Abstract
Firstly described by Robert Douglas Sweet in 1964, febrile neutrophilic dermatosis is a disabling, not only cutaneous disorder, clinically characterised by fever and painful erythematous nodules, with a typical background of neutrophilia. Sweet’s syndrome (SS) is a chronic inflammatory reactive disorder of unknown cause and incompletely established pathogenesis, although an interplay between genetic and environmental factors, including infections, is likely to occur. A significant part of cases has been demonstrated to be linked with malignancies, especially in the hematologic setting. Because of the underlying disease and related therapeutic measures, SS may present atypical clinical course, whereas the response to treatment is strictly dependent on the concurrent hematologic disease. Herein we describe a case of a lady who had a refractory form of SS, resulted in a paraneoplastic cutaneous disease, and AML. Surprisingly, clinical remission of SS followed cytotoxic chemotherapy while hematologic disorder obtained a further complete response.
Keywords: Sweet syndrome, Acute leukaemia, Complete response, Epirubicin
Introduction
Sweet syndrome (SS), or acute febrile neutrophilic dermatosis [1], is a rare inflammatory condition characterised by painful cutaneous nodules and neutrophilic infiltrate in the dermis, in the absence of vasculitis. SS is a potentially disabling disease, significantly associated with malignancies (15-20% of cases). Among these, hematologic neoplasms, particularly acute myeloid leukaemia (AML) and myelodysplastic syndromes are the most commonly reported [2][3].
Herein we describe a case of a lady who had a refractory form of SS, resulted in a paraneoplastic cutaneous disease, and AML. Surprisingly, clinical remission of SS followed cytotoxic chemotherapy while hematologic disorder obtained a further complete response.
Case report
A 72 – year - old woman was admitted in 2015 because of several erythematous lesions on the dorsal surfaces of both the lower extremities. They were initially suspicious for pyoderma gangrenosum, so she was treated with oral prednisone and gentamicin ointment topically, with partial response. Six months later similar lesions recurred at the same anatomical sites, now presenting as erythematous, painful, popular and nodular skin lesions, showing pustule sporadically on the top (Fig 1a, 1b). The patient was afebrile, with slight leucocytosis, Erythrocyte Sedimentation Rate >20mm/hour and positive CRP protein. Anamnestic personal data were positive for COPD and Charcot Marie-Tooth disease, whereas a family history of leukaemia was referred. A skin biopsy was performed, showing a dense neutrophilic dermal infiltrate without vascular aspects of leukocytoclasia; the morphological findings covered the highest number of criteria for the diagnosis of acute neutrophilic dermatosis (Figs 1c, 1d, 1e 1f).
Figure 1.
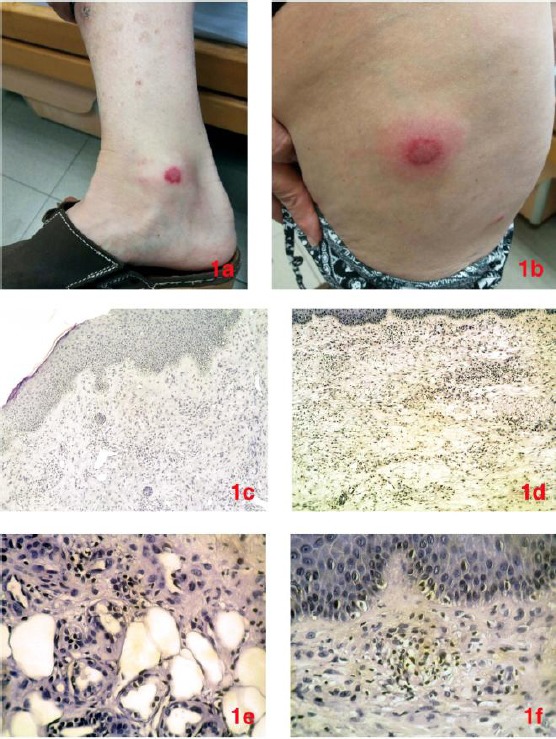
Nodular skin lesions localised at legs (a) and thighs (b). Incisional biopsy revealed normal epidermis and dense inflammatory infiltrate in the dermis (c), mainly composed of lymphocytes and histiocytes (d) [Haematoxylin and eosin stain, x40]. The infiltration extended to the subcutaneous adipose tissue (e), showing a pale neutrophilic background (f) [Haematoxylin and eosin stain, x100]
In the meanwhile, she was commenced on oral prednisone (0.75 mg/kg for five days and gradual tapering of the dosage) achieving only temporary relief of signs and symptoms with recurrence of manifestations. A hematologic consultation was also obtained together with blood exams: anemia [RBC at 2,600,000 mmc (n.r. 4,200,000-5,400,000), HGB at 89 g/L (n.r. 120 - 160), thrombocytopenia [PLT at 42,000 (n.r. 130,000-260,000)] and the presence of blasts, prompted a bone marrow examination that revealed 43% of blasts and multilineage dysplasia. A diagnosis of acute myelogenous leukaemia was made, cytogenetics also supporting the diagnosis through the evidence of deletion of 5q [- 5/del (5q)]. Gene mutations in fms - related tyrosine kinase - 3 (FLT3) gene [FLT3 - internal tandem duplication] were also found.
