Abstract
BACKGROUND:
Postmenopausal women experience undesired symptoms that adversely affect their quality of life. In the recent years, a specific 12 - week fractional CO2 laser treatment has been introduced, with highly significant relief of symptoms.
AIM:
The aim of this paper is the identification of the early modifications of structural components of atrophic vaginal mucosa induced by laser irradiation, which is responsible for the restorative processes.
MATERIAL AND METHODS:
We investigated by microscopical, ultrastructural and biochemical methods the modifications of the structural components of postmenopausal atrophic vaginal mucosa tissues after 1 hour following a single fractional laser CO2 application.
RESULTS:
In one hour, the mucosal epithelium thickens, with the maturation of epithelial cells and desquamation at the epithelial surface. In the connective tissue, new papillae indenting the epithelium with newly formed vessels penetrating them, new thin fibrils of collagen III are also formed in a renewed turnover of components due to the increase of metalloproteinase - 2. Specific features of fibroblasts support stimulation of their activity responsible of the renewal of the extracellular matrix, with an increase of mechanical support as connective tissue and stimulation of growth and maturation to epithelium thanks to new vessels and related factors delivered.
CONCLUSION:
We found the activation of regenerative mechanisms expressed both in the connective tissue - with the formation of new vessels, new papillae, and new collagen - and in the epithelium with the associated thickening and desquamation of cells at the mucosal surface.
Keywords: Vaginal atrophy, Postmenopausal, Fractional CO2 laser, Laser treatment, Regenerative
Introduction
Vaginal atrophy (VA) is common clinical condition due to a decline in ovarian estrogens’ production. In women with VA, it is possible to observe a macroscopic narrowing and shortening of the vaginal canal and a microscopic thinning of the vaginal mucosa (often associated with inflammation).
VA is a physiological process occurring after menopause, but it can develop in some other conditions such as after bilateral ovariectomy, chemotherapy for breast cancer, pelvic radiotherapy, and also during breast - feeding.
Moderate and severe VA can determine genital and urinary symptoms like vaginal dryness, dyspareunia, vaginal burning, dysuria, urinary urgency, urinary incontinence and a higher likelihood to develop urinary tract infections (UTIs).
Different authors have reported the clinical safety and efficacy of a fractional CO2 laser treatment protocol (MonaLisa Touch™), including three office sessions performed at an interval of 30 days one after the other, with a significant improvement in the genital and urinary symptoms [1][2][3][4][5][6] as well as in the sexual function [7]
Salvatore and Zerbinati [1][2][3] described the following important histological changes after this treatment protocol:
a thickening of the epithelium, with the maturation of epithelial cells and desquamation at the epithelial surface as in premenopause;
a new formation of papillae indenting the epithelium with newly formed and extended small vessels;
in the connective tissue underlying the epithelium, the formation of new thin fibrils and morphological features of fibroblasts supporting a renewal of the extracellular matrix with functional restoration.
All these events are part of the regenerative effect on the vaginal tissue of the fractional CO2 laser treatment. However, no - one has yet described its onset, and when and how the initial tissue modifications occur.
In this study, we aimed to identify the first modifications features (1 hour following one laser application) responsible for the starting regenerative mechanisms in vaginal postmenopausal atrophic mucosa after fractional CO2 laser treatment.
Methods
In this study, during a surgical procedure for prolapse in a postmenopausal woman (63 years) and with the informed consent of the patient, we evaluated atrophic vaginal mucosa biopsies, before treatment and 1 hour after a single fractional CO2 laser application. The CO2 laser (SmartXide2 V2LR, Monalisa Touch®, DEKA, Florence, Italy) was set to a single spot size of 200 μm, and an optomechanical scanner was used to induce a spot sequential targeting in each energy pulse (D - pulse) with dot power 30 W, dwell time 1000 μs, dot spacing 1000 μm and stack parameter 2.
Mucosal biopsies (before any treatment as a control, and one hour after laser irradiation) were taken, immediately immersed in the fixative solutions and processed according to standard procedure with paraffin and epoxy resin embedding, respectively for light and electron microscopy. Particular care was used for correct orientation of biopsy samples, both in the embedding and sectioning phases of the preparative procedure, to prevent incorrect observations. The sectioning plane for all preparations was rigorously perpendicular to the epithelial surface, the thickness of all sections (pre - and post-treatment biopsies) was the same (5 µm), and the staining of sections was made at the same time and in the same solutions of dyes.
For light microscopy, some sections were stained with Hematoxylin and Eosin for verifying the correct orientation of mucosa samples, others with trichromic for a general view of the mucosal structural organisation, others with Picrosirius red (see above).
For high resolution light microscopy and electron microscopy, semithin (0.2 μm) and ultrathin sections were cut from epoxy resin (Epon 812) embedded specimens, after fixation with a glutaraldehyde/paraformaldehyde (2.5/2%) solution in Sodium cacodylate buffer followed by Osmium tetroxide 1.33% solution in s - Collidine buffer and dehydration before embedding. Semithin and ultrathin sections were obtained by an ultramicrotome Reichert Ultracut. Semithin and ultrathin sections were stained, respectively, with toluidine blue and uranyl acetate/lead citrate.
