Abstract
Background
The aim of the study was to assess the association between chronic kidney disease (CKD) and obesity in predicting CKD among Chinese adults, distinguishing between 5 different adiposity indices: visceral fat index (VFI), percentage body fat (PBF), body mass index (BMI), waist circumference (WC) and waist-to-height ratio (WHtR).
Methods
A total of 29,516 participants aged 35 years or above were selected using a stratified multistage random sampling method across China during 2012–2015. CKD was defined as an estimated glomerular filtration (eGFR) < 60 ml/min/1.72m2.
Results
The overall weighted prevalence of CKD was 3.94% (3.62% in males and 4.25% in females). All five adiposity indices had significant negative correlations to eGFR (P < 0.05). The area under the ROC (receiver operating characteristic) curves (AUC) for PBF was almost significantly larger than the other adiposity indices (P < 0.001). In addition, PBF yielded the highest Youden index in identifying CKD (male: 0.15; female: 0.20). In the logistic analysis, PBF had the highest crude odds ratios (ORs) in both males (OR: 1.819, 95% CI 1.559–2.123) and females (OR: 2.268, 95% CI 1.980–2.597). After adjusted for age, smoking status, alcohol use, education level, marital status, rural vs. urban area, geographic regions, and diagnosis of hypertension, diabetes mellitus, myocardial infarction and stroke, the ORs on PBF remained significant for both genders (P < 0.05).
Conclusions
Obesity is associated with an increased risk of CKD. Furthermore, PBF was a better predictor for identifying CKD than other adiposity indices (BMI, WC, WHtR, and VFI).
Electronic supplementary material
The online version of this article (10.1186/s12882-018-0837-1) contains supplementary material, which is available to authorized users.
Keywords: Chronic kidney disease, Adiposity indices, Percentage body fat, Chinese adults, Cross-sectional study
Background
Chronic kidney disease (CKD) has been a major global health problem. It also plays an important role in development of end-stage renal disease (ESRD) [1], all-cause mortality [2], and non-vascular health outcomes [3]. Currently, CKD is the 12th highest cause of death (14% of all deaths) worldwide [4]. A large representative survey in the United States has shown that the prevalence of CKD among adults aged ≥45 years’ was 11.6% [5]. In China, the overall prevalence of CKD among adults aged 18 years or older was 10.8% [6].
Obesity is becoming a global health concern. Over the past decade, changes in dietary and physical activity patterns has led to an increase in obesity prevalence, especially in large cities [7]. Previous studies have shown that the prevalence of obesity increased from 4.0% in 1993 to 10.7% in 2009 among Chinese adults [8]. An increasing body of evidence suggests that obesity, a risk factor of kidney disease, had a direct impact on the development of CKD and end stage renal disease (ESRD) [9]. Among many indicators of obesity, body mass index (BMI) and waist circumference (WC) have been widely used to define general and abdominal obesity, respectively [10]. However, BMI cannot differentiate between lean and fat mass, and WC does not account for the effect of height on risk [11]. Waist-to-height ratio (WHtR) was introduced as an alternative to WC. Additional obesity indicators include percentage body fat (PBF), which is defined as the percentage of individual fat mass over body weight [12]; and visceral fat index (VFI), an accurate and reliable indicator for evaluating body fat stored around important organs, such as the liver, intestines and pancreas [11].
The identification of CKD risk factors is essential, especially in the face of an increasing prevalence of CKD and obesity. Currently, it is unknown which of the existing adiposity indices is best for predicting CKD. Previous studies have only considered one or two types of adiposity measurement in relation to CKD [13–15]. Thus, we conducted this cross-sectional study in Chinese adults to assess the association between obesity and CKD, and compared the performance of five adiposity indices (BMI, WC, WHtR, PBF and VFI) to predict CKD.
Methods
Study population
This cross-sectional investigation was conducted from 2012 to 2015. This cross-sectional study was a part of a 2012–2015 survey on the prevalence of hypertension in China, spanning 31 provinces with 262 cities/counties; a detailed description of sampling methods has been previously published [13]. The 262 cities and counties were stratified into Eastern, Middle and Western regions, based on economic development status. Using simple random sampling (SRS), 16 cities and 17 counties were selected for this study, including 7 cities and 7 counties in the Eastern region, 6 cities and 6 counties in the Middle region, and 3 cities and 4 counties in the Western region. To meet the designed sample size of 35,000 participants aged ≥35 years and take non-response into account in the survey 56,000 subjects were randomly selected from the eligible sites. As a result, 34,994 participants completed the survey, the overall response rate was 62.5%. After excluded the subjects without blood pressure (n = 414), demographic information (n = 485), adiposity indices (n = 1836), laboratory test values (n = 2743), 29,516 were included in the final analysis. All the participants gave written informed consent prior to data collection. The ethical review committee of Fuwai Hospital (Beijing, China) and each participating center (Additional file 1) approved the study’s protocol.
