Abstract
Development of a closed circulatory system requires that large arteries adapt to the mechanical demands of high, pulsatile pressure. Elastin and collagen uniquely address these design criteria in the low and high stress regimes, resulting in a nonlinear mechanical response. Elastin is the core component of elastic fibers, which provide the artery wall with energy storage and recoil. The integrity of the elastic fiber network is affected by component insufficiency or disorganization, leading to an array of vascular pathologies and compromised mechanical behavior. In this review, we discuss how elastic fibers are formed and how they adapt in development and disease. We discuss elastic fiber contributions to arterial mechanical behavior and remodeling. We primarily present data from mouse models with elastic fiber deficiencies, but suggest that alternate small animal models may have unique experimental advantages and the potential to provide new insights. Advanced ultrastructural and biomechanical data are constantly being used to update computational models of arterial mechanics. We discuss the progression from early phenomenological models to microstructurally motivated strain energy functions for both collagen and elastic fiber networks. Although many current models individually account for arterial adaptation, complex geometries, and fluid–solid interactions (FSIs), future models will need to include an even greater number of factors and interactions in the complex system. Among these factors, we identify the need to revisit the role of time dependence and axial growth and remodeling in large artery mechanics, especially in cardiovascular diseases that affect the mechanical integrity of the elastic fibers.
Keywords: elastin, aorta, vascular mechanics, extracellular matrix, collagen
Introduction
Elastic fibers provide energy storage and recoil to the large elastic arteries. These mechanical properties are critical due to the high, cyclic pressure environment found in the conducting arteries. Although the main constituent of the elastic fiber is elastin, other components play major roles in the fiber's functionality. Genetic mutations causing elastic fiber insufficiency or disruption, such as supravalvular aortic stenosis (SVAS) and Marfan syndrome (MFS), result in cardiovascular defects linked to arterial stiffening and wall remodeling that compromise cardiovascular function. Therefore, an understanding of the impact of elastic fiber biology on arterial mechanics is imperative for advancements in vascular diagnosis, treatment, and tissue engineering.
This review summarizes advances in arterial biomechanics and provides new insights and directions for future work. Key points include an updated model of elastic fiber assembly, arterial remodeling in development and disease, and microstructurally based constitutive modeling of elastic fibers. These points are discussed in light of improved imaging technologies, mechanical testing, and computational modeling, which have allowed for collaborative advances in the field. Because the circumferential, elastic properties of the large arteries are so important for proper cardiovascular function, other properties, such as time-dependent and axial mechanical behavior, are often neglected. We posit that these properties play an important role in large artery mechanics and should be addressed in future work.
Arterial Wall Structure
The structure of large elastic arteries distinguishes them from other vessels, such as muscular arteries, capillaries, and veins, and allows for much of their physiological function. The wall, shown in Fig. 1, consists of three layers: the intima, media, and adventitia. Each of these layers uniquely contributes to the wall's overall function and is further discussed below.
Fig. 1.
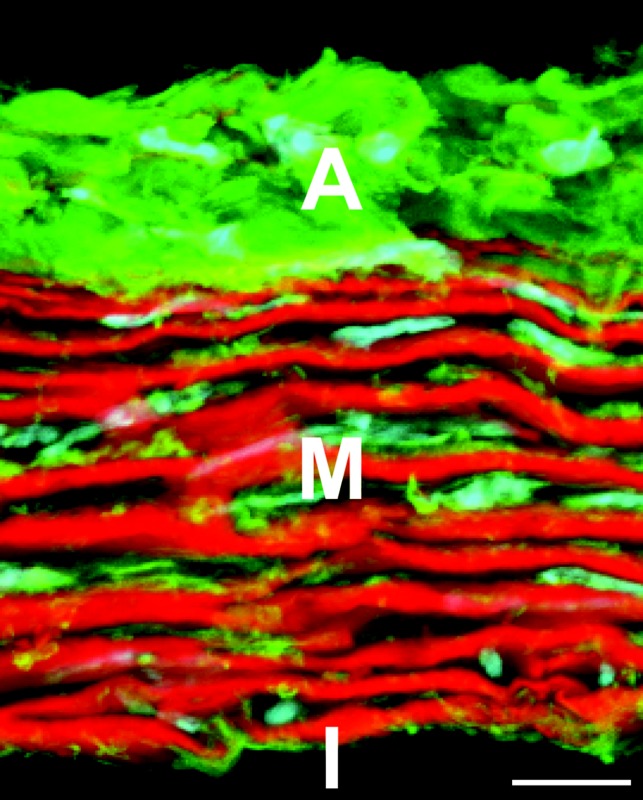
Cross section of the mouse ascending aorta stained with fluorescent probes for elastin (red), collagen (green), and cell nuclei (cyan). Adventitia (A), media (M), and intima (I) are shown. The image is a maximum projection of several z-planes for a slice that is not a perfect circumferential cross section, which provides the illusion of depth. Scale bar = 10 μm.
Intima.
The intima is located on the luminal side of the arterial wall, directly in contact with circulating blood. It is predominantly populated by endothelial cells (ECs), which synthesize proteins, such as collagen IV and laminin, to create the basal lamina [1,2]. Initially, most research was focused on the permeability of the intimal layer [3,4]. More recent studies have shown that the endothelium does not merely serve a barrier function. For example, ECs respond to hemodynamic shear stress by elongating and aligning in the direction of flow [5] and releasing signals that control vascular tone [6–8]. Despite the intimal layer's roles in various processes, such as inflammation [9] and angiogenesis [10], it has a minimal contribution to the artery's passive mechanical properties.
Media.
The media is the thickest layer and is found between the internal and external elastic laminae. The main components of the media are vascular smooth muscle cells (SMCs), elastin, and collagen. The contribution of SMCs to the artery's passive mechanical properties is frequently considered to be negligible, but atomic force microscopy has shown that both the extracellular matrix (ECM) and SMCs become stiffer in arteries from older monkeys and may collectively contribute to increased arterial stiffness with aging [11]. Additionally, atomic force microscopy studies in our laboratory showed that SMCs have an increased elastic modulus while in a synthetic phenotype [12]. In this phenotype, SMCs produce ECM proteins that are arranged into the characteristic circumferentially orientated lamellar units [13]. These units consist of SMCs surrounded by elastic fiber sheets with collagen fiber bundles in nearby spaces [14]. Extensive crosslinking within elastic fibers imparts elasticity to the arterial wall, while collagen fibers provide strength and limit wall extension. Enzymatic digestion of arteries highlights each of these contributions. During mechanical testing, elastase and collagenase-treated carotid arteries dilate at early and later points along their pressure–diameter curves, respectively (Fig. 2). This supports the tenet that elastin is associated with the low stress regime, while collagen fibers only begin to engage at high stresses. This sequential recruitment of ECM proteins is responsible for the artery's nonlinear stress–strain curve [15].
Fig. 2.
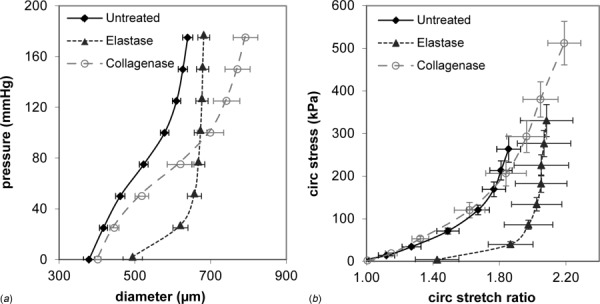
Mechanical contributions of elastin and collagen in the mouse carotid artery. Diameter–pressure (a) and circumferential stretch ratio–Cauchy stress (b) relationships are shown. Arteries were untreated or treated with elastase or collagenase to digest elastin or collagen, respectively, and then mounted in a pressure myograph. Elastase treated arteries dilate at low pressures and then become very stiff at high pressures, while collagenase treated arteries show little change at low pressures and dilate at high pressures, becoming less stiff. The results are consistent with the idea that elastin and collagen dominate the circumferential mechanical behavior at low and high pressures, respectively. N = 6–8/group. Mean ± SEM.
Adventitia.
The adventitia is the outermost layer of the arterial wall and is characterized by its heterogeneity. The adventitia mainly consists of fibroblasts and a collagen-rich ECM, but also includes several other important components, such as the vasa vasorum, nerves, and immune cells [16]. Given the diverse milieu surrounding adventitial fibroblasts, they respond to a wide variety of chemical and mechanical cues. For example, hypertensive environments result in increased fibroblast proliferation and collagens I and III synthesis [17,18]. Inflation tests of isolated adventitial layers show that the adventitia bears over half of the load at high pressure and highlight collagen's role as structural reinforcement [19]. Investigations into the adventitia's microstructure, such as collagen fiber orientation and waviness [20], have been used to develop more detailed constitutive models and improve understanding of the layer's contribution to wall mechanics [21].
Elastic Fibers
Components
Elastin.
The elasticity of arterial tissue is in large part due to the presence of elastin as the core component of the elastic fiber [22]. Initially, vascular SMCs secrete elastin in its soluble form: tropoelastin [23]. Repeating and alternating hydrophobic and lysine-containing amino acid sequences in tropoelastin result in the many interactions and crosslinks that transform it into the insoluble and mature form of elastin [24]. Although tropoelastin self-assembles to some degree via coacervation [25,26], associated proteins, such as fibulins-4 and -5, play important roles in the crosslinking and arrangement of tropoelastin multimers during elastic fiber assembly [27], [28]. The assembly processes lead to a conformational change that provides the molecular basis for elastin's elasticity [29].
Elastin's role in large artery function is of paramount importance. Mutations in the elastin gene cause SVAS, William's syndrome, and cutis laxa [30]. SVAS, which may also occur as a condition of William's syndrome, is a narrowing of the aorta often seen with SMC hyperplasia and hypertrophy, ECM remodeling, and hypertension, potentially leading to heart failure and death [31]. Elastin haploinsufficient (Eln+/−) mice are a viable model for SVAS and demonstrate how large arteries adapt to the consequences of reduced elastin. In Eln+/− mice, diffuse arterial stenosis and increased stiffness causes hypertension, which is compensated for by an increase in the number of lamellar units, with a similar tension per lamellar unit as wildtype mice [32]. Although Eln+/− mice live a normal life span, the complete absence of elastin in Eln−/− mice results in perinatal death due to SMC overproliferation and consequently, aortic obstruction [33]. Recent experiments in our laboratory show that ascending aortae from newborn Eln−/− mice not only have altered elastic properties but also have increased hysteresis with cyclic pressurization (Fig. 3), indicative of a change in time dependent mechanical properties [34].
Fig. 3.
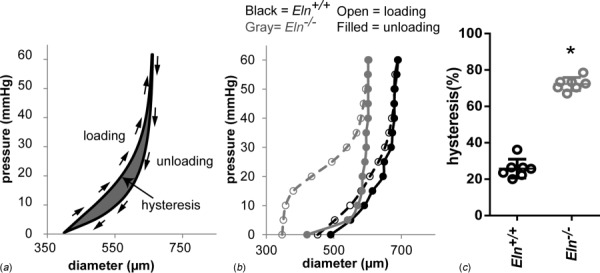
Hysteresis is increased in the absence of elastin. Example loading and unloading curves showing the hysteresis, or area between the loading and unloading diameter–pressure curves (a). Representative diameter–pressure curves for loading and unloading of a newborn aorta from Eln+/+ and Eln−/− mice show the increased hysteresis area in Eln−/− aorta (b). Quantification of the hysteresis area as a percent of the total area under the loading curve shows a three-fold increase in hysteresis for Eln−/− aorta compared to Eln+/+ (c). Hysteresis is indicative of the energy loss during cyclic loading. N = 6–7/group. * = P <0.05 by students t-test [34].
Fibrillins.
The microfibril structure within elastic fibers is primarily made of fibrillins. The fibrillin family consists of three isoforms [35]. In microfibrils, fibrillin-1 molecules have a “beads on a string” structure and are arranged in parallel and unstaggered relative to each other [36]. Although fibrillin-1 monomers are about 150 nm long, bead-to-bead distances are much smaller due to helical packing [37]. These compacted regions can be extended while under tension and can augment arterial elasticity. Fibrillin-1 and -2 are highly homologous, sharing similar domains responsible for the molecule's bending and flexibility [38]. Their tissue distributions are similar, but fibrillin-2 expression significantly decreases in adulthood.
Mutations in the fibrillin-1 gene cause MFS, which affects fibrous connective tissue and includes a potentially fatal vascular phenotype [39,40]. Fbn1−/− mice have thinner and disorganized elastic lamellae and die shortly after birth, often due to aneurysm rupture [41]. Another MFS model, the mgR/mgR mouse, underexpresses fibrillin-1 without affecting tropoelastin crosslinking, which results in fragmented elastic fibers and increased arterial stiffness [42]. The reduction of fibrillin-1 appears to accelerate fatigue-induced damage to elastic fibers, underscoring its importance in the ageing process [43]. Fibrillin-2 mutations result in an MFS-like condition called congenital contractural arachnodactyly [44]. Interestingly, the congenital contractural arachnodactyly model, Fbn2−/− mice show normal aortic wall maturation [41].
Fibrillin-3 is the most recently identified member of the fibrillin family and is absent in rodents, making it more difficult to study. In early human vascular development (sixth to tenth gestational week), fibrillin-3 weakly correlates with elastic fiber deposition, but is more abundantly found in areas lacking elastin [45]. At this time, its role in elastic fiber assembly and function is not known.
Fibulins.
The fibulins are a family of seven glycoproteins known to interact with various components of basement membranes and ECM. Structurally, they all have tandem repeats of epidermal growth factor-like domains with a C-terminal fibulin-type module [46].
Fibulins 1–5 bind to tropoelastin, but only some of these appear to be involved in elastic fiber formation [46–48]. Fibulin-2, -4, and -5 also bind to fibrillin-1, implying that they may function to link tropoelastin to the microfibrillar scaffold. Suppression of fibulins-4 or -5, but not -2, with siRNA in cell culture leads to a reduction in mature elastic fibers [49]. Fbln2−/− mice have no apparent phenotype, lending support to the idea that fibulin-2 may have a redundant function compensated for by other protein family members [46].
The importance of fibulins-4 and -5 with regard to elastic fibers suggests that they have an impact on large artery mechanics. Fibulin-4 mutations are linked to autosomal recessive cutis laxa syndrome, causing loose skin, vascular tortuosity, ascending aortic aneurysms, and other complications [50]. A number of fibulin-4 deficient mouse models have been developed that all have elastic fiber fragmentation, altered arterial mechanics, and aortic aneurysms [51–53]. Fibulin-5 mutations are also associated with cutis laxa, SVAS, and poorly developed elastic fibers [54]. Fbln5−/− mice have a thicker arterial wall with disrupted elastic fibers. Central arteries from these mice show increased stiffness and decreased energy storage [55,56].
Lysyl Oxidase.