Specific analysis of sections for light microscopy was made using a staining method based on Picrosirius red, a dye which not only stains specifically collagen fibres but also enhances the collagen birefringence [8][9][10]. Picrosirius red was used as a solution 0.1% of Sirius red F3B in a saturated aqueous solution of picric acid for one hr at room temperature. The sections to be compared, all with the same thickness, were stained for the same time in the same dye solutions. Fainty staining with Hematoxylin was also used for nuclei.
At the light microscope provided with a setting of circularly polarised light, Picrosirius red stained fibres can be differentiated through a scale of different colours. Birefringent structures (collagen fibres) in the connective tissue of lamina propria were highly visible not only as brilliant structures, but some of them appeared Red/Orange, and others appeared Green/Yellow. In the scale of colours, Red/Orange structures are representing thick collagen fibres, while Green/Yellow is representing thin fibres [11].
On this basis, in the sections of the same thickness (5 µm) observed with circularly polarized light, it has been possible to apply a computerized morphometric analysis through a specific thresholding of the colour scale, permitting the identification and the evaluation of thick collagen fibres (mature), and thin collagen fibres (immature, the most recently formed), these last as expression of the onset of regenerative mechanisms of the extracellular matrix.
Microscopic observations were made at a light microscope Carl Zeiss Axiophot provided, for circular polarising microscopy, with suitable filters in the condenser stage and the microscope tube. Images were recorded through a microscope digital 5 megapixels CCD camera Nikon DS - Fi2.
Computerized morphometric analysis was also performed. As the most reliable tool, it was used ImageJ (NIH, version 1.51a), a well - known software recognised as the standard tool by the international scientific community.
The following different steps were used as ImageJ tools, operative sequence and related meanings are reported: - File open; - Image, Type RGB color; - Adjust, Color Threshold: adjust Hue, Saturation, Brightness for the interactive selection of the Red/Orange or Yellow/Green threshold values to have a mask exactly superposed to the structures of interest to be saved in two different channels.
The Hue upper value for Red/Orange must be separated by a significant wider cleft from the lower Hue value for Yellow/Green to prevent superposition of data and a double evaluation into the two channels of the same structures (a value of 8 was evaluated as good).
In this study the values were:
Red channel: Hue 0 - 34, Saturation 106 - 255, Brightness 106 - 186.
Green channel: Hue 44 - 118, Saturation 106 - 255, Brightness 106 - 186.
- Save as: Red/Orange and Yellow/Green were saved as the single images of the two different channels.
For a correct comparison of results, obtained from sections of the same thickness and stained at the same time and in the same staining solutions, the values of Hue, Saturation, Brightness thresholds were maintained the same for all the microscopic preparations.
In the following step, each one of the images representing the two channels has to be converted into a digital one.
- Image, Type, 8bit before the following step.
- Image, Adjust, Threshold: it means to extract from each selected continuous tone image, a binary image. So the areas of interest corresponding to the defined masks (Red or Green) are constituted by black pixels (signal) on a white background. These images are ready for the measurements.
- Edit, Selection, Select all
- Analyze, Select particles. Select the following parameters: Display results, Summarize, Add to Manager, In situ show. The area % occupied in the reference frame of 2560 x 1920 sq pixels is so obtained and registered for comparison.
Zymography. Vaginal mucosa samples collected by biopsy were suspended in 10 mM phosphate buffer, pH 7.4 (1 mL) and then submitted to homogenization by using a Potter - Elvehjem homogeniser. The homogenate obtained was centrifuged at 12000 rpm for 10 min and the supernatant transferred into Eppendorf tubes and stored at - 80°C until the moment of use. The protein concentration of cellular extracts was determined using the bicinchoninic acid assay [12]. Aliquots of cellular extracts containing 30 µg of proteins were run on 10% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS - PAGE) containing 0.1% gelatin. After electrophoresis and washing with 2.5% Triton X - 100 (30 min), gels were incubated 24 hr at 37°C with 100 mM Tris - HCl, 10 mM CaCl2, pH 7.4. Gels were stained with Coomassie Brilliant Blue and destained with 20% methanol, 10% acetic acid. The proteolytic activities were quantified after gels scan using Image J software.
Results
In this paper, we were able to identify very early structural modifications, at 1 hour after treatment, of vaginal mucosa in a postmenopausal woman treated with one fractional CO2 laser. These modifications will be enhanced and stabilised with more applications in clinical practice, restoring a healthy condition to vaginal mucosa with relief of undesired symptoms affecting women quality of life.
The comparison between two slices from two biopsies samples of the same postmenopausal woman is represented in Figure 1, before (1a) and 1 hour (1b) after fractional CO2 application.
Figure 1.