Adiposity indices
All staff involved in the study were trained and certified prior to survey implementation, according to a uniform protocol and operation manual. Height was measured to the nearest 5 mm with a standard stadiometer without shoes in the standing position. WC was measured to the nearest 5 mm directly touching the participant’s skin using a cloth tape. Weight was measured to the nearest 0.1 kg with light clothing and no shoes. PBF and VFI were measured using an OMRON body fat and weight measurement device (V-body HBF-371, OMRON, Kyoto, Japan). BMI was calculated as weight (in kilograms) divided by the square of height (in meters). WHtR was calculated as WC in centimeters divided by height in centimeters.
Glomerular filtration rate assessment
Fasting blood samples were collected in the morning after 10-12 h fasting. The samples were processed properly and refrigerated immediately. All samples were analyzed in a designated central laboratory (Beijing Adicon Clinical Laboratories, INC, Beijing, China). The serum creatinine was measured using Jaffe’s kinetic method. Estimated glomerular filtration rate (eGFR) was calculated using the equation originating from the modified Modification of Diet in Renal Disease formula for Chinese patients [14]: eGFR = 175*Scr-1.234*age-0.179 [if female, *0.79], where Scr is serum creatinine concentration (mg/dL) and age is in years. CKD was defined as eGFR< 60 ml/min/1.73m2.
Covariate measurements
Eligible participants completed the standardized questionnaire through face-to-face interview by trained staff to obtain information on demographic characteristics, lifestyle factors (i.e. smoking and alcohol use) and comorbidities (hypertension, diabetes mellitus, myocardial infarction and stroke). Smoking status was defined as a person who smoked in the past month or total cigarette consumption more than 20 packages in the lifetime. Alcohol use was defined as a person who consumed more than one alcoholic drink per week in the last 30 days. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, and/or diastolic blood pressure (DBP) ≥ 90 mmHg, and/or self-reported history of antihypertensive medications for treatment of hypertension in the last 2 weeks. Diabetes mellitus was defined as participants with previously diagnosed diabetes and used insulin or hypoglycemic agents, or had a fasting blood glucose ≥7.0 mmol/L.
Statistical analysis
Continuous variables were presented as means ± standard deviations (SDs) and compared using the Student’s t-test or Wilcoxon rank-test. Categorical variables were presented as numbers (percentages) and compared using Chi-square test. The Pearson correlation coefficient was used to assess the correlation between each of the adiposity indices (BMI, WC, WHtR, PBF and VFI) and eGFR.
Receiver operating characteristics curve (ROC) analyses for both genders were used to determine the optimal cut-off values of each adiposity index for CKD with the maximum Youden index (sensitivity+specificity-1). Additionally, the areas under the ROC curve (AUCs) of these five adiposity indices were used to determine their reliability as predictive markers of CKD; statistical significance determined by applying the method of DeLong et al. [15] using MedCalc version 11.4.2.0.
Each adiposity index was divided into lower and higher level groups according to optimal gender- specific cut-off values performed in following ROC analyses. The association between the adiposity and CKD in both genders was calculated using multiple logistic regression analyses after adjusting age, education level, marital status, areas (rural/urban), region (eastern, middle and western region), smoking status, alcohol use, hypertension, diabetes mellitus, myocardial infarction and stroke.
The data analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina, USA). Sample weights were adjusted for non-response, total population (according to 2010 Chinese census) and region to obtain Chinese nationally representative prevalence estimates. All P values were two-sided, P < 0.05 was considered statistically significant.
Results
A total of 29,516 participants (13,410 males, and 16,106 females) were included in this study with a mean age of 56.48 ± 13.13 years. The overall weighted prevalence of CKD was 3.94% (3.62% in male and 4.25% in female).
Table 1 presents the characteristics of individuals included in the current analysis. The prevalence of CKD in both male and female tended to increase with age (P for trend < 0.001). Furthermore, we found that participants who had hypertension, diabetes mellitus, myocardial infarction or stroke had a higher risk of CKD in both genders (all P < 0.001). In addition, the values of BMI, WC, WHtR, PBF, and VFI almost consistently higher among participants with CKD, while only WHtR, PBF and VFI had statistical significant in both genders (all P < 0.001).
Table 1.
