Abstract
Free-living flatworms, such as the planarian Schmidtea mediterranea, are extensively used as model organisms to study stem cells and regeneration. The majority of flatworm studies so far focused on broadly conserved genes. However, investigating what makes these animals different is equally informative for understanding its biology and might have biomedical value. We re-analyzed the neoblast and germline transcriptional signatures of the flatworm M. lignano using an improved transcriptome assembly and show that germline-enriched genes have a high fraction of flatworm-specific genes. We further identified the Mlig-sperm1 gene as a member of a novel gene family conserved only in free-living flatworms and essential for producing healthy spermatozoa. In addition, we established a whole-animal electron microscopy atlas (nanotomy) to visualize the ultrastructure of the testes in wild type worms, but also as a reference platform for different ultrastructural studies in M. lignano. This work demonstrates that investigation of flatworm-specific genes is crucial for understanding flatworm biology and establishes a basis for such future research in M. lignano.
Introduction
Animal models inspired researchers for hundreds of years. In biomedicine, a variety of organisms is employed to study e.g. development, ageing, and mechanistic underpinnings of diseases, with the aim of translating these findings to humans. While most research focusses on the use of established models, such as yeast, nematodes, fruit flies, and mice, it is sometimes the unique feature of a non-standard experimental model that brings the breakthrough. For example, squalamine, a compound isolated from dogfish sharks, exhibits strong anti-fungal and anti-bacterial activity. It was shown to be very efficient in fighting a broad spectrum of human pathogens, strengthening its therapeutic potential1. Another example is a recent study deciphering the remarkable resistance of tardigrades to X-ray radiation, which led to the discovery of a novel DNA protector, the Dsup protein. When expressed in human cells, this protein shows the ability to protect human DNA as well2. These examples indicate the power of exploring nature’s biodiversity, with a focus on organisms demonstrating extreme characteristics, such as a remarkable resistance to environmental factors, astonishing regeneration abilities, or an extremely long lifespan3–6.
One of such organisms is the free-living, hermaphrodite flatworm Macrostomum lignano (Fig. 1a), which has a number of interesting features such as the ability of whole-body regeneration7, a high resistance to ionizing irradiation up to 210 Gray8, and the presence of a population of actively proliferating neoblasts, which represent the stem cells and progenitors of flatworms9–11. Recently, we established the transcriptional signatures of the proliferating somatic neoblasts and germline cells, and demonstrated the role of several genes conserved between M. lignano and human in stem cell and germline biology12. To understand the biology of this model organism, it will, however, be crucial to also perform functional studies of non-conserved genes. This is illustrated for example by the recent identification of three novel Mlig-stylet genes, which are required for the differentiation of the male copulatory apparatus during tail-regeneration13. Importantly, investigating non-conserved genes may also lead to discoveries translatable to human health, such as improved wound healing or novel anthelminthic drugs. The present study demonstrates the groundwork we laid for this novel research direction. As a first step, we reanalyzed the published dataset using an improved transcriptome assembly, and reassessed the conservation level of genes enriched in the neoblasts and germline cells. In addition, we present the characterization of a previously uncharacterized gene which we named Mlig-sperm1. Knockdown of this gene results in aberrations in the gonads and sperm structure, and leads to a reduced fertility. This serves as a case study to illustrate the recently developed techniques and resources allowing in depth gene characterization of M. lignano.
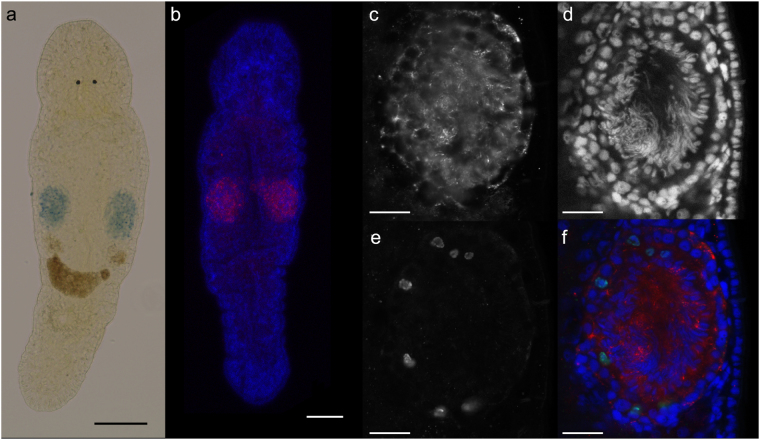
Figure 1.
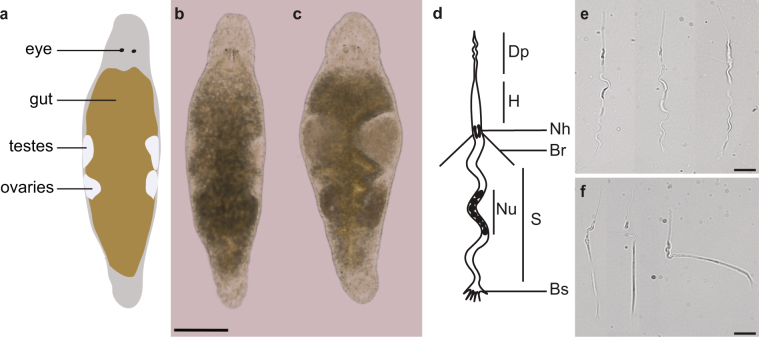
The structure of testes and sperm cells in healthy control and Mlig-sperm1 knockdown M. lignano animals. (a) Illustration of the simplified morphology of M. lignano, indicating the location of the eyes, gut, testes, and ovaries. (b,c) Comparison of the overall morphology between control (b) and Mlig-sperm1(RNAi) (c) individuals. (d) Schematic structure of an adult sperm cell. Dp, distal process (feeler); H, head; Nh, notch; Br, bristle; Nu, nucleus; S, shaft; Bs, brush. For a detailed description of sperm cells in M. lignano see Ref.16. (e,f) Comparison of sperm morphology between control (e) and knockdown (f) worms using DIC microscopy. Scale bars are 100 µm (b,c) and 10 µm (e,f).
