Abstract
Background:
Infertility is described as not receiving pregnancy despite unprotected and regular sexual intercourse in a 1 yr period. It is detected by 15% of the couples. Male and female factor in the etiology may be detected in similar rates.
Objective:
The present study aims to investigate ion channel gene expression in semen samples of infertile male compared with fertile men.
Materials and Methods:
A total of 150 men who applied to the urology clinic due to infertility were divided into five equal groups: asthenozoospermia, oligozoospermia, oligoasthenoteratozoospermia, teratozoospermia, and normozoospermia (control). All paticipants were evaluated with Cation Channel Spermia (CatSper) 1, 2, 3, 4, Proton Voltage Gated Ion Channel1 (Hv1), Potassium Channel Subfamily U1 (KCNU1), and transmembrane protein (TMEM16A) gene expression in semen samples.
Results:
“CatSper1, 4, HV1, KCNU1, and TMEM16A gene expression were detected higher in the oligozoospermia group compared to the controls. CatSper1, 2, 3, 4, KCNU1, and TMEM16A gene expression in the asthenozoospermia group and CatSper1, 2, 3, 4, KCNU1, and TMEM16A gene expression in the teratozoospermia group were detected lower compared to the controls. CatSper1, 4, HV1, and TMEM16A gen expression were higher in the oligoasthenoteratozoospermia men than the controls while CatSper3 gen expression was detected as lower.”
Conclusion:
It was detected that these ion channels have an effect on sperm progressive motility and morphology. It may be considered that mutations in these ion channels may result in infertility.
Key Words: Infertility, Male, Genes, Ion channels, Sperm
Introduction
The etiology of infertility remains still unclear by more than 50% of the couples wishing to achieve pregnancy (1). Elements, such as zinc, magnesium, copper, chlorine, potassium and calcium, are important for the maintenance of normal spermatogenesis, sperm maturation, DNA metabolism and repair and gene expression in germ cells (2). Spermatozoa mainly perform the transport of Na+, K+, Cl- and Ca+2 ions through voltage gated and ligand gated ion channels. Recent investigations in knockout mouse models have identified many ion channel genes involved in spermatogenesis, and their mechanisms of action are currently being clarified. However, some of these findings have been directly applicable to humans because phenotype in mice does not always have direct counterparts in humans (1, 3). After ejaculation of the sperm cells into the female reproductive tract, the sperm cells enter a different micromilieu where ion concentrations, pH and osmotic pressures are changed (4). By the adaptation period, the sperm cells get involved in processes like capacitation, acrosome reaction and fertilization, where normal function of Ca+2, K+, Cl-, Gamma-Amino Butyric Acid and ion channels is required (5).
The major ion channels which may be found in the sperm cell are Cation Channel Spermia (CatSper), Potassium Channel Subfamily U1 (KCNU1), Proton Voltage-Gated Ion Channel1 (Hv1), transmembrane protein (TMEM16). CatSper 1, 2, 3, 4 are weakly voltage dependent and pH sensitive ion channels. They are permeable for Ca2+ ions and demonstrate sperm specific features. It was demonstrated that the localization of the CatSper ion channels are at the main part of the sperm flagellum. These ion channels are necessary for progressive sperm motility, sperm decomposition in the female reproductive tract, oocyte penetration and fertilization (3). Human spermatozoa are immotile in the male reproductive system and get activated by entering the female reproductive tract. Alcalization of the sperm cytoplasm is necessary for this activation process. The Hv1 which is also located at the main part of the sperm's flagellum like CatSper plays a role by the induction of the intracellular alcalization and the activation of the spermatozoa.
The Hv1 also helps activation of CatSperion channels. SLO3 /KCNU1 is potassium voltage-gated ion channel and is also located in the main part of the sperm's flagellum. It was reported that SLO3 is sensitive to both pH and the voltage level, plays a role by sperm capacitation and/or acrosome reaction and a mutation in the gene coding this ion channel may result in defective sperm activation and fertility. SLO3 is shown to facilitate the potassium ions to move out of the sperm cell and to decrease the membrane voltage into more negative values. SLO3 has a vital function in the acquisition of normal morphology and sperm motility (1). TMEM16 is a calcium activated chloride channel and is located in the head part of the spermatozoa, it is thought to have a role by induced acrosome reaction (6).
In this study CatSper1, 2, 3, 4, KCNU1, Hv1, and TMEM16 ion channel expressions were studied in infertile men with oligoasthenospermia, isolated asthenozoospermia, teratozoospermia, and oligozoospermia in comparison with fertile individuals with normal sperm parameters.
