Abstract
Background:
Spermatogonial stem cells (SSCs) are undifferentiated cells which are highly reproducible and expandable. Several studies have been conducted to reproduce these cells in culture. They used growth factors, hormones and different feeder cells to improve survival and proliferation of SSCs.
Objective:
This study was conducted to evaluate the effects of follicular stimulating hormone (FSH) on gene expression of fibroblast growth factor (FGF2) and glial cell-derived neurotrophic factor (GDNF) in Sertoli cells.
Materials and Methods:
Sertoli cells and SSCs were isolated from 3-5 month-old calves. Bovine testicular cells were cultured for 15 days with or without FSH. Identification of these cells was confirmed by immunocytochemistry analysis. Colony formation of SSCs was evaluated using an inverted microscope. The gene expression of FGF2 and GDNF and the gene markers bcl6b, thy-1, and C-kit were evaluated using the quantitative RT-PCR technique.
Results:
The results indicated that FSH increased colonization of SSCs. the expression of GDNF, FGF2, and markers of undifferentiated spermatogonia was increased following culture in control and FSH groups (p<0.05), this increase was more in FSH group. Conversely, the expression of C-kit was decreased in both groups (p<0.05).
Conclusion:
The results showed that FSH can increase the self-renewal of SSCs in vitro via upregulation of GDNF and FGF2 expression in Sertoli cells.
Key Words: Sertoli cell, SSCs, FSH, Self-renewal
Introduction
Spermatogenesis is a complex developmental process of cell proliferation and differentiation that originates from spermatogonial stem cells (SSCs) and produce mature spermatozoa (1). SSCs have two main features including self-renewing and differentiation properties, that maintains spermatogenesis (1). In this context thy1 (1-3) and bcl6b (3-5) are known undifferentiated markers of spermatogonia, whereas C-kit (6) considered as an SSCs differentiation marker. The balance between SSCs self-renewal and differentiation requires a specific microenvironment, called niche (1). In mammalian testes, Sertoli cells, basal membrane and, interstitial cells are the main components of SSCs niche (7). Sertoli cells generate growth factors required for self-renewing of SSCs such as glial cell derived neurotrophic factor (GDNF), Basic fibroblast growth factor (bFGF) and Kit (8-10). GDNF is a critical factor for self-generation of SSCs. GDNF is a protein secreted after generation of Sertoli cells and is responsible for maintaining and proliferating SSCs in both body and culture (9). Fibroblast growth factor 2 (FGF2) is secreted and expressed by Sertoli cells, Leydig cells and differentiated germ cells and stimulates self-renewing of SSCs (11). SSCs are undifferentiated cells which are highly reproducible and expandable. Biotechnologically, these cells are particularly important, because they are the only adult stem cells which can transfer genetic information to the next generation (12). To study characteristics and function of SSCs, it is essential to achieve a sufficient number of them (13). For this purpose, several studies have been conducted successfully to proliferation these cells in vitro. They have used growth factors, hormones and different feeder cells to improve survival, proliferation and occasionally differentiation of SSCs (14-17). Hormonal control of spermatogenesis is through follicle stimulating hormone (FSH) and testosterone activity on Sertoli cells. FSH is an essential prerequisite for maintaining spermatogenesis in adult mammals (18, 19). FSH stimulates GDNF production by the Sertoli cells and consequently increases the SSCs self-renewal (20). This study was conducted to evaluate the effect of FSH on the expression of the growth factors FGF2 and GDNF in Sertoli cells.
Materials and methods
This experimental study was performed at Stem Cell Research Center, Faculty of Veterinary Medicine, University of Tehran between November 2015 and July 2016.
Animals and testicular biopsy
Testicular biopsies were obtained from 8 Holstein calves (4 calves were used for evaluation of colonization and 4 calves for gene expression) aged between 3 to 5 months, as previously described (21). Following testicular biopsy, samples taken from testicle were placed on ice and transferred to the laboratory within 2 hr, before they could be used for experiments.
