Abstract
AIM:
A multi - centre two years the long prospective open clinical study was conducted in five countries located in four different continents from May 2015 to evaluate the clinical safety and efficacy of Automatic Biofibre hair implant in male and female androgenetic alopecia. Biofibre®is a CE/TGA certified medical grade polyamide fibre suitable for implantation.
MATERIAL AND METHODS:
A total of 213 patients were enrolled in the study. Patients were assessed pre -operatively by Hamilton scale grading and the percentage of scalp covered by hair. All the patients underwent Biofibre hair implantation by a standardised surgical technique followed by adequate post-operative care. Efficacy of the implant was evaluated by surgeons and patients bimonthly for the first year and trimonthly during the second year. Any adverse effects were recorded during these visits.
RESULTS:
At the completion of the study period, a total of 194 patients concluded the trial and the results were statistically evaluated. Both Hamilton scale grading and covered area percent improved at the end of the study, and subjective and objective evaluations revealed satisfactory results. Side effects were reported in only 18 cases (9.27%) which were easily controlled by either topical or systemic treatment in 8 to 10 days.
CONCLUSION:
Overall a successful result was noticed in 97.94% of patients with great psychological satisfaction.
Keywords: Biofibre, Hair implant, Alopecia, Baldness, Treatment
Introduction
Clinical experience with synthetic hair implantation began in Japan in the ‘70s and rapidly spread all over the world as the correction of male pattern baldness has always been a much sought-after cosmetic surgical procedure. However, various complications occurred due to the rampant use of this technique by non - qualified personnel, poor patient selection, unsterile operating conditions, and non -biocompatible implant material [1]. Subsequently, in 1983, an FDA ban was issued against the implantation of synthetic fibres, such as monoacrylic, polyacrylic, polyester and natural processed human hair [2], owing to the rising unpopularity for this procedure amongst various dermatologists and surgeons due to the many adverse outcomes encountered [3].
With the advancements in biomedical technology, many biocompatible inorganic materials have come up, which have been more successful and have resulted in many fewer complications. Subsequent studies from Europe focused on biocompatible synthetic fibres and a better technique of insertion with proper medical protocols was conducted to prevent complications such as secondary infection or inflammation [4]. Investigations on suture materials revealed that a specific polyamide mixture resulted in fewer foreign body reactions. These medical-grade polyamides were therefore selected to develop a biocompatible fibre for scalp implantation, a human hair-like, melt dyed fibre suitable for human use (Biofibre®, Medicap srl, Italy).
The UE in 1996 recognised the artificial hair implant technique as medical act and included artificial hair in the medical devices list, therefore submitting it all safety standard requirements [5, 6]. In the same year also Australian TGA approved artificial hair use by qualified doctors in suitable clinics.
Materials and Methods
Biofibre®is a sterile, inert, UV - resistant, highly bio - compatible, medical-grade polyamide fibre which is 0.08 - 0.09 mm thick and 160 - 460 mm long suitable for male and female implantation [7].
Approved colouring agents are incorporated at the molecular level during the liquid extrusion phase by creating a stable compound with no colour migration. Biofibre® hair is available in 13 colours, three different shapes (straight, wave, curly) and three different lengths (15 cm, 30 cm, 45 cm). The fibres can be washed and dried like natural hair, but they should not be bleached or permanently waved. One end of the fibre carries an open knot to anchor it to the scalp tissue and to allow fibrosis. The special reversible knot allows total fibre extraction in case of need with no residues in the scalp. Histological studies have shown that a keratin shield surrounds the implanted fibres facing bacterial introduction [8]. This study began in May 2015, and 213 patients were enrolled in the trial from five investigational centres in agreement with the principles of the Declaration of Helsinki. All patients signed an informed consent form and were included according to the following inclusion and exclusion criteria (Table 1). The patients came from 4 different continents to have trials, resulting in having different climate, habits and ethnicity.
Table 1.