Materials and methods
A total of 120 infertile men (19-55 yr) referred to the urology outpatient clinic between June 2014 and June 2015 were enrolled the study. And also 30 participants enrolled the study as control group Participants were divided into 5 groups (n=30/each) according to their semen analysis (2010 WHO criteria) (7): normozoospermia (control) oligozoospermia, asthenozoospermia, teratozoospermia (According to Kruger’s strict criteria normal morphology <4%), and oligoasthenoteratozoospermia groups.
All men with varicocele, reproductive pathology in family history, and abnormal genetic profile were excluded from the study. The detailed anamnesis of the patients were recorded and physical examination and laboratory findings were evaluated.The age, duration and type of the infertility, method of contraception and frequency of weekly sexual intercourse, history of smoking, mumps, orchitis, prostatitis, epididymitis, and using assisted reproductive techniques was recorded for all participants.
General physical examination and scrotal examination including epididymis, vas deferens, and plexus pampiniformis examination were performed by the same physican. Laboratory tests included blood follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL) levels, total testosterone (TT), and semen analysisFSH, LH, PRL,TT hormone parameters are measured with Advia Centaur XP ((Simens Healthcare Diagnostics, Germany) device with Siemens commercial kits by using chemo-luminescence method.
Patients with abnormal sperm parameters underwent a second semen analysis after a period of one month. Semen analysis was performed after 3 days of abstinence by the same andrology laboratory technician. Samples were scanned under 100X magnification with phase-contrast microscopy (Olympus CX41, USA) Number, motility and morphology of the sperms were studied under 200X magnification with the same microscope.
Methods used for ion channel assessment
Total RNA isolation
RNA isolation is completed according to Georgiadis et al (8). Semen samples were centrifuged by 5000 g at a temperature of 4oC. The supernatant part was removed and the pellet was suspended in 1ml 0.5% Triton-X solution. The samples were kept in ice for 10 min for the lysis of the somatic cells. Then, the hypotonic Triton X solution was removed by centrifugation for 5 min at 5000 g and 4oC and the pellet was washed with PBS. Then, they were resolved in 1 ml Trizol. The solution was aspirated and ejected through a 30 G needle for 3 times, vortexed for 30 sec and then incubated at room temperature (24oC) for 5 min. The homogenised samples were centrifuged at 4oC by 12.000 g for 10 min and the supernatant part was removed. Lastly, 200 µl chloroform was added. The samples were vortexed for 30 sec and incubated at room temperature for 5 min. The liquid phase was transferred to a new tube and equal amount of isopropanol, one-tenth amount of 3M ammonium acetate and 1 µl glycogen was added. The samples were incubated at room temperature for 30 min and then centrifuged by 20.000 g at 4oC for 30 min. The supernatant part was removed and the pellet was washed with 75% ethanol. The samples were centrifugated by 12.000 g at 4oC for 5 min, ethanol was removed and the pellet was dried at room temperature then resolved in 20 µl water and a temperature of 58oC was applied for 15 min. RNA Assay Kit For Use With The Qubit® 2.0 Fluorometer (Invitrogen/ Molecular Probes) was used. The RNA amount was measured in μg/ml.
Complementary DNA S ynthesis
High- Capacity cDNA Reverse Transcription Kit (P/N: 4387406, Applied Biosystem, USA) was applied for cDNA synthesis. Reverse transcriptase reactions including RNA specimens with purified total RNA, 1× RT buffer, 0.25 mM each of dNTPs, 1 U/µl MultiScribe reverse transcriptase and 0.25 U/µl RNase inhibitor. The specimens were put into thermal cycler and incubated at 25oC for 10 min, at 37oC for 120 min, at 85oC for 5 min and were preserved at 4ºC. The consisting cDNA specimens were kept at-20oC (8).
cDNA Amplif ication with Real Time Polymerase Chain Reaction (RT-PCR)
The cDNA fragments generated by reverse transcription were amplified with RT-PCR by the presence of sequence-specific primers. The RT-PCR was performed on repeated cycling of three steps. By the preparation of the RT-PCR plate, cDNA samples of 2 μl were put into the wells. For every sample on ice 5 μl TaqMan Master Mix, 2.5 μl nuclease-free water and 0.5 μl primer hybridisation probe and component amounts calculated according to the sample size were placed into the Eppendorf tubes and vortexed. Eight μl of the prepared mixture was put on the cDNA samples on the plate and the plate was covered with optic adhesive tape. The samples were centrifuged with miniplate spinner for one minute in order to reduce generated bubbles and to achieve precipitation.