Cell Isolation
Cell isolation was implemented using a two-step enzymatic isolation procedure as previously used by our lab (22). Briefly, the obtained testis tissue was washed three times in DMEM containing antibiotics and Fetal Bovine Serum (FBS) 10% (Sigma, USA). They were then minced into small pieces as much as possible by a sterile scissor. The minced testicular tissue was suspended in DMEM containing 1 mg/ml collagenase (Sigma-Aldrich, USA), 1 mg/ml hyaluronidase (Sigma-Aldrich, USA), 1 mg/ml trypsin (Sigma-Aldrich, USA) and 5 µg/ml DNase (Fermentas, Germany) at 37°C in a shaker incubator operated at 80 cycles per minute for approximately 60 min. After three times washing in DMEM and removal the interstitial cells, during the second step of enzymatic digestion, the seminiferous tubules were again incubated at 37°C in DMEM containing 1 mg/ml collagenase, 1 mg/ml hyaluronidase and 5 µg/ml DNase. In this step, seminiferous tubules were deconstructed and their cells were separated. Finally, obtained cellular suspension was centrifuged at 30×g for 2 min to achieve population individual cells. Following filtration through 77 and 55 mm nylon filters, the cells were pelleted. The pellet was re-suspended in DMEM containing antibiotics and 10% fetal bovine serum (FBS).
Cell culture
Cell culture for colonization and gene expression were conducted as the procedure which was previously used by our lab (23). To assess colonization and gene expression 24-well and 6-well plates (TPP, Switzerland) were used, respectively. To evaluate the gene expression and colonization, cells were seeded at the concentrations of 10*105 and 3*104 per well contacting DMEM, respectively.
The plates were incubated at 37oC in the presence of 5% CO2 for 15 days. DMEM (Sigma-Aldrich, St. Louis, MO, USA) containing 10% FBS, 4 mM L-glutamine, 0.1 mM non-essential amino acids, 100 IU/mL penicillin and 100 mg/mL streptomycin was used for culturing cells. The cells were cultured in two groups including group1 (Control) and group2 (FSH 30 IU/ml). Culture medium together with the FSH was refreshed every 3 days.
Cells identification
Vimentin is a cytoskeleton protein in Sertoli cell cytoplasm. At day 6 of culture, for Sertoli cells identification, Vimentin was stained, as described by Anway et al and Tajik et al (24, 25) and the specific marker Oct-4 was assessed in colonies of SSCs by the method proposed by Kubota et al (9).
Colony assay
Four cell populations from different calves were used for evaluation of colony formation. The colonization of SSCs in the control and FSH groups was assessed using an inverted microscope (IX71, Olympus, Japan).
Gene expression assessment (qRT-PCR)
Expression of the considered genes was assessed in the days 0, 6 and 12. Following trypsinization of the cultured cells (n=4 cell population from different calves), total RNA existing in the cells was extracted using Trizol reagent (Fermentas, Germany). In order to eliminate contamination of DNA, the extracted RNA was treated by DNase І (Fermentas, Germany). The concentration of the extracted RNA was determined by using spectrophotometry (Eppendorff, Germany). cDNAs were built by using 500ng RNA extracted and oligo-primers and cDNA synthesis kit (Fermentas, Germany).
Table I lists the primers of the considered genes. qRT-PCR was done by using SYBR Green mastermix (Fermentas, Germany) and by thermocycler (Applied Biosystems, USA). qRT-PCR started with a primary melting stage for 5 min at 95°C to activate polymerase and continued with 40 cycles including melting (30 s at 95°C), synthesis (30 s at 58°C) and formation (30 s at 72°C). Quality of qRT-PCR reactions was determined by melting curve analysis.
Table I.
Antibodies introduction for SSC
| AB | Name | Concentration | Company |
|---|---|---|---|
| Primary AB | Rabbit anti Oct 4 | 1/100 | Abcam |
| Secondary AB | Goat anti rabbit IGg | 1/100 | Abcam |
For each sample, qRT-PCR was done for reference gene (β-actin) and target gene simultaneously. Cycle threshold (Ct) of the reference gene was subtracted from cycle threshold of the target gene to obtain ΔCt. In each nteraction interaction, Ct on time point 0 was considered as calibrator. Consequently, the relative gene expression was obtained by using Livak and Schmittgen (26) and calculation of ΔΔCt.