Patient inclusion and exclusion criteria
| Patient inclusion and exclusion criteria | |
|---|---|
| Inclusion Criteria | Exclusion criteria |
| Age 25 to 65 | psychological disorders |
| Clinical diagnosis of androgenetic alopecia and grading with Hamilton scoring | dermatitis or any dermatosis of the scalp |
| Good general health without any other pathology of the scalp | chronic metabolic disorders, immunodeficiencies, allergies |
| Patients willing to return for follow up | patients not willing to return for follow up, or with reduced therapeutic compliance |
| Informed consent | jobs where hygiene could not be guaranteed and maintained |
Demographic and anamnestic information was collected with special regards to previous medical or surgical treatments and any known allergies. All patients underwent a preliminary dermatological checkup followed by biochemical blood profiles. All patients were tested for hypersensitivity by implanting only 100 fibres as a test implant. If there were no hypersensitivity reported in six weeks, 500 - 1200 fibres were further implanted per session at a gap of minimum three weeks till a satisfactory cosmetic result was obtained.
A standard operative technique was used for all the patients in all the centres and a similar post- operative care was provided to avoid bias due to different operators. A special needle- containing automatic implanter was used to implant the fibre. This instrument allows the operator to reach the right depth in the scalp implanting the fibre until the knot reaches the galea capitis. The fibres were spaced at a minimum interval of 2 mm. Special attention and care were paid by the operating surgeon to avoid implanting two fibres at the same point and giving any traction to the implanted fibre to avoid displacement of the fiber superficially. Topical as well as systemic antibiotics were prescribed to all cases for the next seven days. In addition to this, all patients were asked to use an antiseptic shampoo on alternate days for seven days and avoid tar - based shampoos.
The post - operative evaluation included: efficacy (as judged by Hamilton scale grading, covered area percent, surgeon and patient’s subjective evaluation) and safety (as judged by adverse events). Clinical examination and scalp hygiene assessment were done bimonthly for the first year and every three months for the second year. The additional assessment was also done in case of any adverse events.
Results
A total of 213 cases of different origins and ethnicities were enrolled in the study, out of which 194 cases completed the trial. Only 19 cases underwent test implantation and were excluded from the study for the following reasons (three cases developed a hypersensitivity reaction to test implants, six patients opted out due to personal reasons, and ten cases were lost to follow up).
Male patients represented the majority of the study population: 165 men (85.05%) vs 29 female (14.95%). The average age was 42 (± 4.78), with an age range of 25 - 65 years, 133 patients (68.56%) had taken previous treatments for alopecia (Table 2):
Table 2.
Patients with previous alopecia treatments. Is it possible to move this table under the relative text reference
| Topical treatments | 91 patients (46.91%) |
| Systemic treatments | 24 patients (12.37%) |
| Surgery | 18 patients (9.28%) |
Food and/or respiratory allergies were detected in 19 subjects (9.79%). However, in these patients, no hypersensitivities were reported to the test implants. The average number of implanted fibres was 2302 (SD2.805; size 200.9) ranging from 300 to 16000. The average duration of pain and tenderness in the implant area was 2.3 days (SD 4.096; se 0.2933) ranging from 1 - 20 days. A diagrammatic comparison of the Hamilton scale grading before and after the trial showed dramatic improvement (Fig. 1) with the majority of the patients being in Hamilton grade II after implantation.
Figure 1.
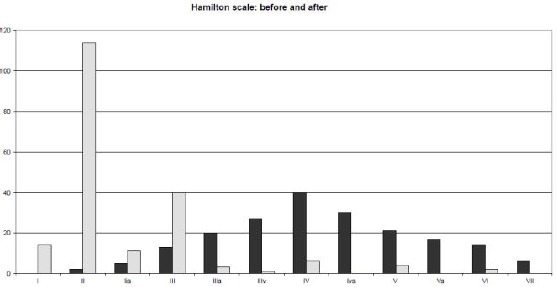
Grey - before; black – after hair implantation
Both subjective (physicians and patients’) and efficacy evaluation data which were recorded on a three-grade scale (1 = slight improvement, 2 = moderate improvement, 3 = marked improvement) showed moderate to marked improvement at the end of the study. Average patients, surgeons’ and efficacy evaluation grades were 2.54, 2.53, and 2.34 respectively.