The normalization of the RNA specimens based on the TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous controls. Real-time PCR was applied by using a standard TaqMan® PCR kit (P/N: 4370074, Applied Biosystem, USA) protocol on an Applied Biosystems 7500 Fast Thermal Cycler. The 10 µl PCR contained 1 µl RT product, 1× TaqMan® Universal PCR Master Mix, 0.5 µM TaqMan® gene specific assay mix (Applied Biosystems, Foster City, CA, USA). Gene expression levels were assesed by using Applied Biosystems 7500 Real-Time PCR system. GAPDH was applied as the control gene. The reactions were kept in a 96-well plate at 95oC for 10 min, followed by 40 cycles of 95oC for 15 sec and 60oC for 1 min. All reactions were repeated 3 times (8). Table I shows features and ID number of investigated genes..
Table I.
Properties and applied biosystem ID codes of ion channels
| Gene symbols | Localization | Number of exons | Number of aminoacids | Permeable ion | Assays IDs |
|---|---|---|---|---|---|
| CatSper1 | 11p13.1 | 12 | 780 | Ca++ | Hs00364950_m1 |
| CatSper2 | 15q15.3 | 14 | 528 | Ca++ | Hs00542505_m1 |
| CatSper3 | 5q31.1 | 8 | 398 | Ca++ | Hs00604374_m1 |
| CatSper4 | 1p36.11 | 10 | 472 | Ca++ | Hs01374398_m1 |
| Hv1/HVCN | 12q24.11 | 7 | 273 | H+ | Hs01032838_m1 |
| KCNU1/ SLO3 | 8p11.23 | 27 | 1149 | K+ | Hs00380426_m1 |
| TMEM16A/ ANO1 | 2q13.1 | 9 | 743 | K+ | Hs00216121_m1 |
| GAPDH | 8p11.23 | 27 | 1149 | K+ | Hs02758991_g1 |
CatSper: Cation channel, sperm associated
Hv1: Hydrogen voltage 1
HVCN: Hydrogen voltage gated channel
KCNU1/SLO3: Potassium channel subfamily U1
TMEM16A: Transmembrane protein 16A
ANO1: Anoctamin 1, calcium activated chloride channel
GADPH: Glyceraldehyde-3-phosphate dehydrogenase
Ca: Calcium
H: Hydrogen
K: potassium
Ethical consideration
Firat university local ethical committee approved the study protocol (30.10.2013/06/14). All participants signed the informed consent form.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package for Windows (version 22.0; IBM SPSS Inc., Chicago, IL, USA). SPSS package programme licensed by our institution (License Nr: 193.255.124.131). Post Hoc and student t-test were used to compare the groups according to demographic data, hormone levels, sperm motility and morphology. Correlation analysis was determined by using Pearson correlation analysis. The p˂0.05 was considered as statistically significant.
Results
The mean age of the 120 patients and 30 controls in the study was detected as 32.7±6.7 yr. Table II was given laboratory findings of the study groups.
Table II.
Laboratory findings of the study groups
| Control | Oligozoospermia | Asthenozoospermia | Teratozoospermia |
Oligoastheno-
teratozoospermia |
p-value | |
|---|---|---|---|---|---|---|
| Age (yr) | 31.6 ± 6.1 | 34.2 ± 7.0 | 33.2 ± 8.1 | 31.5 ± 4.9 | 34.8 ± 7.7 | P= 0.369 F= 1.082 |
| FSH (mIU/ml) | 3.4 ± 1.4 | 8.7 ± 5.5a | 5.0 ± 2.9 a ,b | 5.2 ± 2.5 a | 6.8 ± 3.1 a, c | P= 0.001 F= 5.342 |
| LH (mIU/ml) | 3.3 ± 1.1 | 5.6 ± 3.9 a | 4.5 ± 1.7 a | 4.6 ± 2.0 a | 5.1 ± 2.9 a | P= 0.128 F= 1.854 |
| PRL (ng/ml) | 8.3 ± 4.2 | 8.4 ± 2.4 | 7.8 ± 2.9 | 9.6 ± 4.9 | 8.1 ± 4.7 | P= 0.751 F= 0.478 |
| T.T. (ng/dl) | 428.6 ± 125.7 | 410.5 ± 236.4 | 402.7 ± 130.1 | 426.0 ± 141.4 | 369.6 ± 107.3 | P= 0.875 F= 0.303 |
| Sperm Count (106) | 63.0 ± 27.02 | 5.5 ± 3.3 | 54.5 ± 31.1 a, b | 41.0 ± 21.9 a, b,c | 9.5 ± 4.0 a, c,d | P <0.001 F= 28.764 |
| Motility (%) | 43.2 ± 7.1 | 43.9 ± 9.9 a | 21.2 ± 6.8 b | 41.0 ± 7.2 a, b, c | 15.6 ± 8.5 b, d | P<0.001 F= 54.91 |
| Kruger (%) | 4.4 ± 0.5 | 3.9 ± 0.6 a | 4.0 ± 0.7 a | 1.5 ± 0.5 a, b, c | 0.8 ± 0.8 a, b, c, d | P<0.001 F= 133.939 |
One Way ANOVA was used to compare the groups according to demographic data, hormone levels, sperm motility and morphology.
