Having split early from Gondwana, Zealandia (now modern New Zealand) escaped discovery until the late 13th century, and therefore remains an important glimpse into a human-free world. Without humans or other land mammals, diverse and peculiar birds evolved in isolation, including several flightless moa species, the giant pouakai eagle (Harpagornis moorei), the kiwi (Apteryx mantelli), and the kakapo parrot (Strigops habroptila). This unique community has fascinated paleoecologists, who have spent almost two centuries devising new ways to glean information from ancient bird remains. In PNAS, Boast et al. (1) apply one recent technological advance, ancient DNA (aDNA) metabarcoding, to confirm previous discoveries and report new details about moa and kakapo diets, parasites, and niches. Their efforts confirm Zealandia was a lot different before humans arrived.
Zealandia’s most diverse avian oddities were the moa. Moa research goes back to the early 1800s, when the Māori told legends to colonizing Europeans about giant birds and showed them fossilized moa bones in caves. Those fossils belonged to at least nine moa species ranging from 1 to 4 m tall, some with notable sexual dimorphism (2). The moa species have different fossil distributions, morphology, and gizzard contents, suggesting they partitioned Zealandia’s resources into distinct ecological niches. To better understand how moas coexisted and interacted with other species, paleontologists have turned to another abundant deposit that moas left behind: coprolites (fossilized dung). The 2,000 moa coprolites that have been collected thus far contain plants, fungi, microbes, and intestinal parasites (3), a priceless resource for creating ecological snapshots from Zealandia. Until the aDNA revolution, however, these snapshots were blurry (4).
Sequencing aDNA allows paleontologists to identify dung-encased organisms at finer resolution than they see with a microscope. Studying aDNA is nerve-wracking, because it degrades over time and each sample can become contaminated where it lies or during sample handling, transport, and storage. However, dedicated aDNA facilities and rigorous protocols help reduce contamination (5). Even then, authentic aDNA fragments can be hard to decipher, because short diagnostic DNA barcodes are still unavailable for most plants and animal species (1). Thus, scientists who work with aDNA need to be careful, both in their techniques and their interpretation. When it works, the effort pays off with insights not imaginable when paleontologists cracked open that first moa coprolite.
Boast’s research group has been around the aDNA block before, having used first-generation sequencing techniques on plant, microbe, and parasite aDNA from moa coprolites to yield family- and even genus-level taxonomic resolution for several taxa that the moa interacted with (6, 7). However, first-generation techniques are too time-consuming and expensive for compiling a comprehensive ancient food web. To make the most from their samples, Boast et al. (1) switched to high-throughput sequencing, which confirmed previous first-generation results and unearthed several hitherto unknown ecological interactions between the moa and plants, fungi, and parasites. In addition to describing these new results, Boast et al. (1) synthesize past findings to add resolution to the Zealandia food web.
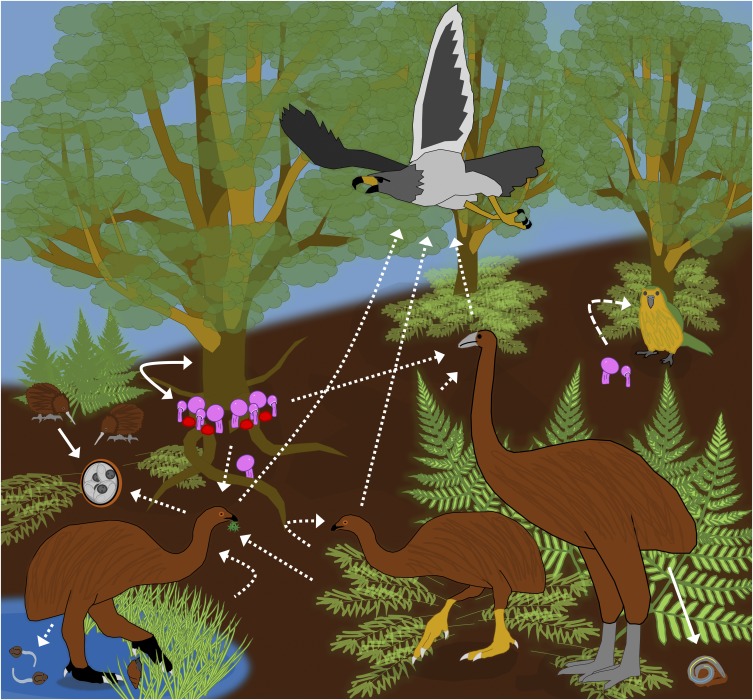
In this enhanced snapshot from Zealandia (Fig. 1), an upland moa (Megalapteryx didiformis) stops to eat moss under a southern-beech tree (Nothofagaceae) and then defecates feces laced with parasite eggs on the forest floor. As with many other upland moa, it acquired parasitic trematodes when feeding at a small alpine pond and heterakoid nematodes when eating contaminated ferns and other vegetation. However, the moa’s real concern is the pouakai, the world’s largest eagle and the only moa predator. A nearby South Island giant moa (Dinornis robustis) has no interest in the upland moa’s moss. Instead, it lunges forward and snaps up a tasty-looking mushroom near a southern-beech trunk. It is lucky to have found this gem, because kakapos and upland moas also seek out Cortinarius mushrooms. The fungal spores will pass through the giant moa’s digestive system, in which live apicomplexan parasite oocysts (Eimeriidae) and an ascarid nematode or two. Later, defecated fungal spores will have the chance to establish a mycorrhizal relationship with a new host tree, and a kiwi might accidentally eat the defecated oocysts. Ancient paradise, it turns out, had abundant dung and parasites.
