Abstract
The heparan sulfate-degrading enzyme heparanase promotes the progression of many cancers by driving tumor cell proliferation, metastasis and angiogenesis. Heparanase accomplishes this via multiple mechanisms including its recently described effect on enhancing biogenesis of tumor exosomes. Because we recently discovered that heparanase expression is upregulated in myeloma cells that survive chemotherapy, we were prompted to investigate the impact of anti-myeloma drugs on exosome biogenesis. When myeloma cells were exposed to the commonly utilized anti-myeloma drugs bortezomib, carfilzomib or melphalan, exosome secretion by the cells was dramatically enhanced. These chemotherapy-induced exosomes (chemoexosomes) have a proteome profile distinct from cells not exposed to drug including a dramatic elevation in the level of heparanase present as exosome cargo. The chemoexosome heparanase was not found inside the chemoexosome, but was present on the exosome surface where it was capable of degrading heparan sulfate embedded within an extracellular matrix. When exposed to myeloma cells, chemoexosomes transferred their heparanase cargo to those cells, enhancing their heparan sulfate degrading activity and leading to activation of ERK signaling and an increase in shedding of the syndecan-1 proteoglycan. Exposure of chemoexosomes to macrophages enhanced their secretion of TNF-α, an important myeloma growth factor. Moreover, chemoexosomes stimulated macrophage migration and this effect was blocked by H1023, a monoclonal antibody that inhibits heparanase enzymatic activity. These data suggest that anti-myeloma therapy ignites a burst of exosomes having a high level of heparanase that remodels extracellular matrix and alters tumor and host cell behaviors that likely contribute to chemoresistance and eventual patient relapse.
Keywords: chemoexosome, chemotherapy, exosome, heparanase, macrophage, myeloma
Introduction
Heparanase is an endo-β-D-glucuronidase that cleaves heparan sulfate chains. Downstream of heparanase enzyme activity is an increase in the expression and activity of growth factors, proteases and RANKL that together promote multiple myeloma growth, metastasis and osteolysis [1–4]. By cleaving heparan sulfate at the cell surface and within the ECM, heparanase also releases many growth factors and cytokines that promote tumor progression [1, 5–8]. Heparanase expression is enhanced in many cancers including multiple myeloma where it is associated with poor prognosis [6]. Recently our lab discovered that frontline anti-myeloma drugs bortezomib and carfilzomib activate the nuclear factor-kappa B (NF-kB) pathway which promotes heparanase expression by myeloma cells [9]. In addition we have shown that chemotherapy causes release of high levels of heparanase by tumor cells [9]. This soluble heparanase can be taken up by chemosensitive tumor cells leading to the activation of ERK and Akt signaling pathways and establishment of chemoresistance [9, 10]. These findings parallel what we found in myeloma patients where heparanase expression was dramatically enhanced in the tumor cells that survived high dose chemotherapy [9]. In addition, we have shown that inhibition of heparanase-driven ERK signaling significantly decreased the resistance of myeloma cells to anti-myeloma drugs in vitro [9].
Exosomes play an important role in driving tumor progression by altering gene expression, cell signaling and increasing immune suppression within tumors [11–18]. Exosomes influence angiogenesis and metastasis where they contribute to formation of pre-metastatic niches [11–13, 19]. Emerging evidence indicates that exosomes also play a role in promoting tumor chemoresistance [20]. This can occur through exosome-mediated shuttling of anti-cancer drugs out of the tumor cell or by delivering cargo to tumor cells including miRNAs that render cells more resistant to drug therapy [21, 22]. In multiple myeloma, we discovered that heparanase expression enhances tumor cell secretion of exosomes and alters their cargo and function in ways that promote myeloma progression [17].
Because heparanase plays a role in regulating both exosomes and chemoresistance, we examined the impact of anti-myeloma drugs on the secretion and function of multiple myeloma tumor cell-derived exosomes. We find that anti-myeloma drugs bortezomib, carfilzomib and melphalan substantially increase tumor cell secretion of exosomes. These chemotherapy-induced exosomes, which we refer to as chemoexosomes, have elevated levels of heparanase on their surface. This endows intact exosomes with the capacity to degrade heparan sulfate within the ECM. When exposed to tumor cells or macrophages, chemoexosomes transfer their heparanase to those cells leading to activation of cell signaling, cell migration and cytokine expression, which together may facilitate tumor chemoresistance and eventual post-therapy relapse.