Overall a successful result was noticed in 97.94% of the patients with psychological satisfaction. Paired T-test for Hamilton Scale Grading and Covered Area Percent gave statistically significant results (Table 3, 4).
Table 3.
Paired T-test for Hamilton Scale Grading
| Paired T-test for Hamilton Scale Grading |
|---|
| average grade before: 4.52 |
| average grade of 2.33 |
| t = 20. 823 |
| p < 0.0001 |
Table 4.
Paired T-test for Covered Area Percent
| Paired T-test for Covered Area Percent |
|---|
| average % before: 61.5 |
| average % after: 85.3 |
| t = -23.3 |
| p < 0.0001 |
An annual rate of 10.61% was noticed. Careful maintenance of post-operative scalp hygiene and use of appropriate products contributes to reducing the fall rate and recurrent folliculitis. These fibres were found safe in 90.73% of cases. Adverse events were clinically classified into three categories (insignificant, mild, and moderate) and were observed in only 18 cases (9.27%), (Table 5, Fig. 2a).
Table 5.
Complication rate in patients
| No. of patients | % | |
|---|---|---|
| Insignificant | 2 | 1.03 |
| Mild | 12 | 6.18 |
| Moderate | 4 | 2.06 |
Figure 2.
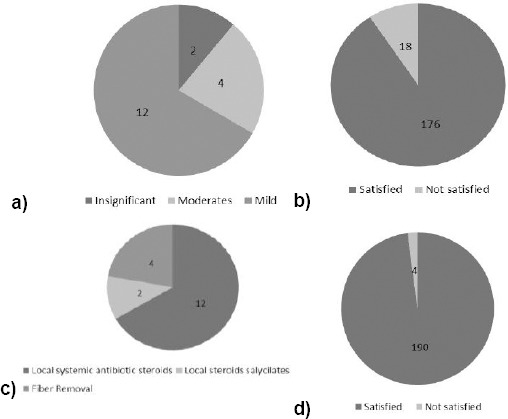
a) Complication; b) results; c) therapy; and d) results after hair implant therapy
Insignificant adverse events like fibre curling were observed in two cases (1.03%). Curling of the fibre is often caused by wrong products application and does not affect the scalp.
Mild side - effects comprising localised slight inflammation and infection were recorded in 12 cases (6.18%) which improved thanks to topical anti-inflammatories and antibiotics with an average healing time of 10.8 days. Staphylococci (aureus and epidermoids), Streptococcus pyogenes, and corynebacteria (acnes and other strains) were the microbial species repeatedly grown in the cultures in these cases. Poor scalp hygiene, excessive perspiration, dirty headgears, secondary seborrhea, inadequate skin care were identified as the main risk factors.
Moderate adverse events observed in four cases (2.06%) also required some fibre removal, when frank abscess or pustular inflammation were not controlled by topical and systemic therapy. In these cases, microbiological cultures revealed Staphylococcus aureus infection and systemic antibiotic therapy (teicoplanin 400 mg. daily, im) was added to the local treatment in order to control infections. No residual damage or permanent scarring was observed during the follow up period in any of these cases. In none of the cases did we record any further complications after healing (Fig. 2 b, c, d).
The results of this clinical trial demonstrate that Biofibre® hair implantation provides satisfactory results in both male and female cases of androgenetic alopecia. Patients with psychological disorders, autoimmune diseases, immunodeficiencies, lack of personal hygiene have to be excluded [9][10].
Figure 3.