Fig. 1.
The ancient Zealandia food web was much different from that in present-day New Zealand, and it included several ecological interactions that Boast et al. (1) revealed by sequencing aDNA from moa coprolites. For instance, the upland moa (M. didiformis) ate moss, ferns, mycorrhizal fungi that require animal-assisted dispersal, and aquatic vegetation. The upland moa also accidentally ate (and was infected by) aquatic trematode larvae (Notocotylidae) and apicomplexan parasites (Eimeriidae), which still exist in extant kiwi. Other parasites that once infected moas, like several heterakoid nematode species, have not been seen since the moa extinctions. Dashed arrows indicate interactions lost since the moa extinctions.
Such interactions continued within this robust Zealandia ecosystem, despite environmental and climate fluctuations (8, 9), until the first Māori landed their canoes on Zealandia and named it Aotearoa. The Māori hunted the moa, which had only feared the pouakai, and drove the nine moa species extinct within ∼200 y (10).
Boast et al. (1) show that some species that interacted with the moa survived their loss. For instance, the upland moa’s Eimeriid coccidians still parasitize extant kiwi. Likewise, animal-dispersed mycorrhizal fungi still exist in New Zealand’s modern forests even without extant animal dispersers (11). These fungi might be ecological anachronisms, like the giant fruits in the Americas that rot under their trees without extinct mammalian megafauna to eat them and disperse their seeds (12). Just as the long-lived trees in the Americas have slowly declined without their ancient seed dispersers, so too might the moa-dispersed fungi and their southern-beech tree hosts decline unless they adapt to use new spore dispersers or moa deextinction ideas become reality (13).
Other species were not robust. Without moa, the pouakai starved. Pouakai is the most obvious secondary extinction to follow the moa, but Boast et al. (1) suggest that other species fell like a teetering Jenga tower after a critical piece is pulled. In particular, several heterakoid nematode species had coevolved to specialize on moa, and have not been seen since the moa disappeared. Other moa and pouakai parasites (most likely several never-seen, host-specific lice) were probably lost. These secondary extinctions result because coevolved complex systems cannot evolve robustness to swift, unpredictable events like the Māori’s arrival and rapid domination (14). How many secondary extirpations or extinctions occurred is not yet clear, and will remain unknown until there are larger sequence libraries from extant species that can be used to confirm their absences. To understand the past with aDNA, one must also sequence the present.
Between the ancient moa extinctions and the present, Europeans and their introductions further altered New Zealand’s biodiversity. Winners included weeds, pests, and people. Their parasites were winners too. On the other hand, specialist parasites, particularly those with complex life cycles, should be least robust to secondary extinction (15) and should go down with the ship. To that end, parasite extinctions have continued in New Zealand. In 1904, scientists discovered the kakapo tapeworm Stringopotaenia psittacea (16), which has not been seen since the kakapo’s dramatic decline toward near extinction. Soon after, a chewing louse (Huiacola extinctus) went extinct (17), along with its host, the huia (a wattlebird hunted for its feathers). More recently, the louse Rallicola (Aptericola) pilgrimi was lost after its host, the smallest kiwi (Apteryx owenii), was translocated to predator-free islands (18). Therefore, most New Zealand extinctions have probably been parasite extinctions.
Society will decide whether we preserve, ignore, or extirpate species we find insignificant, threatening, or disgusting. Some point out that parasites deserve protection because they can play important roles as consumer species (19). Others consider that ignoring these associated species is taxonomic chauvinism (20); it is like counting sunken ships in a naval battle, without mourning the sailors that go down with those ships. The extent to which we value host vessels or their parasite passengers is most often answered by veterinarians. When veterinarians treated the last remaining California condors in 1987, they found the condor louse, Colpocephalum californici, a symbiont whose ancestors had flown with condors over plains populated by mammoths and saber-toothed tigers; they then exiled the condor louse to extinction with an insecticide treatment (20). Had moa survived to face Europeans, New Zealand veterinarians would have likely done the same to moa parasites.