Supportive care for cytopenia was established, using erythropoietin, red blood cells transfusions with iron chelation therapy and platelet transfusions.
Treatment with cytarabine (200 mg daily for seven days) plus epirubicin (75 mg daily for three days) was started. Methylprednisolone 40 mg/day intravenously was also prescribed and tapered over two weeks. Supportive antibiotics, including vancomycin and meropenem, were added during the hospital stay.
Cytostatic therapy was well tolerated without serious adverse effect, except for neutropenia, which required occasional dose reduction. After the first cycle, a complete remission of cutaneous manifestations of SS happened, with the improvement of blood values and marrow histology after three cycles. The patient is also undergoing the fourth cycle of treatment with further hematologic improvement and no new skin lesions.
Discussion
Sweet’s syndrome, or acute febrile neutrophilic dermatosis, is an uncommon, severe cutaneous condition, characterized by the abrupt development of painful, tender, erythematous plaques, fever greater than 38°C, and a nodular perivascular neutrophilic dermal infiltrate without evidence of vasculitis on histologic examination [1][3].
Although frequently idiopathic, similarly to other diseases in this ‘reactive’ setting4, SS may develop in association with other systemic disorders or in the presence of identifiable triggers, including underlying autoimmune disorders, pregnancy, antecedent vaccination, drug assumption, inflammatory bowel disease, or infections [5][6].
The so-called ‘malignancy-associated Sweet’s syndrome’ accounts for 15-20% of total cases of SS, and occurs in the association, or as a consequence, with both haematological and visceral malignancies [2].
In this setting, AML constitutes the most reported in clinical practice [2][7].
In fact, SS could present as a paraneoplastic cutaneous manifestation or as a drug-induced SS due to medications commonly used in the treatment of AML [8].
The aberrant production of both pro-inflammatory cytokines (IL - 6, TNF - alpha) and signalling molecules, that has been demonstrated in AML and SS, may affect neutrophil function, finally contributing to the dermal clumping of the mature neutrophils [7][9].
According to with literature, SS is relatively rare in general population whereas it occurs in about 1% of patients with AML [6].
It also occurs more commonly in females and at various stages, including pre-diagnosis, at diagnosis, at the commencement of therapy, during remission and with relapse [7][10], thus representing such as a “marker” of AML disease activity.
Dealing with the data by Kazmi et al., [7], about 33% of patients receive the diagnoses of SS and AML at the same time, whereas another third of them during treatment of relapsed disease, and 29% during primary induction of chemotherapy. About diagnostic criteria, anaemia and thrombocytopenia are present in almost all patients, with fever being reported only in two-thirds of cases, as well as neutrophilia and elevated erythrocyte sedimentation rate. Clinical manifestations were classically multifocal, diffuse and asymmetrical. Cytogenetic characterisation revealed mainly changes in chromosome 5 (38% of cases) [7].
Glucocorticoids, either topical or systemic, together with antibiotics and wound care, represent the mainstays of SS therapy [11].
Although they showed to be highly efficacious, some recalcitrant cases may significantly benefit from the treatment of the underlying AML, and of infections, through the restoration of normal granulocyte function.
Our case was consistent with the above-mentioned characteristics. Initial cutaneous manifestations were probably underestimated, because of not typical features of the lesions, and the diagnosis of leukaemia might be obscured by steroid administration.