Lysyl oxidase (LOX) is a copper-dependent enzyme with diverse roles in intra- and extracellular spaces. Its best known function is the oxidation of lysine residues in elastin and collagen to form crosslinks [28]. Additionally, a related family of four LOX-like (LOXL) proteins has been identified [57]. Of these, LOXL1 shares the most structural and expression pattern similarities to LOX [58]. Both enzymes are mostly localized to collagen- and elastin-rich tissues.
Deficiencies in LOX activity yield connective tissue abnormalities [59] and are associated with vascular defects causing arterial dissection [60]. Recently, our laboratory investigated the microstructural and mechanical consequences of lysyl oxidase deficiency in Lox−/− mice. Fluorescent staining of Lox−/− aortas at P0 shows severe elastic fiber fragmentation, consistent with that previously observed in embryonic aortas [61]. Lox−/− aortas have altered opening angles, circumferential stresses, circumferential stretch ratios [62], and increased energy loss with cyclic loading and unloading, highlighting the importance of properly crosslinked elastic fibers in determining time-dependent properties of the aortic wall [34].
LOXL1 mutations are associated with exfoliation syndrome, a disorder characterized by fibril ECM accumulation in the eye and a leading cause of glaucoma [63]. In addition to a similar, but not identical, ocular phenotype, Loxl1−/− mice develop pelvic organ prolapse a few days postpartum, enlarged alveoli, and fragmented and diffuse elastic fibers in the skin and aorta [64]. The delay in pelvic organ prolapse suggests that LOXL1 may not only play a role in elastic fiber development but also maintenance in adulthood. As the field of vascular regenerative medicine continues to expand, we would be remiss to not consider LOXL1's role in elastic fiber homeostasis.
Others.
There are four members of the elastin microfibrillar interface (EMILIN)/multimerin protein family with a wide range of functions dependent on ECM and/or cellular interactions [65]. All members share structural similarities, including a long region prone to coiling. EMILIN-1 is the most extensively studied. EMILIN-1 is localized between microfibrils and tropoelastin fiber cores and may assist with elastic fiber adhesion to cells [66]. In vivo and in vitro studies show that EMILIN-1 deficiency results in elastic fiber fragmentation. Emilin1−/− mice have hypertension caused by reduced SMC proliferation, narrower arteries, and increased peripheral resistance, but do not display altered mechanical properties as determined by pressure–diameter curves [67]. The structural and functional changes in Emilin1−/− arteries could be caused by elastic fiber disruption or by changes in cell adhesion mediated by EMILIN-1 that alter TGF-β signaling.
The microfibril-associated glycoprotein (MAGP) family consists of two small, cysteine-rich proteins that covalently bind to fibrillin in microfibrils [68]. MAGP-1 is of particular interest because its distribution is almost identical to that of fibrillin-1. It is specifically localized on fibrillin-1's beads and its N-terminal regions contain a tropoelastin binding sequence that could facilitate elastic fiber assembly [69]. Surprisingly, MAGP-1-deficient mice do not have altered vessel structure, compliance, or hemodynamic loads, suggesting that their impaired thrombotic response to injury is mediated by MAGP-1 through a separate mechanism [70].
Due to their abundance in ECM and basement membranes, proteoglycans are also of interest in the study of elastic fibers. Structurally, proteoglycans consist of a protein core with at least one glycosaminoglycan (GAG) chain [71]. Specifically, decorin and biglycan form ternary complexes with elastic fiber components, such as tropoelastin, fibrillin-1, and MAGP-1 [72,73], suggesting potential roles in elastogenesis. Proteoglycans with heparan sulfate GAGs, such as perlecan, mediate interactions within fibrillin molecules [74]. Increased proteoglycans are present in aortic aneurysms and in the aortas of mice that do not express fibulin-4 in the SMCs (Fbln4SMKO) [75]. Given proteoglycan binding affinity to water, changes to their quantity or activity may contribute to the observed changes in time-dependent mechanical properties in mouse models of different elastinopathies.
Assembly.
Several models of elastic fiber assembly have been presented and are continuously updated as new data become available. Many of these models include the interactions between components, their locations relative to the cell and ECM, and the sequence of events [25,76,77]. Using these and incorporating the most recent data on the subject, we describe a current and comprehensive model of elastic fiber formation in Fig. 4.
Fig. 4.
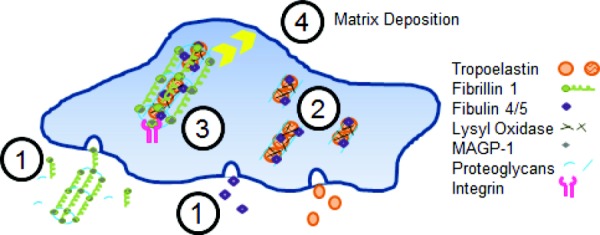
Model of elastic fiber assembly. Microfibrils consisting mostly of fibrillin-1, but also including MAGP-1 and interacting with proteoglycans, are assembled in the extracellular space. At the same time, fibulin-4/5 and tropoelastin are secreted from the SMC (1). Tropoelastin coacervates and then interacts with fibulin-4/5 and is crosslinked by LOX on the SMC surface (2). Integrins link microfibrils to the cell surface where they interact with the tropoelastin/fibulin aggregates and allow further crosslinking by LOX (3). The crosslinked, fully assembled elastic fiber is then deposited into the ECM (4).
The microfibrillar scaffold is constructed in tandem with tropoelastin synthesis and secretion. The same SMCs secrete fibrillin-1 and -2, though fibrillin-2 is only expressed in prenatal development [38]. Following secretion, fibrillin-1 undergoes proteolytic processing resulting in the “beads on a string” fibril structure [78]. Further assembly into a joined lateral structure may be proteoglycan assisted [37,74]. MAGP-1 associates with fibrillin-1 beads in preparation for tropoelastin integration [79]. Fibrillin-1's interaction with α5β1 and αvβ3 integrins brings the fiber structure closer to the cell surface [25,80].
Tropoelastin is secreted and coacervates to form aggregates on the cell surface [77]. Heparan and chondroitin sulfate-containing GAGs on the cell surface align these aggregates as they continue to grow [81]. Fibulin-4 and -5 mediate (either facilitating or limiting) tropoelastin crosslinking by LOX and bind to fibrillin-1 to transfer tropoelastin aggregates onto the microfibril [76]. As the process progresses, latent TGF-β binding proteins (LTBPs) bind to fibrillin-1 and tropoelastin-fibulin-5 complexes, promoting elastic fiber assembly while mediating TGF-β related signaling cascades [76,82]. Lastly, a stable and thorough integration of the tropoelastin aggregates is established by additional LOX mediated crosslinking.
The deposition of mature elastic fibers into the existing ECM is less well understood, but is a topic of on-going study. Immunostaining shows that fibronectin fibers precede and colocalize with elastic fibers [25]. Knockdown of fibronectin expression reduces the accumulation of fibrillin-1 in the matrix [83]. More recent work has shown that LTBPs act as physical anchors between fibronectin and fibrillin-1 [84]. These observations suggest that the fibronectin matrix is a necessary precursor to elastic fiber integration.
Further understanding of elastic fiber assembly in large arteries will have to consider the effects of biochemical and biophysical signals. Many models of elastic fiber assembly already consider the role of chemical interactions. Although many of these chemical interactions are discussed above, there are still unanswered questions and opportunities for further research. One example is the recent work on elastogenesis in adult reproductive tissue. Elastic fiber formation in cervical tissue is highly regulated by hormones and their downstream effects in pregnant mice [85]. Abnormalities in both collagen and elastic fiber structure predictably translate into reduced mechanical function. These and similar studies may provide insight into the kind of biochemical processes necessary to catalyze elastic fiber synthesis in other tissues, such as the vasculature. The contribution of mechanical signals to this complex process is not well understood. A recent study compared tissue engineered constructs seeded with vascular SMCs exposed to either pulsatile flow or static conditions [86]. Although there was no difference in LOX or soluble elastin quantities, there was a significant increase in mature insoluble elastin in the pulsatile flow condition. The authors surmise that pulsatile flow stretches tropoelastin molecules and exposes additional binding sites for increased crosslinking. Overall, the more robust and organized ECM in the pulsatile flow condition resulted in improved mechanical properties, such as linear modulus and ultimate tensile strength, as measured by uniaxial tensile tests. As the fields of tissue engineering and mechanobiology continue to grow and interact, rigorous mechanical stimulation, testing, and analyses may be used to better recreate and evaluate elastic fiber assembly in tissue-engineered arteries.
Physiologic Adaptation in Growth and Development
Vascular Morphogenesis.
Early development of the large arteries begins with the formation of the endothelium. This occurs with the migration of presumptive ECs from the mesoderm to the sites of vasculogenesis, as observed and described in the Japanese quail embryo [87]. The first large conducting artery, the dorsal aorta (DA), develops concurrently with the heart and initially appears as two parallel EC cords. After continued EC aggregation, the two structures develop lumens and eventually fuse [88].
The ECs in this precursor vessel are directly involved in the recruitment of SMCs, the next step in vascular maturation. Self-recruitment may occur as some ECs transdifferentiate into SMCs [89,90]. More often, EC-mediated signaling encourages migration of SMCs from an already existing pool of SMCs or differentiation of surrounding undifferentiated cells [91–93]. Signaling of these precursor cells may depend on their proximity to specific locations throughout the arterial tree. Each region recruits its cells from a different embryonic cell population, creating a diversity of cellular origins and developmental histories [94]. Deficiencies in key signaling molecules disrupt arterial development and result in atretic aortas, disorganized ECs, and the absence of SMCs [95].
Arterial wall maturation and proper elastic fiber assembly requires SMC phenotypic fluidity. Synthetic SMCs are prone to proliferation and protein synthesis, while contractile SMCs are relatively quiescent and develop intercellular contractile filaments and cytoskeletal structures [13]. SMC-specific markers have been used to determine the presence of SMCs in the developing artery and identify their phenotype. Medial SMC phenotypic changes were tracked in bovine arteries starting from an early fetal time point (60 days out of a 280 day gestational period) through birth and into adulthood [96]. Initially, all SMCs begin with a synthetic phenotype. As development advances, most SMCs shift toward a contractile phenotype. Approaching birth, many SMCs in the medial layer revert back to a synthetic phenotype. The temporal pattern of phenotypic changes provides a blue print for ECM assembly.
Elastic Fiber Development.
The chick embryo model has been used to study elastogenesis in early development. Like mice, chicks have a 21-day gestation period. Tropoelastin is first identified in the heart at embryonic day (E) 4-4.5 [97]. Tropoelastin expression progresses distally toward the dorsal aorta by E6 and is distributed throughout the arterial tree by E12. In addition to soluble elastin expression, the development of microfibrillar scaffolds is also important for vascular maturation. Our laboratory imaged temporal changes in the arterial microfibrillar scaffold using a fibrillin-2 antibody generously provided by Dr. Charles Little [98]. Figure 5 shows nuclear and fibrillin-2 staining in the DA at the level of the heart in chick embryos at E6 and E10. At E6, fibrillin-2 is largely disorganized and is distributed diffusely throughout the DA wall thickness. At E10, fibrillin-2 fibers are densely packed within the DA wall thickness and are circumferentially aligned.
Fig. 5.
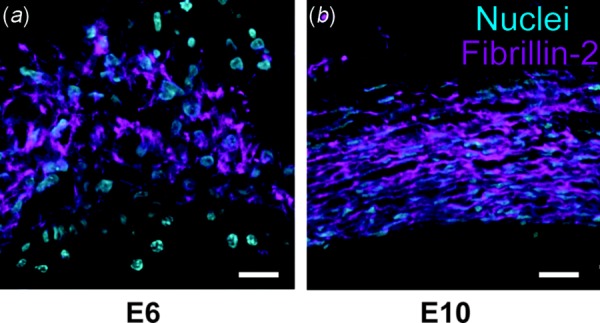
Microfibrillar organization in developing chick DA. Chick embryos at embryonic day 6 (a) and 10 (b) were sectioned at the level of the heart and the DA was stained with fluorescently tagged antibodies to cell nuclei (blue) and fibrillin-2 (purple). The density and circumferential orientation of the fibrillin-2 microfibrillar scaffold increase over the four day developmental period. The DA lumen is at the bottom of the images. Scale bar = 10 μm.
This initial ECM foundation may then provide a new set of signaling instructions causing SMCs to limit the rate of additional ECM production. Our previous work showed that SMCs in an elastin-rich culture environment downregulate synthetic genes and have an increased stiffness, presumably due to a reinforced contractile apparatus [12]. This is in agreement with decreased SMC proliferation rates reported by Karnik et al. [99] in the presence of elastin. These results underscore elastin's importance not only as a mechanical substrate but also a signaling molecule. In contrast to the decreased rates of ECM production in early development, elastin and collagen synthesis dramatically increase near the end of gestation [100], pointing to another set of cues that may modulate SMC phenotype. In several species, such as mice and sheep, upregulation of ECM expression coincides with rapid increases in blood flow and pressure observed in late prenatal and early postnatal development [100,101]. As these hemodynamic loads plateau in adulthood, SMCs settle into a contractile phenotype and collagen and elastin amounts remain relatively constant [96,100].
Mechanics.
The purpose of the arterial wall is to withstand and adapt to the mechanical loads inherent in a closed circulatory system [23]. These adaptations begin at the onset of blood flow with the first heartbeat. Early studies show that pressure and shear stress (due to flow) increase vessel wall thickness and lumen diameter, respectively [102,103]. These experiments show that the arterial wall adapts to normalize stresses. This mechanical feedback is not only critical for arterial development but also for the degeneration of extraneous vessels. This phenomenon can be observed in aortic arch patterning. Ink injections into the chick embryo vasculature show differing flow distributions in the aortic arches [104,105]. Low wall shear stress correlates with smaller aortic arch diameters and eventually, regression. These selection events are often preceded by an increase in shear stress, which is then normalized by a stress-dependent growth mechanism.
Although much of the stress normalization can be accounted for by SMC growth (through either hyperplasia or hypertrophy), SMC-mediated ECM deposition is also instrumental in establishing and maintaining stress homeostasis. In addition to hemodynamically imposed stresses, the growing embryo applies axial loads on the developing arteries. This is often ignored, but it has recently been pointed out that axial stress regulation should not be neglected [106]. Elastin's role in the axial remodeling process is evident in Eln+/− mice. In a normal embryo, the aorta's length is determined by both growth and stretch. Given that SMCs and collagen both turn over regularly, it is the elastic fiber that bears much of the tension [107], establishing a target stress that SMC growth and ECM remodeling strives to maintain. For example, reduced elastin in Eln+/− mice shifts some of the axial stress burden onto the SMCs, causing them to grow and reducing the in vivo axial stretch ratio. A similar mechanism can be used to describe elastin's role in circumferential growth and remodeling in response to pressure.
