Abstract
Since the beginning of the twentieth century, there have been attempts at creating artificial hair to treat baldness. Major evolution took place at the end of 1970’s when, unfortunately, artificial hair treatments were applied without appropriate medical controls, resulting in sub-standard results from the use of unsuitable materials and technique. The large improper use of this technique in North America from no medical personnel and with dangerous fibres led the Food and Drug Administration (FDA) to suspend the procedure in 1983. In Europe, a new trial on artificial hair procedure started at the beginning of 1990’s.
In 1995 the European Union (UE) recognised the artificial hair implant as a legitimate medical treatment and outlined the rules related to that procedure. In 1996, biocompatible fibres (Biofibre®) produced by Medicap® Italy were approved by the UE Authorities and by the Australian Therapeutic Goods Administration (TGA) as medical devices for hair implant. An effective medical protocol was developed during the following years to provide correct guidelines for appropriate treatment, and to reduce possible related complications. Automatic Biofibre® hair implant represents the last achievement in this hair restoration technique with significant advantages for the patients.
Keywords: hair implant, artificial hair implant, hair surgery, alopecia; biofibre
Introduction
The very first experiences with artificial hair implants date back to the beginning of the twentieth- century as recorded by a USA patent [1]. In 1930 Dr. M. Sasagawa reported his experiments with the implantation of cut human hair [2]. Nonetheless, its greatest evolution did not take place until the 1970’s. In 1976, Dr S. Yamada and Dr. K. Fukuta presented their technique to JMJ [3]. From 1976 fierce competition within the North American market resulted in a number of different companies offering inadequately tested fibers for human hair replacement with very negative results [4][5][6]. In those years, the technique was often being performed inappropriately by non-medical operators, in non-medical environments and without any medical protocol. That led to frequent complications (severe infection, inflammation, broken hair embedded in the scalp, etc,) resulting from unsuitable materials and techniques. In the USA, the above situation provoked a Government inquiry followed, in 1983, by a ban on the implantation with special reference to the materials utilized at that time as human hair and colored industrial fibers such as polyester, modacrylic and polyacrylic fibers (FDA, June 13, 1983) [7]. During the following years, some European companies specialized in the bio-medical field started researching on artificial hair in cooperation with University Departments. In 1993 biocompatible fibers (Biofibre®) were developed in Italy by Medicap company. From 1993 onward clinical trials [8][9][10] and histological studies [11][12] were performed with encouraging results, leading to additional research on the biocompatible material field and medical protocol application. In 1995 the UE recognized the artificial hair implant technique as a medical act, setting that strict and ethical medical protocols must be followed to ensure the safety of implants and minimize complications [13]. In consequence of this regulation, all fibers to be used for this practice have to be compulsory approved and certified as CE medical devices. The approval of this methodology was a great advantage for patients since it legally prevented the procedure from being performed by unqualified people. From the beginning of the 2000’s many cases of patients treated by artificial hair implant were worldwide presented to the scientific community [14][15][16][17], getting favorable outcome and interest also for USA doctors. On 2007, a study about the use of Biofibre® hair implant to treat scalp scars was published [18] as an additional indication for this technique. In 2011, Biofibre® hair implant was included in an academic text of cosmetic dermatology [19] at the World Congress of Cosmetic Dermatology (WCOCD). In 2013, the first Automatic machine for Biofibre® hair implant was presented by Medicap Italy with significant advantages for the technique [20]. In 2014 was released the new high-density version of Biofibre®, named as MHD® hair, which allows triple hair quantity with the same number of implants. Such fibres are presently used for the crown area only allowing very mild aftercare and the very quick result, while for the front hairline the single Biofibre® is recommended to ensure a more natural aesthetic result. Recent PubMed publications bring additional evidence of the reliability of the present artificial hair implant technique [21].
