NADH dehydrogenases play overlapping roles in the homeostasis of NADH in the growth of Mtb. Further metabolic and biochemical studies will be required to elucidate the specificity of type I and type II NADH dehydrogenases’ metabolic roles, although the growth and virulence attenuation of the ΔndhΔnuoAN mutant suggests that Nuo may be an Achilles heel in the in vivo metabolism of Mtb. The set of these mutants in both virulent and BSL2-safe Mtb strains should provide useful tools for screening of new compounds to disable the electron transport pathways of the tubercle bacilli.
Methods
Bacterial Strains.
The Mtb strains, plasmids, and phages used in this study were obtained from laboratory stocks. mc26230 (Mtb H37Rv ΔRD1ΔpanCD) is an Mtb strain (35) reclassified as a biosafety level 2 strain by the Albert Einstein College of Medicine Institutional Biosafety Committee. The strains were grown at 37 °C in Middlebrook 7H9 (Difco), supplemented with 10% (vol/vol) OADC (oleic acid-albumin-dextrose-catalase; Difco), 0.2% (vol/vol) glycerol, 0.05% (vol/vol) tyloxapol. The solid media used were Middlebrook 7H10 (Difco), supplemented with 10% (vol/vol) OADC and 0.2% (vol/vol) glycerol. Plates were incubated at 37 °C for 4 to 8 wk. D-pantothenate (24 mg/L) was added to the liquid or solid media to grow mc26230. The ndh, ndhA, and the full operon nuoAN were deleted from Mtb CDC1551 and mc26230 using the specialized transduction system (14).
Quantitative Real-Time PCR.
ndh, ndhA, and nuoH relative expression was measured by quantitative real-time PCR (RT-qPCR). Triplicate cultures (10 mL) of Mtb CDC1551, CDC1551 Δndh, CDC1551 ΔndhA, CDC1551 ΔnuoAN, CDC1551 ΔndhΔnuoAN, CDC1551 ΔndhAΔnuoAN, CDC1551 Δndh pMV361::ndh, and CDC1551 ΔndhA pMV361::ndhA were grown to an OD600 nm ≈ 0.1 at 37 °C and centrifuged, and the cell pellets were resuspended in 1 mL Qiagen RNA Protect reagent (Qiagen) for 24 h. RNA was isolated using Qiagen RNeasy kit, and RT-qPCR was performed using protocols previously described (36).
Measurement of NADH and NAD+ Cellular Concentrations.
Cultures (12 mL) were grown at 37 °C to log phase (OD600 nm ≈ 1.0) in Middlebrook 7H9 medium (see above). NAD+ and NADH were extracted as previously described (36), and their concentrations were obtained by measuring spectrophotometrically the rate of 3-[4,5-dimethylthiazol-2-yl]–2,5-diphenyltetrazolium bromide reduction by the yeast type II alcohol dehydrogenase in the presence of phenazine ethosulfate at 570 nm (37, 38).
Minimum Inhibitory Concentration Determination.
The strains were grown to OD600 nm ≈ 0.8–1 and diluted 1/1,000. Serial twofold dilutions of each drug tested were prepared in sterile 96-well plates for a final volume of 0.1 mL before the addition of the diluted bacterial cultures (0.1 mL). The plates were incubated at 37 °C for 7 d. OD590 nm was read on a plate reader, and the MIC was determined as the lowest concentration of drug that prevented growth.
Murine Macrophage Infection.
J774A.1 macrophage cells (ATCC) were subcultured according to the supplier’s recommendations in Dulbecco’s modified Eagle medium (DMEM; Invitrogen), supplemented with 10% FBS (Invitrogen). Macrophages (∼100,000 cells per well) were seeded into 24-well tissue culture plates and cultured for 3 d. At the time of the infection, cell density was ∼3.6 × 105 cells per well. The Mtb strains were grown at 37 °C to OD600 nm ≈ 0.8, washed twice in PBS, and sonicated twice for 10 s. The bacterial suspensions were diluted in DMEM, supplemented with 10% FBS, and used to infect the J774 cells for 4 h at 37 °C in 5% CO2 at an approximate multiplicity of infection (MOI) of 1 to allow for bacterial uptake. Cell monolayers were washed twice with PBS and incubated in DMEM, supplemented with 10% FBS at 37 °C in 5% CO2. At specific time points, media were removed, and the wells were washed once with PBS and then treated for 5 min with 0.05% aqueous SDS solution to lyse the macrophages. The lysates were serially diluted in PBS and plated for colony-forming unit (cfu) determination.