Pressure loading is distributed by concentric layers of elastic lamellae. In small mammals (i.e., mouse, rabbit, rat), the number of lamellae across the arterial wall is established soon after birth, with wall thickening occurring through deposition of ECM between existing SMC layers [14]. However, in larger mammals (i.e., dog, human, sheep), the number of lamellae continues to increase postnatally [108]. Lamellar addition after birth may be related to the presence of a vasa vasorum in the adventitial layer of larger arteries that is necessary to provide nutrients that cannot diffuse across the thick-walled artery. The number of lamellae increases from 35 to 56 from birth to adulthood in human thoracic aorta that has a vasa vasorum, while the number increases from 25 to 28 units from birth to adulthood in human abdominal aorta that does not have a vasa vasorum [109]. The timeline of lamellae formation may also be related to the life span of each organism. The number of lamellae in rat aorta is unchanged after 5 weeks of age [96], near the time of sexual maturity. The number of lamellae in human thoracic aorta is unchanged after 20 years of age [109], also near the time of sexual maturity. The total number of lamellar units scales with diameter and applied pressure in different organisms, providing a constant tension per lamellar unit across several species [108,110]. Ultrastructural studies on Eln+/− mice reveal increased thickness and number of lamellar units to maintain the constant tension/lamellar units in a hypertensive environment [111].
Developmental adaptations to mechanical stimuli are intertwined and collaborative. We have described how arterial mechanics can both modulate and be affected by SMC phenotype, growth, and remodeling. The presence or absence of wall components, specifically elastic fibers, determines how load sharing occurs and instigates compensatory mechanisms. For now, much of the focus has been on SMCs, collagen, and elastin. Genetically modified mouse models may be used to uncover and further understand how other elastic fiber components may also contribute to developmental mechanical requirements.
Alternatively, the use of other small animal models may supplement already existing data from genetically modified mice and provide key insights. Developmental biologists often rely on the experimental advantages of alternate models, such as zebrafish and chick embryos. Morpholino knockdown of specific genes in zebrafish provides in vivo evidence for the roles of ECM molecules, such as syndecan [112] and magp1 [113], in vasculogenesis and elastic fiber formation, respectively. These approaches may help identify mechanically significant molecules. Unlike the genetic perturbations in mouse and zebrafish models, physical manipulations can be used to affect developmental processes in the chick embryo. Historically, the chick embryo model has been used to show how mechanical perturbations cause cardiac defects in early development [114]. These techniques have not yet been extended to the study of arterial ECM maturation. The ability to impose physical interventions and subsequently measure mechanical properties sets chick embryo-based studies apart from the typical genetic or chemical approaches.
Pathological Adaptation in Disease and Aging
Hereditary Disease.
In addition to developmental studies, genetically modified mice have been used to study the effects of hereditary diseases on the mature vasculature. Of the mouse models previously mentioned here, Eln+/−, Fbln4SMKO, Fbln5−/−, and mgR/mgR are all viable into adulthood and serve as models for SVAS, cutis laxa, and MFS [42,111,115], respectively. The adaptation to elastin haploinsufficiency has already been discussed above within the context of development. In the fibulin deficient models, elastic fiber fragmentation and associated vascular abnormalities are observed prior to developmental maturity and lead to cardiovascular problems in adulthood. The mgR/mgR genetic modification, however, only begins to take its toll in adulthood [42]. Histology shows that elastic fiber fragmentation is seen after elastin production has ceased, thus disallowing a compensatory increase in elastic lamellae, as seen in Eln+/− mice. Instead, the fibulin- and fibrillin-deficient mouse models compensate for increased systolic or pulse pressure with increased wall thickness, resulting in normalized circumferential wall stresses [42,115,116].
Many congenital defects in elastic fiber proteins are associated with dissection and aneurysm development. A dissection is a tear across the intima and into the medial layer, creating a second “lumen” for blood flow within the arterial wall. The circumferential orientation of the media's structures provides little resistance to longitudinal progression of the dissection. Many dissections ultimately result in hemodynamic alterations, luminal blockage, and rupture [117]. Aneurysms are a bulging or distension of the arterial wall, often resulting in thinning and susceptibility of dissection and rupture. These may occur in various locations, such as the cerebral arteries and the thoracic and abdominal aorta.
The initiation and progression of dissections and aneurysms is the subject of intense study. As shown in the Fbln2 and Fbln5 double knockout mouse, elastic fiber disruptions, specifically in the inner elastic laminae, may contribute to dissection initiation [118]. Histological analysis of dissections in MFS patients and in fibrillin-deficient mice show both elastic fiber defects and altered TGF-β signaling, suggesting that together these factors play a role in dissection formation [117]. Recall that fibrillin-1's LTBP interacts with and sequesters TGF-β [82]. In the absence of fibrillin-1, the hallmark of MFS, TGF-β signaling is dysregulated and potentially encourages dissection.
TGF-β signaling is also involved in SMC mechanotransduction. While the ability of SMCs to sense and respond to mechanical cues results in stress homeostasis, it can also exacerbate vascular pathobiology. This maladaptation has dire consequences for the mechanical integrity of large arteries. This is observed in Fbln4SMKO mice, which are prone to thoracic aortic aneurysms. They have increased pulse pressure, increased elastic fiber fragmentation, and decreased SMC-elastic fiber interactions, all contributing to a mechanically altered environment surrounding the vascular SMCs [116,119]. Egr, a mechanosensitive transcription factor known to increase TGF-β signaling, is significantly higher in Fbln4SMKO aortas compared to controls and may be a molecular transducer for the mechanical perturbations.
Acquired Disease.
Although genetic mutations cause some cardiovascular diseases, other cardiovascular diseases occur due to environmental factors that can also be studied in animal models. Aortic arch banding in wild type mice has been used to study arterial remodeling due to nonhereditary hypertension [120,121]. Hypertensive carotid arteries have reduced circumferential and axial stretch ratios at in vivo pressures, due to SMC growth and ECM remodeling. Specifically, increases in proteoglycans and collagen were observed [121]. Ultimately, this led to a stiffer artery capable of less energy storage. Effective blood transport through stiffer arteries places greater load on the heart and feeds into a positive feedback loop of worsening hypertension.
Another disease that can arise from extrinsic conditions is atherosclerosis. Previous studies focused on the role of turbulent flow or SMC lineage [122], but elastic fibers may also play a more immediate role. SMCs and, potentially, macrophages are found in and produce elastic fibers in plaques [123]. These are the site of calcium and lipid deposits, both which cause stiffening. Aside from their own physical contributions to stiffness, lipid deposits additionally increase elastic fiber susceptibility to enzymatic digestion. Plaque cells are participants in the same TGF-β signaling pathways that influence arterial mechanics. This makes a potential link between atherosclerosis, elastic fiber integrity, and artery wall mechanics worth investigating.
Our laboratory recently investigated the effects of reduced elastin amounts and the resulting cardiovascular changes on atherosclerotic plaque size and stability. Eln+/− mice have hypertension and increased arterial stiffness, both of which are associated with plaque accumulation in humans. We measured plaque accumulation in Eln+/− mice crossed with atherosclerosis susceptible Ldlr−/− (low density lipoprotein receptor knockout) mice and fed a high fat western diet [124]. Surprisingly, there were no differences in plaque size for Eln+/−Ldlr−/− mice compared to the control Eln+/+Ldlr−/− mice, despite increased arterial stiffness and hypertension. There may be confounding factors, such as the fact that western diet alone contributes to arterial stiffening [125], and so does the Ldlr−/− genotype [126]; however, our work suggests that mouse models may be useful for teasing out causal relationships between some of the associative factors identified in human studies.
Aging.
Elastic fibers, produced in early development, have a half-life on the order of the human life span. A lifetime's worth of cyclic loading, proteolytic exposure, and negligible turnover rates are bound to have an effect on elastic fiber integrity and large artery mechanics. Therefore, it is no surprise that human aging is associated with stiffening of elastic tissue.
Much of the literature on elastic fibers and aging focuses on dermal tissue. Electron microscopy shows that elastic fibers become frayed and porous in aged skin [127], but it is unclear how much of this structural change is due to exogenous factors, such as sun exposure or direct trauma. These limitations may be mitigated by controlled experiments with animal models. One approach creates a model of aging from murine common carotid arteries (CA) with a gradient of elastic fiber integrity [128]. Control and elastase-treated CAs serve as upper and lower bounds, respectively, with CAs from three genetic mutants (Eln+/−, mgR/mgR, and Fbln5−/−) in between. As expected, the reductions in intact elastic fibers result in decreased extensibility and energy storage. Another approach compares the aortic mechanics of an MFS mouse model to control mice through 9 months of age [129]. Elastic fiber staining, stress–strain curves, and breaking stresses did not significantly change over this time period. Since these results were observed in relatively young mice (typical mouse life span is slightly over 2 years) and at single locations, data are needed to compare cardiovascular aging at later time points and throughout the cardiovascular tree.
Structure-Function Relationships
Computational modeling of large elastic arteries provides valuable insight for the development of predictions and designs relating to arterial mechanics. Physiologically relevant models of mechanical behavior can probe aspects of developmental and pathological processes that may be challenging or impossible to measure experimentally. In turn, model predictions may guide treatment strategies for cardiovascular disease and point to key design factors in tissue engineering.
Some of the earliest constitutive models, dating back to the 1970s, were defined by strain energy density functions (SEFs) that phenomenologically described arterial stress–strain relationships. Often, these models treated the artery as a single bulk material, though some did examine the individual contributions of the medial and adventitial layers [21]. The biggest drawback to these models is that the constants are the result of numerical fitting and do not have a physical interpretation. Nonetheless, these models have been extensively used due to their good agreement with experimental data and application for finite element models with complex geometry [130].
One of the most well-known SEFs is attributed to Fung. Initially, he proposed a two-dimensional exponential SEF [131], which was later generalized for a three-dimensional, thick-walled tube with a transmural stress distribution [132]. Since then, many proposed SEFs have an exponential form, although a few logarithmic [133] and polynomial [134] forms (and even some combinations of these [135]) have also been presented. The use of these SEFs, intended for elastic materials, to describe viscoelastic biological tissue is incompatible. To address this, Fung referred to tissue as “pseudoelastic” and proposed fitting loading and unloading curves to SEFs separately as if they were two distinct elastic materials [131]. A more complex but accurate way to account for hysteresis is to implement theories of linear or quasilinear viscoelasticity. Although viscoelastic modeling remains largely phenomenological, future efforts should be made to incorporate viscoelastic effects into microstructural models.
Time-dependent mechanical properties associated with viscoelasticity could alternately be caused by fluid movement within a poroelastic solid, such as the arterial wall ECM. Biphasic or poroelastic models have traditionally been used for highly hydrated tissues such as cartilage [136]. Simon et al. [137] developed a biphasic, isotropic model of the arterial wall and performed drained and undrained tests to obtain material parameters and steady-state pressurization tests to determine the hydraulic conductivity. Johnson and Tarbell [138] developed a biphasic, anisotropic model of the aortic wall that was able to reproduce measures of diameter, thickness, and hydraulic conductivity under applied pressure. Although there are different approaches to including time-dependent effects in arterial constitutive modeling, experimental data increasingly suggest that time-dependent properties should not be ignored [56,121,128].
Microstructurally Inspired Models.
Advances in imaging technology, such as confocal [20], two-photon [139], and electron microscopy [140], have provided unprecedented detail of the microstructural architecture within the arterial wall. These technologies allow and motivate a shift away from phenomenological models and toward microstructural models. In these models, wall components, such as SMCs, collagen, elastin, may be described by structurally motivated SEFs.
Smooth muscle cells are usually assumed to have a circumferential orientation and can contribute to both the artery's passive and active mechanical properties. Although their passive mechanical contribution is relatively small and often neglected, SMCs active mechanical behavior maintains a basal tone from which the artery can contract or relax [141]. Hill provides one of the earliest descriptions of muscle contraction [142]. Hill's and other similar models place damped contractile and elastic elements in series and parallel combinations, sometimes including additional branches for passive ECM [130]. More recent formulations of SMC active behavior are dependent on mechanical stimuli, such as shear stress [141,143,144]. Three-dimensional imaging of pressurized mouse carotid arteries shows a helical organization of SMCs, rather than a strictly circumferential orientation, which would induce torsion in the arterial wall when the SMCs contract and could be considered in future modeling efforts [145].
Many microstructural models take into account the complexities of the collagen fiber network in the arterial wall. For example, Holzapfel describes two components: a noncollagenous isotropic neo-Hookean matrix and a two-fiber family collagen mesh [21]. Histological analyses show that collagen fiber alignment is better described as a distribution of orientations, rather than two discrete angles. To reflect this arrangement, new SEFs with four-fiber families [146] or distributions centered about discrete angles [147] were developed. In addition to orientation, confocal microscopy allows for quantitative descriptions of collagen fiber waviness or crimp [20]. Collagen fiber waviness has been included in SEFs as a distribution of strains with a defined engagement strain at which the fibers straighten and begin to carry load [148,149]. Some models consider both the orientation and waviness of collagen fibers with improved fitting to experimental data [150]. Volume averaging theory has been implemented to represent microstructural qualities on a continuum scale in a multiscale model of a decellularized porcine carotid artery [151]. In this same model, fiber interactions were included in the description of the collagen microstructure. Individual fibers, governed by their own constitutive relation, were connected by rigid, freely rotating crosslinks. While not considered in this particular model, collagen fibers may interact not only with themselves but also with other wall components, such as elastic fibers.
Elastic Fiber Microstructural Modeling.
Microstructural SEFs often incorporate detail about collagen fiber structure, but simplify elastin's contribution by describing it as an isotropic solid [131,147,149]. Elastic fiber micrographs show that this is an oversimplification [100]. Modeling elastin as a transversely isotropic material was a first step to introducing anisotropic characteristics. The elastin SEF was divided into an isotropic neo-Hookean component and an anisotropic elastin fiber component oriented in the axial direction [152]. Kao et al. expand on this idea by modeling the pulmonary artery's elastin component as orthotropic, with neo-Hookean fiber families oriented in both the axial and circumferential directions [153]. Like collagen fibers, elastic fiber constitutive models may include both orientation and waviness. One example incorporates fiber distributions for both elastin and collagen, in addition to a recruitment function to describe the late engagement of adventitial collagen at high strains [154]. In all of these cases, elastic fiber anisotropy improved correspondence between the experimental data and the model predictions.
Typically, the total arterial wall SEF is the summation of each constituent's SEF, scaled by a mass or volume fraction, by the rule of mixtures. This decoupling is challenged by potential interactions between components, such as those between elastin and collagen in ligament [155] or collagen and proteoglycans in lung parenchyma [156]. ECM interactions in the artery wall have not been extensively imaged and are not frequently included in microstructural models. Humphrey reports that collagen fibers from elastase treated arteries exhibit reduced undulation, but does not include this interaction in the mechanical analyses [43]. Elastase digestion has also been implicated in the increased circumferential alignment of collagen fibers [157]. Holzapfel proposes that collagen fiber sliding occurs in the absence of elastin and accounts for lengthening in elastase treated tissue [158]. Holzapfel accounted for these observations through the use of a damage variable associated with the collagen SEF, providing good agreement between the model predictions and stress–stretch curves from elastase treated arteries.