Materials
The safety of implant fibres has to be assured. At the same time, good aesthetic quality and durability must also be considered to maintain the expected results over time. The main features required are biocompatibility, resistance to traction, the absence of capillarity, resistance to physical-chemical stress, low tissue trauma, and good aesthetic qualities. Biofibre® medical hair prosthetic fibres meet all the biocompatibility and safety requirements established by international standards committees for medical devices. They are available in 13 colours (Fig.1), with different lengths (15, 30 or 45 centimetres) and in various shapes (straight, wavy, curly and afro) to satisfy different patients requests. Presently, they are also available in the new high-density version, MHD®, which allows for each implant to have three hairs implanted as the final result. This fibre is used for the crown area only, while for the front hairline the single Biofibre® is recommended. The instruments used for the procedure are also very important. The special hooked needles are less traumatic according to diameter and shape. These instruments also ensure easy work for the physician and best comfort for the patient. Automatic Biofibre® hair implant device represents a valuable solution to encounter such requirement.
Figure 1.

Biofibre® is available in 13 colours, with different lengths (15, 30 or 45 cm) and in various shapes (straight, wavy, curly and afro)
Indications
Biofibre® hair implant is carried out thanks to minor surgery. It is indicated for diffuse alopecia or hair thinning both for male and female patients (Fig. 2a-be Fig. 3a-d).
Figure 2.
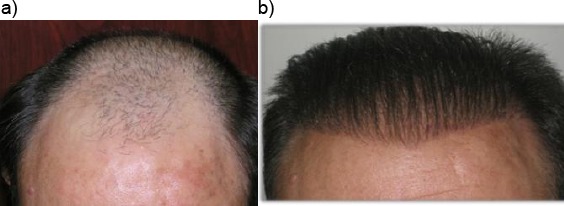
a): Patient with androgenetic alopecia; b): Final results after five implant sessions with 4000 Biofibre® as a whole
Figure 3.
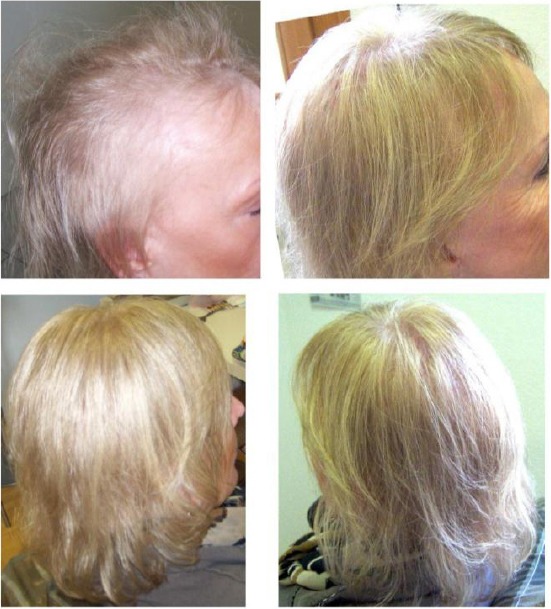
A 53 years old woman with chronic telogen effluvium (upper left); Final results after three implant sessions with 2000 Biofibre® as a whole (upper right, down)
This technique ensures an immediate aesthetic result and sufficient quantity of hair in a short time without requiring any donor area. It is a very soft surgery not requiring hospitalisation and can be used alone or in combination with other hair restoration techniques to improve final aesthetic results when required [15][17] or in case of the poor donor area. It is also performed to correct scars or scalp burns (Fig. 4a-b). This technique is not indicated for implant on the temples, on low frontal hairline, scalp areas with very thin dermal tissue (such as the sideburns) or in case of the pathologically atrophic scalp.
Figure 4.
