Abstract
Objective
We have previously characterized the reproductive hormone profile in infertile women with diminished ovarian reserve (DOR) as being distinct from that seen in age comparable healthy controls. Hypothesizing that DOR reflects accelerated reproductive aging, we herein compare urinary reproductive hormone dynamics between young women with DOR and a population of chronologically older perimenopausal controls.
Methods
In this prospective observational study, urinary levels of pituitary gonadotropins (FSH and LH) and metabolites of estrogen (E1c) and progesterone (Pdg) were assessed in daily morning urine samples collected in a spontaneous menstrual cycle in 8 infertile premenopausal women with DOR and in 11 perimenopausal controls. Areas under the curves (AUC) were calculated for the respective measured hormones, and comparisons were made using Mann-Whitney U test.
Results
Urinary E1c levels were significantly attenuated in premenopausal women with DOR compared to the older perimenopausal cohort. Despite the relatively lower estrogen, a significantly more pronounced LH surge was evident in the younger population. Early follicular FSH was lower in the women with DOR, but luteal urinary Pdg excretion was comparable in the two groups.
Conclusion
Our data suggest distinctions in functioning of the central (hypothalamic-pituitary) and peripheral (ovarian) components of the hypothalamic-pituitary-ovarian axis in premenopausal women with DOR compared to chronologically older perimenopausal controls. Increased hypothalamic-pituitary sensitivity to estrogen positive feedback is suggested in premenopausal women with DOR. Our observations identify DOR as a distinct entity in the paradigm of reproductive senescence.
Keywords: Diminished ovarian reserve, Premenopause, Perimenopause, LH surge, E1c, Pdg, Luteal insufficiency
Introduction
The concept of “ovarian reserve” is a relatively recent appreciation within the continuum of reproductive aging, and alludes to the number of remaining oocytes in any premenopausal female at a given age.1,2 Although ovarian reserve assessment is routinely undertaken in the context of infertility workup, this concept remains relatively underappreciated in the primary care setting. Pre-menopausal women diagnosed with diminished ovarian reserve (DOR) are without clinical stigmata other than sub fertility., Indeed, covert biomarkers and suboptimal quantitative and qualitative responses to attempts at ovarian stimulation during fertility treatment are the hallmarks of DOR in otherwise asymptomatic women.3 Despite strides in infertility management over recent years, suboptimal reproductive successes are recognized in patients with DOR.4–7
Recent studies have demonstrated an increased likelihood of early menopause in infertile women manifesting features of DOR,8–10 suggesting an acceleration of the process of reproductive aging in at least a subset of these young women.11 In a study of reproductive hormones in daily morning urine specimens collected over a spontaneous menstrual cycle, we had previously observed that the urinary reproductive hormone profiles in young women with DOR are distinct from those in age comparable controls.9 Significantly prolonged gonadotropin (FSH and LH) surges and significantly attenuated luteal excretion of estrogen and progesterone metabolites were noted in women with DOR compared to age comparable controls.9 Hypothesizing that DOR reflects accelerated reproductive aging, we herein compare urinary reproductive hormone dynamics between young women with DOR and a sample of chronologically older controls. Our analyses identify the reproductive hormone milieu of DOR as being distinct from that of chronologically advanced perimenopause.
Methods
Participants identified with DOR collected daily first morning voided urine over an entire menstrual cycle, as was previously described.9 The study was approved by the Institutional Review Board (IRB) at the Albert Einstein College of Medicine and Montefiore Medical Center. Daily morning urine samples were available from a historical cohort of perimenopausal controls, also was previously described.10 Of note, informed consent was available for all controls for use of data in future studies.
DOR Cases
Daily urinary reproductive hormone data were available for eight healthy, regularly cycling (21–35 days) infertile patients (age range 32–37 years).9 DOR was diagnosed by 1) early follicular (Cycle Day 1–3) serum FSH concentration of greater than 10 mIU/ml (as per practice standard for the institution), and 2) suboptimal ovarian response to previous attempts at ovarian hyperstimulation (<3 dominant follicles after utilization of gonadotropins in excess of 300 U/day). Patients with abnormal serum level of prolactin, thyroid functions, or chronic diseases were excluded.
Perimenopausal Controls
Previously reported urinary reproductive hormone data10 for 11 healthy regularly cycling, perimenopausal normo-prolactinemic and euthyroid historic controls were utilized for comparison.