Ethical consideration
The research was conducted in accordance with guidelines of the Animal Ethics Committee at the University of Tehran.
Statistical analysis
All data were analyzed using Statistical Package for the Social Sciences, version 24.0, SPSS Inc, Chicago, Illinois, USA (SPSS). Data related to colonization and gene expression was assessed by using paired-samples t-test.
Table II.
Antibodies introduction for Sertoli cells
| AB | Name | Concentration | Company |
|---|---|---|---|
| Primary AB | Mouse anti vimentin | 1/200 | Abcam |
| Secondary AB | Sheep anti-mouse IGg | 1/100 | Abcam |
Table III.
Sequence of the primers used for qRT-PCR
| Gene | Forward primer (3′-5′) | Reverse primer (5′-3′) |
|---|---|---|
| B-ACTIN | TCG CCC GAG TCC ACA CAG | ACC TCA ACC CGC TCC CAA G |
| FGF2 | AAA ACA GGA CCT GGG CAG AA | ATA TAC CTC TTC ATG TAA AAT GAG ATC AGA TG |
| GDNF | GCAGCC GAA ACA ATG TAC GA | AAG GCG ATG GGT CTG CAA |
| THY-1 | TTC ATC TCC TTG TGA CGG GTT | GCA GAG GTG AGG GAA TGG C |
| c-KIT | TAC CAA CCA AGG CAG ACA A | CTT TGA GGC AAG GAA CGC |
| BCL6B | AGG GCA CAG GGA ACT CTT TTC | CCT CCT TTG GCT TGA GTG TTTT |
Results
Immunocytochemical staining of Sertoli cells and SSCs
The expression of Vimentin markers detected in the Sertoli cells (Figure 1) and OCT4 was detected in the colonies of SSCs. (Figure 2).
Figure 1.
Immunocytochemical staining of the bovine Sertoli cell for Vimentin at day 6 of culture. 7ADD staining is used to demonstrate the nuclei of Sertoli cells (magnification×400).
Figure 2.
Immunocytochemical staining of bovine SSCs for Oct-4 at day 6 of culture. DAPI is the nuclear staining of SSCs (magnification ×400).
Numbers of Colonies
The result of this study showed that the number of colonies in two groups increased until the 12th day of co-culture and after 12 days they decreased. Spermatogonial colonies started to appear around day 3 and increased their number through time. Colonies were counted at days 3, 6, 9, 12 and 15. The number of colonies in the FSH group was significantly higher than that of the control group, during in cell culture (p<0.05).
Gene Expression
Expression of FGF2 significantly increased in both group on day 6 and 12 compared to day 0 (p<0.001). Expression of FGF2 was not different in group 2 on day 6 and 12 (p>0.05), while it increased significantly in group 1 during culture (p=0.001). Expression of FGF2 in the FSH group was significantly higher than that of the control group on days 6 and 12 (p<0.05).
Expression of GDNF significantly increased in both groups on days 6 and 12 compared to day 0 (p<0.05); However, it was not different on day 12 compared to day 6 in both groups (p=0.136). On day 6, expression of GDNF in group 2 was higher than group 1 (p=0.001).
Expression of thy1 significantly increased in group 1 on day 6 (p=0.027) and 12 (p<0.0001) compared to day 0 (p<0.05). Expression of thy1 significantly increased on day 12 compared to day 6 in grou1 (p=0.005). Expression of thy1 significantly increased in group 2 on day 6 (p=0.001) and 12 (p<0.0001) compared to day 0 (p<0.01). Moreover, a significant difference was found on day 12 compared to day 6 (p<0.0001). On days 6 and 12, expression of thy1 was higher in group 2 compared to group 1 (p<0.05).
In group 1, expression of C-kit significantly decreased on days 6 and 12 compared to that on day 0 (p<0.05); while no significant difference was found between days 6 and 12 in group 1 (p=0.396). In group 2, expression of C-kit significantly decreased on day 6 (p=0.008) and 12 (p=0.012) compared to day 0 (p<0.05); while no significant difference was found between day 6 and 12 (p=0.365).