Baseline characteristics of study population
| Male (n = 13,410) | Female (n = 16,106) | Total (n = 29,516) | |||||
|---|---|---|---|---|---|---|---|
| eGFR≥ 60 | eGFR< 60 | P | eGFR≥ 60 | eGFR< 60 | P | ||
| Age (year) | |||||||
| 35~ 44 | 2942 (23.11) | 17 (2.49) | < 0.0001 | 3953 (25.99) | 23 (2.57) | < 0.0001 | 6935 (23.50) |
| 45~ 54 | 3001 (23.58) | 40 (5.87) | 3950 (25.97) | 61 (6.82) | 7052 (23.89) | ||
| 55~ 64 | 2938 (23.08) | 95 (13.93) | 3453 (22.70) | 144 (16.11) | 6630 (22.46) | ||
| 65~ 74 | 2476 (19.45) | 237 (34.75) | 2566 (16.87) | 295 (33.00) | 5574 (18.88) | ||
| ≥ 75 | 1371 (10.77) | 293 (42.96) | 1290 (8.48) | 371 (41.50) | 3325 (11.27) | ||
| Urban | 5870 (46.12) | 305 (44.72) | 0.4760 | 6636 (43.62) | 405 (45.30) | 0.3250 | 13,216 (44.78) |
| Region | |||||||
| Eastern | 5464 (42.93) | 302 (44.28) | < 0.0001 | 6549 (43.05) | 339 (37.92) | < 0.0001 | 12,654 (42.87) |
| Middle | 5200 (40.85) | 220 (32.26) | 5814 (38.22) | 283 (31.66) | 11,517 (39.02) | ||
| Western | 2064 (16.22) | 160 (23.46) | 2849 (18.73) | 272 (30.43) | 5345 (18.11) | ||
| Higher Education (> 12 years) | 957 (7.52) | 46 (6.74) | 0.4540 | 847 (5.57) | 24 (2.68) | < 0.0001 | 1874 (6.35) |
| Married | 11,640 (91.47) | 547 (80.21) | < 0.0001 | 13,263 (87.19) | 565 (63.20) | < 0.0001 | 26,015 (88.14) |
| Smoking status | 5861 (46.05) | 226 (33.14) | < 0.0001 | 516 (3.39) | 42 (4.70) | 0.0380 | 6645 (22.51) |
| Alcohol use | 4946 (38.86) | 157 (23.02) | < 0.0001 | 541 (3.56) | 28 (3.13) | 0.5040 | 5672 (19.22) |
| Hypertension | 5184 (40.73) | 440 (64.52) | < 0.0001 | 5603 (36.83) | 589 (65.88) | < 0.0001 | 11,816 (40.03) |
| DM | 1281 (10.06) | 114 (16.72) | < 0.0001 | 1298 (8.53) | 152 (17.00) | < 0.0001 | 2845 (9.64) |
| MI | 97 (0.76) | 17 (2.49) | < 0.0001 | 93 (0.61) | 15 (1.68) | < 0.0001 | 222 (0.75) |
| Stroke | 340 (2.67) | 54 (7.92) | < 0.0001 | 266 (1.75) | 40 (4.47) | < 0.0001 | 700 (2.37) |
| Weight (kg) | 67.61 ± 11.19 | 65.65 ± 11.90 | < 0.0001 | 59.45 ± 9.99 | 57.83 ± 10.78 | < 0.0001 | 63.05 ± 11.36 |
| BMI (kg/m2) | 24.40 ± 3.36 | 24.28 ± 3.64 | 0.3585 | 24.70 ± 3.64 | 24.80 ± 4.03 | 0.4103 | 24.56 ± 3.53 |
| WC (cm) | 85.43 ± 9.93 | 85.73 ± 10.77 | 0.4479 | 82.31 ± 10.04 | 84.75 ± 11.34 | < 0.0001 | 83.81 ± 10.17 |
| WHtR | 0.51 ± 0.06 | 0.52 ± 0.06 | 0.0003 | 0.53 ± 0.07 | 0.56 ± 0.07 | < 0.0001 | 0.52 ± 0.06 |
| PBF | 24.92 ± 5.34 | 26.59 ± 5.51 | < 0.0001 | 33.25 ± 5.23 | 35.16 ± 5.78 | < 0.0001 | 29.56 ± 6.75 |
| VFI | 11.04 ± 4.79 | 12.02 ± 5.59 | < 0.0001 | 7.87 ± 4.08 | 9.12 ± 4.86 | < 0.0001 | 9.37 ± 4.74 |
eGFR estimated glomerular filtration rate, the unit was as ml/min/1.73m2, BMI body mass index, WC waist circumference, WHtR waist-to-height ratio, PBF percent body fat, VFI visceral fat index, DM diabetes mellitus, MI myocardial infarction
Data are expressed as the mean ± SD or as n (%)
Pearson’s correlation coefficients for the 5 adiposity indices each against eGFR stratified by gender are illustrated in Table 2. We found that all these indices had a significant negative correlation to eGFR (P < 0.0001). BMI had the lowest Pearson’s correlation coefficient in both genders (Pearson’s correlation coefficient:− 0.051 in males; − 0.014 in females) which indicated the weakest correlations with eGFR.