Although enzymatic digestion protocols allow for the study of individual constituent contributions and interactions, mouse models with ECM deficiencies may provide supporting evidence or new perspectives, keeping in mind the ability of the mouse arteries to remodel and adapt to genetic deficiencies. As more mouse models are developed and become available, additional control over ECM expression (such as inducible knockout or overexpression of a gene) may attenuate these compensatory effects and further our understanding of elastic fiber remodeling and mechanics.
Growth and Remodeling
Constitutive models, such as those discussed above, can be used in conjunction with growth and remodeling (G&R) theories to predict mechanical changes over the course of developmental, pathological, or aging processes.
SMC Growth.
Skalak describes finite growth as the integrated result of differential growth [159]. Changes in infinitesimal surface and volume elements have been used to model growth in biological tissues, such as bone and muscle, respectively [160,161]. In arteries, growth creates residual stress and reduces stress concentrations at the inner wall [132,162]. Stress-dependent growth laws are frequently used to describe mechanically triggered SMC proliferation, such as that observed in the artery [163]. Rodriguez defines stress as a function of elastic deformation, while deformation due to growth reduces these stresses. Once the tissue returns to its target stress, the growth rate is reduced to zero. As arteries are not simply passive pressurized vessels, elastic deformation growth laws were later expanded to include shear stress dependence [164] and muscle contraction [144].
Remodeling.
Deviations from a homeostatic stress may also modulate the degradation and regeneration of individual constituents into a new configuration that approaches the desired homeostatic condition. Unlike the growth rates in the models describe above, the correlation between turnover rates and stress differences may be described by first-order kinetics [165]. This idea was implemented in a two-dimensional thin-walled constrained mixture model [166] and later in a thick-walled model inclusive of residual stress [144]. Figure 6 illustrates the time-dependent configurations of SMCs, elastin, and collagen during G&R. It is important to note that elastin microstructural properties are only recently being considered in constitutive modeling; therefore both of these models treat elastin as an isotropic neo-Hookean solid. Interestingly, the first model not only examines collagen turnover but also compares results for negligible and complete elastin turnover [166]. Negligible elastin turnover, as observed in adulthood, results in an incomplete restoration of the homeostatic state, restricting the artery's ability to compensate in response to disease and aging. G&R theory has been invoked in models of hypertension [167], cerebral [168] and aortic [52] aneurysms, and aging [169]. Initially, the models only showed that G&R could account for general observations and trends, such as thickening in response to hypertension, but the models are now being used to reproduce phenotypic changes found in genetically modified mice. For example, in a model where elastin is neo-Hookean and collagen is transversely isotropic, the mechanical behavior of an Fbln4SMKO mouse artery after 30 days of G&R is qualitatively reproduced by decreasing the elastin and increasing the collagen material constants, respectively [52]. This model was adapted from an earlier version used to describe early postnatal development, which allows elastin production but maintains negligible degradation [170]. Developmental G&R models can be extended into the embryonic period as more experimental data regarding the temporal and spatial deposition of individual constituents become available.
Fig. 6.
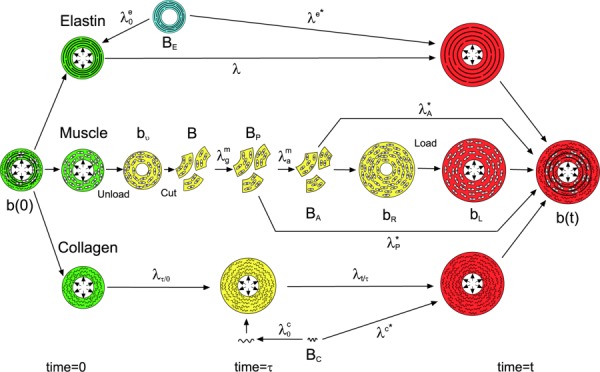
Configurations for arterial growth and remodeling. Each constituent, elastin (E), muscle (SMCs) (m), and collagen (C) has its own stress-free configuration (denoted by capital B). For muscle, BP is the passive stress-free configuration and BA is the active stress-free configuration. Stressed configurations (denoted by lower case b's) vary with time and are color coded. Blue denotes configuration at time <0, green at time = 0, yellow at time = 0 ≤ τ ≤ t, and red at time = t. Muscle undergoes several different stressed configurations including bν = unloaded and intact, bR = unloaded after growth and activation that induces residual stress, and bL = loaded. Stretch ratios between states are denoted by λ's with superscripts denoting the constituent and subscripts denoting the time or mode of deformation: g = growth, a = activation, A = active, and P = passive. λ* for each constituent is the stretch ratio from the stress-free configuration to the loaded configuration of the composite arterial wall. From Alford et al. [144]. Reprinted with permission from Springer @ 2008.
More recent G&R models incorporate both microstructural and large-scale geometric properties. As before, collagen fiber dispersion is described as consisting of four families [171] or a distribution quantified from confocal microscopy [172]. The first study uses a two-layered finite element approach, which can be adapted for more complex structures, such as the aortic arch, or patient-specific geometries. As these models become more generalized and are applied to an increasing number of conditions, there will be a need to include additional microstructural characteristics, evolving tissue-scale geometries, and interactions (fluid, chemical, etc.). Recent models now include descriptions of the elastic fiber microstructure, but still neglect some potentially important features, such as porosity. Compromised elastic fibers and lamellae may affect hydraulic conductivity and alter the arterial G&R response to circulating biochemical factors or pharmaceutical treatments. Armstrong et al. [173] combined theories of mixed poroelasticity with transport and swelling with volumetric growth to demonstrate the effects of time-driven, concentration-driven, and stress-driven growth. Disruption of elastic fibers has already been shown to affect G&R processes in the circumferential direction, while axial responses have been largely ignored. We support recent efforts to highlight the importance of axial remodeling and interactions between circumferential and longitudinal G&R responses [106]. Mouse models of elastic fiber defects often demonstrate arterial tortuosity, which may be linked to mechanical changes in the circumferential direction that are critical for vascular integrity.
Chemical and fluid interactions may also affect the rate and extent to which G&R successfully recreates the appropriate stress state of the artery. Chemical effects on SMC tone, reviewed here [130], are a good starting point from which the field can expand to include G&R. Some models with fluid–solid interactions (FSI) include arterial growth laws [104,105], but only a few include remodeling. Recent advancements in FSI arterial modeling use patient-specific geometries and describe vessel walls as flexible or distensible to identify regions of high wall tension and aneurysmal development [174]. This relatively new approach, coined fluid-solid-growth [175], can be used to improve predictions of aneurysms, but has yet to be widely implemented. Future iterations of these models may need to reconsider idealizations, such as the lack of pulsatile blood flow. Given the sheer number of factors and interactions, the complexity of these problems may require the development or application of novel computational tools and approaches, as well as collaborations between different fields.
Summary and Future Directions
We have summarized the role of elastic fibers and their components in developmental and pathological processes affecting large artery mechanics. Our model of elastic fiber assembly recapitulates the many interactions that occur for proper fiber formation. The temporal pattern of elastogenesis not only makes the developmental time period particularly interesting, but also imposes restrictions on the arterial wall's ability to remodel in response to pathological conditions later in life. Computational modeling has progressed from early phenomenological models of the bulk wall to microstructurally inspired constrained mixture models, with the potential to include additional interactions in more complex geometries.
This overview of the field has revealed areas of untapped potential. We suggest that the following three points receive further attention:
-
(1)
Inclusion of experimentally observed phenomena, such as time dependency and axial G&R, in experimental and computational design and analysis.
-
(2)
Expansion of research with alternate small animal models, besides mice.
-
(3)
Collaboration between the continuously evolving fields of biology and mechanics.
Although it is widely accepted that arteries do not exhibit fully elastic behavior, most experiments and models are not designed to consider time-dependent mechanical behavior, which may be explained by viscoelastic or biphasic theory. Many G&R models ignore arterial adaptations in the axial direction, despite experimental evidence showing decreased axial stretch. While mouse models will continue to be useful in the field, we would be remiss to forego the advantages of other small animal models, such as the chick. As new animal models are considered, their associated experimental techniques may be added to the growing toolbox used in biomechanics. It is clear that already existing tools, such as the various imaging technologies discussed here, have strengthened the interplay between biology and mechanics. Nonetheless, it is imperative that researchers interested in advancing the field keep up with the latest developments in biology, as these may help address unanswered mechanistic questions that still linger. Focusing future research in these three directions may further our understanding of the mechanical contributions of elastic fibers in large artery disease, development, and aging.
Contributor Information
Maria Gabriela Espinosa, Department of Biomedical Engineering, , Washington University, , St. Louis, MO 63130.
Marius Catalin Staiculescu, Department of Mechanical Engineering and , Materials Science, , Washington University, , St. Louis, MO 63130.
Jungsil Kim, Department of Mechanical Engineering and , Materials Science, , Washington University, , St. Louis, MO 63130.
Eric Marin, Department of Biomedical Engineering, , Saint Louis University, , St. Louis, MO 63103.
Jessica E. Wagenseil, Department of Mechanical Engineering and , Materials Science, , Washington University, , One Brookings Drive, CB 1185, , St. Louis, MO 63130 , e-mail: jessica.wagenseil@wustl.edu.
Funding Data
National Heart, Lung, and Blood Institute (Grant Nos. HL106305 and HL115560).
References
- [1]. Yurchenco, P. D. , and O'Rear, J. J. , 1994, “ Basal Lamina Assembly,” Curr. Opin. Cell Biol., 6(5), pp. 674–681. 10.1016/0955-0674(94)90093-0 [DOI] [PubMed] [Google Scholar]
- [2]. Han, S. , Shin, Y. , Jeong, H. E. , Jeon, J. S. , Kamm, R. D. , Huh, D. , Sohn, L. L. , and Chung, S. , 2015, “ Constructive Remodeling of a Synthetic Endothelial Extracellular Matrix,” Sci. Rep., 5, p. 18290. 10.1038/srep18290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Schwartz, S. M. , and Benditt, E. P. , 1972, “ Studies on Aortic Intima—I: Structure and Permeability of Rat Thoracic Aortic Intima,” Am. J. Pathol., 66(2), pp. 241–264.https://www.ncbi.nlm.nih.gov/pubmed/5009972 [PMC free article] [PubMed] [Google Scholar]
- [4]. Gerrity, R. G. , Richardson, M. , Somer, J. B. , Bell, F. P. , and Schwartz, C. J. , 1977, “ Endothelial Cell Morphology in Areas of In Vivo Evans Blue Uptake in the Aorta of Young Pigs—II: Ultrastructure of the Intima in Areas of Differing Permeability to Proteins,” Am. J. Pathol., 89(2), pp. 313–334.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2032231/ [PMC free article] [PubMed] [Google Scholar]
- [5]. Levesque, M. J. , and Nerem, R. M. , 1985, “ The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress,” ASME J. Biomech. Eng., 107(4), pp. 341–347. 10.1115/1.3138567 [DOI] [PubMed] [Google Scholar]