Results
Extended M. lignano transcriptome assembly
As part of the M. lignano genome annotation we have recently published a genome-guided transcriptome assembly Mlig_RNA_3_7_DV1_v114. We noticed that some real but lowly expressed genes, such as TERT15, were not included in this transcriptome assembly because all predicted transcripts with low expression levels (RPKM < 0.5) were filtered out. To mitigate this problem, we extended the transcriptome assembly by keeping low expression transcripts if they contain a predicted open reading frame of at least 100 amino acids. The new transcriptome assembly, Mlig_RNA_3_7_DV1_v3, has 143,648 transcriptional units, which are processed into 153,985 genes after identification of trans-splicing events, and include duplicated gene copies and alternative transcript forms (Supplementary Table 1). Clustering of sequences by 95% global sequence identity results in 53,480 and 88,426 non-redundant transcriptional units and genes respectively (Supplementary Table 1).
Characterization of conservation levels in different gene groups
To identify the conservation levels of genes enriched in proliferating cells, we first re-analyzed the previously established proliferating neoblast and germline transcriptional signatures12 using the extended transcriptome assembly Mlig_RNA_3_7_DV1_v3 (Supplementary Table 2, Supplementary Fig. 1). While the conclusions of the previous work do not change with the new analysis, the number of transcript clusters classified as ‘stringent neoblast’ increased from 357 to 489 (Supplementary Fig. 1k); in contrast, the number of ‘irradiation’ and ‘germline’ transcript clusters decreased from 7,277 to 5,901 and from 2,739 to 2,604 respectively (Supplementary Fig. 1c,g). We assigned the conservation level to each M. lignano gene as ‘conserved’ if there is a significant homology with human genes, as ‘flatworm-specific’ if homologs are identified only in the free-living Schmidtea mediterranea and/or the parasite Schistosoma mansoni, or as ‘M. lignano-specific’ if no homologs are detected (Table 1). The distribution analysis of the conservation levels between different gene categories revealed striking differences between neoblast and germline genes. While overall 47.3% of M. lignano genes are conserved in human, 8.2% are flatworm-specific and 44.5% are Macrostomum-specific (Table 1), for the neoblast genes the fraction of human-conserved genes is substantially higher at 85%, while flatworm-specific and non-conserved gene fractions are only 2.8% and 12.2% respectively (Table 1). At the same time, the fraction of germline genes conserved in human is 37.6%, which is significantly less than overall, while the fraction of Macrostomum-specific genes rises to 54.2% (Table 1). Since in this analysis we used all transcripts from the Mlig_RNA_3_7_DV1_v3 transcriptome assembly, including transcripts without predicted open reading frame (ORF), it is possible that the fraction of Macrostomum-specific transcripts is inflated. We repeated the conservation distribution analysis using only transcripts with ORFs and clustering sequences at 95% amino-acid identity level to exclude biases due to possible expansions of gene families. However, the picture did not change significantly: overall 55.3% of genes are conserved in human, 9.7% are flatworm-specific and 35% are Macrostomum-specific, while the numbers are 86%, 3% and 11% for the neoblast genes and 42.5%, 9% and 48.5% for the germline genes respectively (Table 1).
Table 1.
Conservation of different M. lignano gene groups in human and flatworms.
| Total number | Homologs in human | Homologs in S. mediterranea | Homologs in S. mansoni | Flatworm- specific* | M. lignano- specific | |
|---|---|---|---|---|---|---|
| Transcript clusters | ||||||
| All | 50,673 | 23,969 (47.30%) |
25,716 (50.75%) |
21,581 (42.59%) |
4,159 (8.21%) |
22,545 (44.49%) |
| Neoblasts | 1,062 | 902 (84.93%) |
871 (82.02%) |
838 (78.91%) |
30 (2.82%) |
130 (12.24%) |
| Neoblasts, stringent |
489 | 406 (83.03%) |
379 (77.51%) |
370 (75.66%) |
14 (2.86%) |
69 (14.11%) |
| Germline | 2,604 | 979 (37.60%) |
1067 (40.98%) |
840 (32.26%) |
213 (8.18%) |
1,412 (54.22%) |
| Protein clusters | ||||||
| All | 30,017 | 16,605 (55.32%) |
17,758 (59.16%) |
14,819 (49.37%) |
2,913 (9.70%) |
10,499 (34.98%) |
| Neoblasts | 840 | 723 (86.07%) |
693 (82.50%) |
676 (80.48%) |
25 (2.98%) |
92 (10.95%) |
| Neoblasts, stringent |
404 | 336 (83.17%) |
310 (76.73%) |
307 (75.99%) |
10 (2.48%) |
58 (14.36%) |
| Germline | 1,917 | 815 (42.51%) |
882 (46.01%) |
707 (36.88%) |
173 (9.02%) |
929 (48.46%) |
*Present in S. mediterranea or S. mansoni but not in human.
Knockdown of a flatworm-specific gene Mlig-sperm1 causes abnormal morphology of testes and spermatozoa and decreased fertility
To assess the possible roles of non-conserved and flatworm-specific genes in Macrostomum biology, we randomly chose six candidate genes enriched in proliferating cells for a pilot functional screen (Supplementary Table 3). From the tested candidates, one of the germline genes, Mlig020950, demonstrated a strong phenotype. We named the Mlig020950 gene as Mlig-sperm1 due to severe defects in sperm morphology in Mlig020950(RNAi) animals, as described below.
Gene knockdown of Mlig-sperm1 led to a dramatic enlargement of the testes in all individuals (Fig. 1b,c). Detailed analysis revealed that these oversized testes accumulated large amounts of sperm cells (Fig. 1c–f), characterized by an aberrant morphology (teratozoospermia), such as a very rigid shaft, and often forming contortion at the notch site (Fig. 1f). In contrast to sperm of control animals, which demonstrate undulating movements of the shaft and distal process (Supplementary Video 1), the knockdown worms’ spermatozoa showed atypical motility (asthenozoospermia): cells were not swimming actively and performed twitching movements (Supplementary Video 2).
