Abstract
Elevated nitrogen associated with global change is believed to promote the invasion of many vigorous exotic plants. However, it is unclear how a weak exotic plant will respond to elevated nitrogen in the future. In this study, the competitive outcome of a weak invasive plant (Galinsoga quadriradiata) and two non-invasive plants was detected. The plants were subjected to 3 types of culture (mixed, monoculture or one-plant), 2 levels of nitrogen (ambient or elevated at a rate of 2 g m−2 yr−1) and 2 levels of light (65% shade or full sunlight). The results showed that elevated nitrogen significantly promoted the growth of both the weak invader and the non-invasive plants in one-plant pots; however, growth promotion was not observed for the non-invasive species in the mixed culture pots. The presence of G. quadriradiata significantly inhibited the growth of the non-invasive plants, and a decreased negative species interaction was detected as a result of elevated nitrogen. Our results suggest that competitive interactions between G. quadriradiata and the non-invasive plants were altered by elevated nitrogen. It provides exceptional evidence that an initially weak invasive plant can become an aggressive invader through elevated nitrogen deposition.
Introduction
Exotic species are commonly considered to be harmful to natural ecosystems of the introduced ranges1,2. Many aggressive invaders have received considerable attention from researchers worldwide, e.g., Centaurea solstitialis, Mikania micrantha, and Bromus tectorum3–5. Such species, which are jointly characterized by high competitive and colonizing abilities in natural communities6,7, usually have serious negative consequences, causing great concern. However, the effects of less aggressive exotic species have been consciously or unconsciously neglected by researchers for a long time. The invasive processes of such weak invasive species are poorly documented yet critical to understanding their expansion dynamics and effects in the future.
The high competitive ability of alien species has been suggested as a key factor associated with successful invasive potential, and competitive exclusion by native plant species seems to be a major force resisting exotic invasions7. It is commonly believed that the advantages of competitive ability account for the prevalence of vigorous invasive plants6,7. However, increasing evidence suggests that the competitive relationship between invasive and native plants varies with the invasion process8,9. For example, the dominance and negative effect of an initial aggressive invasive plant Heracleum mantegazzianum decreased with time10. Such a change is presumably attributed to adaptation and coevolution. Additionally, limited evidence suggests that the competitive relationship between invasive and native plants can also be changed by environmental variation, such as global change11. The magnitude of invasive species’ impacts on native plants may be modified by global changes that increase the availability of resources (e.g., nitrogen) and hence potentially alter the competitive dynamics between invasive and native species12. For instance, elevated nitrogen increased the predominance of invasive Duchesnea indica in contrast to native Fragaria vesca, hence environments with increased N deposition (i.e. from anthropogenic sources) could promote the invasive potential of D. indica13.
Nitrogen has been suggested as an important limiting factor to fast-growing plants, especially invasive species, including weak invaders (low competitive ability)14. In past decades, human activity has greatly increased the amount of biologically available N entering the natural environment in China15, and this trend is predicted to continue during the coming decades16. Such changes in nitrogen will certainly affect the competition between invasive and native plants. It has been suggested that vigorous invasive plants are usually more aggressive with respect to resource competition and are more effective at resource use compared to native plants6,17. Consequently, vigorous invasive plants will be promoted by elevated nitrogen deposition in the context of global change. Indeed, a growing body of evidence shows that elevated N deposition leads to a relative decline in native species biomass and a simultaneous increase in the biomass of vigorous invaders18,19. However, with our scope, no reports have dealt with how a weak invader and its competitors respond to elevated nitrogen.
Galinsoga quadriradiata is an annual herbaceous plant from Central and South America20. Although it has already colonized most abandoned land or farmland in Central, Eastern and Southern China21,22, and can decrease agricultural production by approximately 50% according to limited evidence20, G. quadriradiata is generally considered to be a relatively weak invader or weed due to its lack of high competitive and colonizing ability in natural communities. This species was first recorded in the “Flora of China” in 1979. However, its actual invasion history should be no less than 100 years according to local farmers’ descriptions. After such a long-term expansion, it has already reached almost all suitable climatic areas in China (unpublished material), and the habitat it invades is commonly restricted to farmland. Since China is one of ten mega-biodiversity countries in the world that play important roles in ensuring agriculture and food safety23, it is necessary to predict the future expansion of such an invader.
With the abovementioned shortfalls in mind, we conducted a greenhouse experiment to investigate the effect of elevated nitrogen on the competitive relationship between the weak invasive species G. quadriradiata and two confamilial competitors (Heteropappus hispidus and Sonchus oleraceus). Meanwhile, to mimic different light conditions in farmland and abandoned land, light source was also taken into consideration. We hypothesized that elevated nitrogen would allow the weak invader to become more vigorous and would change the relationship with its competitors. The present study addressed the two following questions: (1) How would the weak invasive plant and its competitors respond to elevated nitrogen? (2) Can elevated nitrogen or the synergistic effect of nitrogen and light change the competition outcome?