Results
Chemotherapy enhances secretion of exosomes by tumor cells
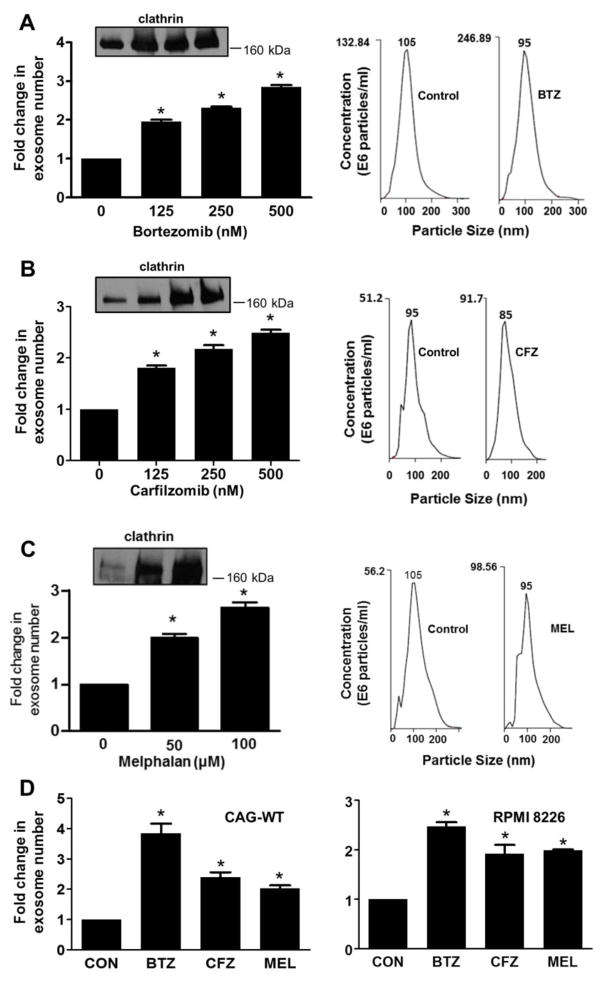
To determine if anti-myeloma drugs impact exosome secretion by tumor cells, cells from the CAG human myeloma cell line expressing heparanase (CAG-HPSE cells) were exposed to increasing concentrations of the proteasome inhibitors bortezomib or carfilzomib or the genotoxic drug melphalan, all being anti-myeloma drugs routinely used in the clinic. Cells were exposed to drug for 16 hours at which time approximately 60% of the cells treated with bortezomib or carfilzomib, or approximately 75% of cells treated with melphalan, had undergone apoptosis and/or cell death. Analysis of the exosomes isolated from the media conditioned by these cells revealed that the number of exosomes secreted by the tumor cells, as determined by NanoSight particle tracking, increased as drug concentrations increased (Fig. 1A–C). To confirm this trend of enhanced exosome secretion following exposure of cells to drugs, extracts of exosomes secreted by an identical number of drug-treated or untreated tumor cells were subjected to western blotting for the protein clathrin, a common component of exosomes. The amount of clathrin detected increased as drug concentration increased, further supporting the finding that exposure to drug enhances exosome secretion (insets, Fig. 1A–C). Plots of NanoSight particle analyses revealed a single peak with a median vesicle size of 105 nm and 95 nm for particles secreted by control and bortezomib or melphalan-treated cells, respectively, and 95 nm and 85 nm for vesicles secreted by control and carfilzomib-treated cells, respectively (Fig. 1A–C, Right panels). Although there is a small difference in median size of the exosomes secreted by the drug treated and untreated cells, there is almost complete overlap of the curves indicating that exosome size is not substantially impacted by the drugs. To further confirm the observation that anti-myeloma drugs enhanced exosome secretion, the number of exosomes secreted by CAG wild-type cells (CAG-WT) and by another human myeloma cell line RPMI 8226 were quantified without or with exposure to bortezomib, carfilzomib or melphalan. NanoSight analysis revealed that each of these drugs enhanced exosome secretion in both cell lines (Fig. 1D). Together these results indicate that exposure of human myeloma cells to chemotherapy stimulates secretion of exosomes.