FSH: Follicle stimulating hormone
LH: Luteinizing hormone
PRL: Prolactin
TT: Total testosterone
: vs control group;
: vs oligozoospermic group;
: vs asthenozoospermic group;
: vs teratozoospermic group.
By comparison of the control with all of the abnormal sperm parameter groups, FSH, LH, levels and kruger, motilite findings was detected significantly differences (p=0.005, p=0.021, p<0.001 and p<0.001 respectively). By the comparison of the control with the oligo group, statistically significant difference is observed regarding to FSH, LH and Kruger parameters (p=0.042, p=0.023 and p=0.025, respectively). The comparison of asthenozoospermia group with the oligozoospermia group revealed a significant differentcence FSH AND sperm count (p<0.001 and p=0.021, respectively). Significanty difference has shown in terms of sperm count and morphology and by comparison of the asthenozoospermia group with the oligoasthenoteratozoospermia group (p<0.001 and p<0.001, respectively).
When comparing asthenozoospermia group with the teratozoospermia group it revealed significant difference kruger (p<0.001) a in terms of sperm count (p>0.05) and kruger was detected significant difference of the oligozoospermia group with the oligoasthenoteratozoospermia group (p=0.003 and p<0.001, respectively). By comparison of oligozoospermia with the teratozoospermia group it revealed a significant difference in FSH, sperm count and kruger (p<0.001, p=0.034 and p<0.001, respectively). A significant difference was found by comparison of oligoasthenoteratozoospermia group with the teratozoospermia group in terms of the sperm count and kruger (p<0.001 and p=0.003, respectively).
There was no correlation detected between age, sperm count, FSH, LH, TT, PRL, morphology and motility levels in the control and oligozoospermia groups. A positive correlation was found between age and FSH, PRL and LH in the asthenozoospermia group (p=0.06; r=0.609, p=0.012; r=0.564, respectively). By the oligoasthenoteratozoospermia group, a positive correlation between age and FSH, morphology and age, kruger and FSH was detected and a positive correlation (p=0.015; r=0.655, p=0.023; r=0.580, p=0.038; r=0.579, respectively) and LH ile testereron arasonda negative korelasyon gözlendi (p=0.047; r=-0.609) a negative correlation between morphology and TT, age and motility and positive sperm count and LH correlation was detected in teratozoospermia group (p=0.12; r=-0.626, pp=0.049; r=-0.376, p=0.05; r=0.514).
In our study, we have detected a decrease in the gene expression of CatSper1, CatSper2, CatSper3, CatSpe4, TMEM16A and KCnu1 gene expression by asthenozoospermia patients were detected as lower compared to the controls (p=0.022, p<0.001, p<0.001, p=0.007, p=0.016, p<0.001, respectively) and no significant difference in the other analyzed gene expression. CatSper1, CatSper3 gene expression by teratozoospermia patients were detected as lower compared to the controls (p<0.001, p<0.001). No significant difference in other gene expressions was detected in teratozoospermic group. By the oligozoospermia group an increase was observed in CatSper1, CatSper4, Hv1, SLO3 controls (p<0.001, p=0.003, p<0.001, p=0.016, respectively). whereas a decrease was found in TMEM16A gene expression. by oligoasthenoteratozoospermia patients were detected as higher (p<0.001 for all genes).