Table 2.
Pearson’s correlation coefficients between adiposity indices and eGFR
| eGFR | ||||
|---|---|---|---|---|
| Male | P | Female | P | |
| BMI (kg/m2) | -0.051 | < 0.0001 | −0.014 | < 0.0001 |
| WC (cm) | −0.061 | < 0.0001 | −0.070 | < 0.0001 |
| WHtR | −0.069 | < 0.0001 | −0.097 | < 0.0001 |
| PBF | −0.129 | < 0.0001 | − 0.151 | < 0.0001 |
| VFI | −0.139 | < 0.0001 | −0.112 | < 0.0001 |
eGFR estimated glomerular filtration rate, BMI body mass index, WC waist circumference, WHtR waist-to-height ratio, PBF percent body fat, VFI visceral fat index
Figure 1 shows the AUC values (95% confidence interval (CI)) and Youden index for each of the five adiposity indices in prediction of CKD. PBF had a significantly higher AUC in both male and female groups (AUC for males: 0.593, 95% CI: 0.584–0.601; AUC for females: 0.617, 95% CI: 0.609–0.624) than the other indices (with WHtR in females being an exception). PBF also yielded the highest Youden index in identifying CKD (male: 0.15; female: 0.20).
Fig. 1.
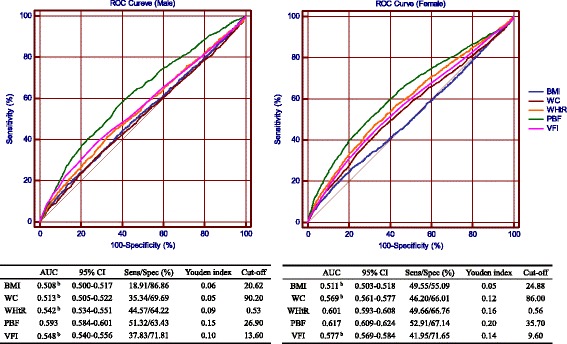
AUC of adiposity indices to identifying subjects with chronic kidney disease according to sex. Sens: sensitivity; Spec: specificity; ROC curve: receiver operating characteristics curve; AUC: area under the ROC curve; BMI: body mass index; WC: waist circumference; WHtR: waist-to-height ratio; PBF: percentage body fat; VFI: visceral fat index
Odds ratios (ORs) for the association between the CKD and high levels of various adiposity indices stratified by gender are shown in Table 3. PBF had the highest crude OR for CKD in male [1.819 (1.559–2.123)] and in female groups [2.268 (1.980–2.597)]. After adjusted for confounding factors, the ORs on PBF were also significant for both genders (P < 0.01). Table 4 shows the performance of the five adiposity indices according to Z-score standardization stratified by gender. As shown, after adjusted for confounding factors, a 1-SD increment change for each adiposity index was associated with higher risk of CKD in both male and female (all P < 0.05). PBF had the highest ORs for CKD in both genders.
Table 3.
Odds ratios and 95% confidence interval for chronic kidney disease according to adiposity indices stratified by gender
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Male | |||
| BMI | 0.650 (0.533–0.792)b | 1.061 (0.857–1.314) | 0.954 (0.768–1.186) |
| WC | 1.248 (1.062–1.466)b | 1.617 (1.355–1.929)b | 1.445 (1.205–1.731)b |
| WHtR | 1.357 (1.163–1.584)b | 1.446 (1.225–1.708)b | 1.295 (1.092–1.535)b |
| PBF | 1.819 (1.559–2.123)b | 1.728 (1.476–2.024)b | 1.409 (1.197–1.657)b |
| VIF | 1.549 (1.321–1.817)b | 1.519 (1.279–1.804)b | 1.357 (1.138–1.617)b |
| Female | |||
| BMI | 1.196 (1.045–1.368)b | 1.434 (1.239–1.660)b | 1.301 (1.121–1.511)b |
| WC | 1.647 (1.439–1.886)b | 1.390 (1.202–1.609)b | 1.262 (1.087–1.464)b |
| WHtR | 1.961 (1.713–2.246)b | 1.303 (1.127–1.507)b | 1.188 (1.025–1.378)a |
| PBF | 2.268 (1.980–2.597)b | 1.956 (1.701–2.249)b | 1.520 (1.315–1.757)b |
| VIF | 1.824 (1.589–2.092)b | 1.422 (1.227–1.648)b | 1.284 (1.105–1.493)b |
BMI body mass index, WC waist circumference, WHtR waist-to-height ratio, PBF percent body fat, VFI visceral fat index
Data are expressed as the odds ratios (95% confidence interval)
Model 1: Non-adjusted
Model 2: Adjusted for age, smoking status, alcohol use, education level, marital status, living area (rural/urban) and region (eastern, middle and western)
Model 3: Adjusted for age, smoking status, alcohol use, education level, marital status, living area (rural/urban), region (eastern, middle and western), hypertension, diabetes mellitus, myocardial infarction and stroke
aP < 0.05, bP < 0.01
Table 4.