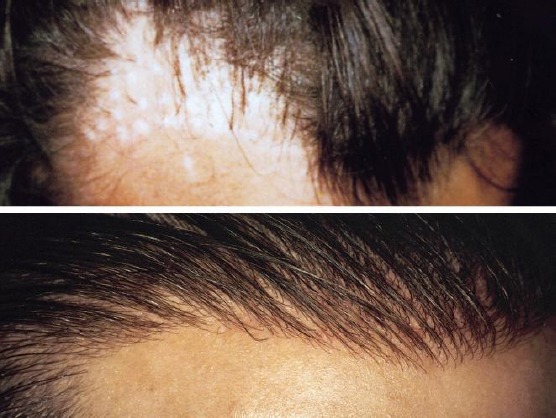
Patient with two frontal scars (upper); Final result after two implant sessions with 1500 Biofibre® as a whole (down)
Patient’s selection
It is important that patients are previously selected and well informed about the correct aftercare protocols and the pros and cons of this procedure. Biofibre® hair implant is not indicated in patients with diabetes mellitus, hepatitis A, B and C, autoimmune diseases, chronic scalp diseases, severe psychosis, not stabilised alopecia areata, when there is lack of personal hygiene, or with employment in dusty or dirty environments. A preliminary screening of the patient, including blood testing, is essential before proceeding with an implanted test. Blood tests list include: Complete blood count, Urea, Creatinine, Bilirubin (total and direct), Gamma-Glutamyl Transferase (GGT), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Fibrinogen, Treponema Pallidum Haemagglutination (TPH), Anti-HIV, Hepatitis A-B and C Markers/Hbsag, Erythrocyte Sedimentation Rate (ESR), Venereal Disease Research Laboratory (VDRL), Prothrombin Time (PT), Partial Thromboplastin Time (PTT), C Reactive Protein, Fasting Blood Sugar Levels, Serum protein electrophoresis, Urinalysis, and Electrocardiogram. Before proceeding with implant sessions, a small number of implant tests must be performed.
They should be observed every one week over a period of at least one month. If not significant problems are observed, larger sessions can be performed at intervals of 1 month each.
Implant Technique and Medical Protocol: From the Stone Age To Now
A correct hair implant procedure requires a combination of safe fibres, suitable implant instruments, trained doctors, careful patient selection, and proper after-care follow-up. Selection of a suitable patient is important. A preliminary visit with tolerance testing allows the exclusion not only of those patients who are not suitable for the fibres implants but also those who have contraindicating skin conditions. Patient must avoid smoking, drinking alcohol for two days before implant, and taking salicylic acid for at least three days before surgery. Before starting the implant, the implant area must be carefully disinfected. The tolerance implant test is performed with 100 fibres and results are evaluated weekly and after four weeks. The implant technique is based on small hooking needles that go out the implanter and hook the Biofibre® root (reversible knot®), placing it under the scalp at galea level. In this way, the root can be held by the fibrous tissue and avoid premature hair loss. The implanter performed by automatic machine allows reaching always the right deepness (Fig. 5). The procedure is performed under local anaesthesia and in one hour up to 600 Biofibre® are usually implanted. A small quantity of local anaesthesia is recommended initially, with repeated administration in case of need. The local anaesthesia commonly used is carbocaine or lidocaine 2% with adrenaline 1:100.000. The suggested quantity of anaesthesia is 1 cc per 200 Biofibre®. The average implant is about 1000 Biofibre® per session, respecting the appropriate distance between each fiber.
Figure 5.
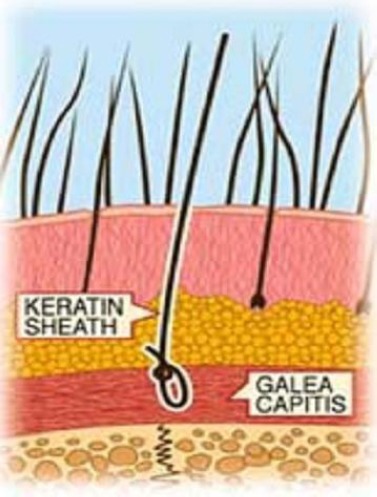
Right implant deepness of Biofibre® performed by automatic machine to allow best retention rate
The implantation is performed to grant the appropriate directional orientation of the fibres to avoid unwished traction and to enhance the final appropriate aesthetic result. Implants performed by the automatic machine minimise implant trauma and hastens cicatrization of the implanted area by allowing a higher degree of fixation, thereby allowing a quicker, better aesthetic result. At the end of the implant procedure, the scalp is disinfected with betadine solution. For large implant sessions, an ice pack protected by sterile gauze is applied for 5 minutes on the scalp. Then the fibres are finally combed with a large toothcomb, holding them at the base to prevent surfacing and the risk of premature fall. The first cleaning with ketoconazole shampoo is suggested after three days. Systemic antibiotic coverage is recommended for one week after the implant, allowing adjustments in therapy depend upon local circumstances and patient’s medical history. The patient must avoid sports, sauna and other activity that can increase sweating for the first three weeks until cicatrisation takes place. Also, should avoid smoking and drinking alcohol for one week after implant session. Before proceeding with the following implant sessions, a four week of pause has to be respected.