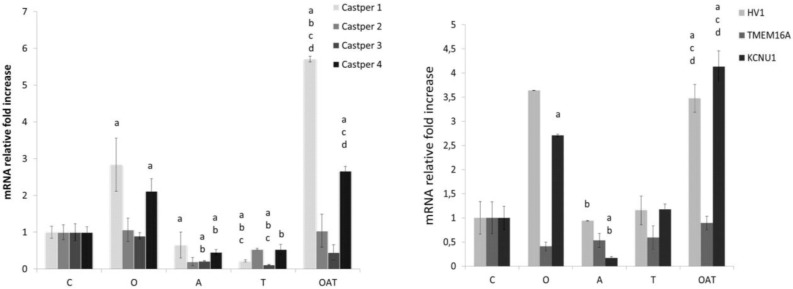
The oligoasthenoteratozoospermia group revealed an increase in CatSper1, Castper4, Hv1 and KCNu1 gen expressions higher (p<0.001 for all genes), no difference in gene expressions of CatSper2 and KCNU1 and a decrease in CatSper3 gene expression. Figure 1 shows mRNA relative fold increase graphic of CatSper 1, CatSper 2, CatSper 3, CatSper 4, Hv1, TMEM16A and SLO3 genes.
Figure 1a.
Quantitative real-time PCR validation of transcriptome data for selected ion channel genes. The mRNA fold increase graphic of Ca+2 permeable CatsPer1, 2, 3, and 4 genes. b. The mRNA fold increase graphic of SLO3 (KCNU1), TMEM16A, and HVv1. GAPDH was used as an internal control (see the Experimental section). Results are means±S.E.M. from three independent experiments. T-test was performed for statistical analysis (n=30 for each group); different superscript letters indicate significant differences (p<0.05), a: vs control group; b: vs oligozoospermic group; c: vs asthenozoospermic group; d: vs teratozoospermic group. (student’s test).
C: Control group
O: Oligozoospermic group
A: Asthenozoospermic group
T: Teratozoospermic group
OAT: Oligoasthenoteratozoospermic group.
Between sperm count and sperm progressive motility with CatSper1 gene expression were found negative correlation (p=0.00 p<0.001, p=0.041). We found positive correlation between CatSper3 gene expression with FSH level, sperm count, progressive motility and Kruger (p=0.040, p=0.002, p=0.001, p<0.001). Between sperm count, progressive motility and TMEM16a expression was detected negative correlation (p=0.026, p=0.00 p<0.001, respectively). Between KCNU1 gene expression and sperm count was detected negative correlation (p=0.023). The other correlations between gene expressions with sperm count, progressive motility and morphology are shown in table III.
Table III.
Correlations between gene expressions and hormones and sperm properties
| FSH | LH | Prolactin | Testosterone | Sperm count (×10 6 /ml) | Progressive motility (%) | Kruger | |
|---|---|---|---|---|---|---|---|
| Casper 1 | 0.184 p= 0.112 |
0.092 p= 0.437 |
-0.065 p= 0.585 |
-0.099 p= 0.400 |
-0.366 p<0.001 |
-0.188 p= 0.041 |
0.046 p= 0.0625 |
| Casper 2 | -0.086 p= 0.462 |
0.027 p= 0.819 |
0.169 p= 0.150 |
0.077 p= 516 |
0.05 P= 0.57 |
-0.015 p= 0.871 |
0.061 p= 0.511 |
| Casper 3 | -0.236 p= 0.040 |
-0.06 p= 0.959 |
0.041 p= 0.730 |
0.178 p= 0.129 |
0.272 p= 0.002 |
0.299 p= 0.001 |
0.357 p<0.001 |
| Casper 4 | 0.201 p= 0.081 |
0.081 p= 0.494 |
-0.075 p= 0.525 |
0.000 p= 0.998 |
-0.300 p= 0.001 |
-0.017 p= 0.857 |
0.076 p= 0.0412 |
| Hv1 | 0.101 p= 0.384 |
-0.46 p= 0.698 |
0.023 p= 0.845 |
0.075 p= 0.523 |
-0.071 p= 0.432 |
0,.092 p= 0.318 |
0.112 p= 0.229 |
| TMEM16A | 0.06 p= 0.609 |
0.030 p= 0.801 |
-0.054 p= 0.650 |
-0.104 p= 0.377 |
-0.198 p= 0.026 |
-0.365 p<0.001 |
0.077 p= 407 |
| KCNU1 | 0.056 p= 0.631 |
0.151 p= 0.198 |
0.143 p= 0.226 |
0.015 p= 0.896 |
-0.202 p= 0.023 |
0.139 p= 0.131 |
-0.118 p= 0.204 |
Correlation analysis was determined by using Pearson correlation analysis. The value p˂0.05 was considered as statistically significant.