In group1, expression of bcl6b significantly increased on day 6 (p=0.004) and 12 (p<0.0001) compared to day 0 (p<0.05). In group 2, expression of bcl6b on day 6 (p=0.005) and 12 (p=0.001) was higher than that on day 0 (p<0.05). However in both groups 1 and 2 no significant difference was found on days 6 and 12 in expression of bcl6b (p>0.05).
Table IV.
Comparison of colony numbers (mean±SD) between control and experimental groups
| Group | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 |
|---|---|---|---|---|---|
| Control | 13.75 ± 3.304034 | 22.00 ± 4.546061 | 23.75 ± 2.217356 | 29.00 ± 1.154707 | 21.00 ± 2.160247 |
| FSH (30 iu/ml) | 28.00 ± 5.830952 | 67.00 ± 4.546061 | 41.00 ± 9.092121 | 86.00 ± 10.70825 | 59.00 ± 4.690416 |
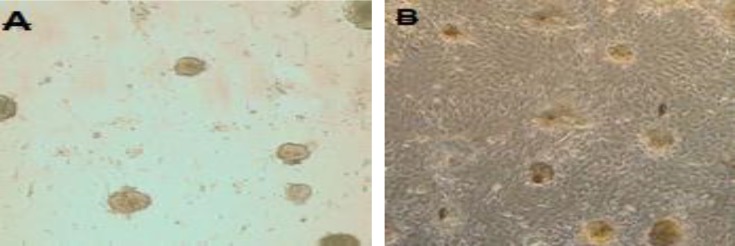
Figure 3.
Colonization of SSCs on day 12 in control (A) and FSH (B) groups (magnification×100).
Figure 4.
Relative gene expression of FGF2 in control and FSH groups on days 0, 6 and 12. Small letter: indicate difference within control group. Capital letter: indicate difference within FSH group. Different letter [(a.b.c) (A, B)] indicates significant difference within groups in different time-points (p<0.05). * Indicate a significant difference between two experimental groups at the determinate time-points (p<0.05).
Figure 5.
Relative gene expression of GDNF in control and FSH groups on days 0, 6 and 12. Small letter: indicate difference within control group. Capital letter: indicate difference within FSH group. Different letter [(a.b.c) (A, B)] indicates significant difference within groups in different time-points (p<0.05). * indicate a significant difference between two experimental groups at the determinate time-points (p<0.05).
Figure 6.
Relative gene expression of thy1 in control and FSH groups on days 0, 6 and 12. Small letter: indicate difference within control group. Capital letter: indicate difference within FSH group. Different letter [(a.b.c) (A, B, C)] indicates significant difference within groups in different time-points (p<0.05). * indicate a significant difference between two experimental groups at the determinate time-points (p<0.05).
Figure 7.
Relative gene expression of C-kit in control and FSH groups on days 0, 6 and 12. Small letter: indicate difference within control group. Capital letter: indicate difference within FSH group. Different letter [(a. b) (A, B)] indicates significant difference within groups in different time-points (p<0.05).
Figure 8.
Relative gene expression of bcl6b in control and FSH groups on days 0, 6 and 12. Small letter: indicate difference within control group. Capital letter: indicate difference within FSH group. Different letter [(a.b) (A,B)] indicates significant difference within groups in different time-points (p<0.05).
Discussion
It seems that FSH stimulates proliferation of Sertoli cells both in vitro and in vivo (27). This hormone is the most important mitogenic factor of Sertoli cells (28). Sertoli cells are stimulated by FSH to secrete growth factors, which stimulate proliferation and colonization of type A SSCs (29). In this study, adding 30 IU/mL FSH to the culture medium has a positive effect on SSC numbers. The number of colonies in FSH treatment group was higher than the control group. This finding is consistent with Anjamrouz et al (10). Hence, proliferative SSCs could colonize and preferred proliferation pathway. This pathway could be activated by growth factors produced by Sertoli cells. Vimentin is a cytoskeleton protein in Sertoli cell cytoplasm. For Sertoli cells identification, Vimentin was stained. This finding is similar to the previous studies by Anway et al and Tajik et al (24, 25). For confirmation of the presence of SSCs, the specific marker Oct-4 was assessed in colonies of SSCs by the method proposed by Kubota et al (9). Thy1 is known as SSCs marker in a wide range of mammals (2, 3, 30-32) and as the best marker for enrichment of SSCs (1).