Follow Up / After Care Protocol
Maintenance of scalp hygiene and periodic medical check-ups are required to keep the expected aesthetic results. Follow-up is very important to check the patient’s scalp conditions and prevent possible complications such as infection or inflammation. Biofibre® after-care protocol consists of regular follow up, proper scalp hygiene, use of suitable products, and avoidance of dangerous products or treatments such as hair bleaching, permanent waving, thermal shocks, and hair curlers. Various behaviours that can lead to side effects with the implant procedure include lack of patient hygiene or lack of asepsis during the procedure, excessive density or quantity of implanted fibres in one session, a traumatic implant procedure, personal fibre intolerance, incorrect choice of implant area, and failure in after-care procedures. Most of these problems can be solved with appropriate therapies and change of habits. When the problem is recurrent or cannot be easily resolved with appropriate antibiotics and/or corticosteroid therapy, it may be necessary to proceed with fibre removal. The reversible knot of Biofibre® does not allow the fibre to fall out from the implant area, but allow it to be pulled out entirely with appropriate traction with no remains (Fig. 6).
Figure 6.

a Reversible knot of Biofibre® after extraction. No remains stay embedded on the scalp, allowing prompt restitution ad integrum of scalp
This ability to remove the implant contributes to the overall safety of the procedure since once removed; the scalp is healed after several days without scars [14]. Special attention has to be reserved to the scalp sebum.
Sebum is an important and useful natural shield for the scalp, but when it is in excess, it must be removed before it leads to sebum accumulation around implanted fibres that can cause early fibre loss and may predispose the patient to a scalp infection. Sebum accumulation can be avoided by gently massaging the scalp with a soft toothbrush during the shower once every two weeks according to the patient scalp, or they can be easily removed periodically with forceps extraction at the implant clinic. After that, the scalp must be cleansed with an antiseptic spray (e.g. chlorhexidine).
Clinical and Histological Studies
The implant technique was validated with clinical studies and scientific research from the 1990’s onwards [8][9][10][18][21]. Differences in the results achieved during these years represent a constant improvement of the technique and related protocols. A recent clinical study about hair implant conducted on 133 patients (95 men and 38 women) for three years has shown very satisfactory results (Fig. 7a-7c).
Figure 7.