Standardized odds ratios and 95% confidence interval for chronic kidney disease according to adiposity indices stratified by gender
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Male | |||
| BMI z-score | 0.964 (0.892–1.042) | 1.241 (1.143–1.347)b | 1.163 (1.068–1.266)b |
| WC z-score | 1.030 (0.954–1.113) | 1.203 (1.109–1.305)b | 1.128 (1.037–1.226)b |
| WHtR z-score | 1.153 (1.067–1.245)b | 1.175 (1.085–1.272)b | 1.103 (1.016–1.197)a |
| PBF z-score | 1.376 (1.272–1.488)b | 1.329 (1.227–1.439)b | 1.186 (1.091–1.289) b |
| VIF z-score | 1.216 (1.129–1.309)b | 1.215 (1.127–1.309)b | 1.145 (1.059–1.237)b |
| Female | |||
| BMI z-score | 1.029 (0.962–1.100) | 1.170 (1.092–1.253)b | 1.108 (1.032–1.190)b |
| WC z-score | 1.267 (1.185–1.354)b | 1.194 (1.116–1.278)b | 1.138 (1.062–1.220)b |
| WHtR z-score | 1.423 (1.332–1.519)b | 1.146 (1.071–1.227)b | 1.093 (1.020–1.172)a |
| PBFz-score | 1.485 (1.380–1.599)b | 1.337 (1.242–1.441)b | 1.153 (1.070–1.244)b |
| VIF z-score | 1.302 (1.227–1.381)b | 1.167 (1.094–1.244)b | 1.108 (1.036–1.185)b |
BMI body mass index, WC waist circumference, WHtR waist-to-height ratio, PBF percent body fat, VFI visceral fat index
Data are expressed as the odds ratios (95% confidence interval)
Model 1: Non-adjusted
Model 2: Adjusted for age, smoking status, alcohol use, education level, marital status, living area (rural/urban) and region (Eastern, middle and Western)
Model 3: Adjusted for age, smoking status, alcohol use, education level, marital status, living area (rural/urban), region (Eastern, middle and Western), hypertension, diabetes mellitus, myocardial infarction and stroke
aP < 0.05, bP < 0.01
Discussion
The prevalence of CKD (eGFR< 60 ml/min/1.73m2) in middle-aged Chinese adults was 3.94% (3.62% in male and 4.25% in female). All of the adiposity indices (BMI, WC, WHtR, PBF and VFI) had a significant negative correlation to CKD in both genders. Obesity is associated with an increased risk of CKD. For the comparison, we found that PBF was a more suitable screening tool for predicting CKD than other adiposity indices for CKD in both males and females.
Several cross-sectional studies demonstrated that the prevalence of CKD differed substantially between geographic regions in China. The prevalence of CKD (eGFR< 60 ml/min/1.73m2) in southern China was 3.2% (2.2% in male vs. 4.1% in female) [16] which was a little lower than the results in our study. While, in the study conducted in Beijing among participants older than 40 years found that the prevalence of reduced renal function (eGFR< 60 ml/min/1.73m2) was 5.2% [17], which was higher than our present study. In addition to methodology, the heterogeneity might be related to differences in lifestyles, medical care and socioeconomic characteristics in different regions of China [18].
Previously, the majority of existing literature has focused on the relationship between BMI and/or WC with CKD. A cross-sectional study conducted in Chinese adults showed that for each increase of 1.0 kg/m2 in BMI, a decline of 0.5 ml/min/1.73m2 [19]. In addition, a prospective cohort study, which included 827 incident cases of ESRD occurred during an average follow-up of 15.5 years, showed that patients had more than 1.1 times higher risk for developing ESRD participants with a higher BMI (BMI ≥ 27.5 kg/m2), compared to a normal BMI (18.5 to 23 kg/m2) [20]. Furthermore, a 7-year population-based cohort study conducted in Iran indicated that, irrespective of general obesity, the risk of developing CKD rose with increasing WC [21]. Even though BMI and WC have been the most widely used measurements to classify obesity, BMI does not account for fat distribution [22]; and WC does not account for height [11]. Thus, other indices which can more accurately measure total body fat quantity and visceral fat, such as WHtR, PBF and VFI, were developed. In our study, we found that WHtR, PBF and VFI correlated much more strongly with eGFR than BMI and WC (according to Pearson’s correlation coefficients in Table 2).
