Abstract
Background
Baculoviral IAP repeat containing 6 (BIRC6), a member of the inhibitors of apoptosis protein (IAP) family, has been reported to be correlated with oncogenesis. The aim of this study was to investigate the prognostic significance of BIRC6 in prostate cancer, as well as its effects on prostate cancer cell lines.
Material/Methods
BIRC6 protein expression was investigated in 112 prostate cancer tissues and 86 benign prostate disease tissues using immunohistochemistry. Overall survival was assessed by Kaplan-Meier analysis with log-rank test. To evaluate the prognostic significance of BIRC6, Cox regression models were applied. The effects of BIRC6 on human prostate cancer cells were confirmed via small interfering RNA.
Results
BIRC6 protein levels were significantly higher in prostate cancer tissues than that in benign prostate tissues (P<0.001). High BIRC6 expression was associated with advanced pathological stage (P=0.011) and positive metastasis (P=0.036). In addition, patients with high BIRC6 expression had worse survival than those with low expression (Log-rank test, P=0.002). Multivariate analysis demonstrated that BIRC6 expression was an independent prognostic factor for prostate cancer (HR=2.771, 95%CI=1.427–5.378, P=0.003). Furthermore, cell experiments suggested that BIRC6 knockdown inhibits cell proliferation and promote apoptosis (P<0.05 for all).
Conclusions
Upregulated BIRC6 predicts aggressive clinical characteristics and poor prognosis in prostate cancer patients. BIRC6 can regulate prostate cancer cell proliferation and apoptosis, which may be a potential therapeutic target.
MeSH Keywords: Betula, Cell Survival, Prognosis, Prostatic Neoplasms
Background
Prostate cancer is one of the most commonly diagnosed cancers among men, and is considered as a major contributor to cancer-related mortality worldwide [1]. Despite various therapeutic choices for prostate cancer patients, such as surgery, hormonal therapy, and radiotherapy, the therapeutic effects are limited for castration-resistant and metastatic prostate cancer [2]. Currently, the evaluation of prostate cancer prognosis mainly depends on clinicopathological characteristics, including tumor stage, grade, and prostate-specific antigen (PSA) level at clinical diagnosis [3]. However, these prognostic predictors have little value in clinical applications, and significantly different clinical outcomes are observed among patients with the same tumor/node/metastasis grade [4]. Therefore, it is necessary to identify novel prognostic biomarkers to guide prostate cancer management.
The inhibitors of the apoptosis protein (IAP) family, which is characterized by 1–3 copies of Baculoviral IAP Repeat (BIR) domain, has been proved to play functional roles in cancer cell apoptosis [5–7]. High levels of IAP are observed in various cancers, such as glioblastoma and pancreatic cancer [8,9]. It has been reported that the IAP family can regulate cell survival via binding to the pro-apoptotic factors in tumors [10]. As an important member of the IAP family, baculoviral inhibition of apoptosis protein repeat containing 6 (BIRC6) has also been reported to be involved in tumor progression via regulating the expression of p53 or its activity [11,12]. Upregulated BIRC6 expression is observed in several cancers, including colorectal cancer [10], hepatocellular carcinoma [11], and human epithelial ovarian cancer [13]. In addition, previous research revealed that BIRC6 is an apoptosis inhibitor implicated in prostate cancer progression and therapy resistance, which might be a potential therapeutic target for this malignancy [14–16]. However, the prognostic value of BIRC6 in prostate cancer patients has not been reported in previous studies.
In the present study, patients pathologically diagnosed with prostate cancer were enrolled. The expression levels of BIRC6 in prostate cancer tissues and normal tissues were investigated, as well as its prognostic significance in prostate cancer patients. The present study may provide a novel indicator for prostate cancer prognosis to guide treatment.
Material and Methods
Patients and tissue samples
Tissue specimens used in the present study were collected from 112 male patients with prostate cancer who underwent surgery at Weifang People’s Hospital. The clinicopathological information of the patients are summarized in Table 1. None of the patients had received pre- or postoperative radiation or hormone therapy before sampling. All the patients were enrolled in a 5-year follow-up investigation, and were followed clinically at regular intervals of 3 months for the first year and of 6 months for the subsequent years. In addition, benign prostate tissue samples were collected as controls from 86 benign prostate hyperplasia patients, whose ages (average age of 63±9.7 years) were matched with the prostate cancer patients.