CatSper: Cation channel, sperm associated
Hv1: Hydrogen voltage 1
TMEM16A: Transmembrane protein 16A
KCNU1: Potassium channel, subfamily U, member 1
FSH: follicle stimulating hormone
LH: luteinizing hormone
Discussion
We have detected statistically significant differences in the ion channel gene expressions by impaired progressive motility and morphology patients compared to the control.The incidence of male factor is reported as 30% by the etiology of infertility (9). Even by normal sperm parameters the etiology of infertility remains still unclear by more than 50% of the couples wishing to achieve pregnancy and this phenomenon is called idiopathic infertility (1). Ion channels are one of the new research topics and are considered to have an important role in the etiology of idiopathic infertility (10). It has been shown that loss of function in the ion channels may impair sperm physiology by causing abnormal capacitation, loss of hyperactivation and impaired acrosome reaction (1). Even by the presence of complete normal sperm parameters, fertilization may fail. For this reason, it is of big importance to understand the ion channel functions on the sperm cell because it may lead to develop new treatment strategies for idiopathic infertility.
CatSper is a sperm-specific, weak voltage-dependent calcium selective channel and is pH sensitive. It acts by the control of the positive loaded calcium ions into the sperm cell and is necessary for sperm hyperactivation and fertilization. It was shown that CatSper ion channels are located on the main part of flagellum by rat and human. CatSper is a heterotetrameric calcium channel and is consisted of 4 different pore forming alfa subunits; CatSper 1, 2, 3, 4, and 3 auxiliary subunits called CatSper β (beta), CatSper γ (gamma) and CatSper δ (delta) (3). Ren and his colleagues have shown the necessity of CatSper presence for normal sperm motility and oocyte penetration. In studies consisting of CatSper-/-transgenic and normal rats it was detected that CatSper-/-sperm revealed weak vitality, decreased motility and curling in the tail part. Studies performing computer-assisted sperm analysis have shown impairment in the forward progressive motility and decrease in swimming speed by CatSper-/-sperm (11).
In another study, fertilisation capacity of CatSper-/- was evaluated in-vitro and it was shown that CatSper-/-sperm were not able to fertilize the oocyte while normal sperm cells revealed a fertilisation rate of 81%. It is reported that some CatSper-/-sperm were able to adhere but not penetrate to the oocyte and the study has also shown that fertilisation occurs in oocytes with enzymatically removed zona pellucida by both CatSper+/+ and CatSper-/-rat sperm which may confirm strongly that CatSper is necessary for penetration (1). In a study of Tamburrino et al, it was reported that CatSper1 gene expression was decreased in spermatozoa from asthenospermic patients. The study also showed a decrease in progressive motility and acrosome reaction (12).
Another study revealed that CatSper1-/-and CatSper2-/-spermia are not able for hyperactivation during capacitation process (3). Avenarius and colleagues have found a correlation between CatSper1 and CatSper2 mutation and asthenoteratospermia (14). In a study of Bhilawadikar and colleagues, it was shown that CatSper2 expression was decreased by asthenozoospermic patients (15). Hildebrand and colleagues have detected that by the absence of CatSper2 expression sperm malformation was present by 88% while short sperm head and curling flagellum was present by 30% which they were correlated with the decrease in sperm motility (16).
Jin and colleagues have stated that CatSper3-/- and CatSper4-/-male rats were infertile even they were showing normal mating attitude. In their study, they have shown that CatSper3-/- and CatSper4-/-rats were infertile despite their normal sperm count and motility. The same study has reported that hyperactivation during capacitation was observed 30 min after incubation at room temperature by normal spermatozoa but first motility was detected at 2 hr after incubation by CatSper3-/- ve CatSper4-/- sperm cell. This result supported that functional CatSper3 and CatSper4 may be necessary for hyperactivation of sperm during capacitation (17). By scanning of current literature only one study about CatSper ion channels in human was found. This study reported a correlation between CatSper1 mutation and idiopathic asthenospermia (10).