Results
Comparisons of harvested mass
The result of the mixed model analysis revealed that Nitrogen (N), Light, Target species (Tsp) and Culture had significant effects on plant mass (Table 1). In general, the growth of the plants increased as a result of nitrogen addition but decreased with shade; the mass of the plants in the one-plant pots was generally higher than that in the monoculture and mixed culture. Moreover, interactive effects between or among some factors were also significant. For example, the interactions among Tsp, Culture, and N were significant. Compared with the mass of the individuals in the one-plant pots, the mass of the invader was not decreased by the mixed culture but was decreased in monoculture under elevated nitrogen (N+Shade and N) (Fig. 1). However, under elevated nitrogen, the mass of the native species H. hispidus in monoculture was not significantly different from that in the one-plant pots but was significantly higher than that in mixed culture; the mass of the exotic non-invasive species S. oleraceus in the one-plant pots was significantly higher than that in mono- and mixed-culture pots with elevated nitrogen. In pots without nitrogen addition (Shade and Control), the masses of both the invasive and non-invasive plants were generally not significantly affected by culture. In other words, the growth of the invader was not significantly inhibited by inter- or intra-specific competitors under the conditions without elevated nitrogen. The presence of invasive G. quadriradiata significantly inhibited the growth of the two non-invasive plants, especially when nitrogen was elevated. However, the presence of the two non-invasive species did not inhibit the growth of the invader regardless of whether nitrogen was added (Fig. 1).
Table 1.
The dependence of total mass on experimental treatments based on mixed model ANOVA.
| Effect | df | F | P |
|---|---|---|---|
| N | 1,165 | 242.9 | <0.001 |
| Light | 1,165 | 74.6 | <0.001 |
| N × Light | 1,165 | 46.0 | <0.001 |
| Tsp | 2,165 | 15.9 | <0.001 |
| Tsp × N | 2,165 | 11.9 | <0.001 |
| Tsp × Light | 2,165 | 2.5 | 0.090 |
| Tsp × N × Light | 2,165 | 4.5 | 0.012 |
| Cul | 2,165 | 43.4 | <0.001 |
| Cul × N | 2,165 | 10.8 | <0.001 |
| Cul × Light | 2,165 | 1.4 | 0.246 |
| Cul × N × Light | 2,165 | 5.1 | 0.007 |
| Tsp × Cul | 4,165 | 18.8 | <0.001 |
| Tsp × Cul × N | 4,165 | 13.1 | <0.001 |
| Tsp × Cul × Light | 4,165 | 1.3 | 0.291 |
| Tsp × Cul × N × Light | 4,165 | 3.7 | 0.006 |
Fixed factors: Nitrogen (N), Light, Target species (Tsp), and culture (Cul); Random factor: Pot number. Significant results are shown in bold.
Figure 1.
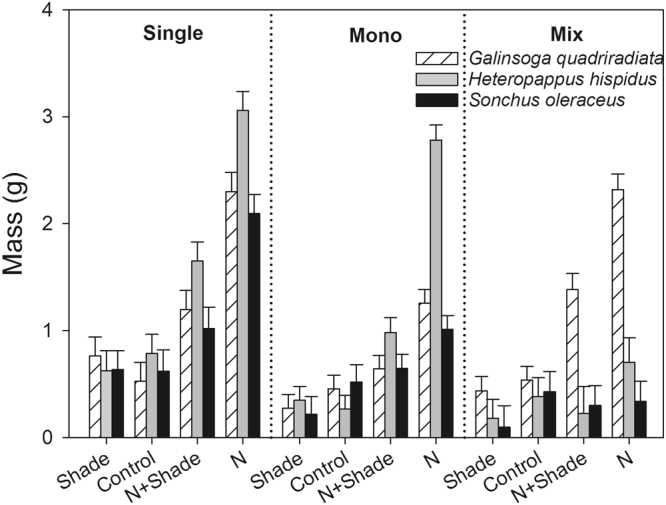
The harvested biomass (g) of the three species in each treatment. The horizontal axis represents nitrogen and light treatments: N+Shade, elevated nitrogen and 65% shaded; N, elevated nitrogen and natural sunlight; Shade, ambient nitrogen and 65% shaded; Control, ambient nitrogen and natural sunlight. Culture: Single, one-plant-culture; Mono, monoculture; Mix, Mixed culture. Values are means ± SE.
Under the one-plant and monoculture scenarios, the total mass of the invader was not greater than those of the two non-invasive species in all treatments. However, in the mixed culture, the total mass of the invader was not lower than those of the two non-invasive species in all treatments (Fig. 1). Nitrogen elevation helped the invader outcompete the non-invasive competitors.
Inter- and intra-specific competition intensity
The relative interaction index (RII) values were generally negative (Fig. 2). RII was significantly affected by Light (F(1,188) = 25.86, P < 0.0001) and Culture (F(1, 188) = 35.00, P < 0.0001). RII value was decreased under Shade treatment (Full sunlight, RII = −0.1187 ± 0.02392; Shade, RII = −0.2907 ± 0.02392). The intra-specific RII (monoculture) value of the invader was significantly lower than that of the inter-specific RII (mixed culture) (Fig. 2). There were significant interactive effects between Light and Nitrogen (F(1,188) = 23.65, P < 0.0001). Shade treatment strengthened while elevated nitrogen alleviated inter- and intra-specific competition. Elevated nitrogen decreased the negative effect of the non-invasive plants on G. quadriradiata in mixed culture pots under light shaded conditions (mixed culture: Shade+N, RII = −0.07221 ± 0.04398; Shade, RII = −0.2696 ± 0.04248).
Figure 2.