Table 1.
Immunohistochemistry staining for BIRC6 in prostate cancer and benign prostate tissue, n (%) (Chi-square test).
| BIRC6 | Prostate cancer tissues | Benign prostate tissues | χ2 | P value |
|---|---|---|---|---|
| Low | 47 (42.0%) | 76 (88.4%) | 13.637 | <0.001 |
| High | 65 (58.4%) | 10 (11.6%) |
The experimental protocols of the current study were in accordance with the guidelines of the Ethics Committee of Weifang People’s Hospital, and informed consent was signed by all participants. All specimens were handled and made anonymous according to ethical and legal standards.
Cell culture
PC-3 cell lines (ATCC, Manassas, VA) were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics. The incubator was set as 5% CO2 humidified atmosphere at 37°C.
Immunohistochemistry
Paraffin-embedded sections of tissues used for immunohistochemistry were deparaffinized with xylene, then treated with graded alcohol for rehydration. Following antigen retrieval with a 10-mM citrate buffer, the tissues samples were incubated with the first rabbit anti-BIRC6 polyclonal antibody (Novus Biologicals, Littleton, CO; NB110-40730) overnight at 4°C, then with the second antibody against HRP-conjugated-rabbit Ig for 30 min. Lastly, the tissue sections were treated with 3, 3′-diami-nobenzidine solution and counterstained with hematoxylin.
BIRC6 protein scoring
BIRC6 staining of tissues was scored by 2 independent pathologists unaware of patient characteristics. The final immunoreactivity was evaluated by intensity and extent of positively stained tumor cells [17]. The intensity of staining was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The extent of positively stained tumor cells was scored as 0 (0%), 1 (<10%), 2 (10–50%), and 3 (>50%). Scores 0–1 were considered as low expression and scores of 2–3 were considered as high expression for subsequent statistical analyses.
Small interfering RNA(siRNA) and cell transfection
BIRC6 siRNA (Lafayette, CO) were used in this study to suppress the expression of BIRC6 with the sequence as follow: 5′-GUUUCAAAGCAGGAUGAUG-dTdR-3′. In addition, vehicle and nontargeting siRNA (siGE-NOME nontargeting siRNA # 3 D-001210-03-05, Dharmancon) were adopted to act as the controls. Cell transfection was performed to modulate the expression of BIRC6 in tumor cells. The cells were transfected with siRNA (80 nM) using Lipofectamine 2000 reagent (Invitrogen, Burlington, ON, Canada) after the incubation in the 6-well plates for 20 h. Briefly, the wells were added with the complex of Lipofectamine 2000 and siRNA and incubated for 6 h. Then the transfection mixture was removed, and the cells were incubated with fresh antibiotic-free DMEM supplemented with FBS (10%) for the subsequent 24 or 72 h for the further analyses.
Western blotting
Cell lines PC-3 were placed in lysis buffer at 4°C for 1 h. Protein samples were electrophoresed using 12% sodium dodecylsulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Cell lysates were transferred to nitrocellulose filter membranes. The membranes were blocked for 1 h in 5% non-fat dry milk diluted with TBST (10 mM Tris-HCl and 0.05% Tween 20). The membranes were then incubated with primary antibodies at 4°C overnight, followed by incubation with appropriate secondary antibodies at room temperature for 2 h. The primary antibodies were mouse monoclonal anti-BIRC6 (1: 500 dilution; Sigma-Aldrich), rabbit polyclonal anti-GAPDH (1: 5000; Abcam). The membranes were washed with PBS 3 times, and the immunoreactive bands were visualized using an ECL plus kit, according to the manufacturer’s instructions.