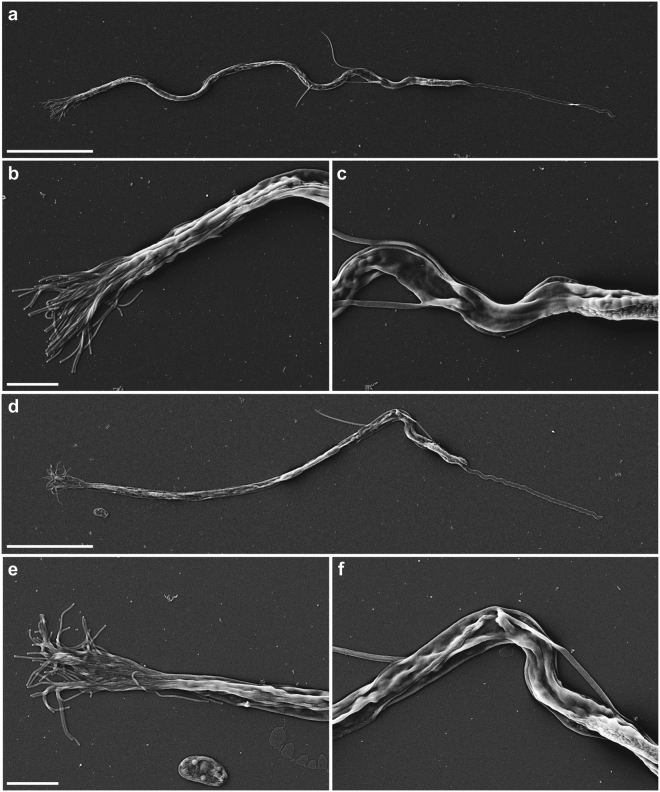
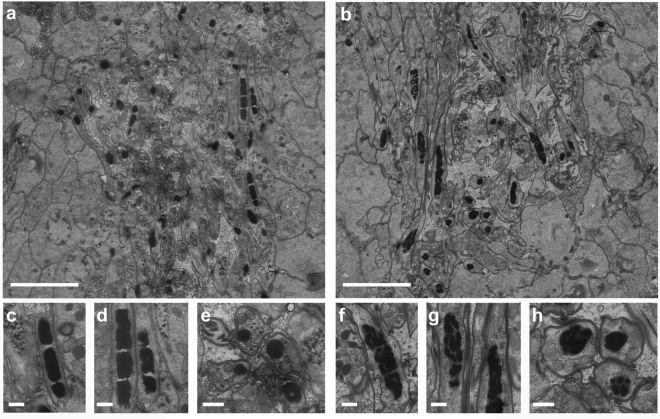
Scanning electron microscopy (EM) confirmed the morphological aberrancy of Mlig-sperm1(RNAi) spermatozoa (Fig. 2). In comparison to control cells (Fig. 2a–c), the shaft of the Mlig-sperm1 knockdown spermatozoa, demonstrates rigidity and lack of curvature (Fig. 2d), and its brush is flattened and inflexible (Fig. 2e). Furthermore, the contortion at the notch site is clearly visible (Fig. 2f). In addition, transmission EM demonstrated that also the nuclei of late spermatids and spermatozoa of Mlig-sperm1(RNAi) worms have an aberrant morphology (Fig. 3). In GFP(RNAi) worms, which are used as negative controls, the nucleus elongates and chromatin condenses into a number of discrete bodies connected by small bridges during the late phase of spermiogenesis (Fig. 3a,c,d,e) as previously described16. In Mlig-sperm1(RNAi) worms, distinct nuclear bodies can be rarely observed and the chromatin has a fragmented appearance (Fig. 3b,f,g,h). To enable comparison of the ultrastructure of GFP(RNAi) and Mlig-sperm1(RNAi) worms with untreated animals, we also provided a nanotomy-atlas of a wild-type individual (Supplementary Fig. 2). In these sections, the fragmented chromatin appearance cannot be observed, indicating that this is indeed a specific characteristic of the phenotype of the Mlig-sperm1 knockdown.
Figure 2.
Scanning electron microscopy of spermatozoa. (a–c) Spermatozoon of a negative control GFP(RNAi) worm. (a) Overview of the complete cell. Note the curved view of the shaft. (b) Detail of the brush, consisting out of separate extensions. (c) Detail of the notch region of the cell. (d–f) Spermatozoon of a Mlig-sperm1(RNAi) worm. (d) Overview of the complete cell. Note the rigidity of the shaft and the contortion at the notch site. (e) Detail of the brush. Compared to the negative control the brush looks more flattened with the base of the extensions being more packed together. (f) Detail of the notch region clearly showing the contortion. Scale bar are 10 µm (a,d) and 2 µm (b,c,e,f).
Figure 3.
Ultrastructure of spermatids and spermatozoa. (a,b) Overview of early and late spermatids and spermatozoa in the testes of a negative control GFP(RNAi) worm (a) and a Mlig-sperm1(RNAi) worm (b). In both images, several longitudinal and cross sections of nuclei of the spermatozoa can be observed as black structures. (c,d) Detail of longitudinal sections of spermatozoa nuclei of a GFP(RNAi) worm. The chromatin of the nucleus is condensed into discrete bodies. (e) Detail of a cross section of spermatozoa nuclei of a GFP(RNAi) worm. (f,g) Detail of longitudinal sections of spermatozoa nuclei of a Mlig-sperm1(RNAi) worm. Compared to the negative control, the chromatin of the nuclei looks fragmented and condensation into discrete bodies is less visible. (h) Detail of a cross section of spermatozoa nuclei of a Mlig-sperm1(RNAi) worm. Compared to the negative control, the chromatin of the nuclei is more fragmented. Scale bars are 5 µm (a,b) and 500 nm (c–h).