Moreover, expression of bcl6b (3-5) has been reported in rich populations of SSCs. Increase in thy1 gene expression in response to ordinary culture is consistent with the study performed by Nasiri et al (33). On the other hand, C-kit is known as differentiated spermatogonial marker; undifferentiated SSCs are negative in terms of C-kit expression (6). GDNF expression increased in response to ordinary culture and removal of SSCs. Sharp increase in GDNF expression stimulates self-renewal and inhibits differentiation of SSCs (34). On the other hand, undifferentiated SSCs gradually disappear in mice with deficient GDNF gene expression and only Sertoli cells remain in seminiferous tubules of these mice (3).
In addition, assessment of GDNF expression during different stages of spermatogenesis has shown that GDNF plays a basic role in proliferation and differentiation of SSCs (35). Studies have shown that addition of GDNF in culture leads to proliferation of SSCs (16). Hence, self-renewal of SSCs in ordinary culture can increase expression of GDNF, as previously reported by He et al (36). Similar to GDNF, FGF2 stimulated SSCs self-renewal in vitro (9) as well as in vivo (37). Synergistic relationship between FGF2 and GDNF through upregulation of ETS variant (10) (Etv5), increase expression of receptor tyrosine kinas Ret, mediating GDNF signals (37). Therefore, it seems that this effect of FSH for SSCs proliferation could be through upregulation of FGF2 and GDNF in Sertoli cells.
Conclusion
In conclusion, the present study showed that follicular stimulating hormone (FSH) increases the self-renewal of SSCs and this effect probably mediated through upregulation of GDNF and FGF2 expression in Sertoli cells.
Acknowledgments
This study was partly supported by Center of Excellence in Application of Stem Cells in Cell Therapy and Tissue Engineering, Faculty of Veterinary Medicine, University of Tehran. We would also thank Dr A. Ghalyanchi, Dr G. JavedaniShahedin, and Dr S. Farzad.
Conflict of interest
The authors declare that there was no conflict of interests regarding the publication of this article.
References
- 1.Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Nati Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reding SC, Stepnoski AL, Cloninger EW, Oatley JM. THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction. 2010;139:893–903. doi: 10.1530/REP-09-0513. [DOI] [PubMed] [Google Scholar]
- 4.Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Nati Acad Sci USA. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type a spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 7.Shetty G, Meistrich , ML The missing niche for spermatogonial stem cells: do blood vessels point the way? Cell Stem Cell. 2007;1:361–363. doi: 10.1016/j.stem.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Lindahl M, Hyvanen , ME , Parvinen , M , de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 9.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Nati Acad Sci USA. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner MK. Growth factors in gonadal development. J Anim Sci. 1992;70 (Suppl.):30–41. [Google Scholar]
- 12.Hill JR, Dobrinski I. Male germ cell transplantation in livestock. Reprod Fertil Dev. 2005;18:13–18. doi: 10.1071/rd05123. [DOI] [PubMed] [Google Scholar]
- 13.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 14.Anjamrooz SH, Movahedin M, Tiraihi T, Mowla SJ. In vitro effects of epidermal growth factor, follicle stimulating hormone and testosterone on mouse spermatogonial cell colony formation. Reprod Fertil Dev. 2006;18:709–720. doi: 10.1071/rd05126. [DOI] [PubMed] [Google Scholar]
- 15.Aponte PM, Soda T, Teerds KJ, Mizrak SC, Van de Kant HJ, De Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–557. doi: 10.1530/REP-07-0419. [DOI] [PubMed] [Google Scholar]