Anyway, although rare and sometimes difficult to be discovered because of the wide spectrum of differential diagnoses, when SS is established, the physician should keep a high index of suspicion to search underlying malignancies. In the same way, sign and symptoms of SS have to be searched for the diagnosis of AML.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349–56. doi: 10.1111/j.1365-2133.1964.tb14541.x. https://doi.org/10.1111/j.1365-2133.1964.tb14541.x PMid:14201182. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PR, Kurzrock R. Sweet's syndrome and malignancy. Am J Med. 1987;82:1220–6. doi: 10.1016/0002-9343(87)90229-4. https://doi.org/10.1016/0002-9343(87)90229-4. [DOI] [PubMed] [Google Scholar]
- 3.Polimeni G, Cardillo R, Garaffo E, Giardina C, Macrì R, Sirna V, Guarneri C, Arcoraci V. Allopurinol-induced Sweet's syndrome. Int J Immunopathol Pharmacol. 2016;29:329–32. doi: 10.1177/0394632015599705. https://doi.org/10.1177/0394632015599705 PMid:26684631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccaro M, Guarneri F, Borgia F, Cannavò SP, Benvenga S. Association of lichen sclerosus and autoimmune thyroiditis:possible role of Borrelia burgdorferi? Thyroid. 2002;12:1147–8. doi: 10.1089/105072502321085261. https://doi.org/10.1089/105072502321085261 PMid:12593730. [DOI] [PubMed] [Google Scholar]
- 5.Vaccaro M, Guarneri F, Guarneri C. Molecular mimicry:a true pathogenetic concept for T-cell-mediated autoimmune and reactive skin diseases? J Am Acad Dermatol. 2005;52(1):E3. doi: 10.1016/j.jaad.2004.05.045. https://doi.org/10.1016/j.jaad.2004.05.045 PMid:15627073. [DOI] [PubMed] [Google Scholar]
- 6.Vaccaro M, Guarneri F, Guarneri C, Borgia F, Cannavò SP. Sweet's syndrome and erythema nodosum after Klebsiella pneumoniae cystitis. Acta Derm Venereol. 2003;83:290–1. doi: 10.1080/00015550310016562. https://doi.org/10.1080/00015550310016562 PMid:12926802. [DOI] [PubMed] [Google Scholar]
- 7.Kazmi SM, Pemmaraju N, Patel KP, Cohen PR, Daver N, Tran KM, Ravandi F, Duvic M, Garcia-Manero G, Pierce S, Nasha A, Borthakur G, Kantarjian H, Cortes J. Characteristics of Sweet's syndrome in patients with acute myeloid leukaemia. Clin Lymphoma Myeloma Leuk. 2015;15:358–63. doi: 10.1016/j.clml.2014.12.009. https://doi.org/10.1016/j.clml.2014.12.009 PMid:25630528 PMCid:PMC4457594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anzalone CL, Cohen PR. Acute febrile neutrophilic dermatosis (Sweet's syndrome) Curr Opin Hematol. 2013;20:26–35. doi: 10.1097/MOH.0b013e32835ad132. https://doi.org/10.1097/MOH.0b013e32835ad132 PMid:23207661. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez Garcia J, Almagro Sanchez M, Fonseca Capdevila E. Multiple neutrophilic dermatoses in myelodysplastic synfrome. Clin Exp Dermatol. 2001;26:398–401. doi: 10.1046/j.1365-2230.2001.00844.x. https://doi.org/10.1046/j.1365-2230.2001.00844.x PMid:11488825. [DOI] [PubMed] [Google Scholar]
- 10.Cho KH, Han KH, Kim SW, Youn SW, Youn JI, Kim BK. Neutrophilic dermatoses associated with myeloid malignancy. Clin Exp Dermatol. 1997;22:269–73. https://doi.org/10.1111/j.1365-2230.1997.tb01093.x PMid:9604452. [PubMed] [Google Scholar]
- 11.Kannan R, Dutta TK, Goel A, Garg BR, Venkateswaran S, Ratnakar C. Sweet syndrome in chronic myeloid leukaemia. Postgrad Med J. 1995;71(836):383. doi: 10.1136/pgmj.71.836.383-a. https://doi.org/10.1136/pgmj.71.836.383-a PMid:7644412 PMCid:PMC2398149. [DOI] [PMC free article] [PubMed] [Google Scholar]