In our study we have reported a decrease in CatSper1 and CatSper2 gene expression by asthenozoospermia and teratozoospermia groups. The gene expression of CatSper1 revealed an increase by oligozoospermia and oligoasthenoteratozoospermia groups where as CatSper2 gene expression revealed no significant change by oligozoospermia and oligoasthenoteratozoospermia groups. The study also revealed that presence of CatSper1 and CatSper2 gene expression may be positively correlated with hyperactive motility and morphology. CatSper3 gene expression has shown a decrease in the asthenozoospermia, teratozoospermia and oligoasthenoteratozoospermia groups compared to the control but no significant change in the oligozoospermia group. CatSper3 ion channel was also positively associated with hyperactive motility and morphology. In our study we detected a decrease of CatSper4 gene expression in asthenozoospermia and teratozoospermia group and an increase in the oligozoospermia and oligoasthenoteratozoospermia groups. Our study also revealed a relation between CatSper4 and hyperactive motility and morphology in a positive way. In the light of all these data revealed in our study we consider that CatSper ion channels may play an important role in the sperm physiology.
In this study, we have detected a significant increase of Hv1 gene expression in the oligozoospermia and oligoasthenozoospermia groups, an insignificant decrease in the asthenozoospermia group and no significant change in the teratozoospermia group compared to the control. As we have mentioned in the introduction part, human spermatozoa are activated by alcalization of the sperm cytoplasm and the Hv1 gene is responsible for the induction of the intracellular alcalization by extracellular proton transfer and the activation of the spermatozoa. Hv1 also activates the CatSper ion channels and plays a role by sperm hyperactivity. Since Hv1 gene may not be found by rats, there is no sufficient data about detailed function of this gene but according to the known mechanisms regarding the function, we can predict that a potential Hv1 ion channel mutation may result in infertility in human (18).
SLO3/KCNU1 is a potassium voltage-gated ion channel and is specific to the testicular tissue regarding some studies made by rats. SLO3/KCNU1 is localized on the main part of the sperm’s flagellum (1). KSper/SLO3 is considered as responsible for the potassium transfer and pH depended potassium conductivity in rat sperm cell. A physiologic ionic gradients of sperm with normal type KSP is SLO3 is hyperpolarized, while SLO3/KCNU1-/-sperm is depolarized (19). Santi and colleagues have stated that SLO3 can only be found by mammals and is specific for testis. In this study, they used SLO3/ KCNU1 knock-out rats and showed a deficit in pH sensitive potassium flow by testicular sperm cell where no fertilization was observed after mating of these mutant homozygote male rats with healthy female rats. Studies revealed that SLO3/KCNQ1 ion channels are the main responsible ion channels for hyperpolarization during capacitation and no other ion channel may substitute them. It has also shown that mutant sperm cells are depolarized after capacitation. SLO3/KCNU1 mutant sperm cells may reach an acrosome reaction after termination of hyperpolarization but could not achieve the zona pellucida penetration. The Osmotic milieu is also an important issue for the sperm flow towards the oocyte. Potassium and SLO3/KCNU1 ion channels play a role in this osmotic volume regulation. By osmotic volume dysregulation, the sperm cell swells and morphological changes occur. These changes may prevent the sperm cell to adhere to the oocyte and result in infertility (20).
In our study, we detected an increase of SLO3/KCNU1 gene expression in the oligozoospermia and oligoasthenoteratozoospermia groups and a decrease in the asthenozoospermia group compared with the control. No significant change was observed in the teratozoospermia group regarding the SLO3/KCNU1 gene expression. SLO3/KCNU1 gene expression decreased in the oligozoospermia, asthenozoospermia and teratozoospermia groups compared to the control but no significant change was observed in the oligoasthenoteratozoospermia group. Regarding these data, we can say that SLO3/KCNQ1 gene expression may be specifically effective by progressive motility of the sperm cell. TMEM16A is a calcium-activated chloride channel located in the head part of the human sperm cell. The role of TMEM16A in the sperm capacitation and zona pellucida induced acrosome reaction was shown in former studies (21). Zanetti and Mayorga stated that TMEM16A is responsible for regulation of cell volume and plays an important role by reducing the distance between the outer acrosomal membrane and plasma membrane and facilitating the acrosome exocytosis (22). In our study, we have observed an increase of TMEM16A gene expression in oligozoospermia and oligoasthenoteratozoospermia groups and a decrease in the asthenozoospermia and teratozoospermia groups compared to the control.
Conclusion
According to the results we have achieved in this study, we assume that a decrease in the function of the ion channels may be related to male infertility especially by asthenozoospermic and teratozoospermic patients. There is a need for detailed evaluation in future studies with larger numbers to investigate this mentioned relation by oligozoospermia and oligoasthenoteratozoospermia patients. We believe that complete clarification of the ion channel functions and developing activator/inhibitor molecules targeting these channels could bring therapeutic options for patients with idiopathic infertility into existence. Also, these activator/inhibitor molecules could affect solely on sperm cells and may be the precursors of drugs providing male contraception.