Cell proliferation assay
The tumor cells were seeded in a 24-well dish with the density of 2.5×104 cells/well and transfected with BIRC6 siRNA or the controls. After the transfection, the wells were added with 50 μL MTT (5 mg/mL) at 0, 24, 48, and 72 h, and then incubated in an incubator with 5% CO2 at 37°C for 4 h. Each of the culture was added 500 μL 20% SDS solution and incubated at room temperature for 1 night without light. The cell proliferation was evaluated with the absorbance measured at 490 nm.
Apoptosis analysis
Cells were harvested at 48 h after transfection, and immunostained with annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s instructions (Apoptosis Detection Kit, KeyGEN), which were then analyzed by flow cytometry (BD FACSCanto II, BD Biosciences, San Jose, USA). Date analysis was carried out using CellQuest software (BD Biosciences).
Statistical analysis
All statistical analyses and graphing were carried out using SPSS 18.0 software and GraphPad Prism 5.0 software. The relationship between the expression of BIRC6 protein and clinicopathologic characteristics of prostate cancer patients was evaluated by chi-square test. Overall survival analysis was performed by the Kaplan-Meier method with log-rank test. The Cox proportional hazard regression model was used to evaluate the prognostic value of BIRC6. P<0.05 was considered statistically significant.
Results
Enhanced BIRC6 expression in prostate cancer tissues
BIRC6 expression levels in 112 prostate cancer tissues and 86 benign prostate disease tissues were evaluated by immunohistochemistry analysis. As shown in Table 1, the expression of BIRC6 was significantly higher in prostate cancer tissues than in benign prostate disease tissues (P<0.001).
Association between BIRC6 expression and clinical pathological characteristics
We investigated the correlation of BIRC6 expression with clinicopathologic features in 112 prostate cancer patients. The patients were divided into 2 groups according to their protein levels of BIRC6. Analysis results suggested that BIRC6 expression was associated with pathological stage (P=0.011) and metastasis (P=0.036). However, there was no significant correlation between BIRC6 expression and age, Gleason score, clinical stage, or PSA level (all, P>0.05) (Table 2).
Table 2.
Correlation between BIRC6 expression and clinicopathological parameters.
| Parameters | No. of cases (n=112) | BIRC6 expression | χ2 | P values | |
|---|---|---|---|---|---|
| Low (n=47) | High (n=65) | ||||
| Age (years) | |||||
| <60 | 46 | 22 | 24 | 1.101 | 0.294 |
| ≥60 | 66 | 25 | 41 | ||
| Gleason score | |||||
| <8 | 34 | 16 | 18 | 0.520 | 0.471 |
| ≥8 | 78 | 31 | 47 | ||
| Clinical stage | |||||
| ≤cT2c | 55 | 26 | 29 | 1.250 | 0.263 |
| ≥cT3a | 57 | 21 | 36 | ||
| Pathological stage | |||||
| ≤pT2 | 58 | 31 | 27 | 6.514 | 0.011 |
| ≥pT3 | 54 | 16 | 38 | ||
| Metastasis | |||||
| No | 49 | 26 | 23 | 4.405 | 0.036 |
| Yes | 63 | 21 | 42 | ||
| PSA level | |||||
| <10 ng/ml | 51 | 25 | 26 | 1.914 | 0.167 |
| ≥10 ng/ml | 61 | 22 | 39 | ||
PSA – prostate specific antigen.
Prognostic value of enhanced BIRC6 expression
Survival analysis was done for the enrolled prostate cancer patients according to their expression levels of BIRC6. Kaplan-Meier curves showed the prostate cancer patients with high BIRC6 expression had worse survival than those with low expression (log-rank test, P=0.002) (Figure 1).
Figure 1.