- [6]. Yoshizumi, M. , Kurihara, H. , Sugiyama, T. , Takaku, F. , Yanagisawa, M. , Masaki, T. , and Yazaki, Y. , 1989, “ Hemodynamic Shear Stress Stimulates Endothelin Production by Cultured Endothelial Cells,” Biochem. Biophys. Res. Commun., 161(2), pp. 859–864. 10.1016/0006-291X(89)92679-X [DOI] [PubMed] [Google Scholar]
- [7]. Buga, G. M. , Gold, M. E. , Fukuto, J. M. , and Ignarro, L. J. , “ Shear Stress-Induced Release of Nitric Oxide From Endothelial Cells Grown on Beads,” Hypertension, 17(2), pp. 187–193. 10.1161/01.HYP.17.2.187 [DOI] [PubMed] [Google Scholar]
- [8]. De Mey, J. G. , and Vanhoutte, P. M. , 1981, “ Role of the Intima in Cholinergic and Purinergic Relaxation of Isolated Canine Femoral Arteries,” J. Physiol., 316(1), pp. 347–355. 10.1113/jphysiol.1981.sp013792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Pober, J. S. , and Cotran, R. S. , 1990, “ The Role of Endothelial Cells in Inflammation,” Transplantation, 50(4), pp. 537–544. 10.1097/00007890-199010000-00001 [DOI] [PubMed] [Google Scholar]
- [10]. Leung, D. W. , Cachianes, G. , Kuang, W.-J. , Goeddel, D. V. , and Ferrara, N. , 1989, “ Vascular Endothelial Growth Factor Is a Secreted Angiogenic Mitogen,” Science, 246(4935), pp. 1306–1309. 10.1126/science.2479986 [DOI] [PubMed] [Google Scholar]
- [11]. Qiu, H. , Zhu, Y. , Sun, Z. , Trzeciakowski, J. P. , Gansner, M. , Depre, C. , Resuello, R. R. , Natividad, F. F. , Hunter, W. C. , Genin, G. M. , Elson, E. L. , Vatner, D. E. , Meininger, G. A. , and Vatner, S. F. , 2010, “ Short Communication: Vascular Smooth Muscle Cell Stiffness as a Mechanism for Increased Aortic Stiffness With Aging,” Circ. Res., 107(5), pp. 615–619. 10.1161/CIRCRESAHA.110.221846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Espinosa, M. G. , Gardner, W. S. , Bennett, L. , Sather, B. A. , Yanagisawa, H. , and Wagenseil, J. E. , 2014, “ The Effects of Elastic Fiber Protein Insufficiency and Treatment on the Modulus of Arterial Smooth Muscle Cells,” ASME J. Biomech. Eng., 136(2), p. 021030. 10.1115/1.4026203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Rensen, S. S. M. , Doevendans, P. A. F. M. , and van Eys, G. J. J. M. , 2007, “ Regulation and Characteristics of Vascular Smooth Muscle Cell Phenotypic Diversity,” Netherlands Heart J., 15(3), pp. 100–108. 10.1007/BF03085963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Clark, J. M. , and Glagov, S. , 1985, “ Transmural Organization of the Arterial Media the Lamellar Unit Revisited,” Arterioscler. Thromb. Vasc. Biol., 5(1), pp. 19–34. 10.1161/01.ATV.5.1.19 [DOI] [PubMed] [Google Scholar]
- [15]. Shadwick, R. E. , 1999, “ Mechanical Design in Arteries,” J. Exp. Biol., 202(23), pp. 3305–3313.http://jeb.biologists.org/content/202/23/3305 [DOI] [PubMed] [Google Scholar]
- [16]. Stenmark, K. R. , Yeager, M. E. , El Kasmi, K. C. , Nozik-Grayck, E. , Gerasimovskaya, E. V. , Li, M. , Riddle, S. R. , and Frid, M. G. , 2013, “ The Adventitia: Essential Regulator of Vascular Wall Structure and Function,” Annu. Rev. Physiol., 75(1), pp. 23–47. 10.1146/annurev-physiol-030212-183802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Herrmann, J. , Samee, S. , Chade, A. , Porcel, M. R. , Lerman, L. O. , and Lerman, A. , 2005, “ Differential Effect of Experimental Hypertension and Hypercholesterolemia on Adventitial Remodeling,” Arterioscler. Thromb. Vasc. Biol., 25(2), pp. 447–453. 10.1161/01.ATV.0000152606.34120.97 [DOI] [PubMed] [Google Scholar]
- [18]. Tozzi, C. A. , Christiansen, D. L. , Poiani, G. J. , and Riley, D. J. , 1994, “ Excess Collagen in Hypertensive Pulmonary Arteries Decreases Vascular Distensibility,” Am. J. Respir. Crit. Care Med., 149(5), pp. 1317–1326. 10.1164/ajrccm.149.5.8173773 [DOI] [PubMed] [Google Scholar]
- [19]. Schulze-Bauer, C. A. J. , Regitnig, P. , and Holzapfel, G. A. , 2002, “ Mechanics of the Human Femoral Adventitia Including the High-Pressure Response,” Am. J. Physiol.—Heart Circ. Physiol., 282(6), pp. H2427–H2440. 10.1152/ajpheart.00397.2001 [DOI] [PubMed] [Google Scholar]
- [20]. Rezakhaniha, R. , Agianniotis, A. , Schrauwen, J. T. C. , Griffa, A. , Sage, D. , Bouten, C. V. C. , van de Vosse, F. N. , Unser, M. , and Stergiopulos, N. , 2012, “ Experimental Investigation of Collagen Waviness and Orientation in the Arterial Adventitia Using Confocal Laser Scanning Microscopy,” Biomech. Model. Mechanobiol., 11(3–4), pp. 461–473. 10.1007/s10237-011-0325-z [DOI] [PubMed] [Google Scholar]
- [21]. Holzapfel, G. A. , Gasser, T. C. , and Ogden, R. W. , 2000, “ A New Constitutive Framework for Arterial Wall Mechanics and a Comparative Study of Material Models,” J. Elast. Phys. Sci. Solids, 61(1–3), pp. 1–48. 10.1023/A:1010835316564 [DOI] [Google Scholar]
- [22]. Carta, L. , Wagenseil, J. E. , Knutsen, R. H. , Mariko, B. , Faury, G. , Davis, E. C. , Starcher, B. , Mecham, R. P. , and Ramirez, F. , 2009, “ Discrete Contributions of Elastic Fiber Components to Arterial Development and Mechanical Compliance,” Arterioscler. Thromb. Vasc. Biol., 29(12), pp. 2083–2089. 10.1161/ATVBAHA.109.193227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Sage, H. , and Gray, W. R. , 1979, “ Studies on the Evolution of Elastin–I. Phylogenetic Distribution,” Comp. Biochem. Physiol. B, 64(4), pp. 313–327. 10.1016/0305-0491(79)90277-3 [DOI] [PubMed] [Google Scholar]
- [24]. Starcher, B. C. , 1986, “ Elastin and the Lung,” Thorax, 41(8), pp. 577–585. 10.1136/thx.41.8.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Wagenseil, J. E. , and Mecham, R. P. , 2007, “ New Insights Into Elastic Fiber Assembly,” Birth Defects Res. C Embryo Today, 81(4), pp. 229–240. 10.1002/bdrc.20111 [DOI] [PubMed] [Google Scholar]
- [26]. Cox, B. A. , Starcher, B. C. , and Urry, D. W. , 1973, “ Coacervation of Alpha-Elastin Results in Fiber Formation,” Biochim. Biophys. Acta, 317(1), pp. 209–213. 10.1016/0005-2795(73)90215-8 [DOI] [PubMed] [Google Scholar]
- [27]. Horiguchi, M. , Inoue, T. , Ohbayashi, T. , Hirai, M. , Noda, K. , Marmorstein, L. Y. , Yabe, D. , Takagi, K. , Akama, T. O. , Kita, T. , Kimura, T. , and Nakamura, T. , 2009, “ Fibulin-4 Conducts Proper Elastogenesis Via Interaction With Cross-Linking Enzyme Lysyl Oxidase,” Proc. Natl. Acad. Sci. U. S. A., 106(45), pp. 19029–19034. 10.1073/pnas.0908268106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Kagan, H. M. , and Li, W. , 2003, “ Lysyl Oxidase: Properties, Specificity, and Biological Roles Inside and Outside of the Cell,” J. Cell. Biochem., 88(4), pp. 660–672. 10.1002/jcb.10413 [DOI] [PubMed] [Google Scholar]
- [29]. Li, B. , and Daggett, V. , 2002, “ Molecular Basis for the Extensibility of Elastin,” J. Muscle Res. Cell Motil., 23(5–6), pp. 561–573. 10.1007/978-94-010-0147-2_15 [DOI] [PubMed] [Google Scholar]
- [30]. Kelleher, C. M. , McLean, S. E. , and Mecham, R. P. , 2004, “ Vascular Extracellular Matrix and Aortic Development,” Curr. Top. Dev. Biol., 62, pp. 153–188. 10.1016/S0070-2153(04)62006-0 [DOI] [PubMed] [Google Scholar]
- [31]. Li, D. Y. , Toland, A. E. , Boak, B. B. , Atkinson, D. L. , Ensing, G. J. , Morris, C. A. , and Keating, M. T. , 1997, “ Elastin Point Mutations Cause an Obstructive Vascular Disease, Supravalvular Aortic Stenosis,” Hum. Mol. Genet., 6(7), pp. 1021–1028. 10.1093/hmg/6.7.1021 [DOI] [PubMed] [Google Scholar]
- [32]. Wagenseil, J. E. , Nerurkar, N. L. , Knutsen, R. H. , Okamoto, R. J. , Li, D. Y. , and Mecham, R. P. , 2005, “ Effects of Elastin Haploinsufficiency on the Mechanical Behavior of Mouse Arteries,” Am. J. Physiol. Heart Circ. Physiol., 289(3), pp. H1209–H1217. 10.1152/ajpheart.00046.2005 [DOI] [PubMed] [Google Scholar]
- [33]. Li, D. Y. , Brooke, B. , Davis, E. C. , Mecham, R. P. , Sorensen, L. K. , Boak, B. B. , Eichwald, E. , and Keating, M. T. , 1998, “ Elastin Is an Essential Determinant of Arterial Morphogenesis,” Nature, 393(6682), pp. 276–280. 10.1038/30522 [DOI] [PubMed] [Google Scholar]
- [34]. Kim, J. , Staiculescu, M. C. , Cocciolone, A. J. , Yanagisawa, H. , Mecham, R. P. , and Wagenseil, J. E. , 2017, “ Crosslinked Elastic Fibers Are Necessary for Low Energy Loss in the Ascending Aorta,” J. Biomech., 61, pp. 199–207. 10.1016/j.jbiomech.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Kielty, C. M. , Sherratt, M. J. , and Shuttleworth, C. A. , 2002, “ Elastic Fibres,” J. Cell Sci., 115(14), pp. 2817–2828.http://jcs.biologists.org/content/115/14/2817.article-info [DOI] [PubMed] [Google Scholar]
- [36]. Handford, P. A. , Downing, A. K. , Reinhardt, D. P. , and Sakai, L. Y. , 2000, “ Fibrillin: From Domain Structure to Supramolecular Assembly,” Matrix Biol., 19(6), pp. 457–470. 10.1016/S0945-053X(00)00100-1 [DOI] [PubMed] [Google Scholar]
- [37]. Reinhardt, D. P. , Keene, D. R. , Corson, G. M. , Pöschl, E. , Bächinger, H. P. , Gambee, J. E. , and Sakai, L. Y. , 1996, “ Fibrillin-1: Organization in Microfibrils and Structural Properties,” J. Mol. Biol., 258(1), pp. 104–116. 10.1006/jmbi.1996.0237 [DOI] [PubMed] [Google Scholar]
- [38]. Zhang, H. , Apfelroth, S. D. , Hu, W. , Davis, E. C. , Sanguineti, C. , Bonadio, J. , Mecham, R. P. , Ramirez, F. , Godfrey, M. , Vitale, E. , Hori, H. , Mattei, M. G. , Sarfarazi, M. , Tsipouras, P. , Ramirez, E. , and Hol, D. W. , 1991, “ Structure and Expression of Fibrillin-2, A Novel Microfibrillar Component Preferentially Located in Elastic Matrices,” J. Cell Biol., 124(5), pp. 855–863. 10.1083/jcb.124.5.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Dietz, H. , and Pyeritz, R. , 1995, “ Mutations in the Human Gene for Fibrillin-1 (FBN1) in the Marfan Syndrome and Related Disorders,” Hum. Mol. Genet., 4(Suppl. 1), pp. 1799–1809. 10.1093/hmg/4.suppl_1.1799 [DOI] [PubMed] [Google Scholar]
- [40]. Krause, K. J. , 2000, “ Marfan Syndrome: Literature Review of Mortality Studies,” J. Insur. Med., 32(2), pp. 79–88.http://aaim.developmentwebsite.ca/journal-of-insurance-medicine/jim/2000/032-02-0079.pdf [PubMed] [Google Scholar]
- [41]. Carta, L. , Pereira, L. , Arteaga-Solis, E. , Lee-Arteaga, S. Y. , Lenart, B. , Starcher, B. , Merkel, C. A. , Sukoyan, M. , Kerkis, A. , Hazeki, N. , Keene, D. R. , Sakai, L. Y. , and Ramirez, F. , 2006, “ Fibrillins 1 and 2 Perform Partially Overlapping Functions During Aortic Development,” J. Biol. Chem., 281(12), pp. 8016–8023. 10.1074/jbc.M511599200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Marque, V. , Kieffer, P. , Gayraud, B. , Lartaud-Idjouadiene, I. , Ramirez, F. , and Atkinson, J. , 2001, “ Aortic Wall Mechanics and Composition in a Transgenic Mouse Model of Marfan Syndrome,” Arterioscler. Thromb. Vasc. Biol., 21(7), pp. 1184–1189. 10.1161/hq0701.092136 [DOI] [PubMed] [Google Scholar]
- [43]. Ferruzzi, J. , Collins, M. J. , Yeh, A. T. , and Humphrey, J. D. , 2011, “ Mechanical Assessment of Elastin Integrity in Fibrillin-1-Deficient Carotid Arteries: Implications for Marfan Syndrome,” Cardiovasc. Res., 92(2), pp. 287–295. 10.1093/cvr/cvr195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Putnam, E. A. , Zhang, H. , Ramirez, F. , and Milewicz, D. M. , 1995, “ Fibrillin–2 (FBN2) Mutations Result in the Marfan–Like Disorder, Congenital Contractural Arachnodactyly,” Nat. Genet., 11(4), pp. 456–458. 10.1038/ng1295-456 [DOI] [PubMed] [Google Scholar]
- [45]. Sabatier, L. , Miosge, N. , Hubmacher, D. , Lin, G. , Davis, E. C. , and Reinhardt, D. P. , 2011, “ Fibrillin-3 Expression in Human Development,” Matrix Biol., 30(1), pp. 43–52. 10.1016/j.matbio.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. De Vega, S. , Iwamoto, T. , and Yamada, Y. , 2009, “ Fibulins: Multiple Roles in Matrix Structures and Tissue Functions,” Cell. Mol. Life Sci., 66(11–12), pp. 1890–1902 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Roark, E. F. , Keene, D. R. , Haudenschild, C. C. , Godyna, S. , Little, C. D. , and Argraves, W. S. , 1995, “ The Association of Human Fibulin-1 With Elastic Fibers: An Immunohistological, Ultrastructural, and RNA Study,” J. Histochem. Cytochem., 43(4), pp. 401–411. 10.1177/43.4.7534784 [DOI] [PubMed] [Google Scholar]
- [48]. Reinhardt, D. P. , Sasaki, T. , Dzamba, B. J. , Keene, D. R. , Chu, M. L. , Göhring, W. , Timpl, R. , and Sakai, L. Y. , 1996, “ Fibrillin-1 and Fibulin-2 Interact and Are Colocalized in Some Tissues,” J. Biol. Chem., 271(32), pp. 19489–19496. 10.1074/jbc.271.32.19489 [DOI] [PubMed] [Google Scholar]