A related study conducted among female subjects aged 65–80 years found that WHtR was a better index associated with CKD when comparing with other common adiposity indices (BMI, WC, waist-to-hip ratio) [23]. Dai et al. suggested that a visceral adiposity index was superior to BMI and WC for predicting CKD in females [24]. Additionally, a cross-sectional study [25] conducted in Chinese adults indicated that body fat percentage was significantly related to increased risk of CKD (OR: 1.049, 95%CI: 1.006–1.093). Our study found that PBF had the highest AUC in both male and female groups. After adjusting for confounding factors, PBF still had the highest ORs for CKD than other adiposity indices in both genders.
There are a few mechanisms that can explain the association between obesity and renal injury, including hemodynamic effects, inflammation and renal lipotoxicity [26]. Angiotensin II is elevated in the blood of obese individuals, leading to vasoconstriction of efferent arterioles; plasma aldosterone is also slightly elevated in obese individuals, causing salt and water retention, and ultimately proteinuria [26, 27]. In regarding to inflammation, cytokines such as interleukin-6 (IL-6) and tumor necrosis factor – alpha (TNF-α) play a major role in CKD development. IL-6 invades the adipose tissue and influences transforming growth factor – beta 1 (TGF-β1) receptor trafficking, leading to renal fibrosis [28]. TNF-α inhibits the activity of the nephron gene promoter in cultured podocytes, resulting in podocyte dysfunction in the obese population [29, 30].
Our study has several strengths. Firstly, we enrolled a large population-based sample, including male and female participants from China. Secondly, to our knowledge, this is the first time a comparison across five adiposity indices (BMI, WC, WHtR, PBF, VFI) has been made to predicting CKD in a large Chinese population. However, there are several potential limitations that should be considered when interpreting the results. First, this is a cross-sectional study and no causal relationship can be established. Further follow-up of the population and more prospective studies will be needed to validate our findings. Secondly, some variables such as physical activity and dietary patterns which might affect renal function were not included in our study. Finally, the gold standards for measurement of fat distribution are micromagnetic resonance imaging and microcomputed tomography [31]; in our investigation, we used the Omron body composition monitor, which may be less accurate than gold standards. However, the Omron device has been used in various studies and has demonstrated reasonable accuracy [32, 33].
Conclusion
Obesity was associated with an increased risk of CKD, with PBF being the best adiposity index predictor for identifying CKD. Given that obesity is modifiable risk factor, CKD patients should manage their weight to prevent CKD progression. Patients should also be screened for high PBF in CKD prevention efforts.
Additional files
List of investigators of the China Hypertension Survey Study. (DOCX 14 kb)
List of IRBs of sub-centers. (DOCX 13 kb)
Acknowledgements
The survey could be accomplished through the fine work of the staff in the national level, and we thank for all the colleagues involving in the survey (Additional file 1). We gratefully acknowledge Tianming Zhao, Guohui Fan, Jingyu Nie, Suning Li for help in maintaining the data, and Dominique Comer Medaglio, Zugui Zhang (from Christiana Care Health System, USA) for help in improving the manuscript.
The authors are grateful to OMRON Corporation, Kyoto, Japan, for supporting Blood Pressure Monitor (HBP-1300) and body fat measurement device (V-body HBF-371); Henan Medical Science & Technology Co., Ltd., China, for Digital ECG device (GY-5000); Microlife, Taipei, China, for Automated ABI device (Watch BP Office device).
Funding
This work was supported by a grant from ministry of Science & Technology, China (No.:2011BAI11B01); from National Health and Family Planning Commission, China (No.:201402002) and from BUCHANG PHARMA, Xian, China, Pfizer China, and Essen Technology Company Limited.
All funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC
Area under the ROC curve
- BMI
Body mass index
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
- PBF
Percentage body fat
- ROC curve
Receiver operating characteristics curve
- VFI
Visceral fat index
- WC
Waist circumference
- WHtR
Waist-to-height ratio
Authors’ contributions
ZW, ZC, XW, LZ, LS, YT and RG participated in the design of the study; ZW, ZC, XW, LZ, JN, CZ, JW, LS, YT and RG, participated in the data collection; YD and ZC performed the statistical analysis; YD wrote the manuscript, ZW supervised and made critical revisions of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The ethical review committee of Fuwai Hospital (Beijing, China) and each participating center (Additional file 2) approved the study’s protocol. And all the participants gave written informed consent prior to data collection.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests from BUCHANG PHARMA, Xian, China, Pfizer China, and Essen Technology Company Limited. All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12882-018-0837-1) contains supplementary material, which is available to authorized users.