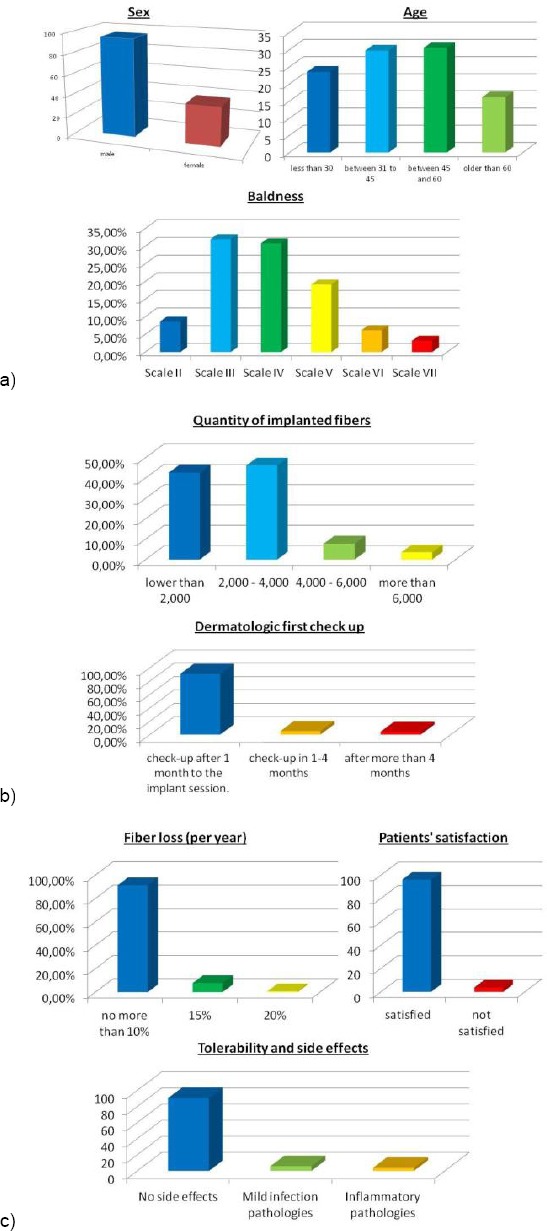
a): clinical features of the patients; b): technique information; c): efficacy and tolerability of implants
The most represented age group of this study was between 30 and 60. These patients underwent implants of up to 6000 Biofibre® (average of 5-6 implants in three months). Fiber loss was not more than 10% per year in 91.4% of the cases, 15% to 7.8% of the cases, and 20% in 0.8% of the cases. 96.2% of the patients stated that they were satisfied with the results of the implant, while 3.8% were not satisfied. As for post-implantation tolerance and complications, 90.3% of the patients recorded no difficulties after surgery. Of the remainder, 5.9% developed mild infections and 3.8% presented with inflammatory changes, mainly from the use of inappropriate chemical substances. Resolution of the scalp infectious and inflammatory issues occurred in an average of 15 days with the use of systemic antibiotics and/or local corticosteroid therapy. In 2.1% of the cases (3 patients), it was necessary to remove the fibres, which was accomplished without leaving any lasting scars.
Histological studies after three years [11][12][21] on Biofibre® (Fig. 8a-8d) have shown that each fibre appears surrounded by a fibrous layer, hindering bacterial penetration. Thin diameters and proper distances between the fibres reduced the occurrence of rejection phenomena. Histopathologically, a sort of infundibulum comprised of Malpighian epithelium, similar to the cutaneous one, formed around the implanted fibre. We can consider it as the basis for adequate fibre anchorage. A moderate, controlled inflammatory infiltrate was noticed. In the middle and deep reticular dermis, the fibre is in contact with collagen. In the hypodermis, no inflammatory infiltrate was noticed.
Figure 8.