Specimen Collection
Details on specimen collection procedures have been published previously.9,10 Briefly, the participants were asked to collect first morning voided urine specimen in supplied containers and transfer a portion of the volume to the provided polypropylene tubes up to the specified mark; the tubes were prefilled with glycerol (final concentration of 7% glycerol was achieved in the collected urine sample).9,10 Glycerol-preserved specimens were used for all assays, because this has been reported to permit measurement of LH and FSH over long storage intervals and does not interfere with E1c or Pdg assay.11–14 The participants were instructed to store the tubes in a provided box within the home freezer. Specimens were transported to the laboratory at the end of the collection period and stored at −20°C.9,10 All subjects were provided with written instructions regarding the procedure of collection and storage of urine samples over an entire menstrual cycle (first day of menses to the first day of next menstrual flow).
Hormone Assays
Urinary LH and FSH were measured using a solid-phase, two-site specific fluoro-immunometric assay (DELFIA; Perkin Elmer, Turku, Finland) and were validated in our laboratory using previously described methods.9,10,14 For the urinary LH, inter-assay coefficient of variation (CV) and intra-assay CV’s were 13.7 and 5.0%, respectively, and 16.4 and 7.6% for FSH, respectively. E1c and Pdg were measured in duplicate by ELISA using antibodies and conjugate tracers provided by Dr. Bill Lasley (University of California, Davis, CA).14,15 The inter-assay CV for E1c was 10.1% and the intra-assay CV was 8.4%. Corresponding CVs for Pdg were 15.0 and 14.0% respectively. Urinary hormone concentrations were adjusted for glycerol and normalized to urinary creatinine (Cr).16 A portion of the historical samples of the control group were assayed concurrently with those from the experimental group, as a quality control measure to assure against assay drift on the available stored control samples. Cycles were centralized to the day of the LH surge (Day Zero), which was defined using established statistical methods.10 Presence of luteal activity was determined by a sustained increase in Pdg concentrations of ≥3µg/mgCr for 3 consecutive days. Days −14 to −1 (inclusive) identified the follicular phase, whereas days −14 to −6, excluding day −6, defined the early follicular phase. Luteal phase was defined as days +2 to +14 (inclusive). Luteal phase adequacy was defined as any day on which urinary Pdg exceeded 3µg/mgCr.9
Data Analyses
Data were organized into Microsoft Excel spreadsheets and inspected for error and completeness. The duration of LH and FSH surges was defined as the number of days of persistent elevation in the respective gonadotropin levels above a threshold of a 3-standard deviations (SD) increase from a follicular phase nadir baseline, which was calculated using a 5-day moving average.17 The integrated urinary excretion of the individual urinary hormones was evaluated for participants with DOR and the healthy perimenopausal controls. Hormone levels for the early follicular as well as the entire follicular and luteal phases, and for the LH and FSH surges in the two groups were compared by. The levels of E1c on day −1, i.e. the day prior to LH surge, the duration of LH and FSH surge (in days) and the duration of adequacy of luteal phase were similarly assessed in the two groups. Area-under-the-curve (AUC) analyses were calculated using Prism 4.0 (GraphPad Software, La Jolla, CA) for comparison. All comparisons were made using non parametric Mann- Whitney U test due to a non-Gaussian distribution of the hormone data. SPSS 16.0 (SPSS Inc, Chicago, IL.) was used for analyses and P < 0.05 was considered as statistically significant.
Results
Data presented reflect the urinary hormone profile over a single menstrual cycle in 8 premenopausal women with DOR (age range 32–37 years) and 11 perimenopausal historical controls (age range 43–52 years). One patient with DOR failed to provide urine samples beyond cycle day #16 (2 days after LH surge), and thus was excluded from analysis evaluating the duration of LH and FSH surges and luteal Pdg levels. All participants displayed adequate FSH and LH surges. All perimenopausal controls and 7 of the DOR patients exhibited luteal activity as reflected by predefined urinary Pdg excursions (excluding the one DOR case who failed to provide post-LH surge urine samples).