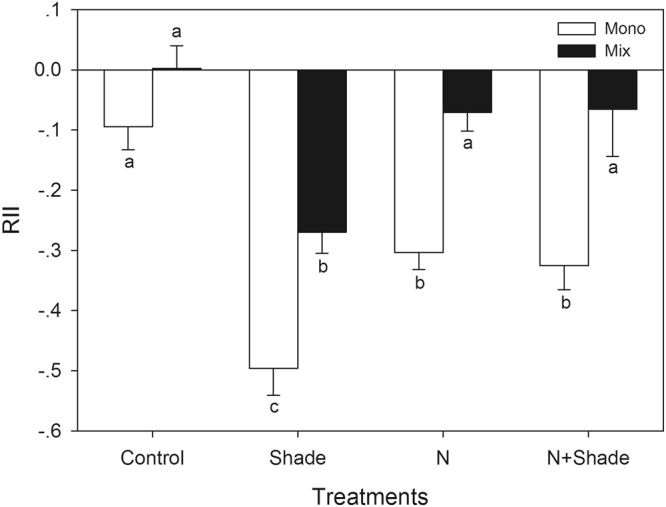
The inter- and intra-specific relative interaction index (RII) of G. quadriradiata. Culture type: Mix, Mixed culture; Mono, monoculture; Nitrogen and light treatments: N+Shade, elevated nitrogen and 65% shaded; N, elevated nitrogen and natural sunlight; Shade, ambient nitrogen and 65% shaded; Control, ambient nitrogen and natural sunlight. Values are means ± SE. Means with the same letter were not significantly different at an alpha level of 0.05.
Trait reaction norms under elevated nitrogen
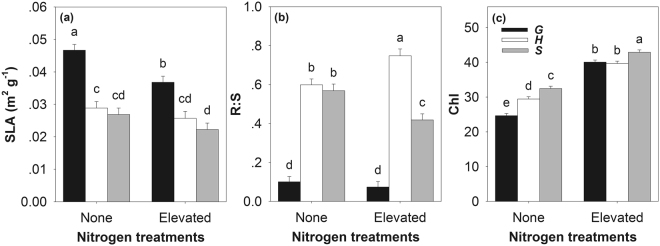
The specific leaf area (SLA) of the plant was significantly influenced by N, Light, Tsp and Culture, the root shoot ratio (R:S) of the plant was significantly influenced by Light and Tsp, and the leaf chlorophyll concentration (Chl) by N, Light, Tsp and Culture (Table 2, and see Supplementary Figs S1–S3). In general, G. quadriradiata exhibited lower R:S (G. quadriradiata, 0.087 ± 0.020; H. hispidus, 0.673 ± 0.023; S. oleraceus, 0.494 ± 0.023; F(2,165) = 220.0, P < 0.001) and Chl (G. quadriradiata, 32.349 ± 0.437; H. hispidus, 34.53 ± 0.477; S. oleraceus, 37.663 ± 0.476; F(2,84) = 36.1, P < 0.001) but higher SLA (G. quadriradiata, 0.042 ± 0.001; H. hispidus, 0.027 ± 0.002; S. oleraceus, 0.025 ± 0.001; F(2,31) = 47.5, P < 0.001) compared with the two non-invasive species. There were significant interactive effects between N and Tsp on the R:S and Chl (except for SLA) (Table 2). Elevated nitrogen significantly decreased the SLA of the invader but did not significantly affect those of the native species (Fig. 3a). Elevated nitrogen did not change the R:S of the invader, but increased and decreased those of H. hispidus and S. oleraceus, respectively (Fig. 3b). Elevated nitrogen significantly increased Chl of all species. The Chl of the invader was significantly lower than those of the other two species in the pots without nitrogen addition. However, in pots with elevated nitrogen, the Chl of the invader was not lower than that of H. hispidus (Fig. 3c).
Table 2.
The dependence of SLA, R:S, Chl, LCC, LNC, or C:N on experimental treatments based on mixed model ANOVA.