Fig. 1.
Chemotherapy enhances exosome secretion by myeloma cells. CAG human myeloma cells that express an elevated level of heparanase (CAG-HPSE) were treated with increasing concentrations of proteasome inhibitors bortezomib or carfilzomib (left panel, A, B) or the genotoxic drug melphalan (C). Exosomes released over 16 hours following exposure to drug were isolated by differential centrifugation and quantified by NanoSight particle tracking. The fold increase in the number of exosomes secreted following exposure of cells to drug is shown. Western blots for clathrin (Insets) of detergent extracts of exosomes secreted by an identical number of control and drug-treated cells (lanes 1–4 in A and B are 0, 125, 250 and 500 nM bortezomib or carfilzomib; lanes 1–3 in C are 0, 50 and 100 μM melphalan). In each case exosomes show increased amount of clathrin with increased drug concentration. Plots shown in the right panel, A, B are from NanoSight analyses of isolated exosomes from cells treated with 250 nM bortezomib or carfilzomib, in C cells were treated with 50 μM melphalan. The number shown at the peak of each plot is the median size of particles in that preparation. (D) CAG wild-type (CAG-WT) and RPMI 8226 human myeloma cells were treated with 250 nM bortezomib (BTZ), 250 nM carfilzomib (CFZ) or 50 μM melphalan (MEL) and the exosomes released over the 16 hours following exposure to drug were isolated by differential centrifugation and quantified by NanoSight particle tracking. The fold increase in the number of exosomes secreted following exposure of cells to drug is shown.
Chemotherapy enhances the amount of heparanase associated with exosomes
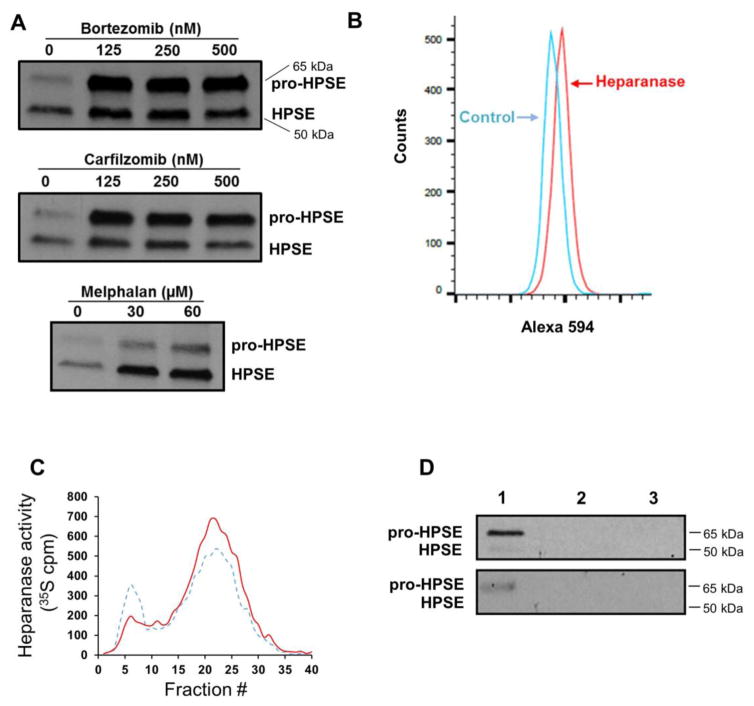
We recently demonstrated that when myeloma cells are exposed to chemotherapy, they upregulate expression and release of heparanase [9]. To determine if some of the heparanase released was associated with exosomes, we isolated exosomes from CAG-HPSE cells without and with prior exposure to bortezomib, carfilzomib or melphalan. When compared to an equal number of exosomes from cells not exposed to drug, the drug-treated cells secreted exosomes having a dramatic increase in both the pro-form (65 kDa) and enzymatically active form (50 kDa) of heparanase (Fig. 2A). To determine if heparanase was present on the exosome surface where it might be available to degrade heparan sulfate present within the ECM or on the cell surface, exosomes secreted by cells treated with bortezomib were captured on beads coated with anti-CD63 antibody. CD63 is a tetraspanin protein present on the surface of most exosomes [23]. The captured exosomes were immunostained for heparanase and analyzed by flow cytometry revealing the presence of heparanase on the exosome surface (Fig. 2B). When exosomes secreted by bortezomib treated cells were exposed to an ECM containing 35S-labeled heparan sulfate, fragments of the heparan sulfate were released thereby confirming the presence of heparanase on the surface of the intact exosome and demonstrating that it can degrade heparan sulfate (Fig. 2C, solid line). Exosomes from CAG-HPSE cells not exposed to bortezomib also degraded heparan sulfate in the ECM but to a lesser degree than did exosomes from cells exposed to bortezomib (Fig. 2C, dashed line). This is consistent with the significant, but lower amount of heparanase present in the exosomes from untreated cells (Fig. 2A).