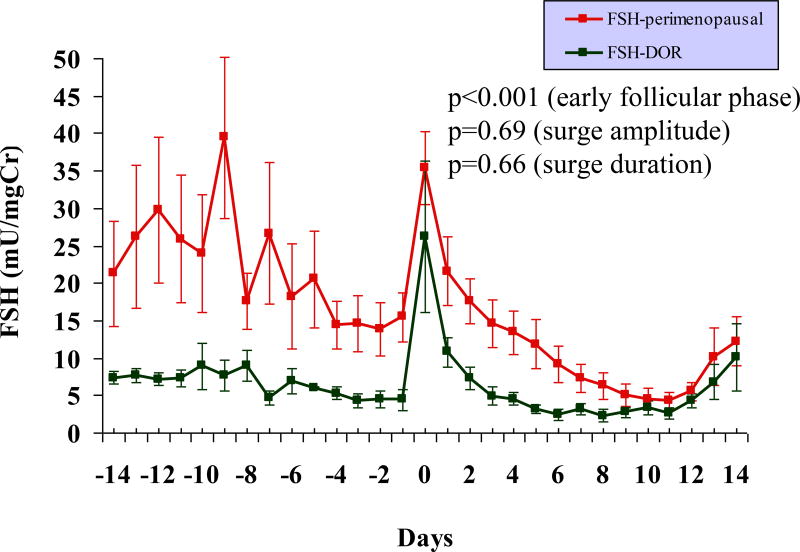
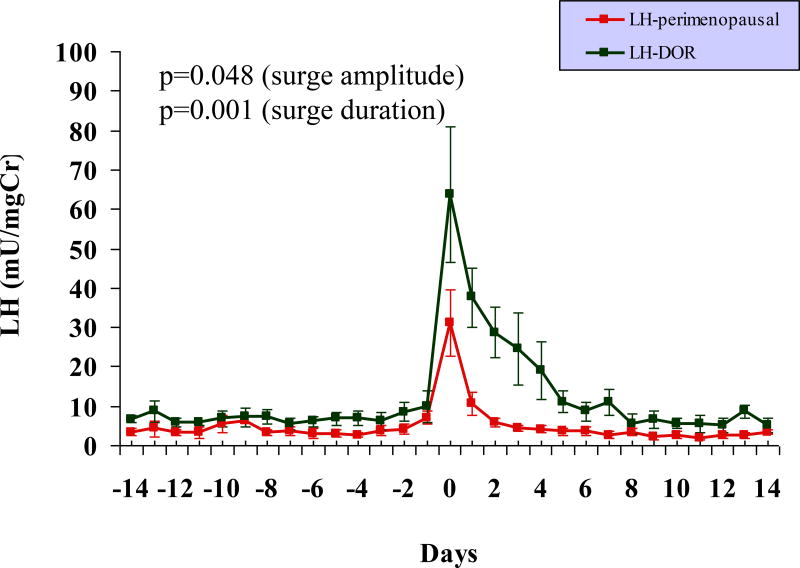
Although early follicular phase FSH level of >10mIU/ml was a diagnostic criterion for DOR (as specified in methods section), the early follicular urinary FSH levels were significantly lower in the DOR group compared to the older perimenopausal population (p < 0.001, Figure 1). The mid cycle urinary FSH surge was comparable in the two groups, both in amplitude and in duration (Table 1). The LH surge, however, were significantly exaggerated in the DOR, in both amplitude and in duration, compared to the older perimenopausal cohort (Table 1, Figure 2).
FIG. 1.
Daily profile of urinary FSH levels across an entire menstrual cycle in 8 premenopausal women with DOR and 11 older perimenopausal controls. Compared with the older controls, the DOR cases demonstrate significantly lower FSH levels in the early follicular phase (P < 0.001). The amplitude and duration of the FSH surge are comparable in the two groups. Note: centering of cycles relative to the LH surge (day 0). FSH, follicle-stimulating hormone; DOR, diminished ovarian reserve; LH, luteinizing hormone.
Table.