| Effect | R:S | SLA | Chl | LCC | LNC | C:N | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | df | F | P | F | P | F | P | |
| N | 1,165 | 0.1 | 0.728 | 1,31 | 13.3 | 0.001 | 1,84 | 460.3 | <0.001 | 1,30 | 6.8 | 0.01 | 140.2 | <0.001 | 114.7 | <0.001 |
| Light | 1,165 | 110.1 | <0.001 | 1,31 | 88.0 | <0.001 | 1,84 | 12.0 | 0.001 | 1,30 | 2.6 | 0.12 | 175.2 | <0.001 | 195.2 | <0.001 |
| N × Light | 1,165 | 2.0 | 0.157 | 1,31 | 0.5 | 0.494 | 1,84 | 19.4 | <0.001 | 1,30 | 0.0 | 0.95 | 37.1 | <0.001 | 0.9 | 0.357 |
| Tsp | 2,165 | 220.0 | <0.001 | 2,31 | 47.5 | <0.001 | 2,84 | 36.1 | <0.001 | 2,30 | 34.4 | <0.001 | 44.2 | <0.001 | 78.4 | <0.001 |
| Tsp × N | 2,165 | 10.8 | <0.001 | 2,31 | 1.7 | 0.201 | 2,84 | 11.5 | <0.001 | 2,30 | 0.0 | 0.99 | 5.9 | 0.007 | 21.3 | <0.001 |
| Tsp × Light | 2,165 | 24.5 | <0.001 | 2,31 | 2.8 | 0.073 | 2,84 | 1.9 | 0.159 | 2,30 | 0.7 | 0.49 | 8.8 | 0.001 | 18.6 | <0.001 |
| Tsp × N × Light | 2,165 | 11.9 | <0.001 | 2,31 | 1.6 | 0.226 | 2,84 | 15.9 | <0.001 | 2,30 | 0.1 | 0.93 | 7.0 | 0.003 | 7.2 | 0.003 |
| Cul | 2,165 | 11.8 | <0.001 | 2,31 | 13.0 | <0.001 | 2,84 | 20.9 | <0.001 | 2,30 | 1.2 | 0.33 | 3.4 | 0.048 | 1.5 | 0.238 |
| Cul × N | 2,165 | 4.6 | 0.011 | 2,31 | 1.0 | 0.376 | 2,84 | 0.9 | 0.395 | 2,30 | 0.5 | 0.63 | 6.3 | 0.005 | 13.6 | <0.001 |
| Cul × Light | 2,165 | 4.1 | 0.019 | 2,31 | 13.4 | <0.001 | 2,84 | 0.4 | 0.663 | 2,30 | 0.3 | 0.73 | 6.0 | 0.006 | 7.1 | 0.003 |
| Cul × N × Light | 2,165 | 9.6 | <0.001 | 2,31 | 1.4 | 0.267 | 2,84 | 1.8 | 0.175 | 2,30 | 0.7 | 0.49 | 3.7 | 0.038 | 3.7 | 0.036 |
| Tsp × Cul | 4,165 | 8.7 | <0.001 | 4,31 | 2.4 | 0.072 | 4,84 | 3.4 | 0.013 | 4,30 | 1.0 | 0.42 | 4.1 | 0.009 | 4.0 | 0.011 |
| Tsp × Cul × N | 4,165 | 6.7 | <0.001 | 4,31 | 0.6 | 0.655 | 4,84 | 2.1 | 0.087 | 4,30 | 0.8 | 0.53 | 5.1 | 0.003 | 10.7 | <0.001 |
| Tsp × Cul × Light | 4,165 | 9.6 | <0.001 | 4,31 | 0.8 | 0.541 | 4,84 | 3.0 | 0.023 | 4,30 | 0.2 | 0.96 | 3.2 | 0.027 | 1.4 | 0.261 |
| Tsp × Cul × N × Light | 4,165 | 9.5 | <0.001 | 4,31 | 0.6 | 0.665 | 4,84 | 2.1 | 0.086 | 4,30 | 1.4 | 0.27 | 2.8 | 0.042 | 4.1 | 0.010 |
SLA, specific leaf area (m2 g−1); R:S, root-shoot ratio; Chl, leaf chlorophyll concentration (a unitless index from 0 to 100); LCC, leaf carbon concentration (%); LNC, leaf nitrogen concentration (%); C:N, leaf carbon-nitrogen ratio. Fixed factors: Nitrogen (N), Light, Target species (Tsp), and culture (Cul); Random factor: Pot number. Significant results are shown in bold.
Figure 3.
The specific leaf area (SLA, m2 g−1), root-shoot ratio (R:S) and leaf chlorophyll concentration (Chl, a unitless index from 0 to 100) of the three species under nitrogen treatments. Species: G, Galinsoga quadriradiata; H, Heteropappus hispidus; S, Sonchus oleraceus. Values are means ± SE. Means with the same letter were not significantly different at an alpha level of 0.05.
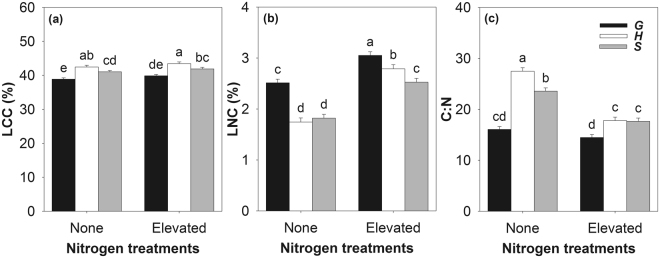
The leaf carbon concentration (LCC) of the plant was significantly influenced by N and Tsp, the leaf nitrogen concentration (LNC) by N, Light, Tsp and Culture, and the leaf carbon-nitrogen ratio (C:N) by N, Light and Tsp (Table 2, and see Supplementary Figs S4–S6). In general, nitrogen addition increased the LCC and LNC but decreased the C:N of the plants. There were significant interactive effects between N and Tsp on LNC and C:N (except for LCC). The LNCs of both the invader and the native species were significantly increased by nitrogen treatment (Fig. 4b). The C:N of the invasive plant was not significantly changed by nitrogen treatment, while those of the non-invasive plants were decreased by nitrogen treatment (Fig. 4c). The invasive plant had higher LNC but lower C:N than the other two species (Fig. 4b and c).
Figure 4.
The leaf carbon concentration (LCC, %), leaf nitrogen concentration (LNC, %) and leaf carbon-nitrogen ratio (C:N) of the three species under nitrogen treatments. Species: G, Galinsoga quadriradiata; H, Heteropappus hispidus; S, Sonchus oleraceus. Values are means ± SE. Means with the same letter were not significantly different at an alpha level of 0.05.