Fig. 2.
Anti-myeloma therapy enhances heparanase protein levels in exosomes. (A) CAG-HPSE cells were treated with increasing concentrations of bortezomib, carfilzomib or melphalan and the secreted exosomes were purified from conditioned media by ultracentrifugation and equal numbers of exosomes were assessed by western blotting for their level of heparanase. The 65 kDa pro-form of the enzyme was elevated the most in exosomes secreted by cells following exposure to proteasome inhibitors, while the active 50 kDa form of the enzyme was predominant in exosomes secreted by cells exposed to melphalan. (B) Chemoexosomes released by bortezomib-treated CAG-HPSE cells were isolated by ultracentrifugation, captured on anti-CD63 magnetic beads and beads were then probed with anti-heparanase polyclonal antibody or rabbit IgG as control followed by Alexa Fluor 594 secondary antibody. Flow cytometry demonstrates the presence of heparanase on the surface of chemoexosomes. (C) To determine if heparanase on the surface of exosomes was active and could degrade heparan sulfate, exosomes secreted by CAG-HPSE cells that were untreated (dashed line) or treated with bortezomib (chemoexosomes, solid line) were incubated with an ECM containing 35S-labeled heparan sulfate. The 35S-labeled material released by heparanase was collected and analyzed by Sepharose 6B chromatography. Heparan sulfate fragments are present in fractions 15–30. (D, upper panel) To determine if heparanase was bound to the exosome surface via heparan sulfate, chemoexosomes bound to beads coated with anti-CD63 antibody were treated with heparinase III and the released material probed for heparanase by western blotting (lane 1). The 65 kDa pro-heparanase was abundant while the 50 kDa active form was present but detected at a low level. Following heparinase III treatment the remaining chemoexosomes were eluted from the beads and both the chemoexosomes (lane 2) and beads (lane 3) probed for heparanase by western blotting. No heparanase was detected, indicating that heparanase is predominantly localized on the chemoexosome surface. Similarly, heparanase was also found on the surface of exosomes isolated from the serum of a myeloma patient (lower panel).
Heparanase is known to bind to heparan sulfate on the surface of cells and we have demonstrated that the heparan sulfate proteoglycan syndecan-1 is present on the surface of exosomes [17, 18]. Thus, we explored whether heparanase was retained at the exosome surface via binding to heparan sulfate. Exosomes secreted by bortezomib-treated cells were bound to beads coated with anti-CD63 antibody and once bound the beads were exposed to heparinase III, a bacterial enzyme that extensively degrades heparan sulfate. This degradation of heparan sulfate released both the pro- and active forms of heparanase from the exosome surface (Fig. 2D, upper panel, lane 1), with the pro- form predominating, consistent with the finding that there is more pro-enzyme than active enzyme cargo in these exosomes (as shown in Fig. 2A). Following treatment with heparinase III, there was no detectable heparanase associated with the exosomes or the beads (Fig. 2D, lanes 2 and 3) indicating that all of the exosome heparanase is associated with the exosome surface. Heparanase was also present on the surface of exosomes isolated from the serum of a multiple myeloma patient (Fig. 2D, lower panel).