Contributor Information
Ying Dong, Email: 437956809@qq.com.
Zengwu Wang, Phone: +86 10 60866161, Email: wangzengwu@foxmail.com.
Zuo Chen, Email: fwhhc@163.com.
Xin Wang, Email: wangxinfw@163.com.
Linfeng Zhang, Email: lf_zh@sina.com.
Jingyu Nie, Email: niejingyu@yeah.net.
Congyi Zheng, Email: zhengcongyi375@163.com.
Jiali Wang, Email: wangjiali.kary@foxmail.com.
Lan Shao, Email: shaolan_pumc@163.com.
Ye Tian, Email: tianye9698@163.com.
Runlin Gao, Email: gaorunlin@citmd.com.
References
- 1.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Smyth A, Glynn LG, Murphy AW, Mulqueen J, Canavan M, Reddan DN, O'Donnell M. Mild chronic kidney disease and functional impairment in community-dwelling older adults. Age Ageing. 2013;42(4):488–494. doi: 10.1093/ageing/aft007. [DOI] [PubMed] [Google Scholar]
- 4.Codreanu I, Perico N, Sharma SK, Schieppati A, Remuzzi G. Prevention programmes of progressive renal disease in developing nations. Nephrology (Carlton) 2006;11(4):321–328. doi: 10.1111/j.1440-1797.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 5.McClellan WM, Abramson J, Newsome B, Temple E, Wadley VG, Audhya P, McClure LA, Howard VJ, Warnock DG, Kimmel P. Physical and psychological burden of chronic kidney disease among older adults. Am J Nephrol. 2010;31(4):309–317. doi: 10.1159/000285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 7.Hu L, Huang X, You C, Li J, Hong K, Li P, Wu Y, Wu Q, Wang Z, Gao R, et al. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS One. 2017;12(9):e0183934. doi: 10.1371/journal.pone.0183934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi B, Liang Y, He T, Reilly KH, Hu Y, Wang Q, Yan Y, Mi J. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993-2009. Obes Rev. 2012;13(3):287–296. doi: 10.1111/j.1467-789X.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Furth S, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Clin Kidney J. 2017;10(1):1–8. doi: 10.1093/ckj/sfw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Q, He Y, Dong S, Zhao X, Chen Z, Song Z, Chang G, Yang F, Wang Y. Optimal cut-off values of BMI, waist circumference and waist:height ratio for defining obesity in Chinese adults. Br J Nutr. 2014;112(10):1735–1744. doi: 10.1017/S0007114514002657. [DOI] [PubMed] [Google Scholar]
- 11.Jiang J, Deng S, Chen Y, Liang S, Ma N, Xu Y, Chen X, Cao X, Song C, Nie W, et al. Comparison of visceral and body fat indices and anthropometric measures in relation to untreated hypertension by age and gender among Chinese. Int J Cardiol. 2016;219:204–211. doi: 10.1016/j.ijcard.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Q, Dong SY, Sun XN, Xie J, Cui Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz J Med Biol Res. 2012;45(7):591–600. doi: 10.1590/S0100-879X2012007500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Zhang L, Chen Z, Wang X, Shao L, Guo M, Zhu M, Gao R. Survey on prevalence of hypertension in China: background, aim, method and design. Int J Cardiol. 2014;174(3):721–723. doi: 10.1016/j.ijcard.2014.03.117. [DOI] [PubMed] [Google Scholar]
- 14.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Chen W, Wang H, Dong X, Liu Q, Mao H, Tan J, Lin J, Zhou F, Luo N, et al. Prevalence and risk factors associated with chronic kidney disease in an adult population from southern China. Nephrol Dial Transplant. 2009;24(4):1205–1212. doi: 10.1093/ndt/gfn604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Zuo L, Xu G, Wang F, Wang M, Wang S, Lv J, Liu L, Wang H. Community-based screening for chronic kidney disease among populations older than 40 years in Beijing. Nephrol Dial Transplant. 2007;22(4):1093–1099. doi: 10.1093/ndt/gfl763. [DOI] [PubMed] [Google Scholar]
- 18.Lin B, Shao L, Luo Q, Ou-yang L, Zhou F, Du B, He Q, Wu J, Xu N, Chen J. Prevalence of chronic kidney disease and its association with metabolic diseases: a cross-sectional survey in Zhejiang province, Eastern China. BMC Nephrol. 2014;15(1):36–42. doi:10.1186/1471-2369-15-36. [DOI] [PMC free article] [PubMed]