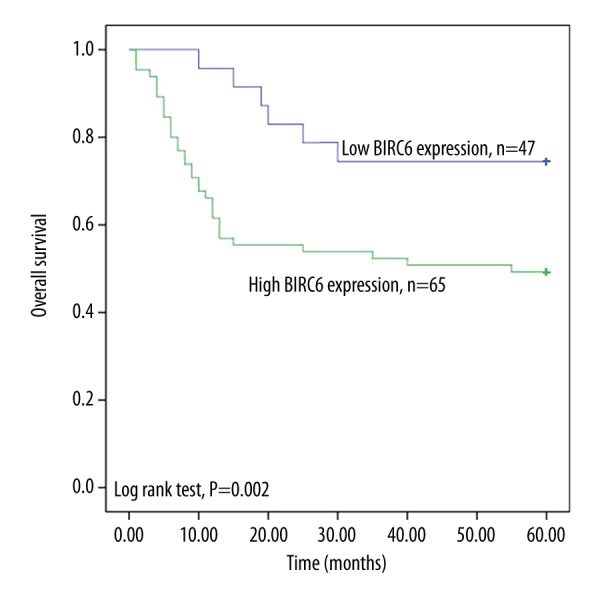
Kaplan-Meier survival curves for the collected prostate cancer patients according to BIRC6 expression. High BIRC6 expression was associated with poor overall survival (log-rank test, P=0.002).
Univariate analysis indicated that elevated BIRC6 protein levels (HR=2.689, 95%CI=1.387–5.214, P=0.003), Gleason score (HR=2.219, 95%CI=1.086–4.610, P=0.033), pathological stage (HR=2.016, 95%CI=1.102–3.686, P=0.023), and PSA level (HR=2.028, 95%CI=1.090–3.774, P=0.026) were significantly associated with overall survival. However, other parameters such as age, clinical stage, and metastasis were not markedly correlated with prognosis of prostate cancer (Table 3).
Table 3.
Univariate and multivariate analysis of clinicopathological parameters associated with overall survival.
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P values | HR (95%CI) | P values | |
| BIRC6 expression (high vs. low) | 2.689 (1.387–5.214) | 0.003 | 1.771 (1.427–5.378) | 0.003 |
| Age (years) (≥60 vs. <60) | 1.054 (0.583–1.904) | 0.862 | – | – |
| Gleason score (≥8 vs. <8) | 2.219 (1.068–4.610) | 0.033 | 2.313 (1.112–4.812) | 0.025 |
| Clinical stage (≥cT3a vs. ≤cT2c) | 1.495 (0.818–2.733) | 0.191 | – | – |
| Pathological stage (≥pT3 vs. ≥pT3) | 2.016 (1.102–3.686) | 0.023 | – | – |
| Metastasis (yes vs. no) | 1.328 (0.732–2.412) | 0.351 | – | – |
| PSA level (≥10ng/ml vs. <10ng/ml) | 2.028 (1.090–3.774) | 0.026 | – | – |
’–’ – indicated no related data.
To further explore the independent factors for prognosis, we carried out the multivariate analysis. The results showed that BIRC6 expression (HR=1.771, 95%CI=1.427–5.378, P=0.003) and Gleason score (HR=2.313, 95%CI=1.112–4.812, P=0.025) were independent indicators for prostate cancer prognosis (Table 3).
Effects of BIRC6 expression on prostate cancer cells
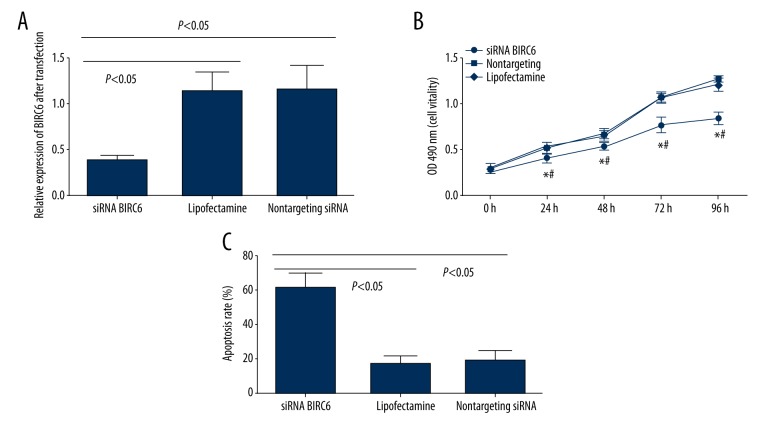
To evaluate the effects of BIRC6 expression on biological processes of prostate cancer cells, the PC-3 cells were transfected by siRNA specific for BIRC6. The expression levels of BIRC6 in the cells transfected with siRNA were assessed by Western blotting. After transfection, decreased levels of BIRC6 protein in PC-3 cells at 72 h were observed compared with transfection with nontargeting siRNA (P<0.05) (Figure 2A). The proliferation of BIRC6 siRNA-transfected PC-3 cells was assessed according to MTT assay. As shown in Figure 2B, siRNA-transfected PC-3 cells revealed decreased growth activity from 24 h compared with controls (P<0.05).