Acknowledgments
Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
The authors declare no conflict of interest.
See companion article on page 1546.
References
- 1.Boast AP, et al. Coprolites reveal ecological interactions lost with the extinction of New Zealand birds. Proc Natl Acad Sci USA. 2018;115:1546–1551. doi: 10.1073/pnas.1712337115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worthy TH, Scofield RP. Twenty-first century advances in knowledge of the biology of moa (Aves: Dinornithiformes): A new morphological analysis and moa diagnoses revised. N Z J Zool. 2012;39:87–153. [Google Scholar]
- 3.Wood JR, Wilmshurst JM. Late Quaternary terrestrial vertebrate coprolites from New Zealand. Quat Sci Rev. 2014;98:33–44. [Google Scholar]
- 4.Cole TL, Wood JR. The ancient DNA revolution: The latest era in unearthing New Zealand’s faunal history. N Z J Zool. 2017;44:1–30. [Google Scholar]
- 5.Cooper A, Poinar HN. Ancient DNA: Do it right or not at all. Science. 2000;289:1139. doi: 10.1126/science.289.5482.1139b. [DOI] [PubMed] [Google Scholar]
- 6.Wood JR, et al. A megafauna’s microfauna: Gastrointestinal parasites of New Zealand’s extinct moa (Aves: Dinornithiformes) PLoS One. 2013;8:e57315. doi: 10.1371/journal.pone.0057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood JR, et al. Resolving lost herbivore community structure using coprolites of four sympatric moa species (Aves: Dinornithiformes) Proc Natl Acad Sci USA. 2013;110:16910–16915. doi: 10.1073/pnas.1307700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlence NJ, et al. The effect of climate and environmental change on the megafaunal moa of New Zealand in the absence of humans. Quat Sci Rev. 2012;50:141–153. [Google Scholar]
- 9.Allentoft ME, et al. Extinct New Zealand megafauna were not in decline before human colonization. Proc Natl Acad Sci USA. 2014;111:4922–4927. doi: 10.1073/pnas.1314972111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry GLW, Wheeler AB, Wood JR, Wilmshurst JM. A high-precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes) Quat Sci Rev. 2014;105:126–135. [Google Scholar]
- 11.Wood JR, et al. Novel interactions between non-native mammals and fungi facilitate establishment of invasive pines. J Ecol. 2015;103:121–129. [Google Scholar]
- 12.Janzen DH, Martin PS. Neotropical anachronisms: The fruits the gomphotheres ate. Science. 1982;215:19–27. doi: 10.1126/science.215.4528.19. [DOI] [PubMed] [Google Scholar]
- 13.Wood JR, Perry GLW, Wilmshurst JM. Using palaeoecology to determine baseline ecological requirements and interaction networks for de-extinction candidate species. Funct Ecol. 2017;31:1012–1020. [Google Scholar]
- 14.Strona G, Lafferty KD. Environmental change makes robust ecological networks fragile. Nat Commun. 2016;7:12462. doi: 10.1038/ncomms12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafferty KD. Biodiversity loss decreases parasite diversity: Theory and patterns. Philos Trans R Soc Lond B Biol Sci. 2012;367:2814–2827. doi: 10.1098/rstb.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beveridge I. A Taxonomic Revision of the Genera Cittotaenia Riehm, 1881, Ctenotaenia, Railliet, 1893, Mosgovoyia Spasskii 1951 and Pseudocittotaenia Tenora, 1976: Cestoda, Anoplocephalidae. Éditions de Muséum; Paris: 1978. [Google Scholar]
- 17.Mey E. Eine neue ausgestorbene vogel-Ischnozere von neuseeland, Huiacola extinctus (Insecta, Phthiraptera) Zool Anz. 1990;224:49–73. German. [Google Scholar]
- 18.Buckley TR, et al. The conservation status of small or less well known groups of New Zealand terrestrial invertebrates. N Z Entomol. 2012;35:137–143. [Google Scholar]
- 19.Dougherty ER, et al. Paradigms for parasite conservation. Conserv Biol. 2016;30:724–733. doi: 10.1111/cobi.12634. [DOI] [PubMed] [Google Scholar]
- 20.Pizzi R. Veterinarians and taxonomic chauvinism: The dilemma of parasite conservation. J Exot Pet Med. 2009;18:279–282. [Google Scholar]