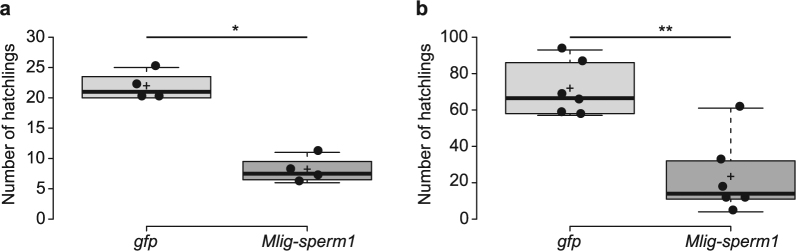
As sperm defects are often reported as a cause of decreased male fertility or even complete sterility17,18, we compared fertility of control and Mlig-sperm1(RNAi) animals. The knockdown of the Mlig-sperm1 gene in adult animals resulted in a significantly lower (p < 0.05, Mann-Whitney U test) number of progeny produced in a period of three weeks (Fig. 4a). To study if knockdown could result in sterility, we performed an additional RNAi experiment of 5 weeks, starting with one-day old hatchlings. In both the control and Mlig-sperm1(RNAi) condition, the first juveniles were observed during the second week of treatment. This experiment confirmed the decreased fertility due to Mlig-sperm1 knockdown (p < 0.01, Mann-Whitney U test), but a complete sterility could not be observed (Fig. 4b).
Figure 4.
Fertility of healthy control and Mlig-sperm1 knockdown M. lignano animals. (a) Fertility of 4 groups of Mlig-sperm1(RNAi) worms and 4 groups of adult negative control GFP(RNAi) worms. The RNAi treatment started with adult animals and was continued for a total of 3 weeks. Data points are indicated by black dots, the black line represents the median, and the cross indicates the mean. The data points represent the number of hatchlings produced by each group of five worms within three weeks. The star represents a significant difference with a p-value < 0.05. (b) Fertility of 6 groups of Mlig-sperm1(RNAi) worms and 6 groups of negative control GFP(RNAi) worms. The RNAi treatment started with one-day old hatchlings and was continued for a total of 5 weeks. The number of hatchlings produced by each group of 5 worms within this period is visualized as a boxplot combined with a beeswarm of the data points. Data points are indicated by black dots, the black line represents the median, and the cross indicates the mean. The double star represents a significant difference with a p-value < 0.01.
Mlig-sperm1 is expressed exclusively in testes
Mlig-sperm1 is categorized as enriched in proliferating germline cells according to the RNA-seq data (Supplementary Table 2). To confirm this, and to study the gene expression pattern in more detail, we performed whole mount in situ hybridization (WISH). In adult animals, strong expression of Mlig-sperm1 was observed in the complete testes (Fig. 5a). Fluorescent in situ hybridization (FISH) and confocal microscopy analysis of adult worms confirmed the pattern observed with WISH (Fig. 5b). Counterstaining the nuclei of all cells with DAPI indicated that Mlig-sperm1 is expressed within testicular cells, since the in situ pattern encircles the DAPI-labeled nuclei (Fig. 5c,d,f, and Supplementary Fig. 3e–g). In addition, this labeling further indicated that the expression is specific for the testes and is not present in the ovaries (Supplementary Fig. 3g). To further confirm the testicular expression, WISH was performed in one day old hatchlings, and 4–5 days old juveniles. Based on light microscopic analysis, no signal was detected in hatchlings (Supplementary Fig. 3a), suggesting that the gene is not expressed in the gonad anlage of primordial germ cells11. In the juveniles, the signal was observed on the level of developing testes in a cluster of several cells (Supplementary Fig. 3b). The expression of Mlig-sperm1 in proliferating testicular cells was confirmed by a FISH combined with a DAPI and mitotic counterstain. Figure 5(c–f) demonstrates Mlig-sperm1 expression around the labeled mitotic nuclei. In conclusion, Mlig-sperm1 is expressed in both proliferating testicular cells and non-proliferating cells of the testes such as spermatids and spermatozoa. Specificity of ISH labelling was confirmed by a Mlig-sperm1 sense probe, for which no signal was detected (Supplementary Fig. 3d).
Figure 5.
Expression pattern of Mlig-sperm1 gene. (a) Whole mount in situ hybridization demonstrating the specific expression of the Mlig-sperm1 gene in the testes of an adult worm (blue precipitation). (b) Fluorescent in situ hybridization of the Mlig-sperm1 gene (red) in an adult worm, combined with a DAPI labeling of all nuclei in the worm (blue). This confirms the testes-specific expression of Mlig-sperm1. (c–f) Triple labeling including Mlig-sperm1 FISH (c), DAPI labeling (d), mitotic labeling with the anti-phospho-histone H3 antibody (e), and the overlay of all three (f). The Mlig-sperm1 (red) clearly encircles DAPI-labeled nuclei (blue) indicating the expression within testicular cells. Importantly, this includes the nuclei of the mitotic cells (green), demonstrating expression in the proliferating testicular cells. Scale bars: 100 µm (a,b), and 25 µm (c–f).
Mlig-sperm1 is a member of a large gene family specific for free-living flatworms
The Mlig-sperm1 gene has two nearly identical loci in the Mlig_3_7_DV1 genome assembly, Mlig020950.g1 and Mlig020950.g2. We next studied the relation of this gene to other Macrostomum genes and homologs in other flatworms. The BLAST search revealed that Mlig-sperm1 has a significant similarity with 35 genes in M. lignano (blastp e-value cutoff 1e-15), which can be grouped into 14 protein clusters based on 95% amino-acid identity cutoff (Supplementary Table 4). Notably, most of the homolog genes are enriched in the proliferating germline cells according to the previous analysis12. Furthermore, the gene is conserved in S. mediterranea, with one and eight contigs identified in asexual (dd_Smed_v6) and sexual (dd_Smes_v1) transcriptome assemblies respectively (Supplementary Table 4), as well as in all 5 other planarian species available in PlanMine19. We did not find significant matches to the Mlig-SPERM1 protein in the transcriptomes of parasitic flatworms or outside flatworm species. Alignment of the representative proteins revealed a conserved domain common to all genes (Supplementary Fig. 4). However, a search against the Pfam database did not reveal homology to any known protein family. The tertiary structure of the Mlig-SPERM1 protein, as predicted by RaptorX20, consists of three domains with 48% of the predicted positions being disorganized. The region conserved between all the genes (Supplementary Fig. 4) corresponds to the first domain, which has the highest organization factor and consists of one beta sheet and several alpha helixes (Supplementary Fig. 5).