Summary of hormonal characteristics in 8 premenopausal women with diminished ovarian reserve (DOR) and 11 healthy perimenopausal controls (mean ± SEM)
| DOR | Perimenopausal | p-value | |
|---|---|---|---|
| FSH | |||
| Early follicular phase (mU/mgCr) | 7.48 ± 0.66 | 26.28 ± 3.0 | < 0.001 |
| Duration of surge (days) | 2.38 ± 0.32 | 1.64 ± 0.31 | 0.066 |
| Magnitude of surge (mU/mgCr) | 26.15 ± 9.45 | 35.39 ± 4.93 | 0.069 |
| LH | |||
| Duration of surge (days) | 3.25 ± 0.45 | 1.45 ± 0.16 | < 0.001 |
| Magnitude of surge (mU/mgCr) | 63.91 ± 16.0 | 31.1 ± 8.43 | 0.048 |
| E1c | |||
| Pre-surge peak (ng/mgCr) | 56.54 ± 10.06 | 106.38 ± 16.71 | 0.062 |
| Follicular phase (ng/mgCr) | 25.17 ± 2.07 | 53.11 ± 5.47 | < 0.001 |
| Luteal phase (ng/mgCr)† | 31.9 ± 2.07 | 81.07 ± 5.98 | < 0.001 |
| Whole cycle AUC (ng/mgCr)† | 654 ± 104 | 1625 ± 324 | 0.041 |
| Pdg | |||
| AUC (µg/mgCr)† | 54.85 ± 11.46 | 77.21 ± 13.08 | 0.272 |
| Luteal adequacy†§ | 7.29 ± 1.34 | 9.7 ± 0.96 | 0.070 |
One patient with DOR failed to provide luteal phase urine samples, and thus, her data was excluded from the analyses.
Luteal adequacy was defined as a Pdg level of greater than 3µg/mgCr.
FIG. 2.
Daily profile of urinary LH levels across an entire menstrual cycle in 8 premenopausal women with DOR and 11 older perimenopausal controls. An exaggerated urinary LH surge is observed in premenopausal women with DOR compared with the older perimenopausal controls; the duration and amplitude of the LH surge are significantly different between the two groups (P < 0.001). Note: centering of cycles relative to the LH surge (day 0). LH, luteinizing hormone; DOR, diminished ovarian reserve.
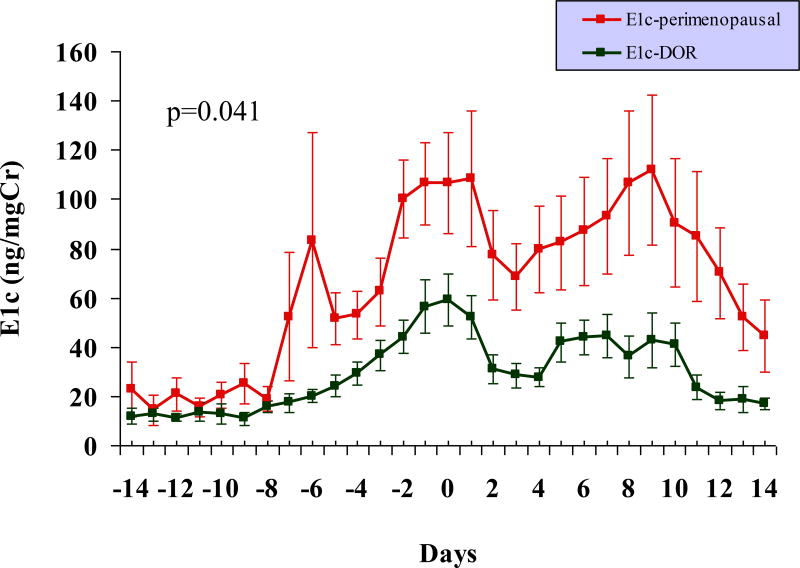
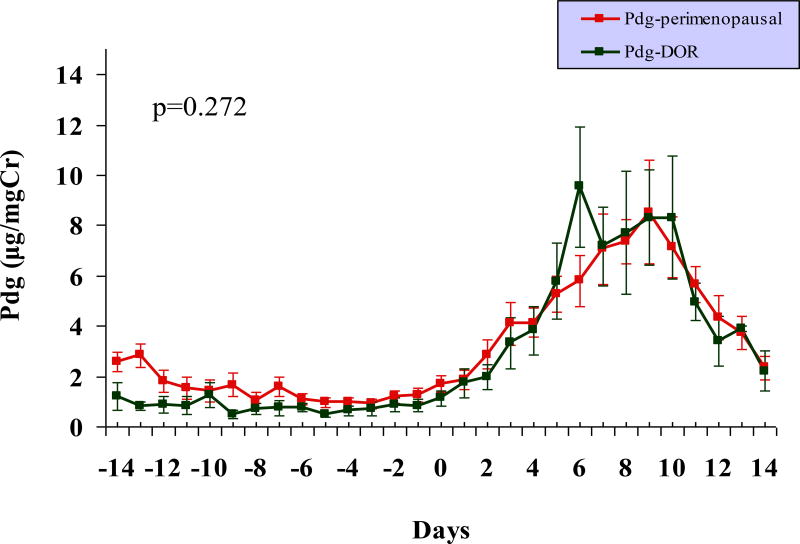
As is demonstrated in Figure 3 and Table 1, significantly attenuated urinary E1c levels, both in the follicular and the luteal phases of the cycle, were observed in the DOR group compared to the perimenopausal older controls. The pre-surge E1c level was lower in premenopausal women with DOR compared to the older perimenopausal group and this difference approached statistical significance (p = 0.062). Although, the luteal urinary Pdg levels were lower and the duration of luteal phase adequacy shorter in young women with DOR compared to the older perimenopausal controls, these differences were not statistically significant (Table 1, Figure 4).