Mouse Challenge Experiments.
The Mtb strains were grown to OD600 nm ≈ 0.8, washed twice with PBS, sonicated (2 × 10 s), and diluted to the appropriate cell densities. C57BL/6 female mice (6–8 wk old) were obtained from the National Cancer Institute. For the i.v. infection, mice were infected with the Mtb strains (∼1 × 106 cfu). Nine mice from each group were kept for the survival experiment. For the aerosol infection, mice were infected with a low dose (100–175 cfus per lung) of the Mtb strains used following a published protocol (39). For each experiment, at the indicated time point, mice were euthanized, and the spleens and right lungs were collected and homogenized in PBS containing 0.05% (vol/vol) tyloxapol. The organ lysates were plated on Middlebrook 7H10 plates to determine cfus per organ.
The animal protocol #20150215 “Evaluation of the safety and the efficacy of attenuated mycobacterial vaccine vectors” was approved by the Einstein Animal Institute, which is accredited by the “American Association for the Use of Laboratory Animals” [DHEW Publication No. (NIH) 78–23, Revised 1978] and accepts as mandatory the NIH “Principles for the Use of Animals.”
More detailed methods are available in Supporting Information.
Supplementary Material
Acknowledgments
We are grateful to Mei Chen and John Kim for technical assistance with the mice work. We thank Drs. Michael Berney and Gregory Cook for helpful discussions and critical reading of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721545115/-/DCSupplemental.
References
- 1.Jnawali H, Ryoo S. 2013 First- and second-line drugs and drug resistance. Available at https://www.intechopen.com/books/tuberculosis-current-issues-in-diagnosis-and-management/first-and-second-line-drugs-and-drug-resistance. Accessed January 20, 2018.
- 2.Pethe K, et al. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med. 2013;19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 3.Kalia NP, et al. Exploiting the synthetic lethality between terminal respiratory oxidases to kill Mycobacterium tuberculosis and clear host infection. Proc Natl Acad Sci USA. 2017;114:7426–7431. doi: 10.1073/pnas.1706139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li K, et al. Multitarget drug discovery for tuberculosis and other infectious diseases. J Med Chem. 2014;57:3126–3139. doi: 10.1021/jm500131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook GM, et al. Oxidative phosphorylation as a target space for tuberculosis: Success, caution, and future directions. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.tbtb2-0014-2016. TBTB2-0014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 7.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2008;105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAdam RA, et al. Characterization of a Mycobacterium tuberculosis H37Rv transposon library reveals insertions in 351 ORFs and mutants with altered virulence. Microbiology. 2002;148:2975–2986. doi: 10.1099/00221287-148-10-2975. [DOI] [PubMed] [Google Scholar]
- 9.Miesel L, Weisbrod TR, Marcinkeviciene JA, Bittman R, Jacobs WR., Jr NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J Bacteriol. 1998;180:2459–2467. doi: 10.1128/jb.180.9.2459-2467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vilchèze C, et al. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother. 2005;49:708–720. doi: 10.1128/AAC.49.2.708-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin JE, et al. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011;7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthy D, Ambady A, Narayana A, Morayya S, Sharma U. Roles of the two type II NADH dehydrogenases in the survival of Mycobacterium tuberculosis in vitro. Gene. 2014;550:110–116. doi: 10.1016/j.gene.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Bardarov S, et al. Specialized transduction: An efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 14.Jain P, et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio. 2014;5:e01245-14. doi: 10.1128/mBio.01245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolfe MD, et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol. 2012;194:686–701. doi: 10.1128/JB.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuny C, Lesbats M, Dukan S. Induction of a global stress response during the first step of Escherichia coli plate growth. Appl Environ Microbiol. 2007;73:885–889. doi: 10.1128/AEM.01874-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilchèze C, Hartman T, Weinrick B, Jacobs WR., Jr Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun. 2013;4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano T, Li LS, Weinstein E, Teh JS, Rubin H. Steady-state kinetics and inhibitory action of antitubercular phenothiazines on mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2) J Biol Chem. 2006;281:11456–11463. doi: 10.1074/jbc.M508844200. [DOI] [PubMed] [Google Scholar]