Discussion
To investigate the extent of conservation of M. lignano genes with neoblast- and germline-enriched expression, we first reanalyzed the transcriptomic signatures of proliferating cells in M. lignano. While all previous RNA-seq based gene expression studies in M. lignano relied on de novo transcriptome assemblies12,13,21,22, we here used the genome-guided transcriptome assembly Mlig_RNA_3_7_DV1_v3, which is based on the recently generated and significantly improved genome assembly for the M. lignano DV1 line14. The new transcriptome assembly resolved many partial transcript issues inherent for de novo transcriptomes, and the decreased fragmentation allows more accurate estimation of gene conservation depth. This explains the change in numbers of transcript clusters of gene groups as ‘stringent neoblast’, ‘irradiation’, and ‘germline’ when the current analysis is compared to the initial one12.
Interestingly, neoblast-enriched genes were found to be deeply conserved, while germline-enriched genes have many non-conserved or flatworm-specific genes. This is in line with published literature on deep conservation of the neoblast regulation program23 and significant variation in evolution of reproductive systems24.
As a next step, we randomly selected six non-conserved candidate genes for a preliminary functional screen, and demonstrated that one of them, Mlig-sperm1, is expressed in all testicular cell types, including proliferating and differentiated cells. Indeed, it should be kept in mind that the enrichment in proliferating germline cells does not give information about the expression in differentiated testicular cell types, a group of cells for which specific transcriptomic analysis is not yet available. All results taken together demonstrate that Mlig-sperm1 plays a role in forming healthy sperm, and consequently the reproductive capacity of M. lignano. Knock down of Mlig-sperm1 resulted in an accumulation of aberrant spermatozoa, leading to enlarged testes. Enlargement of the testes due to accumulating aberrant testicular cells was previously observed during knock down of melav2, a gene essential for spermatid differentiation25. Besides its role in sperm differentiation, Mlig-sperm1 might have additional functions, as is it also expressed in the proliferating testicular cells. Given the conservation level between Mlig-sperm1 and other genes, and the classification of many of the genes as enriched in proliferating germline cells, we suggest that Mlig-sperm1 is a member of a novel protein family specific for free-living flatworms, with important roles in reproduction.
Of note, the transmission electron microscopy analysis of GFP(RNAi) and Mlig-sperm1(RNAi) animals presented in Fig. 3 was performed using the anatomy at the nanoscale (nanotomy) approach, which allows visualization of large specimen areas26 and provides a ‘Google-Earth’ style of data presentation and navigation at different levels of resolution. The nanotomy datasets are available at http://www.nanotomy.org/OA/Macrostomum. In addition to the RNAi worms, the ultrastructure of wild-type testes is also made available. However, instead of focusing on the testes area, we generated 35 cross-sections and a longitudinal section covering a complete animal (Supplementary Fig. 2). While the detailed annotation of these nanotomy images is beyond the scope of this work, we believe that the generated resource will serve as a valuable reference on M. lignano morphology at the ultrastructural level and complements genomic resources available for this developing model organism. The current progress in the available resources and techniques, e.g. the recently developed transgenesis methods14, are making this model increasingly attractive for different research areas.
Our approach of specifically selecting non-conserved or flatworm-specific genes can yield important insight into aspects of flatworm biology, such as germline development. This is illustrated by the Mlig-sperm1, but also the recently published example of Mlig-stylet genes13. Future studies of genes conserved within flatworms, and more specifically in parasitic flatworms, could help to develop treatments for infections caused by parasites, such as Schistosoma. Current research in that area focuses its efforts on dissecting the mechanism behind the maintenance and activity of the germline, as the egg-induced inflammation is the main cause of Schistosoma-associated pathologies27,28. In addition, focusing on somatic neoblast-enriched flatworm-specific genes could contribute to our understanding of e.g. their astonishing regeneration capacity and would create an opportunity to improve such competencies in humans. This is in line with the recent report on improved radiotolerance of human cultured cells by a tardigrade-unique protein2. We therefore advocate that investigating M. lignano genes not conserved in humans is an approach with truly great potential. This paper illustrates several of the current available tools and resources for this type of research.
Methods
Experimental organism and culture conditions
Macrostomum lignano (Macrostomida, Rhabditophora) is a free-living marine flatworm and an obligatory, non-self-fertilizing hermaphrodite reproducing exclusively in a sexual manner29. The combination of a short generation time of about 3 weeks and high fertility rates allows a rapid expansion of cultures30.
The animal is small, about 1 mm long and consists of approximately 25,000 cells9. Worms are transparent and major tissues and organs can be easily distinguished (i.e. eyes, brain, gonads, reproductive organs, gut). Worms are kept in Petri dishes with nutrient-enriched artificial seawater (f/2)31, at 20 °C and a 14/10 hours light/dark cycle and are fed ad libitum with the diatom Nitzschia curvilineata29.
In the present study, the recently collected and introduced in the Berezikov lab, NL10 strain was used. In contrast to DV132, NL10 does not demonstrate chromosomal polymorphism14.
Transcriptome assembly
The transcriptome assembly Mlig_RNA_3_7_v3 was generated exactly as previously described, with the exception that low expressed transcripts (RPKM < 0.5) were retained in the assembly if they contain on open reading frame of at least 100 amino-acids, as predicted by as predicted by TransDecoder33. Furthermore, the transcripts in the assembly were classified by selecting only one representative transcript for each predicted CDS and removing the non-coding transcripts and transcripts overlapping repeat annotations. The resulting transcriptome subset is named ‘Core Genes’ and contains 56,036 non-redundant genes (Supplementary Table 1). This Core Genes subset of the Mlig_RNA_3_7_DV1_v3 transcriptome assembly is useful in cases where representative transcripts rather than full transcriptome diversity is desirable.