Chemoexosomes transfer heparanase to myeloma cells and alter their behavior
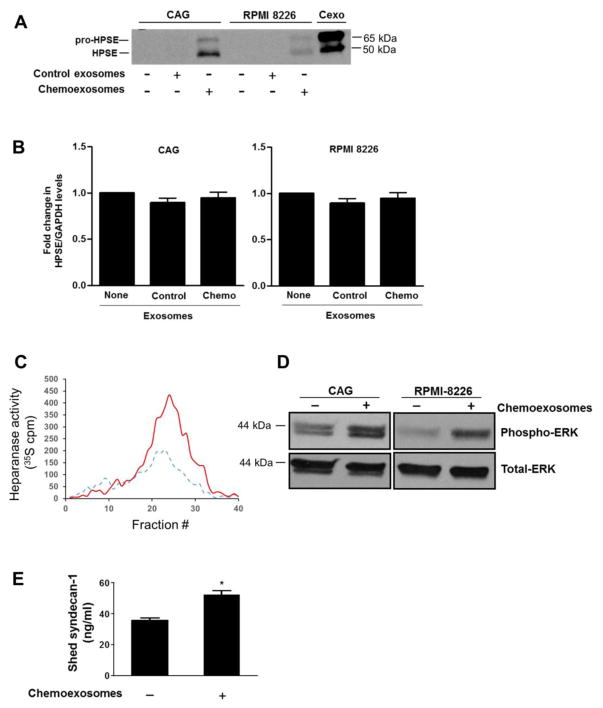
Cells from human myeloma cell lines CAG (wild-type) and RPMI 8226, both of which express low levels of endogenous heparanase, were incubated with chemoexosomes for 16 hours then probed for heparanase expression. Western blotting demonstrates accumulation of heparanase in cells exposed to chemoexosomes but not in cells exposed to control exosomes (exosomes secreted by cells not exposed to bortezomib) (Fig. 3A). Note that in cells exposed to chemoexosomes, the predominant band is the active (50 kDa) form of heparanase while the pro (65 kDa) form of heparanase predominates in the purified chemoexosomes (Cexo). This suggests that the heparanase on chemoexosomes is taken up by the cells and activated. Quantitative PCR indicated that exposure of tumor cells to chemoexosomes did not stimulate endogenous expression of heparanase thereby confirming that the increase in heparanase is due to its transfer from the chemoexosomes to the tumor cells (Fig. 3B). Consistent with the presence of enzymatically active heparanase, when an extract of myeloma cells exposed to chemoexosomes was incubated with ECM containing 35S-labeled heparan sulfate, a much higher level of heparan sulfate fragments were released compared to extracts of cells not exposed to chemoexosomes (Fig. 3C). We previously demonstrated that upregulation of heparanase expression or addition of recombinant heparanase to myeloma cells regulates multiple cell behaviors including enhancing ERK signaling and increasing shedding of syndecan-1 from the tumor cell surface [24–26]. Consistent with those findings, exposure of myeloma cells to chemoexosomes stimulated ERK signaling and syndecan-1 shedding (Fig. 3D, 3E), suggesting that heparanase delivered to the myeloma cells by chemoexosomes is regulating these behaviors.
Fig. 3.
Chemoexosomes transfer heparanase enzyme activity to recipient cells. (A), Wild-type CAG or RPMI 8226 myeloma cells that express very low levels of endogenous heparanase were incubated for 16 hours with exosomes secreted by cells that were untreated (control exosomes) or treated with 250 nM bortezomib (chemoexosomes). Cells were washed to remove exosomes remaining in the medium then lysed and analyzed by western blotting for heparanase. Purified chemoexosomes (Cexo) were loaded in the lane on the far right of the gel. (B) Heparanase transcript levels in wild-type CAG and RPMI 8226 cells were assessed by quantitative PCR in the absence of exosomes or after 16 h exposure to control exosomes or chemoexosomes. (C) Cell lysates were prepared from wild-type CAG cells that had been incubated for 16 h with exosomes secreted by CAG-HPSE cells treated without (control exosomes, dashed line) or with bortezomib (chemoexosomes, solid line). The lysates were then added to a well containing 35S-labeled ECM and the liberated heparan sulfate degradation fragments were separated on a Sepharose 6B column. (D) Wild-type CAG or RPMI 8226 cells growing in the absence of presence of chemoexosomes were extracted and probed for levels of phospho-ERK or total ERK. (E) CAG wild-type cells were grown in the absence or presence of chemoexosomes and the amount of syndecan-1 shed into the culture medium after 16 hours was measured by ELISA. Results are mean ± SD from three separate experiments, *p<0.01 compared to no exosome addition.