Figure 2.
BIRC6 siRNA inhibited cell proliferation and promoted apoptosis in vitro. (A) Relative expression of BIRC6 in prostate cancer cells after siRNA transfections examined by Western blotting. (B) Cell viability assay in siRNA BIRC6, Lipofectamine, and nontargeting siRNA-transfected prostate cancer cells. * Compared with Lipofectamine prostate cancer cells, P<0.05; # compared with nontargeting siRNA-transfected prostate cancer cells, P<0.05. (C) Flow cytometry analysis showed that siRNA BIRC6 significantly promoted apoptosis.
Flow cytometric analysis was applied to evaluate the effects of BIRC6 on prostate cancer cell apoptosis. Analysis results indicated that the apoptosis of BIRC6 siRNA-transfected PC-3 was significantly increased compared with controls (P<0.05) (Figure 2C).
Discussion
Prostate cancer is one of the most prevalent malignancies among men worldwide. However, the treatments are unnecessary for most prostate cancer patients because the cancer may not cause serious clinical symptoms within the patient’s lifetime, and severe adverse effects may be induced by the therapies [18,19]. Only those with aggressive cancer should be treated. However, based on the current diagnostic and prognostic biomarkers, over-diagnosis and over-treatments frequently occur among prostate cancer patients [20]. Therefore, novel molecular biomarkers which can accurately reflect the initiation and aggression of prostate cancer are urgently needed.
In the present study, we investigated the prognostic significance of BIRC6 in prostate cancer patients. BIRC6 is a member of the IAP family, which was reported to be associated with the progression of various cancers. A study by Dong et al. indicated that the expression of BIRC6 protein was increased in non-small cell lung cancer tissues, and its elevated levels were significantly associated with aggressive cancer progression [21]. A study based on hepatocellular carcinoma patients demonstrated that BIRC6 was over-expressed in hepatocellular carcinoma patients and was associated with malignant clinical parameters [11]. In the present study, we investigated the expression levels of BIRC6 in prostate cancer patients; analysis suggested that the expression levels of BIRC6 were significantly upregulated in prostate cancer patients, and increased levels were markedly correlated with advanced pathological stage and positive metastasis. These results suggest that BIRC6 promotes the progression of prostate cancer.
BIRC6 as an apoptosis inhibitor that serves as a prognostic predictor for several cancers, such as non-small cell lung cancer, childhood acute leukemia, and colorectal cancer [10,21,22]. Nevertheless, research on the prognostic significance of BIRC6 in prostate cancer is limited. In the present study, we used Cox regression analysis to evaluate the prognostic value of BIRC6 in prostate cancer. The results suggested that BIRC6 is an independent predictor for prostate cancer prognosis. Due to the low sensitivity and specificity of the present diagnostic and prognostic tools, numerous molecular markers were identified for prostate cancer prognosis. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) has been found to be involved in the progression of prostate cancer, which could be employed as a candidate diagnostic biomarker for this disease [23]. Qu et al. reported that the expression profile of Pim-3 was significantly correlated with pathological parameters of prostate cancer patients, and its elevated expression predicted poor clinical outcomes [24]. Zang et al. reported that ELL-associated factor 2 (EAF2) deletion predicted poor survival status for patients with prostate cancer [25]. Increased expression of Tripartite Motif 47 (TRIM 47) was observed in prostate cancer tissues, suggesting poor outcomes for these patients [26]. A related study by Wang et al. indicated that the expression levels of long non-coding RNA LOC400891 were significantly different between prostate cancer tissues and non-cancerous tissues, which could serve as a prognostic marker for prostate cancer [27]. Although a variety of novel biomarkers were confirmed for prostate cancer, few of them have been applied in clinical practice. Therefore, the value of BIRC6 in clinical application needs to be ascertained in a study with larger sample size.