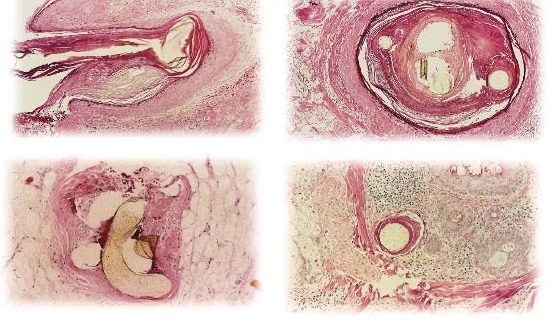
Within the pseudo-infundibula, a compact keratin layer adheres closely to the fibres. In the middle and deep reticular dermis, the fibres are surrounded by a small amount of focally granulomatous chronic infiltrate. In the deepest dermis and hypodermis, the fibres are surrounded by fibroplasia, and no inflammatory infiltrate is noted. (Courtesy of Dr PA Fanti, Lab of Dermatology Histopathology, University of Bologna, Italy)
Discussion
The problem of alopecia affects both sexes and all ages. Between the main surgical techniques for solving the problem of common baldness, the implant of biocompatible fibres Biofibre® has to be listed. This is a non-traumatic technique, which is performed under local anaesthesia and enables the implantation of the desired quantity of hair, the immediate excellent aesthetic result with a natural appearance, and the accompanying psychological and physical rebound. Artificial hair will not age – i.e. it will not turn white. Biofibre® hair implant is indicated to cover thinned and/or bald areas in case of irreversible alopecia. It is also very successful in alopecia of cicatricial origin (scars, burns). Emerging studies are proving its efficacy in case of total alopecia areata. Post-operative patients are advised to avoid hair dyes with ammonia, hair bleaching, permanent waving, excessive heat, hair curler and thermal shocks, and violent brushing. The disadvantages of this technique are that the implanted “hair” will not grow. It needs some periodical re-touches to keep the expected aesthetic result. Patients with unbalanced diabetes, hepatitis, autoimmune diseases, chronic scalp diseases, or unstable forms of alopecia areata are not suitable for this technique. Implant in some scalp areas must be avoided: temples, the low front line on the forehead, and over very thin or atrophic scalp. Spontaneous Biofibre® hair implants loss is very subjective as it is influenced by many factors such as the patient’s scalp and habits, the climate and the implant procedure. The use of automatic Biofibre® hair implant devices reduces the average implant loss since they allow to place the fibres always in the right depth and angle orientation.
Local infections are mostly due to poor hygiene of the patient and lack of the after-care protocol. Local inflammation is normally caused by the use of the patients of improper products. Fiber extractions although very rare, allow a prompt solution of the problem without remains.
Pre-operative patient selection, mutual consent of patient, appropriate implant equipment, asepsis of the operation field, preparation of the patient, correct implant techniques and medical protocols, compatibility tests, implant on suitable implant areas, patient records, post-operative treatment and drug prescription, aftercare patient instructions providing forbidden products and a treatments list, periodical check-ups and post-implant management must be followed.
In conclusion, why Synthetic Hair Implant is approved in many Countries and not in some others is still mysterious. We tried here to provide the main technical changes, which made this technique safe and effective. Biofibre® Hair Implant is a soft surgery technique, performed under local anaesthesia by a manual implanter or by an automatic machine with certified medical fibres. This technique allows immediate aesthetic result, without patient downtime and with relevant psychological comfort for the patient. It can be considered an efficient hair restoration technique for male and female patients in cases of androgenetic alopecia, hair thinning and scars. Conditions for the success of the implant are suitable patient selection, healthy scalp, compliance with the implant and post-implant medical protocol, correct patient after-care and periodical medical check-up. Clinical and histological studies demonstrated that Biofibre® hair Implant is safe and well tolerated by patients, and can be totally reverse if needed. It can be used alone or in combination with other hair restoration techniques. Studies about implant on total alopecia areata are successfully taking place.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.United States Patent Office, Patent #1,059,63, patented on the 22nd of April. 1913 [Google Scholar]