Conservation and alignments
Amino-acid sequences from the Mlig_RNA_3_7_DV1_v3 transcriptome assembly were queried against human, S. mediterranea (dd_Smed_v6 and dd_Smes_v1) and S. mansoni (ASM23792v2) genes using BLAST34 and hits with E-value below 0.01 were considered as homologs for the purpose of conservation analysis. In addition, Mlig-sperm1 gene was search against all species available in PlanMine19 and WormBase35. Sequence alignment and visualization were performed with CLC Main Workbench (QIAGEN Aarhus A/S).
Whole mount in situ hybridization
cDNA synthesis was performed using the SuperScript III First-Strand Synthesis System (Life Technologies) according to the manufacturer’s protocol with 2–3 µg of total RNA as a template for each reaction. Provided oligo(dT) and hexamer random primers were used.
DNA fragments selected as templates for in situ hybridization probes, were amplified from cDNA by standard PCR with GoTaq Flexi DNA Polymerase (Promega), followed by cloning using the pGEM-T vector system (Promega) and sequenced by GATC Biotech. All primers used are listed in Supplementary table 3. DNA templates for producing DIG – labeled riboprobes were amplified from sequenced plasmids using High Fidelity Pfu polymerase (Thermo Scientific). Forward (5′-CGGCCGCCATGGCCGCGGGA-3′) and reversed (5′TGCAGGCGGCCGCACTAGTG-3′) primers binding the pGEM-T vector backbone near the insertion site were designed. Moreover, versions of the same primers with a T7 promoter sequence (5′-GGATCCTAATACGACTCACTATAGG-3′) appended upstream were obtained. The T7 promoter sequence serves as a start site in subsequent in vitro transcriptions. A pair of primers, depending on the orientation of the insert in the vector: forward with T7 promoter and reverse without or vice versa, was used to amplify every ISH probe template.
Digoxigenin (DIG) labeled RNA probes (800 to 1200 bp in length) were generated using the DIG RNA labeling Mix (Roche, Switzerland) and T7 RNA polymerase (Promega, Fitchburg, WI) according to the manufacturer’s protocol for in vitro transcription. The concentration of every probe was measured with the Qubit RNA BR assay (Invitrogen), probes were diluted in Hybridization Mix10 to 20 ng/µl, stored at −80 °C and used within 4 months. The final concentration of the probe and optimal temperature used for hybridization varied for different probes and were optimized for each probe.
Whole mount in situ hybridization (ISH) was performed following the published protocol10. Pictures were made using a standard light microscope with DIC optics and an AxioCam HRC (Zeiss, Germany) digital camera and the EVOS XL Core Imaging System (ThermoFisher).
Fluorescent in situ hybridization and immunofluorescence
Fluorescent in situ hybridization (FISH) was performed following the published FastBlue protocol developed for planarians36, except the 5% NAC treatment and bleaching steps were omitted. FISH combined with immunofluorescence of mitotic cells was performed as described before12 using the primary anti-phopho histone H3 (1:250) (Millipore, Billerica, MA) and secondary goat anti-rabbit IgF Antibody conjugated with FITC (1:150) (Millipore). The DAPI counterstain was always performed as a last step by incubating labeled worms in NucBlue Fixed Cell ReadyProbes Reagent (ThermoFisher Scientific) for at least 1 hour to ensure penetration in the complete worm. Slides were mounted using 80% glycerol solution, and the labeling was visualized with a Leica TCS SP8 confocal microscope at the UMCG Imaging and Microscopy Center.
RNA interference
In order to generate dsRNA fragments, the same plasmids were used as for making ISH probes. Templates for the synthesis of both sense and antisense RNA strands were amplified from the plasmids containing the insert of interest. The same primers were used as for ISH riboprobe template amplification, and for each fragment, two PCRs were performed – with both pairs of primers (forward with T7 promoter and reversed without and vice versa). High Fidelity Pfu polymerase (Thermo Scientific) in 150 µl of total volume reaction was used. PCR products were then run on 1% agarose gel, PCR product bands were cut out and purified using the QIAquick Gel Extraction Kit (Qiagen, Netherlands). Each template was then used to synthesize the corresponding single strand RNA with the TranscriptAid T7 High Yield Transcription Kit (Thermo Scientific) according to manufacturer’s protocol. The single reaction volume was 50 µl, and tubes were incubated in 37 °C for 5 hours. Afterwards 100 µl of nuclease-free water was added to each tube, sense and antisense RNA strands were mixed to a final volume of 300 µl and annealed by incubating them at 70 °C for 10 min, followed by gradual cooling down to room temperature, taking approximately 90 min. Every sample was then treated with 1U of RNase A (Life Technologies) and 5U of DNase I (Thermo Scientific) for 45 min at 37 °C. Samples were alcohol precipitated overnight at −80 °C. dsRNA was pelleted by centrifugation at 12,000 g for 15 min at 4 °C, washed with 75% ethanol, and air-dried for 5 min. dsRNA was resuspended in nuclease-free water and the concentration was measured using Nanodrop ND1000. Freshly autoclaved and filtered f/2 medium was used to adjust the concentration to 10 ng/µl. Samples were aliquoted in 1.5 ml Eppendorf tubes and stored at −80 °C.
Specific knockdown of candidate genes by RNA interference with double-stranded RNA delivered by soaking was performed as previously described37. RNAi soaking experiments were performed in 24-well plates in which algae were grown. Individual wells contained 300 µl of dsRNA solution (10 ng/ml in f/2 medium) in which 15 individuals were maintained. RNAi was performed for three weeks during which dsRNA solution was refreshed daily. Worms were weekly transferred to fresh 24-well plates to ensure sufficient amount of food. As a negative control, GFP dsRNA was used. Photos of randomly selected worms were made between 2 and 3 weeks of treatment.
Fertility experiment
During the RNAi experiment with adults, worms were treated for three weeks with Mlig020950 dsRNA or with GFP dsRNA as a negative control. After that, worms were randomly selected and divided into four groups of five worms. These were cultured in freshly prepared 12-well plates for three weeks while RNAi treatment was ongoing. As a measure of fertility, the number of hatchlings produced by each group were counted twice a week.