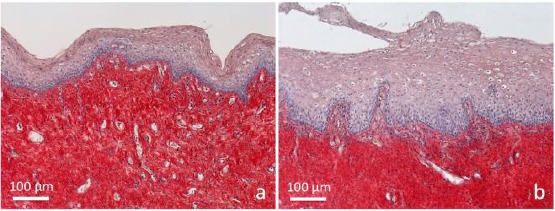
Samples of vaginal mucosa respectively a) before and b) 1 hour after laser application. a) The epithelium does not present any superficial desquamation, and its basal surface appears relatively smooth. The connective tissue is intensely stained; b) the epithelium is thicker, formed by bigger cells distributed in very numerous layers. At the surface, it appears desquamating in the vaginal lumen. The connective tissue is penetrating into epithelial indentations as deep bell-shaped structures constituting newly formed papillae. Papillae are formed by a loose connective tissue with small and thin fibrils inside, faintly stained by Picrosirius red. Many penetrating small vessels, are observable inside them. Light microscopy (not polarised light), sections 5 µm thick. Picrosirius red and fainty Hematoxylin staining
Before treatment (Figure 1a) the vaginal epithelium was constituted by few layers of cells, densely packed, with dark nuclei and few cytoplasm. No signs of epithelial desquamation were detectable. The connective tissue appeared formed by thick and densely packed bundles of collagen fibres. No papillae were detectable.
At 1 hour after laser application (Figure 1b), the epithelium appeared much thicker, formed by numerous layers of cells, bigger and with clearly observable desquamation at the epithelial surface. The connective tissue was also different, with numerous papillae deeply indenting the epithelium. The most superficial part of the connective tissue underlying the epithelium and constituting the papillae appeared lighter; inside it a rich network of small blood vessels was detectable. Inside papillae at higher magnification (Figure 2), blood capillaries appeared differently oriented, some longitudinal, some obliquous, others transversally sectioned.
Figure 2.
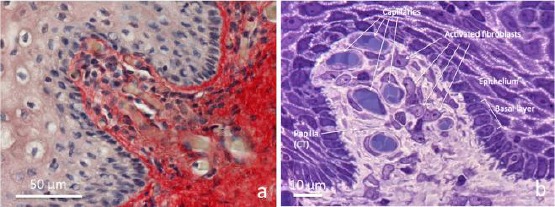
Connective tissue bell-shaped papillae in vaginal mucosa samples 1 hour after laser application. a) Paraffin section (5 µm) stained with Picrosirius red and light Hematoxylin, b) semithin section (0.2 μm) from resin embedded specimen, stained with toluidine blue. a) at the centre towards the high left, the connective tissue of papilla seem “pushing” underneath the epithelium by the growing connective tissue rich in vessels. In the loose and faintly stained connective tissue of papilla, very thin red fibrils between blood capillaries are observable. b) The semithin section permits high-resolution light microscopy. Transverse sections of blood capillaries, small pericytes around endothelium and fibroblasts with clear nuclei and well visible nucleoli, and basophilic cytoplasm are observable. Into the epithelium facing the connective tissue, the basal layer appears organised by a single row of cuboidal cells with basophilic cytoplasm. Light microscopy (not polarised light)
The thin endothelial wall of capillaries appeared surrounded by a light extracellular matrix in which very fine fibrils, often intermingled between them, were well identifiable in Picosirius red stained sections (Figure 2a). The cellularity inside papillae was represented mainly by endothelial cells and fibroblasts.
In semithin sections (0.2 µm) from epoxy resin embedded specimens (Figure 2b), further details on the structures inside papillae were observable. The very thin endothelium of capillaries appeared surrounded by flattened pericytes and the extracellular matrix, particularly rich in ground substance, was faintly stained due to the presence of very thin fibrils widely distributed and differently oriented. Here, big and compacted bundles of collagen fibres were absent. Many fibroblasts were easily identifiable inside papillae, due to clear nuclei rich of euchromatin, in which nucleoli were detectable for their intense basophilia. Their cytoplasm also was basophilic, with a uniformly distributed basophilia. At the electron microscope, the cytoplasm of fibroblasts was particularly rich in profiles of the rough endoplasmic reticulum (RER), represented by a lot of membranous cisternae with many ribosomes attached to the cytoplasmic surface of membranes (Figure 3).
Figure 3.
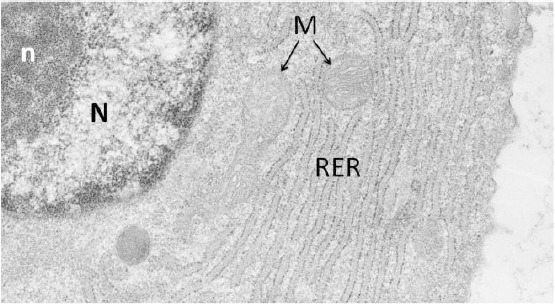
Electron micrograph representing a papillary fibroblast. Part of the nucleus (N) rich in euchromatin is showing inside a compacted nucleolus (n). In the cytoplasm, a highly extended rough endoplasmic reticulum (RER) rich in ribosomes, containing inside cisternae a finely filamentous material, is well visible. M: mitochondria
In the epithelium, the basal layer was well recognisable (Figure 2b), as formed by highly ordered cuboidal cells, which in the living tissue are continuously providing cells both to the same basal layer and to the upper layers of the epithelium for maturation and superficial desquamation. In the lower part of epithelium facing the represented papilla (Figure 2b), the basal surface of the epithelial cells of the basal layer appeared provided with many protrusions, as small feet anchoring to the basement membrane, as into the most of the functionally active premenopausal vaginal epithelium. In the upper third of papilla (Figure 2b), the basal part of epithelial cells appeared smoother, less or not provided with small basal protrusions, as a sign of the dynamic growth of the epithelium in the apical part of the newly formed and still developing papilla.