FIG. 3.
Profile of daily urinary E1c excretion across a spontaneous menstrual cycle suggests relative hypoestrogenism in premenopausal women with DOR (n = 8) compared with the older perimenopausal controls (n = 11). The differences in urinary E1c levels in the two groups are of statistical significance across the different phases of the menstrual cycle (see Table 1 for details). Note: centering of cycles relative to the LH surge (day 0). E1c, estrogen conjugate; LH, luteinizing hormone; DOR, diminished ovarian reserve.
FIG. 4.
Comparable urinary levels of progesterone metabolite Pdg are observed in premenopausal women with DOR (n = 8) and older perimenopausal controls (n = 11; P > 0.05). Note: centering of cycles relative to the LH surge (day 0). Pdg, progesterone; LH, luteinizing hormone; DOR, diminished ovarian reserve.
Discussion
We had previously described distinctions in the reproductive hormone milieu of infertile women with DOR compared to healthy reproductive age controls with normal ovarian reserve.9 Our current study extends our earlier observations and identifies distinctions between DOR and chronologically advanced perimenopause. DOR is thus appreciated as a distinct entity in the paradigm of reproductive aging. Elevation in FSH is a recognized hallmark of reproductive aging18–20 and is appreciated concomitantly with declining ovarian reserve. Gonadal signals such as inhibin, activin and follistatin are recognized modulators of FSH secretion.21–23 In this context, the blunted elevation in follicular phase urinary FSH in premenopausal women with DOR compared to the older perimenopausal controls may reflect a higher level of counter-regulatory gonadal hormones, e.g., inhibin, activin, and follistatin, and hence a relatively replete ovarian reserve in the younger population compared to the older perimenopausal cohort. This is consistent with the fact that the women with DOR were still cycling regularly, while the perimenopausal controls had experienced some cycle irregularity10 (i.e., at least one skipped menstrual period within the 3 months prior to enrollment).
The significantly higher urinary E1c level in the chronologically older perimenopausal cohort was previously reported, and identifies the perimenopause as a state of relative estrogen excess.10,24–28 Conversely, these comparative analyses suggest premenopausal DOR as a state of relative hypoestrogenism compared to the more advanced stage of reproductive aging, i.e. perimenopause. Indeed, we have previously identified DOR as a risk for premenopausal bone loss and low bone mass8 and have suggested relative hypoestrogenism as a pathophysiological mechanism in this regard.
Our findings of significantly more prominent LH surges in the setting of DOR, compared to perimenopause, especially in the light of the observed blunted E1c excretion observed in the former, was unexpected. In contrast to the previously reported failure to elicit an LH surge despite normal estrogen exposure in some perimenopausal women,29 we observed an exaggerated and protracted urinary LH surge in premenopausal women with DOR in the setting of relative hypoestrogenism. Indeed, a dampening of the positive hypothalamic-pituitary feedback response to estrogen is recognized as a feature of the menopausal transition. However, premenopausal women with DOR displayed robust gonadotropin surges, suggesting an enhanced hypothalamic-pituitary sensitivity to the relatively hypoestrogenic milieu. Thus, women with DOR appear to have hormonal feedback responses that are distinct from both the healthy young women with normal ovarian reserve9, as well as from a chronologically older cohort of perimenopausal women. The observed differences in the reproductive hormone dynamics between premenopausal women with DOR and the older perimenopausal controls may be attributable, in part, to the residual ovarian reserve in the former, as reflected by regular menstrual cyclicity in all women diagnosed with DOR. Regularly menstruating premenopausal women with DOR may thus be earlier in the process of progressive ovarian senescence compared to the older perimenopausal cohort. Alternatively, chronological differences between the two groups may underlie the observed differences.