Discussion
Our study found that elevated nitrogen altered the competitive relationship between the weak invader G. quadriradiata and the two non-invasive plant species. It indicates that elevated nitrogen deposition may strongly influence the invasion processes of weak invaders as well as vigorous invasive plants in natural ecosystem. We should be especially cautious of the expansion of weak invasive plants in response to elevated nitrogen deposition in the context of global change. The results showed that the competitive interactions between G. quadriradiata and the non-invasive plant species were not altered by light treatment but by nitrogen enrichment. In the Control and Shade treatments, when we planted the weak invader together with the two non-invasive species, no competitive advantage was exhibited. However, in the treatments with elevated nitrogen (N and N+Shade), the weak invader obtained a considerable advantage and outcompeted the non-invasive plants (Fig. 1). The shade treatment suppressed the growth of both the invader and non-invasive species, especially in pots with elevated nitrogen (Fig. 1). However, it did not change the competitive pattern between G. quadriradiata and the non-invasive species. The results indicate that initially weak invasive plants can become more vigorous under elevated nitrogen deposition associated with global change. As suggested by numerous studies, the invasion of vigorous invasive plants can usually be accelerated by elevated nitrogen3,19,24. Such vigorous invasive plants are commonly characterized by a conspicuous and strong competitive ability with respect to resources6. However, G. quadriradiata is not a vigorous, but rather weak, competitor under ambient conditions (Fig. 1). Although G. quadriradiata is a worldwide invasive plant in farmland, it is generally considered to be a relatively weak invader or weed due to its lack of high competitive and colonizing ability in natural communities which is usually characterized by relatively low soil nitrogen resource and high competition from close relatives. Thus, our results suggest that competition relationships between some originally weak exotic species and their competitors can be changed by elevated nitrogen. Such changes of competition outcome caused by nitrogen resource were also occasionally reported by some researchers13. This suggests that necessary attention should be paid to the expansion of invasive plants which are weak under low nitrogen but powerful under rich nitrogen conditions in the context of global change.
In fact, elevated nitrogen significantly promoted the growth of both the weak invader and the non-invasive plants in the one-plant pots; however, such a promotional effect was not observed for the non-invasive species in the mixed culture pots (Fig. 1 and Table 1). Moreover, a decreased negative species interaction was detected as a result of elevated nitrogen, especially in Shade treatment (Fig. 2). The interactions between the invader and the two competitors were generally negative, and the intra-specific RIIs of the invader were significantly lower than the inter-specific RIIs in most of the treatments (Fig. 2). Taken together, the results suggest that, the negative competitive effect of G. quadriradiata counteracts the positive effect of nitrogen elevation on the growth of the non-invasive plants, and that elevated nitrogen alleviated the negative effects of the non-invasive plants on G. quadriradiata. However, the high intra-specific RII value suggested intense intra-specific competition in the invader’s population. Previous studies suggested that increased nitrogen deposition alleviated, enhanced or even had no effect on the competitive effects of invasive plants on their competitors3,25,26. Our results revealed that elevated nitrogen enhanced the competitive effect of G. quadriradiata on its competitors. Many cases have underlined thel roles of competition for nutrient resources during plants’ invasion process27. It is indeed crucial to examine resource competition dynamics and the impact of global environmental change on competitive interactions between invasive and native species28. The conflicting results suggest that the response of invaders and native competitors may be species-specific.
Changes in the availability of resources associated with plant invasions may thus create conditions that may either increase or decrease the competitive ability of invasive species in contrast to those of native or other alien species28,29. How these changes will affect resource competition between alien and native species is key to improving our understanding of the long-term effects of plant invasions on native communities28. Global change is believed to alter the competitive relationship between invasive and native species in many ways, such as through land cover change, elevated CO2 and the nitrogen fertilization effect11,29. All these factors can enhance environmental resource availability, and nitrogen is especially important for invasive plants. This is probably because nitrogen is crucial for fast-growing plants and is usually difficult to obtain under natural conditions (except for nitrogen-fixing species)30,31. However, as a result of global change, nitrogen may be captured more easily by plants than before. In fact, elevated nitrogen can occur as a result of atmospheric nitrogen deposition as well as anthropogenic activities, such as agricultural fertilization of farmland13. The nitrogen resource of wild field can directly or indirectly be enhanced by such agricultural activities. In China, the large area of farmland and frequent agricultural fertilization32 may unintentionally further accelerate the colonization and expansion of G. quadriradiata into natural plant communities.