- [49]. Yamauchi, Y. , Tsuruga, E. , Nakashima, K. , Sawa, Y. , and Ishikawa, H. , 2010, “ Fibulin-4 and -5, but Not Fibulin-2, Are Associated With Tropoelastin Deposition in Elastin-Producing Cell Culture,” ACTA Histochem. Cytochem., 43(6), pp. 131–138. 10.1267/ahc.10026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Hucthagowder, V. , Sausgruber, N. , Kim, K. H. , Angle, B. , Marmorstein, L. Y. , and Urban, Z. , 2006, “ Fibulin-4: A Novel Gene for an Autosomal Recessive Cutis Laxa Syndrome,” Am. J. Hum. Genet., 78(6), pp. 1075–1080. 10.1086/504304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. McLaughlin, P. J. , Chen, Q. , Horiguchi, M. , Starcher, B. C. , Stanton, J. B. , Broekelmann, T. J. , Marmorstein, A. D. , McKay, B. , Mecham, R. , Nakamura, T. , and Marmorstein, L. Y. , 2006, “ Targeted Disruption of Fibulin-4 Abolishes Elastogenesis and Causes Perinatal Lethality in Mice,” Mol. Cell. Biol., 26(5), pp. 1700–1709. 10.1128/MCB.26.5.1700-1709.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Le, V. P. , Yamashiro, Y. , Yanagisawa, H. , and Wagenseil, J. E. , 2014, “ Measuring, Reversing, and Modeling the Mechanical Changes Due to the Absence of Fibulin-4 in Mouse Arteries,” Biomech. Model. Mechanobiol., 13(5), pp. 1081–1095. 10.1007/s10237-014-0556-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Halabi, C. M. , Broekelmann, T. J. , Lin, M. , Lee, V. S. , Chu, M.-L. , and Mecham, R. P. , 2017, “ Fibulin-4 Is Essential for Maintaining Arterial Wall Integrity in Conduit But Not Muscular Arteries,” Sci. Adv., 3(5), p. e1602532. 10.1126/sciadv.1602532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Loeys, B. , Van Maldergem, L. , Mortier, G. , Coucke, P. , Gerniers, S. , Naeyaert, J.-M. , and De Paepe, A. , 2002, “ Homozygosity for a Missense Mutation in Fibulin-5 (FBLN5) Results in a Severe Form of Cutis Laxa,” Hum. Mol. Genet., 11(18), pp. 2113–2118. 10.1093/hmg/11.18.2113 [DOI] [PubMed] [Google Scholar]
- [55]. Wan, W. , Yanagisawa, H. , and Gleason, R. L., Jr. , 2010, “ Biomechanical and Microstructural Properties of Common Carotid Arteries From Fibulin-5 Null Mice,” Ann. Biomed. Eng., 38(12), pp. 3605–3617. 10.1007/s10439-010-0114-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Ferruzzi, J. , Bersi, M. R. , Uman, S. , Yanagisawa, H. , and Humphrey, J. D. , 2015, “ Decreased Elastic Energy Storage, Not Increased Material Stiffness, Characterizes Central Artery Dysfunction in Fibulin-5 Deficiency Independent of Sex,” ASME J. Biomech. Eng., 137(3), p. 031007. 10.1115/1.4029431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Lucero, H. A. , and Kagan, H. M. , 2006, “ Lysyl Oxidase: An Oxidative Enzyme and Effector of Cell Function,” Cell. Mol. Life Sci., 63(19), pp. 2304–2316. 10.1007/s00018-006-6149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Molnar, J. , Fong, K. S. K. , He, Q. P. , Hayashi, K. , Kim, Y. , Fong, S. F. T. , Fogelgren, B. , Szauter, K. M. , Mink, M. , and Csiszar, K. , 2003, “ Structural and Functional Diversity of Lysyl Oxidase and the LOX-Like Proteins,” Biochim. Biophys. Acta (BBA)-Proteins Proteomics, 1647(1), pp. 220–224. 10.1016/S1570-9639(03)00053-0 [DOI] [PubMed] [Google Scholar]
- [59]. Kuivaniemi, H. , Peltonen, L. , Palotie, A. , Kaitila, I. , and Kivirikko, K. I. , 1982, “ Abnormal Copper Metabolism and Deficient Lysyl Oxidase Activity in a Heritable Connective Tissue Disorder,” J. Clin. Invest., 69(3), pp. 730–733 10.1172/JCI110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Sibon, I. , Sommer, P. , Daniel Lamaziere, J. M. , and Bonnet, J. , 2005, “ Lysyl Oxidase Deficiency: A New Cause of Human Arterial Dissection,” Heart, 91(5), p. e33. 10.1136/hrt.2004.053074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Mäki, J. M. , Räsänen, J. , Tikkanen, H. , Sormunen, R. , Mäkikallio, K. , Kivirikko, K. I. , and Soininen, R. , 2002, “ Inactivation of the Lysyl Oxidase Gene Lox Leads to Aortic Aneurysms, Cardiovascular Dysfunction, and Perinatal Death in Mice,” Circulation, 106(19), pp. 2503–2509. 10.1161/01.CIR.0000038109.84500.1E [DOI] [PubMed] [Google Scholar]
- [62]. Staiculescu, M. C. , Kim, J. , Mecham, R. P. , and Wagenseil, J. , 2017, “ Mechanical Behavior and Matrisome Gene Expression in Aneurysm-Prone Thoracic Aorta of Newborn Lysyl Oxidase Knockout Mice,” Am. J. Physiol. Circ. Physiol., 313(2), pp. H446–H456. 10.1152/ajpheart.00712.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Thorleifsson, G. , Magnusson, K. P. , Sulem, P. , Walters, G. B. , Gudbjartsson, D. F. , Stefansson, H. , Jonsson, T. , Jonasdottir, A. , Jonasdottir, A. , Stefansdottir, G. , Masson, G. , Hardarson, G. A. , Petursson, H. , Arnarsson, A. , Motallebipour, M. , Wallerman, O. , Wadelius, C. , Gulcher, J. R. , Thorsteinsdottir, U. , Kong, A. , Jonasson, F. , and Stefansson, K. , 2007, “ Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma,” Science, 317(5843), pp. 1397–1400. 10.1126/science.1146554 [DOI] [PubMed] [Google Scholar]
- [64]. Liu, X. , Zhao, Y. , Gao, J. , Pawlyk, B. , Starcher, B. , Spencer, J. A. , Yanagisawa, H. , Zuo, J. , and Li, T. , 2004, “ Elastic Fiber Homeostasis Requires Lysyl Oxidase–Like 1 Protein,” Nat. Genet., 36(2), pp. 178–182. 10.1038/ng1297 [DOI] [PubMed] [Google Scholar]
- [65]. Colombatti, A. , Spessotto, P. , Doliana, R. , Mongiat, M. , Bressan, G. M. , and Esposito, G. , 2011, “ The EMILIN/Multimerin Family,” Front. Immunol., 2, p. 93. 10.3389/fimmu.2011.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Zanetti, M. , Braghetta, P. , Sabatelli, P. , Mura, I. , Doliana, R. , Colombatti, A. , Volpin, D. , Bonaldo, P. , and Bressan, G. M. , 2004, “ EMILIN-1 Deficiency Induces Elastogenesis and Vascular Cell Defects,” Mol. Cell. Biol., 24(2), pp. 638–650. 10.1128/MCB.24.2.638-650.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Zacchigna, L. , Vecchione, C. , Notte, A. , Cordenonsi, M. , Dupont, S. , Maretto, S. , Cifelli, G. , Ferrari, A. , Maffei, A. , Fabbro, C. , Braghetta, P. , Marino, G. , Selvetella, G. , Aretini, A. , Colonnese, C. , Bettarini, U. , Russo, G. , Soligo, S. , Adorno, M. , Bonaldo, P. , Volpin, D. , Piccolo, S. , Lembo, G. , and Bressan, G. M. , 2006, “ Emilin1 Links TGF-β Maturation to Blood Pressure Homeostasis,” Cell, 124(5), pp. 929–942. 10.1016/j.cell.2005.12.035 [DOI] [PubMed] [Google Scholar]
- [68]. Weinbaum, J. S. , Broekelmann, T. J. , Pierce, R. A. , Werneck, C. C. , Segade, F. , Craft, C. S. , Knutsen, R. H. , and Mecham, R. P. , 2008, “ Deficiency in Microfibril-Associated Glycoprotein-1 Leads to Complex Phenotypes in Multiple Organ Systems,” J. Biol. Chem., 283(37), pp. 25533–25543. 10.1074/jbc.M709962200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Gibson, M. A. , Leavesley, D. I. , and Ashman, L. K. , 1999, “ Microfibril-Associated Glycoprotein-2 Specifically Interacts With a Range of Bovine and Human Cell Types Via αVβ3 Integrin,” J. Biol. Chem., 274(19), pp. 13060–13065. 10.1074/jbc.274.19.13060 [DOI] [PubMed] [Google Scholar]
- [70]. Werneck, C. C. , Vicente, C. P. , Weinberg, J. S. , Shifren, A. , Pierce, R. A. , Broekelmann, T. J. , Tollefsen, D. M. , and Mecham, R. P. , 2008, “ Mice Lacking the Extracellular Matrix Protein MAGP1 Display Delayed Thrombotic Occlusion Following Vessel Injury,” Blood, 111(8), pp. 4137–4144. 10.1182/blood-2007-07-101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Iozzo, R. V. , and Murdoch, A. D. , 2016, “ Proteoglycans of the Extracellular Environment: Clues From the Gene and Protein Side Offer Novel Perspectives in Molecular Diversity and Function,” FASEB J., 10(5), pp. 598–614.http://www.fasebj.org/content/10/5/598.abstract [PubMed] [Google Scholar]
- [72]. Trask, B. C. , Trask, T. M. , Broekelmann, T. , and Mecham, R. P. , 2000, “ The Microfibrillar Proteins MAGP-1 and Fibrillin-1 Form a Ternary Complex With the Chondroitin Sulfate Proteoglycan Decorin,” Mol. Biol. Cell, 11(5), pp. 1499–1507. 10.1091/mbc.11.5.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Reinboth, B. , Hanssen, E. , Cleary, E. G. , and Gibson, M. A. , 2002, “ Molecular Interactions of Biglycan and Decorin With Elastic Fiber Components: Biglycan Forms a Ternary Complex With Tropoelastin and Microfibril-Associated Glycoprotein 1,” J. Biol. Chem., 277(6), pp. 3950–3957. 10.1074/jbc.M109540200 [DOI] [PubMed] [Google Scholar]
- [74]. Sabatier, L. , Djokic, J. , Hubmacher, D. , Dzafik, D. , Nelea, V. , and Reinhardt, D. P. , 2014, “ Heparin/Heparan Sulfate Controls Fibrillin-1, -2 and -3 Self-Interactions in Microfibril Assembly,” FEBS Lett., 588(17), pp. 2890–2897. 10.1016/j.febslet.2014.06.061 [DOI] [PubMed] [Google Scholar]
- [75]. Papke, C. L. , Tsunezumi, J. , Ringuette, L. J. , Nagaoka, H. , Terajima, M. , Yamashiro, Y. , Urquhart, G. , Yamauchi, M. , Davis, E. C. , and Yanagisawa, H. , 2015, “ Loss of Fibulin-4 Disrupts Collagen Synthesis and Maturation: Implications for Pathology Resulting From EFEMP2 Mutations,” Hum. Mol. Genet., 24(20), pp. 5867–5879. 10.1093/hmg/ddv308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Yanagisawa, H. , and Davis, E. C. , 2010, “ Unraveling the Mechanism of Elastic Fiber Assembly: The Roles of Short Fibulins,” Int. J. Biochem. Cell. Biol., 42(7), pp. 1084–1093. 10.1016/j.biocel.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Kozel, B. A. , Rongish, B. J. , Czirok, A. , Zach, J. , Little, C. D. , Davis, E. C. , Knutsen, R. H. , Wagenseil, J. E. , Levy, M. A. , and Mecham, R. P. , 2006, “ Elastic Fiber Formation: A Dynamic View of Extracellular Matrix Assembly Using Timer Reporters,” J. Cell. Physiol., 207(1), pp. 87–96. 10.1002/jcp.20546 [DOI] [PubMed] [Google Scholar]
- [78]. Milewicz, D. M. , Pyeritz, R. E. , Stanley Crawford, E. , and Byers, P. H. , 1992, “ Marfan Syndrome: Defective Synthesis, Secretion, and Extracellular Matrix Formation of Fibrillin by Cultured Dermal Fibroblasts,” J. Clin. Invest., 89(1), pp. 79–86. 10.1172/JCI115589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Rock, M. J. , Cain, S. A. , Freeman, L. J. , Morgan, A. , Mellody, K. , Marson, A. , Shuttleworth, C. A. , Weiss, A. S. , and Kielty, C. M. , 2004, “ Molecular Basis of Elastic Fiber Formation. Critical Interactions and a Tropoelastin-Fibrillin-1 Cross-Link,” J. Biol. Chem., 279(22), pp. 23748–23758. 10.1074/jbc.M400212200 [DOI] [PubMed] [Google Scholar]
- [80]. Bax, D. V. , Bernard, S. E. , Lomas, A. , Morgan, A. , Humphries, J. , Shuttleworth, C. A. , Humphries, M. J. , and Kielty, C. M. , 2003, “ Cell Adhesion to Fibrillin-1 Molecules and Microfibrils Is Mediated by Alpha 5 Beta 1 and Alpha v Beta 3 Integrins,” J. Biol. Chem., 278(36), pp. 34605–34616. 10.1074/jbc.M303159200 [DOI] [PubMed] [Google Scholar]
- [81]. Broekelmann, T. J. , Kozel, B. A. , Ishibashi, H. , Werneck, C. C. , Keeley, F. W. , Zhang, L. , and Mecham, R. P. , 2005, “ Tropoelastin Interacts With Cell-Surface Glycosaminoglycans Via Its COOH-Terminal Domain,” J. Biol. Chem., 280(49), pp. 40939–40947. 10.1074/jbc.M507309200 [DOI] [PubMed] [Google Scholar]
- [82]. Noda, K. , Dabovic, B. , Takagi, K. , Inoue, T. , Horiguchi, M. , Hirai, M. , Fujikawa, Y. , Akama, T. O. , Kusumoto, K. , Zilberberg, L. , Sakai, L. Y. , Koli, K. , Naitoh, M. , von Melchner, H. , Suzuki, S. , Rifkin, D. B. , and Nakamura, T. , 2013, “ Latent TGF-β Binding Protein 4 Promotes Elastic Fiber Assembly by Interacting With Fibulin-5,” Proc. Natl. Acad. Sci. U. S. A., 110(8), pp. 2852–2857. 10.1073/pnas.1215779110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Kinsey, R. , Williamson, M. R. , Chaudhry, S. , Mellody, K. T. , McGovern, A. , Takahashi, S. , Shuttleworth, C. A. , and Kielty, C. M. , 2008, “ Fibrillin-1 Microfibril Deposition Is Dependent on Fibronectin Assembly,” J. Cell. Sci., 121(16), pp. 2696–2704. 10.1242/jcs.029819 [DOI] [PubMed] [Google Scholar]
- [84]. Zilberberg, L. , Todorovic, V. , Dabovic, B. , Horiguchi, M. , Couroussé, T. , Sakai, L. Y. , and Rifkin, D. B. , 2012, “ Specificity of Latent TGF-β Binding Protein (LTBP) Incorporation Into Matrix: Role of Fibrillins and Fibronectin,” J. Cell. Physiol., 227(12), pp. 3828–3836. 10.1002/jcp.24094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Nallasamy, S. , Yoshida, K. , Akins, M. , Myers, K. , Iozzo, R. , and Mahendroo, M. , 2017, “ Steroid Hormones Are Key Modulators of Tissue Mechanical Function Via Regulation of Collagen and Elastic Fibers,” Endocrinology, 158(4), pp. 950–962. 10.1210/en.2016-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Eoh, J. H. , Shen, N. , Burke, J. A. , Hinderer, S. , Xia, Z. , Schenke-Layland, K. , and Gerecht, S. , 2017, “ Enhanced Elastin Synthesis and Maturation in Human Vascular Smooth Muscle Tissue Derived From Induced-Pluripotent Stem Cells,” Acta Biomater., 52, pp. 49–59. 10.1016/j.actbio.2017.01.083 [DOI] [PubMed] [Google Scholar]