Chemoexosomes stimulate macrophage migration and secretion of TNF-α
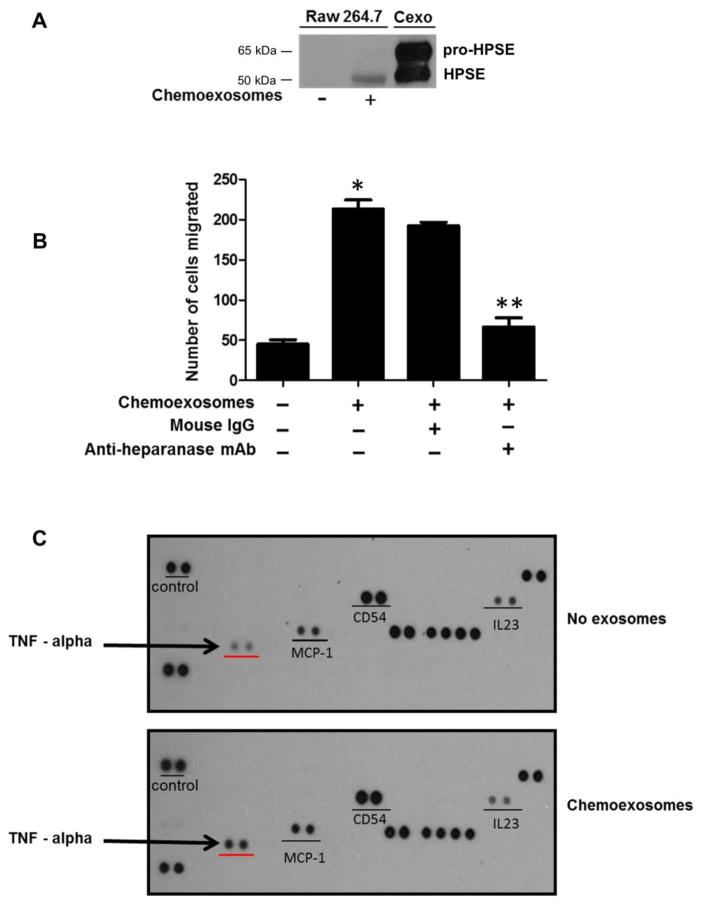
To determine if chemoexosomes impact host cells that are important in promoting myeloma progression, the behavior of macrophages was investigated. Similar to myeloma cells, active heparanase was detected in macrophage lysates following their exposure to chemoexosomes (Fig. 4A). Because heparanase is known to stimulate cell migration and invasion, macrophages were plated in the upper chamber of a transwell insert in the absence or presence of chemoexosomes. Following overnight incubation, cells that had migrated through the 8 μm pores of the transwell filter were counted (Fig. 4B). Chemoexosomes enhanced macrophage migration greater than four-fold vs. cells not exposed to chemoexosomes. Importantly, the ability of chemoexosomes to stimulate migration was blocked by a monoclonal antibody that inhibits heparanase enzyme activity, indicating that exosome heparanase is mediating enhanced macrophage migration. Lastly, probing of a cytokine array with extracts of macrophages that had been exposed to chemoexosomes indicated little change in overall cytokine profile except that TNF-α, a cytokine important in promoting myeloma growth, was substantially upregulated (Fig. 4C). Upregulation of TNF-α expression by exposure of macrophages to chemoexosomes was confirmed by quantitative PCR (Fig. 4D).
Fig. 4.