- 19.Yano T, et al. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: A pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011;286:10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hards K, Cook GM. Targeting bacterial energetics to produce new antimicrobials. Drug Resist Updates. 2018;36:1–12. doi: 10.1016/j.drup.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Velmurugan K, et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann SH, et al. The BCG replacement vaccine VPM1002: From drawing board to clinical trial. Expert Rev Vaccines. 2014;13:619–630. doi: 10.1586/14760584.2014.905746. [DOI] [PubMed] [Google Scholar]
- 23.Gengenbacher M, et al. Deletion of nuoG from the vaccine candidate Mycobacterium bovis BCG ΔureC:hly improves protection against tuberculosis. MBio. 2016;7:e00679-16. doi: 10.1128/mBio.00679-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstein EA, et al. Inhibitors of type II NADH:menaquinone oxidoreductase represent a class of antitubercular drugs. Proc Natl Acad Sci USA. 2005;102:4548–4553. doi: 10.1073/pnas.0500469102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monod J. The growth of bacterial cultures. Annu Rev Microbiol. 1949;3:371–394. [Google Scholar]
- 26.Hartman T, et al. Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1004510. doi: 10.1371/journal.ppat.1004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bald D, Villellas C, Lu P, Koul A. Targeting energy metabolism in Mycobacterium tuberculosis, a new paradigm in antimycobacterial drug discovery. MBio. 2017;8:e00272-17. doi: 10.1128/mBio.00272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhana A, et al. Reductive stress in microbes: Implications for understanding Mycobacterium tuberculosis disease and persistence. Adv Microb Physiol. 2010;57:43–117. doi: 10.1016/B978-0-12-381045-8.00002-3. [DOI] [PubMed] [Google Scholar]
- 29.Hong WD, et al. Rational design, synthesis, and biological evaluation of heterocyclic quinolones targeting the respiratory chain of Mycobacterium tuberculosis. J Med Chem. 2017;60:3703–3726. doi: 10.1021/acs.jmedchem.6b01718. [DOI] [PubMed] [Google Scholar]
- 30.Rozwarski DA, Grant GA, Barton DH, Jacobs WR, Jr, Sacchettini JC. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science. 1998;279:98–102. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 32.Vilcheze C, Jacobs WR., Jr Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: Genes, mutations, and causalities. Microbiol Spectr. 2014;2:MGM2-0014-2013. doi: 10.1128/microbiolspec.MGM2-0014-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang S, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: Prospective, multicenter, randomized controlled study in China. Clin Infect Dis. 2015;60:1361–1367. doi: 10.1093/cid/civ027. [DOI] [PubMed] [Google Scholar]
- 34.Van Deun A, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 35.Sambandamurthy VK, et al. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: A safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006;24:6309–6320. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 36.Vilchèze C, Weinrick B, Wong KW, Chen B, Jacobs WR., Jr NAD+ auxotrophy is bacteriocidal for the tubercle bacilli. Mol Microbiol. 2010;76:365–377. doi: 10.1111/j.1365-2958.2010.07099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonardo MR, Dailly Y, Clark DP. Role of NAD in regulating the adhE gene of Escherichia coli. J Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.San KY, et al. Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng. 2002;4:182–192. doi: 10.1006/mben.2001.0220. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, et al. Einstein contained aerosol pulmonizer (ECAP): Improved biosafety for multi-drug resistant (MDR) and extensively drug resistant (XDR) Mycobacterium tuberculosis aerosol infection studies. Appl Biosaf. 2011;16:134–138. doi: 10.1177/153567601101600302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.