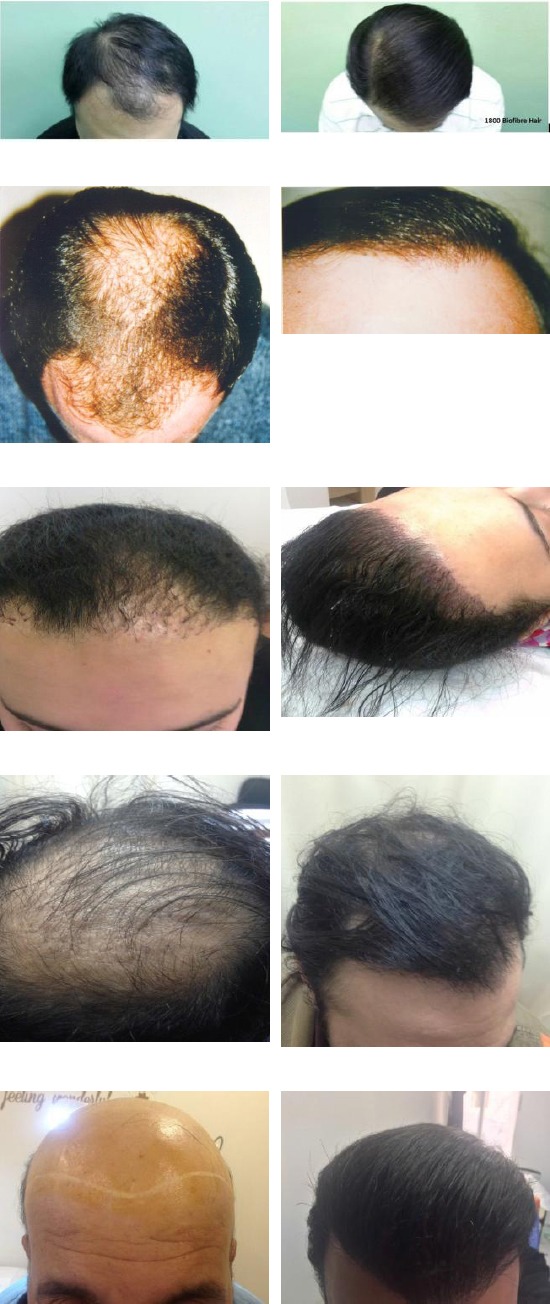
Several patients before (left) and after hair implantation (right)
Use of biocompatible hair implant fibres, modern automatic equipment, careful selection of patients, respect of pre - and post-implant protocol and correct after care are essential requirements to achieve the expected results. If the medical protocol indications are respected, this technique represents a safe and efficacious method to treat androgenetic alopecia with immediate aesthetic results providing immense psychological comfort [11][12][13] to the male and with particular attention to female patients [14]. Use of wrong substances, of unsuitable treatment and a lack of hygiene or correct after-care can compromise the expected result. If problems appear and can’t be successfully treated, a complete fibre removal is performed without residues [15][16]. The Biofibre hair implant procedure can also be performed in combination with other hair restoration techniques such as follicular unit hair transplantation to maximize final aesthetic result or in case of scarce donor - area [17][19]. It is also an appropriate technique to cover scalp scars such as post-burn and post-traumatic scars [20][21]. Several implant tests on alopecia total are and alopecia areata cases are taking place with encouraging results.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Hanke CW, bergfeld WF. Fiber implantation for pattern baldness. JAMA. 1979;241:146–8. https://doi.org/10.1001/jama.1979.03290280026020. [PubMed] [Google Scholar]
- 2.Food and Drug Administration:Listing of banned devices, prosthetic hair fibers, Code of federal regulations. 21:895–101. [Google Scholar]
- 3.Schwartz RS, Downham TF. Dangers of synthetic fiber implantation for male pattern baldness. Cutis. 1980;25:491. PMid:6991215. [PubMed] [Google Scholar]
- 4.D'Ugo A. Hair implantation, the exact method to carry it out. Brasilia, Brazil: 32°Congresso Brasileiro de Cirurgia Plastica; 1995. Nov 11-15, [Google Scholar]
- 5.Directive 93/42/EEC of the Council concerning Medical Devices [Google Scholar]
- 6.Universal Medical Device Nomenclature System, Prostheses, Hair, Universal Medical Device Code, 16611 [Google Scholar]
- 7.Palmieri B, Griselli G, D'ugo A, Palmieri G, Salti G. Evaluation of polyamide synthetic hair. A long-term clinical study. Panminerva Med. 2000;42(1):49–53. PMid:11019605. [PubMed] [Google Scholar]