During the RNAi experiment starting during development, six groups of five randomly selected one-day old hatchlings were made. These groups were cultured in freshly prepared 12-well plates for a total of five weeks, including the developmental time (2–3 weeks) and early adulthood. During this time worms were treated with Mlig020950 dsRNA or with GFP dsRNA as a negative control. As a measure of fertility, the number of hatchlings produced by each group within these five weeks were counted on a daily basis.
Electron Microscopy (EM)
Scanning EM to define surface structure using secondary electron detection
To isolate spermatozoa, Mlig020950(RNAi) and GFP(RNAi) worms were relaxed in 7.14% MgCl.6H2O and cut through the testes on a glass slide, using a surgical blade. The cells within the testes were then squeezed out and pipetted onto a poly-l-lysine coated coverslip. After fixation in 2% glutaraldehyde plus 2% paraformaldehyde in 0.1 M sodium cacodylate, samples were postfixed with 1% Osmium tetroxide for 30 minutes at 4 °C. Slides were rinsed three times with water, and dehydrated through increasing concentrations of ethanol. Samples were incubated for 10 min in a 1:1 mixture of absolute ethanol and tetramethylsilane on ice, followed by 10 minutes incubation in pure tetramethylsilane on ice. Samples were air dried, glued on aluminium stubs using double sided carbon tape, sputter coated with 10 nm Pd/Au and imaged in a Zeiss Supra55 Scanning Electron Microscope operated at 5 KV using secondary electron detection (Fig. 2).
Transmission EM for ultrastructural analysis of spermatogenesis
Mlig020950(RNAi) and GFP(RNAi) worms were relaxed in 7.14% MgCl.6H2O and fixed in 2% glutaraldehyde plus 2% paraformaldehyde in 0.1 M sodium cacodylate buffer for 24 hours at 4 °C. After postfixation in 1% osmium tetroxide/1.5% potassium ferrocyanide for 2 hours at 4 °C, worms were dehydrated using ethanol and embedded in EPON epoxy resin. Sections of 60 nm were collected on single slot grids and contrasted using 5% uranyl acetate in water for 20 min, followed by Reynolds lead citrate for 2 min. The longitudinal sections were scanned as described before38 (Fig. 3).
Transmission EM for transversal nanotomy of Macrostomum (back scatter detector)
M. lignano was fixed following the recommended chemical fixation method described by Salvenmoser39, using a simultaneous fixation with glutaraldehyde and osmium tetroxide. Worms were dehydrated using ethanol and embedded in EPON epoxy resin. Thin sections (~100 nm) were collected every 30 µm on silicon wafers as described before40. Data was acquired on a Zeiss Supra 55 STEM microscope using a back scatter detector (BSD) at 5 kV with 5 nanometer pixel size, 5 µs dwell time using an external scan generator ATLAS 5 (Fibics, Canada) and stitched as described before38,41. After tile stitching the data were exported as an html file and uploaded to the online image database (Supplementary Fig. 2). Data are available at http://www.nanotomy.org/OA/Macrostomum. Supplementary Fig. 2.
Data availability
The extended transcriptome assembly Mlig_RNA_3_7_DV1_v3 is available at http://gb.macgenome.org/downloads. The re-analyzed gene expression data are available at http://neoblast.macgenome.org. The generated nanotomy images are available at http://www.nanotomy.org/OA/Macrostomum.
Electronic supplementary material
Acknowledgements
We would like to acknowledge Fleur Broek for the preliminary work within the project. We thank Aziz Aboobaker for the help with identification of the M. lignano TERT gene, leading to the improvements in the M. lignano transcriptome assembly. Part of the work has been performed in the UMCG Microscopy and Imaging Center (UMIC), sponsored by ZonMW grant 91111.006. This work was supported by the European Research Council Starting Grant (MacModel, grant no. 310765) to EB.
Author Contributions
M.Gru, S.M., E.B., Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article; M.Gre, J.K., A.W., B.G., Acquisition and analysis of E.M. data.
Competing Interests
The authors declare no competing interests.
Footnotes
Magda Grudniewska and Stijn Mouton contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21107-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zasloff M, et al. Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential. Proc. Natl. Acad. Sci. 2011;108:15978–15983. doi: 10.1073/pnas.1108558108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto T, et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016;7:12808. doi: 10.1038/ncomms12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Magalhães JP. The Bad and the Ugly? EMBO Rep. 2015;16:771–776. doi: 10.15252/embr.201540606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenzano DR, Aboobaker A, Seluanov A, Gorbunova V. Non‐canonical aging model systems and why we need them. EMBO J. 2017;36:959–963. doi: 10.15252/embj.201796837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austad, S. N. Cats, ‘Rats,’ and Bats: The comparative biology of aging in the 21st century. In Integrative and Comparative Biology50, 783–792 (2010). [DOI] [PMC free article] [PubMed]
- 6.Austad SN. Is there a role for new invertebrate models for aging research? Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2009;64:192–194. doi: 10.1093/gerona/gln059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egger B, Ladurner P, Nimeth K, Gschwentner R, Rieger R. The regeneration capacity of the flatworm Macrostomum lignano - On repeated regeneration, rejuvenation, and the minimal size needed for regeneration. Dev. Genes Evol. 2006;216:565–577. doi: 10.1007/s00427-006-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Mulder K, et al. Potential of Macrostomum lignano to recover from gamma-ray irradiation. Cell Tissue Res. 2010;339:527–542. doi: 10.1007/s00441-009-0915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladurner P, Rieger R, Baguñà J. Spatial distribution and differentiation potential of stem cells in hatchlings and adults in the marine platyhelminth macrostomum sp.: a bromodeoxyuridine analysis. Dev. Biol. 2000;226:231–241. doi: 10.1006/dbio.2000.9867. [DOI] [PubMed] [Google Scholar]