We evaluated the effects of BIRC6 on prostate cell lines. The results of cell experiments suggested that knockdown of BIRC6 in prostate cancer cell lines suppresses cell proliferation and promotes apoptosis. The results were consistent with previous studies. Lamers et al. revealed that upregulated BIRC6 in neuroblastoma cell lines binds and degrades pro-apoptotic protein DIABLO, thus inhibiting cell apoptosis [28]. However, some studies reported different results. Luk et al. indicated that members of the IAP family were co-upregulated in prostate cancer cell lines, and knockdown of one member did not inhibit cell proliferation [29]. Therefore, the mechanisms by which BIRC6 is involved in tumor progression should be verified in further research.
Conclusions
Our study shows that BIRC6 is upregulated in prostate cancer and is associated with aggressive tumor progression. Moreover, elevated level of BIRC6 predicts poor prognosis for prostate cancer patients, and BIRC6 can regulate prostate cancer cell proliferation and apoptosis in vitro. However, the detailed mechanism by which BIRC6 is involved in prostate cancer needs further investigation.
Footnotes
Source of support: Departmental sources
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Yin X, Xiao Y, Li F, et al. Prognostic role of neutrophil-to-lymphocyte ratio in prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2016;95(3):e2544. doi: 10.1097/MD.0000000000002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braeckman J, Michielsen D. Prognostic factors in prostate cancer. Recent Results Cancer Res. 2007;175:25–32. doi: 10.1007/978-3-540-40901-4_3. [DOI] [PubMed] [Google Scholar]
- 4.Deng QK, Lei YG, Lin YL, et al. Prognostic value of Protocadherin10 (PCDH10) methylation in serum of prostate cancer patients. Med Sci Monit. 2016;22:516–21. doi: 10.12659/MSM.897179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulda S. Inhibitor of Apoptosis (IAP) proteins as therapeutic targets for radiosensitization of human cancers. Cancer Treat Rev. 2012;38(6):760–66. doi: 10.1016/j.ctrv.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S. Exploiting inhibitor of apoptosis proteins as therapeutic targets in hematological malignancies. Leukemia. 2012;26(6):1155–65. doi: 10.1038/leu.2012.4. [DOI] [PubMed] [Google Scholar]
- 7.Fulda S. Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: molecular mechanisms and therapeutic opportunities. Leukemia. 2014;28(7):1414–22. doi: 10.1038/leu.2014.56. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Berglund A, Kenchappa RS, et al. BIRC3 is a novel driver of therapeutic resistance in Glioblastoma. Sci Rep. 2016;6:21710. doi: 10.1038/srep21710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon JH, Shin JS, Hong SW, et al. A novel small-molecule IAP antagonist, AZD5582, draws Mcl-1 down-regulation for induction of apoptosis through targeting of cIAP1 and XIAP in human pancreatic cancer. Oncotarget. 2015;6(29):26895–908. doi: 10.18632/oncotarget.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu T, Weng S, Tang W, et al. Overexpression of BIRC6 is a predictor of prognosis for colorectal cancer. PLoS One. 2015;10(5):e0125281. doi: 10.1371/journal.pone.0125281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W, Xue R, Weng S, et al. BIRC6 promotes hepatocellular carcinogenesis: Interaction of BIRC6 with p53 facilitating p53 degradation. Int J Cancer. 2015;136(6):E475–87. doi: 10.1002/ijc.29194. [DOI] [PubMed] [Google Scholar]
- 12.Lopergolo A, Pennati M, Gandellini P, et al. Apollon gene silencing induces apoptosis in breast cancer cells through p53 stabilisation and caspase-3 activation. Br J Cancer. 2009;100(5):739–46. doi: 10.1038/sj.bjc.6604927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Chen YJ, Hou J, et al. Expression and clinical significance of BIRC6 in human epithelial ovarian cancer. Tumour Biol. 2014;35(5):4891–96. doi: 10.1007/s13277-014-1641-6. [DOI] [PubMed] [Google Scholar]