- 19.He Y, Liu D, Tan W, Ma X, Lian F, Xu X. Association between body mass index and mildly decreased estimated glomerular filtration rate in Chinese adults with early chronic kidney disease. J Ren Nutr. 2016;26(6):367–372. doi: 10.1053/j.jrn.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Lew QJ, Jafar TH, Talaei M, Jin A, Chow KY, Yuan JM, Koh WP. Increased body mass index is a risk factor for end-stage renal disease in the Chinese Singapore population. Kidney Int. 2017;92(4):979–87. [DOI] [PMC free article] [PubMed]
- 21.Noori N, Hosseinpanah F, Nasiri AA, Azizi F. Comparison of overall obesity and abdominal adiposity in predicting chronic kidney disease incidence among adults. J Ren Nutr. 2009;19(3):228–237. doi: 10.1053/j.jrn.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Muller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes Rev. 2012;13(Suppl 2):6–13. doi: 10.1111/j.1467-789X.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaroszynski A, Derezinski T, Jaroszynska A, Zapolski T, Wasikowska B, Wysokinski A, Jawien A, Zaluska W, Horoch A. Association of anthropometric measures of obesity and chronic kidney disease in elderly women. Ann Agric Environ Med. 2016;23(4):636–640. doi: 10.5604/12321966.1226859. [DOI] [PubMed] [Google Scholar]
- 24.Dai D, Chang Y, Chen Y, Chen S, Yu S, Guo X, Sun Y. Visceral adiposity index and lipid accumulation product index: two alternate body indices to identify chronic kidney disease among the rural population in Northeast China. Int J Environ Res Public Health. 2016;13(12):1231–41. [DOI] [PMC free article] [PubMed]
- 25.Yang S, Xiao F, Pan L, Zhang H, Ma Z, Liu S, Liu Y, Zhang W, Zeng X, Liu C, et al. Association of serum irisin and body composition with chronic kidney disease in obese Chinese adults: a cross-sectional study. BMC Nephrol. 2015;16:16–26. doi:10.1186/s12882-015-0009-5. [DOI] [PMC free article] [PubMed]
- 26.Soltani Z, Washco V, Morse S, Reisin E. The impacts of obesity on the cardiovascular and renal systems: cascade of events and therapeutic approaches. Curr Hypertens Rep. 2015;17(2):1–14. doi:10.1007/s11906-014-0520-2. [DOI] [PubMed]
- 27.Snyder S, Turner GA, Turner A. Obesity-related kidney disease. Prim Care. 2014;41(4):875–893. doi: 10.1016/j.pop.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J Biol Chem. 2005;280(13):12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 29.Takano Y, Yamauchi K, Hayakawa K, Hiramatsu N, Kasai A, Okamura M, Yokouchi M, Shitamura A, Yao J, Kitamura M. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett. 2007;581(3):421–426. doi: 10.1016/j.febslet.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Ikezumi Y, Suzuki T, Karasawa T, Kawachi H, Nikolic-Paterson DJ, Uchiyama M. Activated macrophages down-regulate podocyte nephrin and podocin expression via stress-activated protein kinases. Biochem Biophys Res Commun. 2008;376(4):706–711. doi: 10.1016/j.bbrc.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Wilson JL, Khaksari M, Cowley MA, Enriori PJ. Abdominal fat analyzed by DEXA scan reflects visceral body fat and improves the phenotype description and the assessment of metabolic risk in mice. Am J Physiol Endocrinol Metab. 2012;303(5):E635–E643. doi: 10.1152/ajpendo.00078.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballesteros-Pomar MD, Calleja-Fernandez A, Diez-Rodriguez R, Vidal-Casariego A, Blanco-Suarez MD, Cano-Rodriguez I. Comparison of different body composition measurements in severely obese patients in the clinical setting. Nutr Hosp. 2012;27(5):1626–1630. doi: 10.3305/nh.2012.27.5.5989. [DOI] [PubMed] [Google Scholar]
- 33.Martin Moreno V, Gomez Gandoy B, Antoranz Gonzalez M, Fernandez Herranz S, Gomez De La Camara A, de Oya Otero M. Validation of the OMRON BF 300 monitor for measuring body fat by bioelectric impedance. Aten Primaria. 2001;28(3):174–181. doi: 10.1016/S0212-6567(01)78927-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of investigators of the China Hypertension Survey Study. (DOCX 14 kb)
List of IRBs of sub-centers. (DOCX 13 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