Morphological traits are suggested to be directly associated with plant competitive ability33. The R:S of the invader was significantly lower than that of the non-invasive competitor, and it was not changed by nitrogen, light or culture type (Fig. 3b and Table 2). This indicates that the weak competitive ability of G. quadriradiata in the Control treatment may be attributed to its lower root allocation, which has been suggested as a main cause for failure in soil nutrient competition34. However, under the scenario of elevated nitrogen, a low R:S may help plants to increase biomass accumulation of aboveground, hence resulting in a competitive advantage13. Other species with a low R:S have also been reported to successfully invade into nutrient-rich habitats13,35. Although nitrogen addition decreased the SLA of all species, that of G. quadriradiata was still higher than those of the native competitors (Fig. 4a). Elevated nitrogen increased the Chl of the invader as well as those of the non-invasive plants (Fig. 4c). It should be noted that the invader obtained highest rate of increase compared to the two native species (G. quadriradiata, H. hispidus and S. oleraceus increased by 53.96%, 30.76% and 28.76% respectively). Higher SLA species invest less dry matter per leaf, and have shorter leaf life spans36. These are associated with lower leaf construction cost, higher nitrogen allocation to photosynthesis, and higher photosynthetic nitrogen use efficiency37,38. The advantages associated with SLA and photosynthetic traits (e.g., chlorophyll concentration) subsequently account for the vigorous invasiveness of exotics37,39,40. In the present case, G. quadriradiata maintained its R:S at lower level and SLA at higher level, and increased chlorophyll concentration in response to elevated nitrogen. Moreover, the invasive plant had higher LNC but lower C:N than the other two species (Fig. 4). Higher leaf nitrogen content is usually related to higher assimilation rate, and is subsequently associated with higher growth rate of plant shoot and higher competitive ability on aboveground resource13. The C:N balance, is also important for regulation of plant growth and development41. For example, C:N plays an important role in regulating leaf senescence42. Plants with lower C:N ratio are usually characterized by higher growth rate43, and subsequently successful invasive plants are commonly characterized by lower C:N compared to non-invasive plants17. In sum, these attributes may help G. quadriradiata successfully outcompete the two competitors. However, it should be acknowledged that the present one-year experiment can not definitely uncover the invasion process of G. quadriradiata in the future. Further studies involving multiple generation under field conditions are necessary to reach a solid conclusion about this issue.
Methods
Experimental design
Three species of the Asteraceae family, i.e., the invasive species G. quadriradiata and the native species H. hispidus and S. oleraceus (which is native to Eurasia, although some researchers take it as a naturalized species in China), were chosen for comparison. All seeds we used in this study were collected from the Qinling-Bashan Mountains in 2014, and were stored in a 4 °C refrigerator.
In early May 2015, the seeds of the three species were sown in nursery pots. Two weeks later, when the seedlings were approximately 3 cm in height, they were transplanted into plastic pots (diameter 12 cm; height 10 cm). The pots were filled with sand and soil in a 1:1 proportion by volume. Two types of culture (Mixed and Monoculture) were conducted. For the mixed-culture, one invasive plant and one of the two non-invasive species were planted in the same pot. For the monoculture, two G. quadriradiata, two H. hispidus, or two S. oleraceus seedlings were planted together. Additionally, to compare the inter- and intra-specific competition intensity, we established a one-plant-culture treatment for each species, i.e., one seedling of each species was transplanted into one pot. After transplanting, all pots were subjected to two environmental treatments based on a factorial design: nitrogen (ambient or elevated) and light (shade or full sunlight) with 10 replicates. For the nitrogen treatments, each pot was watered with 5 ml deionized water (ambient) or 3.783 g L−1 NH4NO3 solution (elevated). The nitrogen addition simulated the current nitrogen deposition rate of 2 g m−2 yr−1 in China15. For the Shade treatment, the radiation intensity was controlled at 65% of the natural level. There were 320 pots and 520 individuals in total. All pots were randomly arranged in an 80 m2 greenhouse to avoid the influence of herbivores, and were watereddaily to keep the soil wet.
Two months later, when the invasive plants were almost mature, the leaf chlorophyll concentration (Chl) of the invasive species was measured using a portable chlorophyll meter SPAD-502 (Spectrum Technologies, Inc., Plainfield, IL, U.S.). The mean of three readings from the chlorophyll meter was calculated from 3 fully expanded leaves. In mid-September 2015, all parts of the plants were harvested and leaf areas were measured immediately. The dry weight of the root, leaf, and stem of each individual was separately measured after the samples had been dried to a constant weight in an oven at 60 °C. Elemental analysis for leaf carbon concentration (LCC, %) and leaf nitrogen concentration (LNC, %) was performed on Vario EL cube CHNOS elemental analyzer (Elementar Analysen Systeme, Hanau, Germany). Five individuals’ leaf sample of each species under each treatment were randomly chosen, and thus a total of 200 sample were measured.
Relative interaction index (RII)
The relative interaction index (RII) between G. quadriradiata and the native or exotic competitors was calculated according to the following equation44:
| 1 |
M1 is the mass of G. quadriradiata under mixed culture or monoculture conditions. M0 is the mass of G. quadriradiata in the one-plant pots. When calculated using data from the mixed culture treatments, the RII represents the intensity of inter-specific competition between the invasive and non-invasive species. When calculated using data from the monoculture treatments, the RII represents the intensity of intra-specific competition of G. quadriradiata. RII ranges from −1 to 1. if RII < 0, the plant experiences a negative impact from its competitor; if RII > 0, the plant experiences a positive impact from its competitor; if RII = 0, no impact is observed44.