Picrosirius red staining did not exhaust its potentiality only with selective staining of collagen and enhancement of its birefringence but using circularly polarised microscopy; it permitted the identification and differentiation of big bundles of collagen (mature) and smaller fibres and fibrils (newly formed) through a scale of colours.
We have applied to digital images of microscopic fields of histological preparations, stained with Picrosirius red and observed at the circularly polarised microscope (Figure 4), a suitable colour channel selection for identification of different collagen fibres (Figure 5). As analytically illustrated in the Methods section, we used ImageJ software and computerized morphometric analysis for extracting from the digital images the percent areas occupied by thick fibres appearing Red/Orange and thin fibres appearing Green/Yellow on identical 2560 x 1920 sq pixels standard reference frames. The segmentation thresholds for both control and treated samples were maintained the same.
Figure 4.

Samples of vaginal mucosa respectively a) and c) before, b) and d) 1 hour after laser application. Picrosirius red stained sections. a) and b) observed with not polarised light. a) atrophic mucosa with a relatively thin epithelium formed by small compacted cells, and connective tissue intensely stained. b) epithelium appears much thicker, with big cells desquamating at the epithelial surface. The connective tissue of papilla is mainly formed by thin fibrils. The same sections, observed at the circularly polarised light microscope, are represented in b) and c) respectively. The visible coloured structures are constituted by collagen fibres which, without any additional filter, are showing different colours, appearing red, or orange, or green or yellow. This is due to different collagens and specific interactions with the Sirius red stain. The epithelium is not visible because it does not contain birefringent structures
Figure 5.
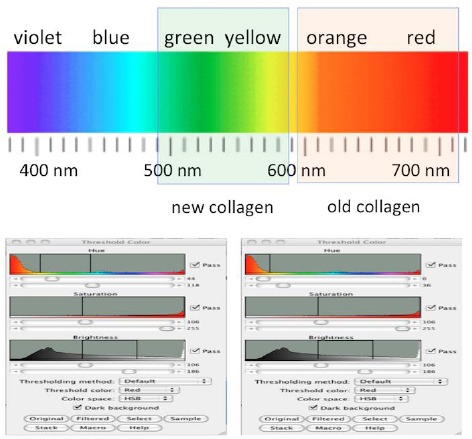
The tool used for computerised morphometric analysis is represented (in detail described in Materials and Methods), with the selected colour bands which have permitted the selected evaluation of the green/yellow and the red/orange fibres. The tool “Adjust colour threshold” was used for segmentation of sampling areas, extracting from each preparation the green/yellow appearing thin fibres and the red/orange appearing thick fibres to be evaluated
The resulting percentage areas of collagen profiles in the Green/Yellow channel and the Red/Orange channel, respectively for controls and treated, are represented in Figure 6.
Figure 6.
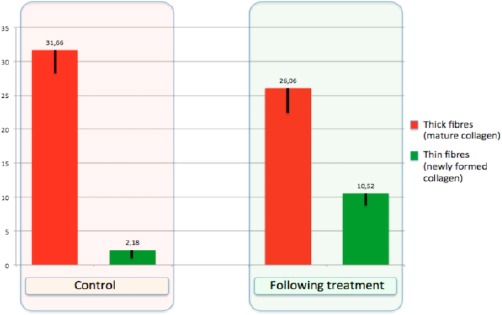
Results from the computerized morphometric analysis performed on the connective tissue of lamina propria in Picrosirius red stained sections. Comparison between the percentage area occupied by thick fibres (red columns, mature collagen) over a reference frame and the percentage of the area occupied by thin fibres (green columns, immature/newly formed collagen) over a corresponding reference frame, respectively in samples controls (before) and in samples 1 hour after fractional CO2 laser treatment
The result of the treatment shows respect to the control, a significant increase of thin fibres (Figure 6), not only in the newly formed papillae but also in the lamina propria of the vaginal mucosa.
At last, the zymographic analysis of MMP - 2 (Figure 7) demonstrated an increase of the active form of MMP - 2 and a corresponding decrease of latent MMP - 2 form, suggesting and confirming the early starting of the enzymatic pathway for collagen turnover.
Figure 7.
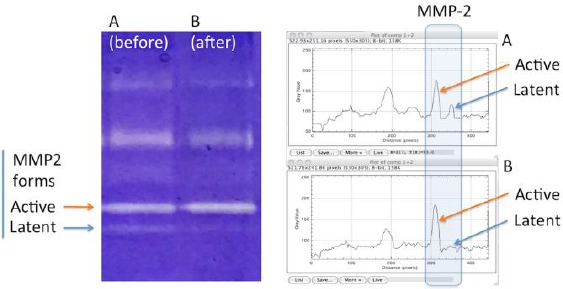
Zymograms obtained by electrophoresis and related densitometry plots of activity bands of active and latent MMP-2 forms from a) control, and b) following treatment
These features are realistically representing the demonstration of the very early onset of the regenerative mechanisms in the connective tissue, with related trophic stimulation of growth and maturation of the epithelium. These regenerative mechanisms, started so early, will be enhanced and then stabilized with following laser applications with relief of symptoms as an effective treatment, consisting in three laser applications with follow up at 12 week [1][2][3][4][5][6], resulting in a long-lasting renewed and rejuvenated mucosa.