- 2.Sasagawa M. Hairshaft implantation method. Jpn J Dermatol Oncol. 1930;30(5):493. [Google Scholar]
- 3.Fukuta K, Narita I, Jodo T. A New procedure of cosmetic prosthetic surgery for baldness. Jpn J Plast Reconstr Surg. 1976;19:6;613. [Google Scholar]
- 4.Hanke CW, Bergfeld WF. Fiber implantation for pattern baldness. JAMA. 1979;241:146–8. https://doi.org/10.1001/jama.1979.03290280026020. [PubMed] [Google Scholar]
- 5.Schwartz RS, Downham TF. Dangers of synthetic fibre implantation for male pattern baldness. Cutis. 1980;25:491–2. PMid:6991215. [PubMed] [Google Scholar]
- 6.Di Gregorio VR, Rauscher G. Experience with the complications of synthetic hair implantation. Plast Reconst Surg. 1981;68:498–504. doi: 10.1097/00006534-198110000-00003. https://doi.org/10.1097/00006534-198110000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration:Proceed Listing of banned devices, prosthetic hair fibres. Code of Federal Regulations. 1983;21:895. [Google Scholar]
- 8.D'ugo A. Follow-up study of 503 patients three years after surgical implantation of artificial fibres on the scalp, XXV Congreso Argentino di Cirugia Plastica, Buenos Aires, Argentina, November 26-30. 1995 [Google Scholar]
- 9.Zhilina NV, Igytyan GG, Antonova LE. Clinical analyses of the results of treatment of alopecia with the Biofibre®implant methodology. Ann of Plastic Reconstr Aesthetic Surg. 2000;1:34–36. [Google Scholar]
- 10.Palmieri B, Griselli G, Dugo A, Palmieri G, Salti G. Evaluation of polyamide synthetic hair. A long-term clinical study. Panminerva Med. 2000;42(1):49–53. PMid:11019605. [PubMed] [Google Scholar]
- 11.Fanti PA, Pastorale T, D'urso C, Misciali C, Tosti A. Histological study on 5 cases of patients who underwent artificial hair implantation without complications. New Orleans, USA: XXXII Annual Meeting, American Society of Dermatopathology; 1995. Feb 01-03, [Google Scholar]
- 12.Santiago M. Estudo histológico de couro cabeludo implantado com fibras artificiais de poliamida. Goiânia, Brazil: XXXV Congreso Brasileiro de Cirurgia Plástica; 1998. Nov 1-4, [Google Scholar]
- 13.Directive 93/42/EEC of the Council concerning Medical Devices, 14th of June 1993 [Google Scholar]
- 14.D'ugo A, Santiago M, Chaker C, Perez Rangel R, Saad Eddin U, Ramponi V. Safety of Biofibre®CE 0373/TGA artificial hair implants:importance of the scalp anchoring system. Chicago, USA: International Society of Hair Restoration Surgery, 10th Annual Meeting; 2002. Oct 9-13, [Google Scholar]
- 15.Santiago M. Extreme artificial hair implant:Biofibre®treatment of total alopecia. Chicago, USA: International Society of Hair Restoration Surgery, 10th Annual Meeting; 2002. Oct 9-13, [Google Scholar]
- 16.Santiago M. Fiber Hair Restoration –A Surgical Option to Treat Female Pattern Baldness. New York, USA: International Society of Hair Restoration Surgery, 11th Annual Meeting; 2003. Oct 15-19, [Google Scholar]
- 17.Brady G. Cases without Hope –Innovative Solutions in Difficult Cases. Sydney, AUSTRALIA: International Society of Hair Restoration Surgery, 13th Annual Meeting; 2005. Aug 24-28, [Google Scholar]
- 18.Santiago M, Perez Rangel R, D'ugo A, Griselli G, Igitian G, Garcia Martin I, Nesheim GB, Saad Eddin U, Smith G, Brady GW, Chaker C. Artificial Hair Fiber Restoration in the Treatment of Scalp Scars. Derm Surg. 2007;33(1):35–44. doi: 10.1111/j.1524-4725.2007.33005.x. [DOI] [PubMed] [Google Scholar]
- 19.Perez Rangel R. Cabello Inorganico Biocompatible, Dermatologia Cosmética, Texto Académico, I°edicion 2011, presentacion February 2012 min 8th WCOCD [Google Scholar]
- 20.Sheta M. Modern Artificial Hair Implant. London, UK: FACE congress; 2014. Jun 20-22, [Google Scholar]
- 21.Serdev N, D'Erme AM, Hercogovaà J, Zarrab Z, Chokoeva AA, Tchernev G, Wollina U, Lotti T. Polyamide hair implant (Biofibre®):evaluation of efficacy and safety in a group of 133 patients. J Biol Regul Homeiostat Agents. 2015;29(Suppl 1):103–9. PMid:26016977. [PubMed] [Google Scholar]