Data analyses
R:S was calculated as the ratio between root dry mass and aboveground dry mass. Specific leaf area (SLA) is defined as the ratio of leaf area to dry mass. C:N was calculated as the ratio between LCC and LNC. A mixed model was used to evaluate the effects of the independent variables of Nitrogen (ambient or elevated N), Light (Shade or Full sunlight) Culture (one-plant, mixed or monoculture), and Target species (G. quadriradiata, H. hispidus or S. oleraceus) on the dependent variables mass, R:S, SLA, Chl, LCC, LNC and C:N respectively. Pot number was used as the random factor of the model. All analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Electronic supplementary material
Acknowledgements
We thank Dr. Zeng-Qiang Qian, and Chenglong Guo, Wentao Peng, Ronghua Zhang and Huili Zhu for their help during the experiment. We thank the editor and two anonymous reviewers for their constructive comments, which helped us to improve the manuscript. This research was funded by the National Natural Science Foundation of China (31600445, 31570425 and 11671243), the Natural Science Basic Research Plan in Shaanxi Province of China (2016JQ3009), the Fundamental Research Funds for the Central Universities (GK201703036), and the Fund of State Key Laboratory of Biocontrol, Sun Yat-sen University (SKLBC14KF04).
Author Contributions
G.L. and Z.Z. conceived and wrote the main text of the manuscript. G.L. designed the experiment. Y.Y. and G.L. analyzed the data and prepared the figures and tables. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21546-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levine JM, et al. Mechanisms underlying the impacts of exotic plant invasions. Proc. Biol. Sci. 2003;270:775–781. doi: 10.1098/rspb.2003.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilà M, et al. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011;14:702–708. doi: 10.1111/j.1461-0248.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 3.Vasquez E, Sheley R, Svejcar T. Nitrogen enhances the competitive ability of Cheatgrass (Bromus tectorum) relative to native grasses. Invasive Plant Sci. Manag. 2008;1:287–295. doi: 10.1614/IPSM-08-062.1. [DOI] [Google Scholar]
- 4.Dukes JS, Chiariello NR, Loarie SR, Field CB. Strong response of an invasive plant species (Centaurea solstitialis L.) to global environmental changes. Ecol. Appl. 2011;21:1887–1894. doi: 10.1890/11-0111.1. [DOI] [PubMed] [Google Scholar]
- 5.Huang F, et al. Rapid evolution of dispersal-related traits during range expansion of an invasive vine Mikania micrantha. Oikos. 2015;124:1023–1030. doi: 10.1111/oik.01820. [DOI] [Google Scholar]
- 6.van Kleunen M, Weber E, Fischer M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010;13:235–245. doi: 10.1111/j.1461-0248.2009.01418.x. [DOI] [PubMed] [Google Scholar]
- 7.Vilà M, Weiner J. Are invasive plant species better competitors than native plant species? - evidence from pair-wise experiments. Oikos. 2004;105:229–238. doi: 10.1111/j.0030-1299.2004.12682.x. [DOI] [Google Scholar]
- 8.Strayer DL. Eight questions about invasions and ecosystem functioning. Ecol. Lett. 2012;15:1199–1210. doi: 10.1111/j.1461-0248.2012.01817.x. [DOI] [PubMed] [Google Scholar]
- 9.Lankau RA. Species invasion alters local adaptation to soil communities in a native plant. Ecology. 2013;94:32–40. doi: 10.1890/12-0675.1. [DOI] [PubMed] [Google Scholar]
- 10.Dostál P, Müllerová J, Pyšek P, Pergl J, Klinerová T. The impact of an invasive plant changes over time. Ecol. Lett. 2013;16:1277–1284. doi: 10.1111/ele.12166. [DOI] [PubMed] [Google Scholar]
- 11.Manea A, Leishman MR. Competitive interactions between native and invasive exotic plant species are altered under elevated carbon dioxide. Oecologia. 2011;165:735–744. doi: 10.1007/s00442-010-1765-3. [DOI] [PubMed] [Google Scholar]
- 12.Dukes JS, Mooney HA. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999;14:135–139. doi: 10.1016/S0169-5347(98)01554-7. [DOI] [PubMed] [Google Scholar]
- 13.Littschwager J, Lauerer M, Blagodatskaya E, Kuzyakov Y. Nitrogen uptake and utilisation as a competition factor between invasive Duchesnea indica and native Fragaria vesca. Plant Soil. 2010;331:105–114. doi: 10.1007/s11104-009-0236-2. [DOI] [Google Scholar]
- 14.Mozdzer TJ, Megonigal JP. Jack-and-Master trait responses to elevated CO2 and N: a comparison of native and introduced Phragmites australis. PLoS ONE. 2012;7:e4279410. doi: 10.1371/journal.pone.0042794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, et al. Enhanced nitrogen deposition over China. Nature. 2013;494:459–462. doi: 10.1038/nature11917. [DOI] [PubMed] [Google Scholar]
- 16.Dentener F, et al. Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem. Cycles. 2006;20:B4003. doi: 10.1029/2005GB002672. [DOI] [Google Scholar]
- 17.Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G. Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann. Bot. 2010;106:371–380. doi: 10.1093/aob/mcq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallano DM, Selmants PC, Zavaleta ES. Simulated nitrogen deposition enhances the performance of an exotic grass relative to native serpentine grassland competitors. Plant Ecol. 2012;213:1015–1026. doi: 10.1007/s11258-012-0061-1. [DOI] [Google Scholar]
- 19.Brooks ML. Effects of increased soil nitrogen on the dominance of alien annual plants in the Mojave Desert. J. Appl. Ecol. 2003;40:344–353. doi: 10.1046/j.1365-2664.2003.00789.x. [DOI] [Google Scholar]
- 20.Kabuce, N. & Priede, N. NOBANIS-Invasive alien species fact sheet Galinsoga quadriradiata. https://www.nobanis.org/globalassets/speciesinfo/g/galinsoga-quadriradiata -/galinsoga-quadriradiata.pdf (2014).