Acknowledgments
This project was supported by grant no TF.14.33 from the Firat University Scientific Research Projects Unit (FUBAP), Elazig, Turkey.
Conflict of interest
None of the authors have declared conflict of interest.
References
- 1.Shukla KK, Mahdi AA, Rajender S. Ion channels in sperm physiology and male fertility and infertility. J Androl. 2012;33:777–788. doi: 10.2164/jandrol.111.015552. [DOI] [PubMed] [Google Scholar]
- 2.Yuyan L, Junqing W, Wei Y, Weijin Z, Ersheng G. Are serum zinc and copper levels related to semen quality? Fertil Steril. 2008;89:1008–1011. doi: 10.1016/j.fertnstert.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Singh AP, Rajender S. CatSper channel, sperm function and male fertility. Reprod Biomed Online. 2015;30:28–38. doi: 10.1016/j.rbmo.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Giojalas LC, Rovasio RA. Mouse spermatozoa modify their motility parameters and chemotactic response to factors from the oocyte microenvironment. Int J Androl. 1998;21:201–206. [PubMed] [Google Scholar]
- 5.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE. The control of male fertility by spermatozoan ion channels. Ann Rev Physiol. 2012;74:453–475. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santi CM, Orta G, Salkoff L, Visconti PE, Darszon A, Treviño CL. K+ and Cl-Channels and Transporters in Sperm Function. Curr Top Dev Biol. 2013;102:385–421. doi: 10.1016/B978-0-12-416024-8.00014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization, Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen. 5th Ed. Switzerland: World Health Organization; 2010. [Google Scholar]
- 8.Georgiadis AP, Kishore A, Zorrilla M, Jaffe TM, Sanfilippo JS, Volk E, et al. High quality RNA in semen and sperm: isolation, analysis and potential application in clinical testing. J Urol. 2015;193:352–359. doi: 10.1016/j.juro.2014.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perdrix A, Travers A, Chelli MH, Escalier D, Do Rego JL, Milazzo JP, et al. Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod. 2011;26:47–58. doi: 10.1093/humrep/deq297. [DOI] [PubMed] [Google Scholar]
- 10.Shu F, Zhou X, Li F, Lu D, Lei B, Li Q, et al. Analysis of the correlation of CATSPER single nucleotide polymorphisms (SNPs) with idiopathic asthenospermia. J Assist Reprod Genet. 2015;32:1643–1649. doi: 10.1007/s10815-015-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, et al. A Sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamburrino L, Marchiani S, Minetti F, Forti G, Muratori M, Baldi E. The CatSper calcium channel in human sperm: relation with motility and involvement in progesterone-induced acrozome reaction. Hum Reprod. 2014;29:418–428. doi: 10.1093/humrep/det454. [DOI] [PubMed] [Google Scholar]
- 13.Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca+2 to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol. 2007;303:214–221. doi: 10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, Kahrizi K, et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet. 2009;84:505–510. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhilawadikar R, Zaveri K, Mukadam L, Naik S, Kamble K, Modi D, et al. Levels of Tektin 2 and CatSper 2 in normozoospermic and oligoasthenozoospermic men and its association with motility, fertilization rate, embryo quality and pregnancy rate. J Assist Reprod Genet. 2013;30:513–523. doi: 10.1007/s10815-013-9972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, Auer J, et al. Genetic male infertility and mutation of CatSper ion channels. Eur J Hum Genet. 2010;18:1178–1184. doi: 10.1038/ejhg.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J, Jin N, Zheng H, Ro S, Tafolla D, Sanders KM, et al. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod. 2007;77:37–44. doi: 10.1095/biolreprod.107.060186. [DOI] [PubMed] [Google Scholar]
- 18.Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;588:4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalizationactivated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA. 2011;108:5879–5884. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santi CM, Martinez-Lopez P, de la Vega-Beltran JL, Butler A, Alisio A, Darszon A, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orta G, Ferreira G, Jose O, Trevino CL, Beltrán C, Darszon A. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrozomal reaction. J Physiol. 2012;590:2659–2675. doi: 10.1113/jphysiol.2011.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanetti N, Mayorga LS. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod. 2009;81:396–405. doi: 10.1095/biolreprod.109.076166. [DOI] [PubMed] [Google Scholar]