- [87]. Coffin, J. , and Poole, T. , 1988, “ Embryonic Vascular Development: Immunohistochemical Identification of the Origin and Subsequent Morphogenesis of the Major Vessel Primordia in Quail Embryos,” Development, 102(4), pp. 735–748.https://www.ncbi.nlm.nih.gov/pubmed/3048971 [DOI] [PubMed] [Google Scholar]
- [88]. Sato, Y. , 2013, “ Dorsal Aorta Formation: Separate Origins, Lateral-to-Medial Migration, and Remodeling,” Dev. Growth Differ., 55(1), pp. 113–129. 10.1111/dgd.12010 [DOI] [PubMed] [Google Scholar]
- [89]. DeRuiter, M. C. , Poelmann, R. E. , VanMunsteren, J. C. , Mironov, V. , Markwald, R. R. , and Gittenberger-de Groot, A. C. , 1997, “ Embryonic Endothelial Cells Transdifferentiate Into Mesenchymal Cells Expressing Smooth Muscle Actins In Vivo and In Vitro,” Circ. Res., 80(4), pp. 444–451. 10.1161/01.RES.80.4.444 [DOI] [PubMed] [Google Scholar]
- [90]. Frid, M. G. , Kale, V. A. , and Stenmark, K. R. , 2002, “ Mature Vascular Endothelium Can Give Rise to Smooth Muscle Cells Via Endothelial-Mesenchymal Transdifferentiation,” Circ. Res., 90(11), pp. 1189–1196. 10.1161/01.RES.0000021432.70309.28 [DOI] [PubMed] [Google Scholar]
- [91]. Hellstrom, M. , Lindahl, P. , Abramsson, A. , and Betsholtz, C. , 1999, “ Role of PDGF-B and PDGFR-Beta in Recruitment of Vascular Smooth Muscle Cells and Pericytes During Embryonic Blood Vessel Formation in the Mouse,” Development, 126(14), pp. 3047–3055.http://dev.biologists.org/content/126/14/3047 [DOI] [PubMed] [Google Scholar]
- [92]. Dickson, M. C. , Martin, J. S. , Cousins, F. M. , Kulkarni, A. B. , Karlsson, S. , and Akhurst, R. J. , 1995, “ Defective Haematopoiesis and Vasculogenesis in Transforming Growth Factor-Beta 1 Knock Out Mice,” Development, 121(6), pp. 1845–1854.http://dev.biologists.org/content/121/6/1845 [DOI] [PubMed] [Google Scholar]
- [93]. Folkman, J. , and D'Amore, P. A. , 1996, “ Blood Vessel Formation: What Is Its Molecular Basis?,” Cell, 87(7), pp. 1153–1155. 10.1016/S0092-8674(00)81810-3 [DOI] [PubMed] [Google Scholar]
- [94]. Carmeliet, P. , 2000, “ Mechanisms of Angiogenesis and Arteriogenesis,” Nat. Med., 6(4), p. 389. 10.1038/74651 [DOI] [PubMed] [Google Scholar]
- [95]. Gale, N. W. , Dominguez, M. G. , Noguera, I. , Pan, L. , Hughes, V. , Valenzuela, D. M. , Murphy, A. J. , Adams, N. C. , Lin, H. C. , Holash, J. , Thurston, G. , and Yancopoulos, G. D. , 2004, “ Haploinsufficiency of Delta-Like 4 Ligand Results in Embryonic Lethality Due to Major Defects in Arterial and Vascular Development,” Proc. Natl. Acad. Sci. U. S. A., 101(45), pp. 15949–15954. 10.1073/pnas.0407290101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Frid, M. G. , Moiseeva, E. P. , and Stenmark, K. R. , 1994, “ Multiple Phenotypically Distinct Smooth Muscle Cell Populations Exist in the Adult and Developing Bovine Pulmonary Arterial Media In Vivo,” Circ. Res., 75(4), pp. 669–681. 10.1161/01.RES.75.4.669 [DOI] [PubMed] [Google Scholar]
- [97]. Rosenquist, T. H. , McCoy, J. R. , Waldo, K. L. , and Kirby, M. L. , 1988, “ Origin and Propagation of Elastogenesis in the Developing Cardiovascular System,” Anat. Rec., 221(4), pp. 860–871. 10.1002/ar.1092210411 [DOI] [PubMed] [Google Scholar]
- [98]. Rongish, B. J. , Drake, C. J. , Argraves, W. S. , and Little, C. D. , 1998, “ Identification of the Developmental Marker, JB3-Antigen, as Fibrillin-2 and Its de Novo Organization Into Embryonic Microfibrous Arrays,” Dev. Dyn., 212(3), pp. 461–471. [DOI] [PubMed] [Google Scholar]
- [99]. Karnik, S. K. , Brooke, B. S. , Bayes-Genis, A. , Sorensen, L. , Wythe, J. D. , Schwartz, R. S. , Keating, M. T. , and Li, D. Y. , 2003, “ A Critical Role for Elastin Signaling in Vascular Morphogenesis and Disease,” Development, 130(2), pp. 411–423. 10.1242/dev.00223 [DOI] [PubMed] [Google Scholar]
- [100]. Wagenseil, J. E. , and Mecham, R. P. , 2009, “ Vascular Extracellular Matrix and Arterial Mechanics,” Physiol. Rev., 89(3), pp. 957–989. 10.1152/physrev.00041.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Bendeck, M. P. , Keeley, F. W. , and Langille, B. L. , 1994, “ Perinatal Accumulation of Arterial Wall Constituents: Relation to Hemodynamic Changes at Birth,” Am. J. Physiol., 267(6), pp. H2268–H2279.http://www.physiology.org/doi/pdf/10.1152/ajpheart.1994.267.6.H2268 [DOI] [PubMed] [Google Scholar]
- [102]. Langille, L. B. , 1993, “ Remodeling of Developing and Mature Arteries: Endothelium, Smooth Muscle, and Matrix,” J. Cardiovasc. Pharmacol., 21, pp. S11–S17. 10.1097/00005344-199321001-00003 [DOI] [PubMed] [Google Scholar]
- [103]. Gerrity, R. G. , and Cliff, W. J. , 1975, “ The Aortic Tunica Media of the Developing Rat. I. Quantitative Stereologic and Biochemical Analysis,” Lab. Invest., 32(5), pp. 585–600.http://europepmc.org/abstract/med/1127878 [PubMed] [Google Scholar]
- [104]. Wang, Y. , Dur, O. , Patrick, M. J. , Tinney, J. P. , Tobita, K. , Keller, B. B. , and Pekkan, K. , 2009, “ Aortic Arch Morphogenesis and Flow Modeling in the Chick Embryo,” Ann. Biomed. Eng., 37(6), pp. 1069–1081. 10.1007/s10439-009-9682-5 [DOI] [PubMed] [Google Scholar]
- [105]. Kowalski, W. J. , Dur, O. , Wang, Y. , Patrick, M. J. , Tinney, J. P. , Keller, B. B. , and Pekkan, K. , 2013, “ Critical Transitions in Early Embryonic Aortic Arch Patterning and Hemodynamics,” PLoS One, 8(3), p. e60271. 10.1371/journal.pone.0060271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Humphrey, J. D. , Eberth, J. F. , Dye, W. W. , and Gleason, R. L. , 2009, “ Fundamental Role of Axial Stress in Compensatory Adaptations by Arteries,” J. Biomech., 42(1), pp. 1–8. 10.1016/j.jbiomech.2008.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Dobrin, P. B. , Schwarcz, T. H. , and Mrkvicka, R. , 1990, “ Longitudinal Retractive Force in Pressurized Dog and Human Arteries,” J. Surg. Res., 48(2), pp. 116–120. 10.1016/0022-4804(90)90202-D [DOI] [PubMed] [Google Scholar]
- [108]. Wolinsky, H. , and Glagov, S. , 1967, “ A Lamellar Unit of Aortic Medial Structure and Function in Mammals,” Circ. Res., 20(1), pp. 99–111. 10.1161/01.RES.20.1.99 [DOI] [PubMed] [Google Scholar]
- [109]. Wolinsky, H. , 1970, “ Comparison of Medial Growth of Human Thoracic and Abdominal Aortas,” Circ. Res., 27(4), pp. 531–538. 10.1161/01.RES.27.4.531 [DOI] [PubMed] [Google Scholar]
- [110]. Gibbons, C. A. , and Shadwick, R. E. , 1989, “ Functional Similarities in the Mechanical Design of the Aorta in Lower Vertebrates and Mammals,” Experientia, 45(11–12), pp. 1083–1088. 10.1007/BF01950164 [DOI] [PubMed] [Google Scholar]
- [111]. Wagenseil, J. E. , Ciliberto, C. H. , Knutsen, R. H. , Levy, M. A. , Kovacs, A. , and Mecham, R. P. , 2009, “ Reduced Vessel Elasticity Alters Cardiovascular Structure and Function in Newborn Mice,” Circ. Res., 104(10), pp. 1217–1224. 10.1161/CIRCRESAHA.108.192054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. Rhodes, J. M. , and Simons, M. , 2007, “ The Extracellular Matrix and Blood Vessel Formation: Not Just a Scaffold,” J. Cell. Mol. Med., 11(2), pp. 176–205. 10.1111/j.1582-4934.2007.00031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Chen, E. , Larson, J. D. , and Ekker, S. C. , 2006, “ Functional Analysis of Zebrafish Microfibril-Associated Glycoprotein-1 (Magp1) In Vivo Reveals Roles for Microfibrils in Vascular Development and Function,” Blood, 107(11), pp. 4364–4374. 10.1182/blood-2005-02-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Midgett, M. , and Rugonyi, S. , 2014, “ Congenital Heart Malformations Induced by Hemodynamic Altering Surgical Interventions,” Front. Physiol., 5, pp. 1–287. 10.3389/fphys.2014.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Papke, C. L. , and Yanagisawa, H. , 2014, “ Fibulin-4 and Fibulin-5 in Elastogenesis and Beyond: Insights From Mouse and Human Studies,” Matrix Biol., 37, pp. 142–149. 10.1016/j.matbio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Huang, J. , Yamashiro, Y. , Papke, C. L. , Ikeda, Y. , Lin, Y. , Patel, M. , Inagami, T. , Le, V. P. , Wagenseil, J. E. , and Yanagisawa, H. , 2013, “ Angiotensin-Converting Enzyme-Induced Activation of Local Angiotensin Signaling Is Required for Ascending Aortic Aneurysms in Fibulin-4-Deficient Mice,” Sci. Transl. Med., 5(183), p. 183ra58. 10.1126/scitranslmed.3005025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Pratt, B. , and Curci, J. , 2010, “ Arterial Elastic Fiber Structure. Function and Potential Roles in Acute Aortic Dissection,” J. Cardiovasc. Surg. (Torino)., 51(5), pp. 647–656.https://www.minervamedica.it/en/journals/cardiovascular-surgery/article.php?cod=R37Y2010N05A0647 [PubMed] [Google Scholar]
- [118]. Chapman, S. L. , Sicot, F. X. , Davis, E. C. , Huang, J. , Sasaki, T. , Chu, M. L. , and Yanagisawa, H. , 2010, “ Fibulin-2 and Fibulin-5 Cooperatively Function to Form the Internal Elastic Lamina and Protect From Vascular Injury,” Arterioscler. Thromb. Vasc. Biol., 30(1), pp. 68–74. 10.1161/ATVBAHA.109.196725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Yamashiro, Y. , Papke, C. L. , Kim, J. , Ringuette, L.-J. , Zhang, Q.-J. , Liu, Z.-P. , Mirzaei, H. , Wagenseil, J. E. , Davis, E. C. , and Yanagisawa, H. , 2015, “ Abnormal Mechanosensing and Cofilin Activation Promote the Progression of Ascending Aortic Aneurysms in Mice,” Sci. Signal., 8(399), p. ra105. 10.1126/scisignal.aab3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Eberth, J. F. , Cardamone, L. , and Humphrey, J. D. , 2011, “ Evolving Biaxial Mechanical Properties of Mouse Carotid Arteries in Hypertension,” J. Biomech., 44(14), pp. 2532–2537. 10.1016/j.jbiomech.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Eberth, J. F. , Gresham, V. C. , Reddy, A. K. , Popovic, N. , Wilson, E. , and Humphrey, J. D. , 2009, “ Importance of Pulsatility in Hypertensive Carotid Artery Growth and Remodeling,” J. Hypertens., 27(10), pp. 2010–2021. 10.1097/HJH.0b013e32832e8dc8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Majesky, M. W. , 2007, “ Developmental Basis of Vascular Smooth Muscle Diversity,” Arterioscler. Thromb. Vasc. Biol., 27(6), pp. 1248–1258. 10.1161/ATVBAHA.107.141069 [DOI] [PubMed] [Google Scholar]
- [123]. Katsuda, S. , and Kaji, T. , 2003, “ Atherosclerosis and Extracellular Matrix,” J. Atheroscler. Thromb., 10(5), pp. 267–274. 10.5551/jat.10.267 [DOI] [PubMed] [Google Scholar]
- [124]. Maedeker, J. A. , Stoka, K. V. , Bhayani, S. A. , Gardner, W. S. , Bennett, L. , Procknow, J. D. , Staiculescu, M. C. , Walji, T. A. , Craft, C. S. , and Wagenseil, J. E. , 2016, “ Hypertension and Decreased Aortic Compliance Due to Reduced Elastin Amounts Do Not Increase Atherosclerotic Plaque Accumulation in Ldlr-/- Mice,” Atherosclerosis, 249, pp. 22–29. 10.1016/j.atherosclerosis.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Henson, G. D. , Walker, A. E. , Reihl, K. D. , Donato, A. J. , and Lesniewski, L. A. , 2014, “ Dichotomous Mechanisms of Aortic Stiffening in High‐Fat Diet Fed Young and Old B6D2F1 Mice,” Physiol. Rep., 2(3), p. e00268. 10.1002/phy2.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Du, B. , Ouyang, A. , Eng, J. S. , and Fleenor, B. S. , 2015, “ Aortic Perivascular Adipose-Derived Interleukin-6 Contributes to Arterial Stiffness in Low-Density Lipoprotein Receptor Deficient Mice,” Am. J. Physiol. Circ. Physiol., 308(11), pp. H1382–H1390. 10.1152/ajpheart.00712.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127]. Pasquali‐Ronchetti, I. , and Baccarani‐Contri, M. , 1997, “ Elastic Fiber During Development and Aging,” Microsc. Res. Tech., 38(4), pp. 428–435. [DOI] [PubMed] [Google Scholar]
- [128]. Ferruzzi, J. , Bersi, M. R. , Mecham, R. P. , Ramirez, F. , Yanagisawa, H. , Tellides, G. , and Humphrey, J. D. , 2016, “ Loss of Elastic Fiber Integrity Compromises Common Carotid Artery Function: Implications for Vascular Aging,” Artery Res., 14, pp. 41–52. 10.1016/j.artres.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129]. Chung, A. W. Y. , Au Yeung, K. , Sandor, G. G. S. , Judge, D. P. , Dietz, H. C. , and van Breemen, C. , 2007, “ Loss of Elastic Fiber Integrity and Reduction of Vascular Smooth Muscle Contraction Resulting From the Upregulated Activities of Matrix Metalloproteinase-2 and -9 in the Thoracic Aortic Aneurysm in Marfan Syndrome,” Circ. Res., 101(5), pp. 512–522. 10.1161/CIRCRESAHA.107.157776 [DOI] [PubMed] [Google Scholar]