- 10.Pfister D, et al. The exceptional stem cell system of Macrostomum lignano: screening for gene expression and studying cell proliferation by hydroxyurea treatment and irradiation. Front. Zool. 2007;4:9. doi: 10.1186/1742-9994-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfister D, et al. Flatworm stem cells and the germ line: Developmental and evolutionary implications of macvasa expression in Macrostomum lignano. Dev. Biol. 2008;319:146–159. doi: 10.1016/j.ydbio.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 12.Grudniewska M, et al. Transcriptional signatures of somatic neoblasts and germline cells in Macrostomum lignano. Elife. 2016;5:e20607. doi: 10.7554/eLife.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengerer, B. et al. Organ specific gene expression in the regenerating tail of Macrostomum lignano. Dev. Biol. 1–13, 10.1016/j.ydbio.2017.07.021 (2017). [DOI] [PubMed]
- 14.Wudarski J, et al. Efficient transgenesis and annotated genome sequence of the regenerative flatworm model Macrostomum lignano. Nat. Commun. 2017;8:2120. doi: 10.1038/s41467-017-02214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai AG, et al. The protein subunit of telomerase displays patterns of dynamic evolution and conservation across different metazoan taxa. BMC Evol. Biol. 2017;17:107. doi: 10.1186/s12862-017-0949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willems M, et al. Ontogeny of the complex sperm in the macrostomid flatworm Macrostomum lignano (macrostomorpha, rhabditophora) J. Morphol. 2009;270:162–174. doi: 10.1002/jmor.10675. [DOI] [PubMed] [Google Scholar]
- 17.Wosnitzer M, Goldstein M, Hardy MP. Review of Azoospermia. Spermatogenesis. 2014;4:e28218. doi: 10.4161/spmg.28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yatsenko AN, Iwamori N, Iwamori T, Matzuk MM. The Power of Mouse Genetics to Study Spermatogenesis. J. Androl. 2010;31:34–44. doi: 10.2164/jandrol.109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandl H, et al. PlanMine - A mineable resource of planarian biology and biodiversity. Nucleic Acids Res. 2016;44:D764–D773. doi: 10.1093/nar/gkv1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng J, Xu J. Raptorx: Exploiting structure information for protein alignment by statistical inference. Proteins Struct. Funct. Bioinforma. 2011;79:161–171. doi: 10.1002/prot.23175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbore R, et al. Positional RNA-Seq identifies candidate genes for phenotypic engineering of sexual traits. Front. Zool. 2015;12:14. doi: 10.1186/s12983-015-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasik K, et al. Genome and transcriptome of the regeneration-competent flatworm, Macrostomum lignano. Proc. Natl. Acad. Sci. 2015;112:12462–12467. doi: 10.1073/pnas.1516718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Önal P, et al. Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO J. 2012;31:2755–2769. doi: 10.1038/emboj.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwander T, Marais G, Roze D. Sex uncovered: The evolutionary biology of reproductive systems. J. Evol. Biol. 2014;27:1287–1291. doi: 10.1111/jeb.12424. [DOI] [PubMed] [Google Scholar]
- 25.Sekii K, Salvenmoser W, De Mulder K, Scharer L, Ladurner P. Melav2, an elav-like gene, is essential for spermatid differentiation in the flatworm Macrostomum lignano. BMC Dev. Biol. 2009;9:62. doi: 10.1186/1471-213X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravelli RBG, et al. Destruction of tissue, cells and organelles in type 1 diabetic rats presented at macromolecular resolution. Sci. Rep. 2013;3:1804. doi: 10.1038/srep01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer H, Collins JJ, Newmark PA. NF-YB Regulates Spermatogonial Stem Cell Self-Renewal and Proliferation in the Planarian Schmidtea mediterranea. PLoS Genet. 2016;12:1–18. doi: 10.1371/journal.pgen.1006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins JJ, Newmark PA. It’s No Fluke: The Planarian as a Model for Understanding Schistosomes. PLoS Pathog. 2013;9:1–5. doi: 10.1371/journal.ppat.1003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieger, R. et al. Laboratory cultures of marine Macrostomida (Turbellaria). Fortschr Zool36 (1988).
- 30.Ladurner, P. et al. In Stem cells: from Hydra to man 75–94 (Bosh, Th.C.G., 2008).
- 31.Anderson, R. A., Berges, R. A., Harrison, P. J. & Watanabe, M. M. In Algal Culturing Techniques 596 (2005).
- 32.Zadesenets KS, et al. Evidence for karyotype polymorphism in the free-living flatworm, macrostomum lignano, a model organism for evolutionary and developmental biology. PLoS One. 2016;11:e0164915. doi: 10.1371/journal.pone.0164915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas BJ, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, R. Y. N. et al. WormBase 2017: molting into a new stage. Nucleic Acids Res. (2017). [DOI] [PMC free article] [PubMed]
- 36.Currie KW, et al. HOX gene complement and expression in the planarian Schmidtea mediterranea. Evodevo. 2016;7:7. doi: 10.1186/s13227-016-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Mulder K, et al. Stem cells are differentially regulated during development, regeneration and homeostasis in flatworms. Dev. Biol. 2009;334:198–212. doi: 10.1016/j.ydbio.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Kuipers J, de Boer P, Giepmans BNG. Scanning EM of non-heavy metal stained biosamples: Large-field of view, high contrast and highly efficient immunolabeling. Exp. Cell Res. 2015;337:202–207. doi: 10.1016/j.yexcr.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Salvenmoser W, Egger B, Achatz JG, Ladurner P, Hess MW. Electron microscopy of flatworms. Standard and cryo-preparation methods. Methods Cell Biol. 2010;96:307–330. doi: 10.1016/S0091-679X(10)96014-7. [DOI] [PubMed] [Google Scholar]
- 40.Kuipers J, et al. FLIPPER, a combinatorial probe for correlated live imaging and electron microscopy, allows identification and quantitative analysis of various cells and organelles. Cell Tissue Res. 2015;360:61–70. doi: 10.1007/s00441-015-2142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokol E, et al. Large-Scale Electron Microscopy Maps of Patient Skin and Mucosa Provide Insight into Pathogenesis of Blistering Diseases. J. Invest. Dermatol. 2015;135:1763–70. doi: 10.1038/jid.2015.109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The extended transcriptome assembly Mlig_RNA_3_7_DV1_v3 is available at http://gb.macgenome.org/downloads. The re-analyzed gene expression data are available at http://neoblast.macgenome.org. The generated nanotomy images are available at http://www.nanotomy.org/OA/Macrostomum.