- 8.Santiago M. Estudo histológico de couro cabeludo implantado com fibras artificiais de poliamida. Goiânia, BRAZIL: XXXV Congreso Brasileiro de Cirurgia Plástica; 1998. Nov 1-4, [Google Scholar]
- 9.Brady G, Fukuta K, Santiago M, Shiell R. Synthetic Fibers in Hair Restoration:Potential Benefits, Patient Selection, Possible Complications. New York, USA: International Society of Hair Restoration Surgery, 11th Annual Meeting; 2003. Oct 15-19, [Google Scholar]
- 10.Rateb A. Twenty years experience with artificial hair insertion for the treatment of alopecia. Paris, France: 19th IMCAS international congress; 2017. Jan 26-29, [Google Scholar]
- 11.Lotti T. Aesthetic Disability. Rome, Italy: SIME 38TH congress; 2017. May 12-14, [Google Scholar]
- 12.Franca K. Current and Emerging Therapies in Hair Transplantation and Hair Implants, Oxford Clinical Handbook, May 2017 [Google Scholar]
- 13.Ramos Et Al. Biofibre Hair Implant – Impact on the Quality of Life. Journal of Biological Regulators & Homeostatic Agents. 2016;30(Suppl 2):21–5. PMid:27373130. [PubMed] [Google Scholar]
- 14.Santiago M. Fiber Hair Restoration –A Surgical Option to Treat Female Pattern Baldness. New York, USA: International Society of Hair Restoration Surgery, 11th Annual Meeting; 2003. Oct 15-19, [Google Scholar]
- 15.Perez Rangel R. Cabello Inorganico Biocompatible, Dermatologia Cosmética , Texto Académico, I°edicion 2011, presentacion February, 2012 min d8th WCOCD [Google Scholar]
- 16.Tchernev G, et al. Biofibre Hair Implant:What is New, What is True? JBRHA. 2016;30(Suppl 2):27–34. PMid:27373131. [PubMed] [Google Scholar]
- 17.Brady G. Cases without Hope – Innovative Solutions in Difficult Cases. Sydney, Australia: International Society of Hair Restoration Surgery, 13th Annual Meeting; 2005. Aug 24-28, [Google Scholar]
- 18.Seery G. Hair Repair. ISHRS Hair Transplant Forum International. 2003;13(1):260–261. [Google Scholar]
- 19.Careno N. Injerto de pelo:quéhacer cuando no hay pelo donante? Santiago, Chile: Jornadas Universitarias de la Asociacion de Dermatologia de Chile; 2015. Jun 23, [Google Scholar]
- 20.Rahoui M. Biofibre et Cicatrice de Brulure du cuir Chevelu, 6°Congres National Algérienne de Chirurgie Esthétique. Algérie: Alger; 2016. Avril. [Google Scholar]
- 21.Santiago M, Perez Rangel R, D'ugo A, Griselli G, Igitian G, Garcia Martin I, Nesheim GB, Saad Eddin U, Smith G, Brady GW, Chaker C. Artificial Hair Fiber Restoration in the Treatment of Scalp Scars. Derm Surg. 2007;33(1):35–44. doi: 10.1111/j.1524-4725.2007.33005.x. [DOI] [PubMed] [Google Scholar]