- 16.Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- 17.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 18.Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD. Identification of testosterone-regulated genes in testes of hypogonadal mice using oligonucleotide microarray. Mol Endocrinol. 2004;18:422–433. doi: 10.1210/me.2003-0188. [DOI] [PubMed] [Google Scholar]
- 19.Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, et al. Regulation of sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 20.Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the gdnf/fsh pathway. Mech Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 21.Akbarinejad V, Tajik P, Movahedin M, Youssefi R. Effect of removal of spermatogonial stem cells (SSCs) from in vitro culture on gene expression of niche factors in bovine. Avicenna J Med Biotechnol. 2016;8:133–138. [PMC free article] [PubMed] [Google Scholar]
- 22.Shafiei Sh, Tajik P, Ghasemzadeh Nava H, Movahedin M, Talebkhan Garoussi M, Qasemi Panahi B, et al. Isolation of bovine spermatogonial cells and co-culture with prepubertal Sertoli cells in the presence of colony stimulating factor-1. Iran J Vet Med. 2013;7:83–90. [Google Scholar]
- 23.Akbarinejad V, Tajik P, Movahedin M, Youssefi R, Shafiei S, Mazaheri Z. Effect of extracellular matrix on bovine spermatogonial stem cells and gene expression of niche factors regulating their development in vitro. Anim Reprod Sci. 2015;157:95–102. doi: 10.1016/j.anireprosci.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Anway MD, Folmer J, Wright WW, Zirkin BR. Isolation of sertoli cells from adult rat testes: An approach to ex vivo studies of sertoli cell function. Biol Reprod. 2003;68:996–1002. doi: 10.1095/biolreprod.102.008045. [DOI] [PubMed] [Google Scholar]
- 25.Tajik P, Barin A, Movahedin M, Zarnani AH, Hadavi R, Moghaddam G, et al. Nestin, a neuroectodermal stem cell marker, is expressed by bovine sertoli cells. Comp Clin Pathol. 2012;21:395–399. [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Meehan T, Schlatt S, O’Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development byactivin, follistatin, and FSH. Dev Biol. 2000;220:225–237. doi: 10.1006/dbio.2000.9625. [DOI] [PubMed] [Google Scholar]
- 28.França LR, Avelar GF, Almeida FF. Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology. 2005;63:300–318. doi: 10.1016/j.theriogenology.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Lamb DJ, Spotts GS, Shubhada S, Baker KR. Partial characterization of a unique mitogenic activity secreted by rat Sertoli cells. Mol Cell Endocrinol. 1991;79:1–12. doi: 10.1016/0303-7207(91)90089-b. [DOI] [PubMed] [Google Scholar]
- 30.Ryu BY, Orwig KE, Kubota H, Avarbock MR, Brinster RL. Phenotypic and functional characteristics of spermatogonial stem cells in rats. Dev Biol. 2004;274:158–170. doi: 10.1016/j.ydbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbasi H, Tahmoorespur M, Hosseini SM, Nasiri Z, Bahadorani M, Hajian M, et al. THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology. 2013;80:923–932. doi: 10.1016/j.theriogenology.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Nasiri Z, Hosseini SM, Hajian M, Abedi P, Bahadorani M, Baharvand H, et al. Effects of different feeder layers on short-term culture of prepubertal bovine testicular germ cells in-vitro. Theriogenology. 2012;77:1519–1528. doi: 10.1016/j.theriogenology.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Yomogida K, Yagura Y, Tadokoro Y, Nishimune Y. Dramatic expansion of germinal stem cells by ectopically expressed human glial cell line-derived neurotrophic factor in mouse Sertoli cells. Biol Reprod. 2003;69:1303–1307. doi: 10.1095/biolreprod.103.015958. [DOI] [PubMed] [Google Scholar]
- 35.Johnston DS, Olivas E, DiCandeloro P, Wright WW. Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia. Biol Reprod. 2011;85:763–769. doi: 10.1095/biolreprod.110.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Z, Jiang J, Hofmann MC, Dym M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod. 2007;77:723–733. doi: 10.1095/biolreprod.107.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139:1734–1743. doi: 10.1242/dev.076539. [DOI] [PubMed] [Google Scholar]