Discussion
In the premenopausal fertile age women, the vaginal mucosa is presenting as a well-structured bilayer of tissues, with a squamous stratified, not keratinised epithelium formed by many layers of cells and a lamina propria of connective tissue. Numerous projections of the connective tissue are expanding in the underface of the epithelium forming characteristic indentations as papillae. Papillae constitute very important mechanical and trophic structures. They are representing not only a significant increase of structural link between the epithelium and the connective tissue but also a functional link permitting a much better diffusion of nutrients to the stratified epithelium through a much more extended surface. Through the basement membrane, the connective tissue results firmly anchored to the epithelium through different collagens, fibronectin, proteoglycans, glycosaminoglycans, and glycoproteins synthesised by fibroblasts.
Another highly significant function of papillae is closely related to a rich presence of blood capillaries, permitting a high rate of metabolic support (water, ions, mineral salts, oxygen, etc.) to the epithelial cells, particularly to the intermediate and superficial layers of the very thick stratified epithelium.
In premenopause, the structure of the epithelium is undergoing to cyclic changes during the ovarian cycle, and it is maintained by the influence of estrogens, particularly during the follicular phase. For the most of cycle, epithelial cells are synthesising glycogen, which is stored in the cells and, through maturation and migration toward the surface is delivered by the shedding cells at the epithelial surface, constituting a sort of secretion. The delivery of glycogen, considering that vaginal wall is lacking glands, is very important for maintaining a correct physiological microenvironment supporting vagina health.
When in postmenopausal women estrogens secretion declines and almost completely stops, vaginal mucosa undergoes to dramatic changes, both structural and functional. The epithelium becomes much thinner, constituted by few layers of small cells, the synthesis and storage of glycogen are highly reduced, and shedding rate is very low or absent.
The benefits of fractional CO2 laser treatment of vulvovaginal atrophy in postmenopausal women have been clearly demonstrated in some recent papers concerning a 12 - week treatment with histological analysis at time 0, after 1 month and after 2 months [1][2][3][4][5][6][13]. Also, the effectiveness of this treatment has been demonstrated even in women with a history of breast cancer [14][15].
But very early modifications on atrophic vaginal mucosa treated with laser irradiation - as in this study (1 hour after one fractional CO2 laser application) - have never been reported in the literature.
In this paper, early structural modifications of atrophic vaginal mucosa in postmenopausal women after a very short time (1 hour after one fractional CO2 application) have been studied, to identify the structures as the first involved in the starting of regenerative mechanisms. Also, we have also considered the role of metalloproteinase MMP - 2, well known as an interstitial collagenase [16], by zymography technique, an efficient method based on electrophoresis of bis-acrylamide gels copolymerized with a protein substrate [17]. The degree of digestion of the substrate into the zymograms by purified gelatinase - A (MMP - 2, the matrix metalloprotease of interest extracted from bioptic samples) is proportional to the enzyme loading, and varying the incubation time results in a shift in the linear range of the assay [18]. In this assay system, active and latent forms of MMP - 2 show the same degree of digestion, allowing reliable comparability of quantitative assessment of MMP - 2 activity, as modifications of the of the latent/active forms. In our observations, the increase of MMP - 2 active forms and the corresponding decrease of the latent form in the biopsy 1 hour following treatment, constitutes a significant indication of production and degradation of collagen, as the stimulation of a new equilibrium occurring in the regenerative process of the connective tissue. These results are in some respect similar to those obtained following photobiomodulation energy transfer in the skin [19], even if in the reported paper it was used a photosensitizer and the evaluation of the effects was made after a longer time. The results of zymography analysis concerning MMP - 2, indicating higher expression level of MMP - 2 active form and lower MMP - 2 latent form in the biopsy of mucosa 1 hour following fractional CO2 laser treatment, are in agreement with our histological and morphometric findings showing an increase of thin collagen III fibres and a decrease of the thick collagen I fibres (Figure 6).
Our observations on vaginal mucosa samples are demonstrating that vaginal mucosa tissues were modified, involving both connective tissue and epithelium. The connective tissue, particularly in the most superficial part, appeared as a loose connective tissue rich in ground substance and very fine collagen fibrils (finely stained by Picrosirius red), containing a lot of small vessels. Newly formed well detectable papillae - absent in the atrophic mucosa - expanding underneath the epithelium, are constituting a striking modification of the connective tissue after fractioned CO2 laser application. Realistically, the presence of a lot of vessels into papillae is an important factor stimulating epithelial metabolism. The epithelium, following treatment, is much thicker than control, with intermediate and superficial layers constituted by bigger cells. At the epithelial surface, evident desquamation is detectable (Figure 1b), with cells shedding in the vaginal lumen. These features are consistently supporting a very early restoration onset of the differentiative/maturative mechanisms of epithelial cells.