- 21.Liu G, Zhang L, Kong B, Wei X, Zhu Z. The population growth dynamic of Galinsoga quadriradiata Ruiz & Pav. on Qinling-Bashan Mountain. Acta Ecol. Sin. 2016;36:3350–3361. [Google Scholar]
- 22.Li C, Qi S, Yao J, Yang L. Genetic diversity and differentiation of invasive plant Galinsoga quadriradiata populations in China. Chin. J. Ecol. 2015;34:3306–3312. [Google Scholar]
- 23.Huang J, et al. Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv. 2016;198:104–112. doi: 10.1016/j.biocon.2016.04.007. [DOI] [Google Scholar]
- 24.Rao LE, Allen EB. Combined effects of precipitation and nitrogen deposition on native and invasive winter annual production in California deserts. Oecologia. 2010;162:1035–1046. doi: 10.1007/s00442-009-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Y, et al. Increased nitrogen deposition alleviated the competitive effects of the introduced invasive plant Robinia pseudoacacia on the native tree Quercus acutissima. Plant Soil. 2014;385:63–75. doi: 10.1007/s11104-014-2227-1. [DOI] [Google Scholar]
- 26.Thomsen MA, Corbin JD, D’Antonio CM. The effect of soil nitrogen on competition between native and exotic perennial grasses from northern coastal California. Plant Ecol. 2006;186:23–35. doi: 10.1007/s11258-006-9109-4. [DOI] [Google Scholar]
- 27.Abbas AM, et al. Competition from native hydrophytes reduces establishment and growth of invasive dense-flowered cordgrass (Spartina densiflora) PeerJ. 2015;3:e1260. doi: 10.7717/peerj.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioria M, Osborne BA. Resource competition in plant invasions emerging patterns and research needs. Front. Plant Sci. 2014;5:501. doi: 10.3389/fpls.2014.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol. 2000;88:528–534. doi: 10.1046/j.1365-2745.2000.00473.x. [DOI] [Google Scholar]
- 30.Bajpai, D. & Inderjit Impact of nitrogen availability and soil communities on biomass accumulation of an invasive species. AoB Plants5, t45 (2013).
- 31.Lowe PN, Lauenroth WK, Burke IC. Effects of nitrogen availability on competition between Bromus tectorum and Bouteloua gracilis. Plant Ecol. 2003;167:247–254. doi: 10.1023/A:1023934515420. [DOI] [Google Scholar]
- 32.Zhang F, et al. Nutrient use efficiencies of major cereal crops in China and measures for improvement. Acta Pedol. Sin. 2008;45:915–924. [Google Scholar]
- 33.van der Werf A, van Nuenen M, Visser AJ, Lambers H. Contribution of physiological and morphological plant traits to a species’ competitive ability at high and low nitrogen supply: A hypothesis for inherently fast- and slow-growing monocotyledonous species. Oecologia. 1993;94:434–440. doi: 10.1007/BF00317120. [DOI] [PubMed] [Google Scholar]
- 34.Craine JM. Reconciling plant strategy theories of Grime and Tilman. J. Ecol. 2005;93:1041–1052. doi: 10.1111/j.1365-2745.2005.01043.x. [DOI] [Google Scholar]
- 35.Liao ZY, Zhang R, Barclay GF, Feng YL. Differences in competitive ability between plants from nonnative and native populations of a tropical invader relates to adaptive responses in abiotic and biotic environments. PLoS ONE. 2013;8:e71767. doi: 10.1371/journal.pone.0071767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dwyer JM, Hobbs RJ, Mayfield MM. Specific leaf area responses to environmental gradients through space and time. Ecology. 2014;95:399–410. doi: 10.1890/13-0412.1. [DOI] [PubMed] [Google Scholar]
- 37.Feng YL, Fu GL, Zheng YL. Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta. 2008;228:383–390. doi: 10.1007/s00425-008-0732-2. [DOI] [PubMed] [Google Scholar]
- 38.Grotkopp E, Rejmánek M. High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. Am. J. Bot. 2007;94:526–532. doi: 10.3732/ajb.94.4.526. [DOI] [PubMed] [Google Scholar]
- 39.Leishman MR, Haslehurst T, Ares A, Baruch Z. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol. 2007;176:635–643. doi: 10.1111/j.1469-8137.2007.02189.x. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher RV, Randall RP, Leishman MR. Trait differences between naturalized and invasive plant species independent of residence time and phylogeny. Conserv. Biol. 2015;29:360–369. doi: 10.1111/cobi.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin T, Oswald O, Graham IA. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon: nitrogen availability. Plant Physiol. 2002;128:472–481. doi: 10.1104/pp.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wingler A, Purdy S, MacLean JA, Pourtau N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006;57:391–399. doi: 10.1093/jxb/eri279. [DOI] [PubMed] [Google Scholar]
- 43.Nie M, Bell C, Wallenstein MD, Pendall E. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci. Rep. 2015;5:9212. doi: 10.1038/srep09212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armas C, Ordiales R, Pugnaire FI. Measuring plant interactions: a new comparative index. Ecology. 2004;85:2682–2686. doi: 10.1890/03-0650. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.