- 14.Low CG, Luk IS, Lin D, et al. BIRC6 protein, an inhibitor of apoptosis: role in survival of human prostate cancer cells. PLoS One. 2013;8(2):e55837. doi: 10.1371/journal.pone.0055837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low CGM. The role of BIRC6, a member of the inhibitor of apoptosis protein (IAP) family, in the survival of human prostate cancer cells. University of British Columbia; 2010. Retrieved from https://open.library.ubc.ca/cIRcle/collections/24/items/1.0071420. [Google Scholar]
- 16.Luk IS, Shrestha R, Xue H, et al. BIRC6-targeting as potential therapy for advanced, enzalutamide-resistant prostate cancer. Clin Cancer Res. 2017;23(6):1542–51. doi: 10.1158/1078-0432.CCR-16-0718. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Fu D, Xi J, et al. Expression and clinical significance of UCH37 in human esophageal squamous cell carcinoma. Dig Dis Sci. 2012;57(9):2310–17. doi: 10.1007/s10620-012-2181-9. [DOI] [PubMed] [Google Scholar]
- 18.Popiolek M, Rider JR, Andrén O, et al. Natural history of early, localized prostate cancer: A final report from three decades of follow-up. Eur Urol. 2013;63(3):428–35. doi: 10.1016/j.eururo.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Canfield SE, Kibel AS, Kemeter MJ, et al. A guide for clinicians in the evaluation of emerging molecular diagnostics for newly diagnosed prostate cancer. Rev Urol. 2014;16(4):172–80. [PMC free article] [PubMed] [Google Scholar]
- 20.Haldrup C, Lynnerup AS, Storebjerg TM, et al. Large-scale evaluation of SLC18A2 in prostate cancer reveals diagnostic and prognostic biomarker potential at three molecular levels. Mol Oncol. 2016;10(6):825–37. doi: 10.1016/j.molonc.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong X, Lin D, Low C, et al. Elevated expression of BIRC6 protein in non-small-cell lung cancers is associated with cancer recurrence and chemoresistance. J Thorac Oncol. 2013;8(2):161–70. doi: 10.1097/JTO.0b013e31827d5237. [DOI] [PubMed] [Google Scholar]
- 22.Ismail EA, Mahmoud HM, Tawfik LM, et al. BIRC6/Apollon gene expression in childhood acute leukemia: Impact on therapeutic response and prognosis. Eur J Haematol. 2012;88(2):118–27. doi: 10.1111/j.1600-0609.2011.01734.x. [DOI] [PubMed] [Google Scholar]
- 23.Shen H, Zhang L, Zhou J, et al. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) acts as a potential diagnostic biomarker for prostate cancer. Med Sci Monit. 2018;24:216–22. doi: 10.12659/MSM.898809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Y, Zhang C, Du E, et al. Pim-3 is a critical risk factor in development and prognosis of prostate cancer. Med Sci Monit. 2016;22:4254–60. doi: 10.12659/MSM.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang Y, Dong Y, Yang D, et al. Expression and prognostic significance of ELL-associated factor 2 in human prostate cancer. Int Urol Nephrol. 2016;48(5):695–700. doi: 10.1007/s11255-015-1210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimura T, et al. Increased expression of Tripartite Motif (TRIM) 47 is a negative prognostic predictor in human prostate cancer. Clin Genitourin Cancer. 2016;14(4):298–303. doi: 10.1016/j.clgc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Cheng G, Li X, et al. Overexpression of long non-coding RNA LOC400891 promotes tumor progression and poor prognosis in prostate cancer. Tumour Biol. 2016;37(7):9603–13. doi: 10.1007/s13277-016-4847-y. [DOI] [PubMed] [Google Scholar]
- 28.Lamers F, Schild L, Koster J, et al. Identification of BIRC6 as a novel intervention target for neuroblastoma therapy. BMC Cancer. 2012;12:285. doi: 10.1186/1471-2407-12-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luk SU, Xue H, Cheng H, et al. The BIRC6 gene as a novel target for therapy of prostate cancer: Dual targeting of inhibitors of apoptosis. Oncotarget. 2014;5(16):6896–908. doi: 10.18632/oncotarget.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]