Related to the possible mechanisms involved in such striking modifications of atrophic vaginal mucosa, some papers on skin treated with fractional CO2 laser - where a connective tissue is closely facing a stratified epithelium (in that case keratinized) and where papillae are constitutive elements - were published supporting the increase in growth factors following CO2 laser application [20][21][22]. One of these reports interesting immunohistochemical evaluations of cytokines delivered in the skin following fractional CO2 irradiation [22]. More specifically, in that paper have been reported increases of TGF - β (stimulating synthesis of matrix proteins, such as collagen), bFGF stimulating angiogenetic activity with endothelial cell migration and proliferation), EGF (stimulating the re-epithelization), PDGF (stimulating fibroblasts to produce extracellular matrix components), and VEGF (regulating vasculogenesis and angiogenesis), starting immediately after laser irradiation, with evaluations performed until to 30 days. Though observed in the skin, the increase in these factors following fractional CO2 laser application, starting immediately after irradiation, well couples with our findings reported in this paper on the very early modifications of atrophic vaginal mucosa following fractional CO2 laser irradiation.
Our microscopic and ultrastructural findings demonstrate the formation of new vessels and the morphological features related to the stimulation of fibroblast activity, with a new production of connective tissue matrix components (as the increase of newly formed thin collagen fibrils), the growth of epithelium and the activation of epithelial cell differentiative mechanisms (glycogen synthesis). In particular, as evident by our observations, the expression of these mechanisms is observed in the epithelium in the suprabasal and in the intermediated layers of cells (big cells with an extended cytoplasm in which begins to be stored newly synthesised glycogen), and in the connective tissue. Inside papillae, particularly, fibroblasts are presenting features - such as an extended rough endoplasmic reticulum (the site of synthesis of procollagen molecules [23], an euchromatic nucleus provided with a clearly detectable nucleolus, and mitochondria (Figure 3) - supporting an active engagement of these cells in the renewal of collagen (and other molecular components of the extracellular matrix).
Owing to our use of the dye Sirius red, Picrosirius red is intensely staining collagen and enhances collagen birefringence [11]. Other Authors demonstrated the advantages of this technique compared to traditional methods [9][11][24][25][26], also useful for studying the differential distribution of the structurally distinct collagen types [11] or the collagenolysis with related stimulation of new collagen synthesis, into vesical prolapse lesions [27].
Due to the variability of the traditional trichrome staining which does not always stain collagen fibres with the same colour and some fibres do not stain at all [26], in contrast, the enhancement of collagen’s natural birefringence by Picrosirius red stain reveals more collagen than can be seen after trichrome staining. With this method, even fine collagen fibres can also be identified due to their enhanced birefringence [26].
The enhancement of birefringence is highly specific for collagen because many Sirius red dye molecules link parallel to the long axis of each collagen molecule, both thick and very thin as single or few tropocollagen molecules [27].
In our study, we used circularly polarised light rather linear polarised light. The advantage of this choice is that all collagen fibres appear bright regardless of their orientation within the section plane [10][26], permitting a reliable quantitative analysis. Owing to the different colours observed, red-orange or green-yellow (Figure 4 c, d), this is due to the degree of polymerisation and packing of collagen molecules and their 3D organisation. Big bundles of highly packed collagen molecules (red/orange) or thin bundles of few molecules of collagen (green/yellow), are reflecting distinct patterns of physical aggregation and the different content of mature, old collagen (collagen I), and immature, newly formed collagen (collagen III) [11][19][27].
Due to the enhancement of natural birefringence of collagen by Picrosirius red staining, it has been possible to detect even very thin collagen fibres, not detectable with other staining methods, as a trichrome stain for example [26]. As thickness and compactness of collagen increases, the colour of the fibre changes from green to yellow, to orange to red. Due to the thickening of collagen fibres as they mature, as in the wound healing, a prevalence of green fibres realistically is indicating a remodelling or a relatively newly formed matrix [26][28][29][30].
Our findings obtained by circular polarising microscopy applied to Picrosirius red stained sections from vaginal mucosa biopsies taken 1 hour after one fractional CO2 laser application, are furtherly supporting the very early starting of regenerative mechanisms. They realistically begin firstly in the connective tissue with the renewal of the extracellular matrix and the formation of new vessels and papillae and develop stimulating the thickening of the epithelium, supporting at the same time the maturation of epithelial cells. A restored synthesis and storage of glycogen occur, followed by desquamation with the delivery of glycogen in the vaginal lumen. Here glycogen is hydrolyzed to glucose, feeding Lactobacilli vaginalis with the production of lactic acid. Lactic acid acidifies the inner vaginal environment restoring the healthy acidic vaginal pH [31][32]. The mucosal surface is so moistened, and at the same time, the colonization of yeasts and potentially pathogenic bacteria are inhibited [31][32].
In conclusion, microscopic modifications of postmenopausal vaginal mucosa 1 hour after fractional CO2 laser treatment have never been reported in the literature.
In this paper, we have presented the onset of very early structural modifications of the epithelium and connective tissues of postmenopausal vaginal mucosa 1 hour following one fractional CO2 laser application. Such modifications are suggesting the activation of regenerative mechanisms expressed both in the connective tissue - with the formation of new vessels, new papillae, and new collagen - and in the epithelium with the associated thickening and desquamation of cells at the mucosal surface. Furthermore, the specific properties of the PicroSirius Red staining which enhances the natural birefringence of collagen, using circular polarising microscopy, permitted to obtain a further reliable demonstration of an effective early remodelling of the extracellular matrix.