Our finding of a protracted LH surge in premenopausal women with DOR is novel and not previously reported. An extensive search of published English literature on Medline and Pubmed from years 1990 to 2010 using search terms “protracted LH surge”, “prolonged LH surge”, “LH surge duration”, and “duration of LH surge” yielded no similar finding in either human or animal studies. Fowler and colleagues suggested that the ovarian gonadotropin surge attenuating factor (GnSAF) play a role in the negative regulation of pulsatile LH secretion.30 A decline in GnSAF was previously reported in both poor responders to ovarian stimulation and in aging women,31 and hence may not be the unifying mediator for the observed differences in our data. GnRH pulses were known to stimulate the synthesis and secretion of LH and FSH from the anterior pituitary. Rapid GnRH pulse frequencies of more than one pulse per hour favor LH secretion while slow pulse frequencies of less than one pulse in 2 to 3 hours favor FSH secretion.32 A recent study by Christian and colleagues on ovariectomized mice suggested the switch of estrogen negative feedback to positive feedback in LH surge initiation is related to the pattern and level of individual GnRH neuron firing.33 Dysregulation of GnRH pulsatility, or a failure of down regulation in the number and function of the available GnRH receptors in the gonadotropes, or a failure to terminate the GnRH positive feedback may be theorized as additional plausible mechanisms contributing to the observed protracted LH surge in premenopausal women with DOR. An examination of the minute-to-minute characteristics of pulsatile GnRH/LH secretion in women with DOR may be helpful in elucidating the underlying pathophysiology.
The overall urinary E1c excretion was observed to be significantly lower in premenopausal women with DOR compared to the perimenopausal cohort. The magnitude of this differential in urinary E1c excretion in the two groups is likely influenced in part by the relative estrogen excess of perimenopause,10,24–28 and by the relative hypoestrogenism of DOR.9 Urinary E1c levels are recognized to reliably reflect ovarian estrogen production, as reflected by paired analyses of urine and serum samples.34 A difference in estrogen metabolic rates between the two groups is additionally plausible and may theoretically be contributory to the observed group differences in urinary E1c levels, a conjecture that cannot be elaborated upon, given the constraints of our study design. Serum estrogen levels are recognized to relate to body mass, particularly in postmenopausal women.35 While differences in body mass may be theorized to influence estrogen metabolites, urinary E1c levels are recognized to reflect ovarian rather than peripheral estrogens34 and are unlikely to be influenced by body mass index, as has previously been described.36
Luteal insufficiency is recognized as contributory to the reproductive compromise of aging37, and we have previously demonstrated evidence of luteal inefficiency in the context of DOR.9 The observed luteal Pdg profiles suggest comparable degrees of luteal compromise in the setting of premature (i.e. DOR) and age appropriate (i.e. perimenopause) ovarian senescence. Although the underlying pathophysiological mechanisms remain far from clear, based on these observations, one may hypothesize that inadequate luteal progesterone secretion may be a common denominator to the reproductive compromise of aging and that seen in the context of compromised ovarian reserve9, 34, a conjecture that merits substantiation by future studies.
The small sample size of our study is an obvious limitation that is a likely contributory to the relatively large standard errors observed in some measurements, such as of LH. This small sample highlights recruitment constraints that are appreciable in studies of infertile women with DOR, an emotionally fragile population that is reluctant to defer pursuit of fertility attempts in favor of participation in clinical research.38 Although historic perimeopausal controls were used, stored urinary samples were tested using established methods39–40 to ensure stability of hormone levels and consistency in assays. FSH receptor polymorphism is a recognized pathophysiologic mechanism for compromised ovarian reserve, albeit only in a subset of DOR population.41 This information unfortunately is not available for the studied cohort. Finally, an observational study such as this precludes the establishment of any cause-effect relationship; these observations however offer meaningful direction for future studies.