- [130]. Kim, J. , and Wagenseil, J. E. , 2015, “ Bio-Chemo-Mechanical Models of Vascular Mechanics,” Ann. Biomed. Eng., 43(7), pp. 1477–1487. 10.1007/s10439-014-1201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Fung, Y. C. , Fronek, K. , and Patitucci, P. , 1979, “ Pseudoelasticity of Arteries and the Choice of Its Mathematical Expression,” Am. J. Physiol. Circ. Physiol., 237(5), pp. H620–H631. 10.1152/ajpheart.1979.237.5.H620 [DOI] [PubMed] [Google Scholar]
- [132]. Chuong, C. J. , and Fung, Y. C. , 1983, “ Three-Dimensional Stress Distribution in Arteries,” ASME J. Biomech. Eng., 105(3), pp. 268–274. 10.1115/1.3138417 [DOI] [PubMed] [Google Scholar]
- [133]. Takamizawa, K. , and Hayashi, K. , 1987, “ Strain Energy Density Function and Uniform Strain Hypothesis for Arterial Mechanics,” J. Biomech., 20(1), pp. 7–17. 10.1016/0021-9290(87)90262-4 [DOI] [PubMed] [Google Scholar]
- [134]. Vaishnav, R. N. , Young, J. T. , and Patel, D. J. , 1973, “ Distribution of Stresses and of Strain-Energy Density Through the Wall Thickness in a Canine Aortic Segment,” Circ. Res., 32, pp. 577–583. 10.1161/01.RES.32.5.577 [DOI] [PubMed] [Google Scholar]
- [135]. Kas'yanov, V. A. , and Rachev, A. I. , 1980, “ Deformation of Blood Vessels upon Stretching, Internal Pressure, and Torsion,” Mech. Compos. Mater., 16(1), pp. 76–80. 10.1007/BF00618816 [DOI] [Google Scholar]
- [136]. Klika, V. , Gaffney, E. A. , Chen, Y.-C. , and Brown, C. P. , 2016, “ An Overview of Multiphase Cartilage Mechanical Modelling and Its Role in Understanding Function and Pathology,” J. Mech. Behav. Biomed. Mater., 62, pp. 139–157. 10.1016/j.jmbbm.2016.04.032 [DOI] [PubMed] [Google Scholar]
- [137]. Simon, B. R. , Kaufmann, M. V. , McAfee, M. A. , Baldwin, A. L. , and Wilson, L. M. , 1998, “ Identification and Determination of Material Properties for Porohyperelastic Analysis of Large Arteries,” ASME J. Biomech. Eng., 120(2), pp. 188–194. 10.1115/1.2798301 [DOI] [PubMed] [Google Scholar]
- [138]. Johnson, M. , and Tarbell, J. M. , 2001, “ A Biphasic, Anisotropic Model of the Aortic Wall,” ASME J. Biomech. Eng., 123(1), pp. 52–57. 10.1115/1.1339817 [DOI] [PubMed] [Google Scholar]
- [139]. Zoumi, A. , Lu, X. , Kassab, G. S. , and Tromberg, B. J. , 2004, “ Imaging Coronary Artery Microstructure Using Second-Harmonic and Two-Photon Fluorescence Microscopy,” Biophys. J., 87(4), pp. 2778–2786. 10.1529/biophysj.104.042887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140]. O'Connell, M. K. , Murthy, S. , Phan, S. , Xu, C. , Buchanan, J. , Spilker, R. , Dalman, R. L. , Zarins, C. K. , Denk, W. , and Taylor, C. A. , 2008, “ The Three-Dimensional Micro- and Nanostructure of the Aortic Medial Lamellar Unit Measured Using 3D Confocal and Electron Microscopy Imaging,” Matrix Biol., 27(3), pp. 171–181. 10.1016/j.matbio.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. Zulliger, M. A. , Rachev, A. , and Stergiopulos, N. , 2004, “ A Constitutive Formulation of Arterial Mechanics Including Vascular Smooth Muscle Tone,” Am J. Physiol. Heart Circ. Physiol., 287(3), pp. H1335–H1343. 10.1152/ajpheart.00094.2004 [DOI] [PubMed] [Google Scholar]
- [142]. Hill, A. V. , 1938, “ The Heat of Shortening and the Dynamic Constants of Muscle,” Proc. R. Soc. B Biol. Sci., 126(843), pp. 136–195. 10.1098/rspb.1938.0050 [DOI] [Google Scholar]
- [143]. Rachev, A. , and Hayashi, K. , 1999, “ Theoretical Study of the Effects of Vascular Smooth Muscle Contraction on Strain and Stress Distributions in Arteries,” Ann. Biomed. Eng., 27(4), pp. 459–468. 10.1114/1.191 [DOI] [PubMed] [Google Scholar]
- [144]. Alford, P. W. , Humphrey, J. D. , and Taber, L. A. , 2008, “ Growth and Remodeling in a Thick-Walled Artery Model: Effects of Spatial Variations in Wall Constituents,” Biomech. Model. Mechanobiol., 7(4), pp. 245–262. 10.1007/s10237-007-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Spronck, B. , Megens, R. T. A. , Reesink, K. D. , and Delhaas, T. , 2016, “ A Method for Three-Dimensional Quantification of Vascular Smooth Muscle Orientation: Application in Viable Murine Carotid Arteries,” Biomech. Model. Mechanobiol., 15(2), pp. 419–432. 10.1007/s10237-015-0699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146]. Baek, S. , Gleason, R. L. , Rajagopal, K. R. , and Humphrey, J. D. , 2007, “ Theory of Small on Large: Potential Utility in Computations of Fluid-Solid Interactions in Arteries,” Comput. Methods Appl. Mech. Eng., 196(31–32), pp. 3070–3078. 10.1016/j.cma.2006.06.018 [DOI] [Google Scholar]
- [147]. Gasser, T. C. , Ogden, R. W. , and Holzapfel, G. A. , 2006, “ Hyperelastic Modelling of Arterial Layers With Distributed Collagen Fibre Orientations,” J. R. Soc. Interface, 3(6), pp. 15–35. 10.1098/rsif.2005.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148]. Zulliger, M. A. , Fridez, P. , Hayashi, K. , and Stergiopulos, N. , 2004, “ A Strain Energy Function for Arteries Accounting for Wall Composition and Structure,” J. Biomech., 37(7), pp. 989–1000. 10.1016/j.jbiomech.2003.11.026 [DOI] [PubMed] [Google Scholar]
- [149]. Cacho, F. , Elbischger, P. J. , Rodríguez, J. F. , Doblaré, M. , and Holzapfel, G. A. , 2007, “ A Constitutive Model for Fibrous Tissues Considering Collagen Fiber Crimp,” Int. J. Non. Linear. Mech., 42(2), p. 391. 10.1016/j.ijnonlinmec.2007.02.002 [DOI] [Google Scholar]
- [150]. Driessen, N. J. B. , Cox, M. A. J. , Bouten, C. V. C. , Baaijens, F. P. T. , Rufenacht, D. , and Stergiopulos, N. , 2008, “ Remodelling of the Angular Collagen Fiber Distribution in Cardiovascular Tissues,” Biomech. Model. Mechanobiol., 7(2), pp. 93–103. 10.1007/s10237-007-0078-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151]. Stylianopoulos, T. , and Barocas, V. H. , 2007, “ Multiscale, Structure-Based Modeling for the Elastic Mechanical Behavior of Arterial Walls,” ASME J. Biomech. Eng., 129(4), pp. 611–618. 10.1115/1.2746387 [DOI] [PubMed] [Google Scholar]
- [152]. Rezakhaniha, R. , and Stergiopulos, N. , 2008, “ A Structural Model of the Venous Wall Considering Elastin Anisotropy,” ASME J. Biomech. Eng., 130(3), p. 031017. 10.1115/1.2907749 [DOI] [PubMed] [Google Scholar]
- [153]. Kao, P. H. , Lammers, S. R. , Tian, L. , Hunter, K. , Stenmark, K. R. , Shandas, R. , and Qi, H. J. , 2011, “ A Microstructurally Driven Model for Pulmonary Artery Tissue,” ASME J. Biomech. Eng., 133(5), p. 051002. 10.1115/1.4002698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Wang, Y. , Zeinali-Davarani, S. , and Zhang, Y. , 2016, “ Arterial Mechanics Considering the Structural and Mechanical Contributions of ECM Constituents,” J. Biomech., 49(12), pp. 2358–2365. 10.1016/j.jbiomech.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155]. Brown, R. E. , Butler, J. P. , Rogers, R. A. , and Leith, D. E. , 1994, “ Mechanical Connections Between Elastin and Collagen,” Connect Tissue Res., 30(4), pp. 295–308. 10.3109/03008209409015044 [DOI] [PubMed] [Google Scholar]
- [156]. Faffe, D. S. , and Zin, W. A. , 2009, “ Lung Parenchymal Mechanics in Health and Disease,” Physiol. Rev., 89(3), pp. 759–775. 10.1152/physrev.00019.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157]. Chow, M. J. , Turcotte, R. , Lin, C. P. , and Zhang, Y. , 2014, “ Arterial Extracellular Matrix: A Mechanobiological Study of the Contributions and Interactions of Elastin and Collagen,” Biophys. J., 106(12), pp. 2684–2692. 10.1016/j.bpj.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158]. Schriefl, A. J. , Schmidt, T. , Balzani, D. , Sommer, G. , and Holzapfel, G. A. , 2015, “ Selective Enzymatic Removal of Elastin and Collagen From Human Abdominal Aortas: Uniaxial Mechanical Response and Constitutive Modeling,” Acta Biomater., 17, pp. 125–136. 10.1016/j.actbio.2015.01.003 [DOI] [PubMed] [Google Scholar]
- [159]. Skalak, R. , Dasgupta, G. , Moss, M. , Otten, E. , Dullemeijer, P. , and Vilmann, H. , 1982, “ Analytical Description of Growth,” J. Theor. Biol., 94(3), pp. 555–577. 10.1016/0022-5193(82)90301-0 [DOI] [PubMed] [Google Scholar]
- [160]. Cowin, S. C. , and Firoozbakhsh, K. , 1981, “ Bone Remodeling of Diaphysial Surfaces Under Constant Load: Theoretical Predictions,” J. Biomech., 14(7), pp. 471–484. 10.1016/0021-9290(81)90097-X [DOI] [PubMed] [Google Scholar]
- [161]. Rodriguez, E. K. , Omens, J. H. , Waldman, L. K. , and McCulloch, A. D. , 1993, “ Effect of Residual Stress on Transmural Sarcomere Length Distributions in Rat Left Ventricle,” Am. J. Physiol., 264(4), pp. H1048–H1056.http://www.physiology.org/doi/pdf/10.1152/ajpheart.1993.264.4.H1048 [DOI] [PubMed] [Google Scholar]
- [162]. Chuong, C.-J. , and Fung, Y.-C. , 1986, “ Residual Stress in Arteries,” Frontiers in Biomechanics, Springer, New York, pp. 117–129. [Google Scholar]
- [163]. Rodriguez, E. K. , Hoger, A. , and McCulloch, A. D. , 1994, “ Stress-Dependent Finite Growth in Soft Elastic Tissues,” J. Biomech., 27(4), pp. 455–467. 10.1016/0021-9290(94)90021-3 [DOI] [PubMed] [Google Scholar]
- [164]. Taber, L. A. , 1998, “ A Model for Aortic Growth Based on Fluid Shear and Fiber Stresses,” ASME J. Biomech. Eng., 120(3), pp. 348–354 10.1115/1.2798001 [DOI] [PubMed] [Google Scholar]
- [165]. Humphrey, J. D. , and Rajagopal, K. R. , 2002, “ A Constrained Mixture Model for Growth and Remodeling of Soft Tissues,” Math. Model. Methods Appl. Sci., 12(3), pp. 407–430. 10.1142/S0218202502001714 [DOI] [Google Scholar]
- [166]. Gleason, R. L. , Taber, L. A. , and Humphrey, J. D. , 2004, “ A 2-D Model of Flow-Induced Alterations in the Geometry, Structure, and Properties of Carotid Arteries,” ASME J. Biomech. Eng., 126(3), pp. 371–381. 10.1115/1.1762899 [DOI] [PubMed] [Google Scholar]
- [167]. Gleason, R. L. , and Humphrey, J. D. , 2004, “ A Mixture Model of Arterial Growth and Remodeling in Hypertension: Altered Muscle Tone and Tissue Turnover,” J. Vasc. Res., 41(4), pp. 352–363. 10.1159/000080699 [DOI] [PubMed] [Google Scholar]
- [168]. Vena, P. , Gastaldi, D. , Socci, L. , and Pennati, G. , 2008, “ An Anisotropic Model for Tissue Growth and Remodeling During Early Development of Cerebral Aneurysms,” Comput. Mater. Sci., 43(3), pp. 565–577. 10.1016/j.commatsci.2007.12.023 [DOI] [Google Scholar]
- [169]. Valentín, A. , Humphrey, J. D. , and Holzapfel, G. A. , 2011, “ A Multi-Layered Computational Model of Coupled Elastin Degradation, Vasoactive Dysfunction, and Collagenous Stiffening in Aortic Aging,” Ann. Biomed. Eng., 39(7), pp. 2027–2045. 10.1007/s10439-011-0287-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170]. Wagenseil, J. E. , 2011, “ A Constrained Mixture Model for Developing Mouse Aorta,” Biomech. Model. Mechanobiol., 10(5), pp. 671–687. 10.1007/s10237-010-0265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171]. Valentín, A. , Humphrey, J. D. , and Holzapfel, G. A. , 2013, “ A Finite Element-Based Constrained Mixture Implementation for Arterial Growth, Remodeling, and Adaptation: Theory and Numerical Verification,” Int. J. Numer. Method Biomed. Eng., 29(8), pp. 822–849. 10.1002/cnm.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172]. Wan, W. , Hansen, L. , and Gleason, R. L. , 2010, “ A 3-D Constrained Mixture Model for Mechanically Mediated Vascular Growth and Remodeling,” Biomech. Model. Mechanobiol., 9(4), pp. 403–419. 10.1007/s10237-009-0184-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173]. Armstrong, M. H. , Buganza Tepole, A. , Kuhl, E. , Simon, B. R. , and Vande Geest, J. P. , 2016, “ A Finite Element Model for Mixed Porohyperelasticity With Transport, Swelling, and Growth,” PLoS One, 11(4), p. e0152806. 10.1371/journal.pone.0152806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174]. Bazilevs, Y. , Hsu, M. C. , Zhang, Y. , Wang, W. , Liang, X. , Kvamsdal, T. , Brekken, R. , and Isaksen, J. G. , 2010, “ A Fully-Coupled Fluid-Structure Interaction Simulation of Cerebral Aneurysms,” Comput. Mech., 46(1), pp. 3–16. 10.1007/s00466-009-0421-4 [DOI] [Google Scholar]
- [175]. Alberto Figueroa, C. , Baek, S. , Taylor, C. A. , and Humphrey, J. D. , 2009, “ A Computational Framework for Fluid-Solid-Growth Modeling in Cardiovascular Simulations,” Comput. Methods Appl. Mech. Eng., 198(45–46), pp. 3583–3602. 10.1016/j.cma.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]