In clinical practice, these early restoring modifications will be enhanced and stabilised with a long-lasting effect by subsequent laser applications in an effective, experienced plan as a clinical treatment that ensures to vaginal mucosa of postmenopausal women a renewed healthy condition.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Salvatore S, Nappi RE, Zerbinati N, Calligaro A, Ferrero S, Origoni M, Candiani M, Leone Roberti Maggiore U. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy:a pilot study. Climacteric. 2014;17(4):363–9. doi: 10.3109/13697137.2014.899347. https://doi.org/10.3109/13697137.2014.899347 PMid:24605832. [DOI] [PubMed] [Google Scholar]
- 2.Perino A, Calligaro A, Forlani F, Tiberio C, Cucinella G, Svelato A, Saitta S, Calagna G. Vulvo-vaginal atrophy:a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80(3):296–301. doi: 10.1016/j.maturitas.2014.12.006. https://doi.org/10.1016/j.maturitas.2014.12.006 PMid:25596815. [DOI] [PubMed] [Google Scholar]
- 3.Zerbinati N, Serati M, Origoni M, Candiani M, Iannitti T, Salvatore S, Marotta F, Calligaro A. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30(1):429–36. doi: 10.1007/s10103-014-1677-2. https://doi.org/10.1007/s10103-014-1677-2 PMid:25410301. [DOI] [PubMed] [Google Scholar]
- 4.Sokol ER, Karram MM. An assessment of the safety and efficacy of a fractional CO2 laser system for the treatment of vulvovaginal atrophy. Menopause. 2016;23:1102–7. doi: 10.1097/GME.0000000000000700. https://doi.org/10.1097/GME.0000000000000700 PMid:27404032. [DOI] [PubMed] [Google Scholar]
- 5.Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause:1-year outcomes. Menopause. 2017;24:810–814. doi: 10.1097/GME.0000000000000839. https://doi.org/10.1097/GME.0000000000000839 PMid:28169913. [DOI] [PubMed] [Google Scholar]
- 6.Behnia-Willison F, Sarraf S, Miller J, Mohamadi B, Care AS, Lam A, Willison N, Behnia L, Salvatore S. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39–44. doi: 10.1016/j.ejogrb.2017.03.036. https://doi.org/10.1016/j.ejogrb.2017.03.036 PMid:28419911. [DOI] [PubMed] [Google Scholar]
- 7.Salvatore S, Nappi RE, Parma M, Chionna R, Lagona F, Zerbinati N, Ferrero S, Origoni M, Candiani M, Leone Roberti Maggiore U. Sexual function after fractional microablative CO₂laser in women with vulvovaginal atrophy. Climacteric. 2015;18(2):219–25. doi: 10.3109/13697137.2014.975197. https://doi.org/10.3109/13697137.2014.975197 PMid:25333211. [DOI] [PubMed] [Google Scholar]
- 8.Putchler H, Waldrop FS, Valentine LS. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beith Path. 1973;150:174–187. doi: 10.1016/s0005-8165(73)80016-2. https://doi.org/10.1016/S0005-8165(73)80016-2. [DOI] [PubMed] [Google Scholar]
- 9.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. https://doi.org/10.1007/BF01002772 PMid:91593. [DOI] [PubMed] [Google Scholar]
- 10.Frohlich MW. Birefringent objects visualized by circular polarization microscopy. Stain Technol. 1986;61:139–143. doi: 10.3109/10520298609110723. https://doi.org/10.3109/10520298609110723 PMid:3523835. [DOI] [PubMed] [Google Scholar]
- 11.Junqueira LCU, Cossermelli W, Brentani R. () Differential staining of collagens type I, II and III by sirius red and polarization microscopy. Arch. Histol. Jap. 1978;41:267–274. doi: 10.1679/aohc1950.41.267. https://doi.org/10.1679/aohc1950.41.267 PMid:82432. [DOI] [PubMed] [Google Scholar]
- 12.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. https://doi.org/10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 13.Salvatore S, Leone Roberti Maggiore U, Athanasiou S, Origoni M, Candiani M, Calligaro A, Zerbinati N. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue:an ex vivo study. Menopause. 2015;22(8):845–849. doi: 10.1097/GME.0000000000000401. https://doi.org/10.1097/GME.0000000000000401 PMid:25608269. [DOI] [PubMed] [Google Scholar]
- 14.Pagano T, De Rosa P, Vallone R, Schettini F, Arpino G, De Placido S, Nazzaro G, Locci M, De Placido G. Fractional microablative CO2 laser for vulvovaginal atrophy in women treated with chemotherapy and/or hormonal therapy for breast cancer:a retrospective study. Menopause. 2016;23:1108–13. doi: 10.1097/GME.0000000000000672. https://doi.org/10.1097/GME.0000000000000672 PMid:27648595. [DOI] [PubMed] [Google Scholar]
- 15.Pieralli A, Fallani MG, Becorpi A, Bianchi C, Corioni S, Longinotti M, Tredici Z, Guaschino S. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch Gynecol Obstet. 2016;294:841–6. doi: 10.1007/s00404-016-4118-6. https://doi.org/10.1007/s00404-016-4118-6 PMid:27170261. [DOI] [PubMed] [Google Scholar]