Conclusion
Our data indicate distinct reproductive hormone dynamics in young infertile women with DOR compared to older perimenopausal women. Distinctions (significantly lower E1c in addition to higher amplitude and duration of LH surge evident in premenopausal women with DOR compared to the perimenopausal cohort) as well as similarities (comparable luteal Pdg urinary excretion in the two groups) are identified in the urinary reproductive hormone profiles of the two population samples. Further investigations are needed to better appreciate and help disentangle the mechanisms that may underlie the observed differences in the reproductive hormone milieu, and better elucidate a place for premenopausal DOR in the paradigm of reproductive aging.
Acknowledgments
Supported in part by National Institutes of Health grants K12 (to L.P.) and (CD 41978) to N.S. This work was presented at the 65th Annual Meeting of ASRM, New Orleans, November 2009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None reported
References
- 1.Tietze C. Reproductive span and rate of reproduction among Hutterite women. Fertil Steril. 1957;8(1):89–97. doi: 10.1016/s0015-0282(16)32587-0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. Federation CECOS. N Engl J Med. 1982;306(7):404–6. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 3.Stovall DW, Toma SK, Hammond MG, Talbert LM. The effect of age on female fecundity. Obstet Gynecol. 1991;77(1):33–6. [PubMed] [Google Scholar]
- 4.Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulation hormone in the prediction of poor ovarian response and failure to become pregnant after in vitriol fertilization: a meta-analysis. Fertil Steril. 2003;79(5):1091–1100. doi: 10.1016/s0015-0282(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 5.de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE. OMEGA-project group. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77:978–985. doi: 10.1016/s0015-0282(02)02972-2. [DOI] [PubMed] [Google Scholar]
- 6.de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, van Leeuwen FE. OMEGA-project group. Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod. 2003;18:1544–52. doi: 10.1093/humrep/deg278. [DOI] [PubMed] [Google Scholar]
- 7.Woldringh GH, Frunt MH, Kremer JA, Spaanderman ME. Decreased ovarian reserve relates to pre-eclampsia in IVF/ICSI pregnancies. Hum Reprod. 2006;21(11):2948–54. doi: 10.1093/humrep/del155. [DOI] [PubMed] [Google Scholar]
- 8.Pal L, Bevilacqua K, Zeitlian G, Shu J, Santoro N. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause. 2008;15(6):1086–94. doi: 10.1097/gme.0b013e3181728467. [DOI] [PubMed] [Google Scholar]
- 9.Pal L, Zhang K, Zeitlian G, Santoro N. Characterizing the reproductive hormone milieu in infertile women with DOR. Fertil Steril. 2010;93(4):1074–9. doi: 10.1016/j.fertnstert.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 10.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf MG, Donald RA, Livesey JH. Pituitary ovarian function in normal women during the menopausal transition. Clinc Endocrinol (Oxf) 1981;14:245–55. doi: 10.1111/j.1365-2265.1981.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 12.Metcalf MG, Donald RA, Livesey JH. Pituitary ovarian function before, during and after the menopause: a longitudinal study. Clinc Endocrinol (Oxf) 1982;17:489–94. doi: 10.1111/j.1365-2265.1982.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 13.Metcalf MG, Livesey JH. Gonadotropin excretion in fertile women: effect of age and the onset of the menopausal transition. J Endocrinol. 1985;105:357–62. doi: 10.1677/joe.0.1050357. [DOI] [PubMed] [Google Scholar]
- 14.Saketos M, Sharma N, Adel T, Raguwanshi M, Santoro N. Time-resolved immunofluorometric assay and specimen storage conditions for measuring urinary gonadotropins. Clin Chem. 1994;40:749–753. [PubMed] [Google Scholar]
- 15.Munro CJ, Stabenfeldt GH, Cragun JR, Addiego LA, Overstreet JW, Lasley BL. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem. 1991;37:838–844. [PubMed] [Google Scholar]
- 16.Taussky HH. A procedure increasing the specificity of the Jaffe reaction for the determination of creatine and creatinine in urine and plasma. Clin Chim Acta. 1956;1(3):210–24. doi: 10.1016/0009-8981(56)90067-5. [DOI] [PubMed] [Google Scholar]