- 16.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–6. doi: 10.1074/jbc.270.11.5872. https://doi.org/10.1074/jbc.270.11.5872 PMid:7890717. [DOI] [PubMed] [Google Scholar]
- 17.Lisboa RA, Andrade MV, Cunha-Melo JR. Zimography is an effective method for detection of matrix metalloproteinase 2 (MMP-2) activity in cultured human fibroblasts. Acta Cir Bras. 2013;28:216–20. doi: 10.1590/s0102-86502013000300010. https://doi.org/10.1590/S0102-86502013000300010 PMid:23503864. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography:detection of picogram quantities of gelatinases. Anal Biochem. 1994;218:325–9. doi: 10.1006/abio.1994.1186. https://doi.org/10.1006/abio.1994.1186 PMid:8074288. [DOI] [PubMed] [Google Scholar]
- 19.de Jesus PD, Saeki SI, Tedesco AC. An ex vivo study of photobiostimulation in the treatment of skin pathologies. J Biophotonics. 2016;9:1189–1198. doi: 10.1002/jbio.201500288. https://doi.org/10.1002/jbio.201500288 PMid:26992152. [DOI] [PubMed] [Google Scholar]
- 20.Nowak KC, McCormack M, Koch RJ. The effect of superpulsed carbon dioxide laser energy on keloid and normal dermal fibroblast secretion of growth factors:a serum-free study. Plast Reconstr Surg. 2000;105(6):2039–2048. doi: 10.1097/00006534-200005000-00019. https://doi.org/10.1097/00006534-200005000-00019 PMid:10839401. [DOI] [PubMed] [Google Scholar]
- 21.Manolis EN, Kaklamanos IG, Spanakis N, Filippou DK, Panagiotaropoulos T, Tsakris A, Siomos K. () Tissue concentration of transforming growth factor b1 and basic fibroblast growth factor in skin wounds created with a CO2 laser and scalpel:a comparative experimental study, using an animal model of skin resurfacing. Wound Repair Regen. 2007;15:252–257. doi: 10.1111/j.1524-475X.2007.00212.x. https://doi.org/10.1111/j.1524-475X.2007.00212.x PMid:17352758. [DOI] [PubMed] [Google Scholar]
- 22.Prignano F, Campolmi P, Bonan P, Ricceri F, Cannarozzo G, Troiano M, Lotti T. Fractional CO2 laser:a novel therapeutic device upon photobiomodulation of tissue remodeling and cytokine pathway of tissue repair. Dermatol Ther. 2009;22(Suppl 1):S8–15. doi: 10.1111/j.1529-8019.2009.01265.x. https://doi.org/10.1111/j.1529-8019.2009.01265.x PMid:19891690. [DOI] [PubMed] [Google Scholar]
- 23.Stephens DJ. Collagen secretion explained. Nature. 2012;482:474–5. doi: 10.1038/482474a. https://doi.org/10.1038/482474a PMid:22358830 PMCid:PMC3566552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93:27–29. doi: 10.1007/BF00266843. https://doi.org/10.1007/BF00266843 PMid:2482274. [DOI] [PubMed] [Google Scholar]
- 25.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Research in Cardiology. 1994;89:397–410. doi: 10.1007/BF00788278. https://doi.org/10.1007/BF00788278 PMid:7535519. [DOI] [PubMed] [Google Scholar]
- 26.Whittaker P. Polarised light microscopy in biomedical research. Microsc Analysis. 1995;33:13–15. [Google Scholar]
- 27.Borges LF, Gutierrez PS, Marana HRC, Taboga SR. Picrosirius-polarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron. 2007;38:580–583. doi: 10.1016/j.micron.2006.10.005. https://doi.org/10.1016/j.micron.2006.10.005 PMid:17126553. [DOI] [PubMed] [Google Scholar]
- 28.Vidal BC, Mello ML, Pimentel ER. Polarization microscopy and microspectrophotometry of Sirius Red, Picrosirius and Chlorantine Fast Red aggregates and of their complexes with collagen. Histochem J. 1982;14:857–78. doi: 10.1007/BF01005229. https://doi.org/10.1007/BF01005229 PMid:6184330. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker P, Boughner DR, Kloner RA. Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol. 1989;134:879–93. PMid:2705508 PMCid:PMC1879777. [PMC free article] [PubMed] [Google Scholar]
- 30.Rich L, Whittaker P. Collagen and picrosirius red staining:a polarized light assessment of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 2005;22:97–104. [Google Scholar]
- 31.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87–94. doi: 10.4065/mcp.2009.0413. https://doi.org/10.4065/mcp.2009.0413 PMid:20042564 PMCid:PMC2800285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Athanasiou S, Pitsouni E, Antonopoulou S, Zacharakis D, Salvatore S, Falagas ME, Grigoriadis T. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19:512–518. doi: 10.1080/13697137.2016.1212006. https://doi.org/10.1080/13697137.2016.1212006 PMid:27558459. [DOI] [PubMed] [Google Scholar]