- 17.Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, Lasley BL, McConnell D, McGaffigan P, Midgely R, Schocken M, Sowers M, Weiss G. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrnol Metab. 2003;284:E521–30. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 18.Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicule-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651–4. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 19.Klein NA, Battaglia DE, Fujimoto VY. Reproductive aing: accelerated ovarian follicular development associated with a monotropic rise in follicule-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81:1038–45. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 20.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–51. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 21.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the perimenopause transition. Recent Prog Horm Research. 2002;57:257–75. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 22.Reame NE, Wyman TL, Phillips DJ, de Kretser DM, Padmanabhan V. Net increase from a decrease in inhibin B and an increase in activin A may contribute in pat to the rise in follicular phase follicle stimulating hormone of aging cycling women. J Clin Endocrinol Metab. 1998;83:3302–07. doi: 10.1210/jcem.83.9.5130. [DOI] [PubMed] [Google Scholar]
- 23.de Kretser DM, Hedger MP, Loveland KL, Phillips DJ. Inhibins, activins and follistatin in reproduction. Hum Reprod Update. 2002;8(6):529–41. doi: 10.1093/humupd/8.6.529. [DOI] [PubMed] [Google Scholar]
- 24.Santoro N, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women’s Health Across the Nation. Menopause. 2007;14(3):415–24. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]
- 25.Sherman BM, West JH, Korenman SG. The menopausal transition analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42:629–36. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Lenton EA, Sexton L, Cooke ID. The effect of age on the cyclical patterns of plasma LH, FSH, estradiol and progesterone in women with regular menstrual cycles. Hum Reprod. 1988;3:851–55. doi: 10.1093/oxfordjournals.humrep.a136796. [DOI] [PubMed] [Google Scholar]
- 27.Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L. Prospectively measured levels of serum follicule-stimulationg hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–30. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 28.Prior JC. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- 29.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 30.Fowler PA, Sorsa-Leslie T, Harris W, Mason HD. Ovarian gonadotrophin surge-attenuating factor (GnSAF): where are we after 20 years of research? Reproduction. 2003;126(6):689–99. doi: 10.1530/rep.0.1260689. [DOI] [PubMed] [Google Scholar]
- 31.Martinez F, Barri PN, Coroleu B, Tur R, Sorsa-Leslie T, Harris WJ, Groome NP, Knight PG, Fowler PA. Women with poor response to IVF have lowered circulating gonadotrophin surge-attenuating factor (GnSAF) bioactivity during spontaneous and stimulated cycles. Hum Reprod. 2002;17(3):634–40. doi: 10.1093/humrep/17.3.634. [DOI] [PubMed] [Google Scholar]
- 32.Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP. Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res. 1999;47:155–87. doi: 10.1016/b978-0-12-571147-0.50009-3. [DOI] [PubMed] [Google Scholar]
- 33.Christian CA, Mobley JL, Moenter SM. Diurnal and estradio-dependent changes in gonadotropin-releasing hormone neuron firing activity. PNAS. 2005;102(43):15682–7. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munro CJ, Stabenfeldt GH, Cragun JR, Addiego LA, Overstreet JW, Lasley BL. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem. 1991;37(6):838–44. [PubMed] [Google Scholar]
- 35.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150(2):161–71. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 36.Santoro N, Lasley B, McConnell D, Allsworth J, Crawford S, Gold EB, Finkelstein JS, Greendale GA, Kelsey J, Korenman S, Luborsky JL, Matthews K, Midgley R, Powell L, Sabatine J, Schocken M, Sowers MF, Weiss G. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89:2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 37.Gleicher N, Weghofer A, Barad DH. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: direct evidence from preimplantation genetic screening (PGS) Reprod Biol Endocrinol. 2010 Nov 10;8:140. doi: 10.1186/1477-7827-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88:5502–9. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 39.Zhang K, Pollack S, Ghods A, Dicken C, Isaac B, Adel G, Zeitlian G, Santoro N. Onset of ovulation after menarche in girls a longitudinal study. J Clin Endocrinology & Metab. 2008;93:1186–94. doi: 10.1210/jc.2007-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livesey JH, Roud HK, Metcalf MG, Donald RA. Glycerol prevents follicle-stimulating hormone and luteinizing hormone from frozen urine. J Endocrinol. 1983;93:381–4. doi: 10.1677/joe.0.0980381. [DOI] [PubMed] [Google Scholar]
- 41.Falconer H, Andersson E, Aanesen A, Fried G. Follicle-stimulating hormone receptor polymorphisms in a population of infertile women. Acta Obstet Gynecol Scand. 2005;84(8):806–11. doi: 10.1111/j.0001-6349.2005.00736.x. [DOI] [PubMed] [Google Scholar]