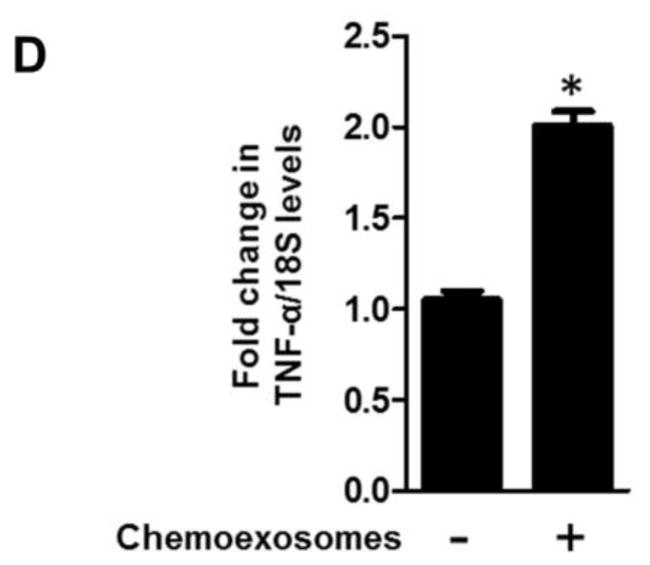
Chemoexosomes released by myeloma cells upregulate macrophage migration and TNF-α expression. (A) RAW 264.7 murine macrophages were incubated for 16 hours without or with chemoexosomes secreted by CAG-HPSE cells. Cells were washed to remove exosomes remaining in the medium then lysed and analyzed by western blotting for heparanase. Purified chemoexosomes were loaded in the lane on the far right (Cexo). (B) Cell migration was assessed using transwell inserts having 8 μm pores. Macrophages were added to the insert and following their attachment and spreading, 100 μg of chemoexosomes was added to the upper well and incubated overnight in the presence or absence of anti-heparanase monoclonal antibody H1023 or control isotype-matched mouse IgG. Cells that migrated through the pores and bound to the underside of the transwell filter were stained and counted. Results are mean ± SD from three separate experiments (*p<0.002 chemoexosomes vs. no exosome control; **p<0.008 chemoexosomes vs. anti-heparanase antibody). (C) Macrophages were incubated for 12 hours without or with chemoexosomes. Cells were washed then lysed and lysates analyzed for cytokine expression using a Proteome Profiler Cytokine array (R&D systems) or (D) by quantitative PCR (*p<0.05). The level of TNF-α was substantially and specifically enhanced in macrophages exposed to chemoexosomes compared to controls.
Chemotherapy alters exosome composition and enriches the exosomes in proteins that regulate the cell cycle
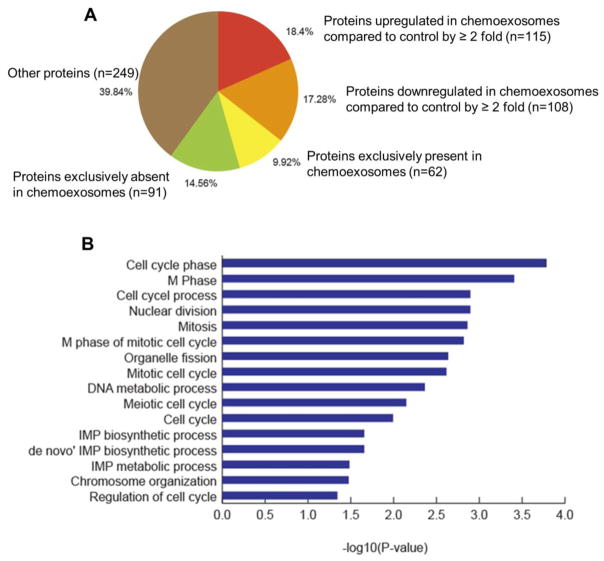
Because heparanase protein level was enhanced in the exosomes secreted by cells exposed to chemotherapy (Fig. 1), we explored whether chemotherapy had a significant impact on overall exosome protein composition. Results revealed that compared to control exosomes, chemoexosomes exhibited greater than a two-fold increase in the level of 115 proteins and greater than a two-fold decrease in the level of 108 other proteins (Fig. 5A). Also there were 91 proteins found to be exclusively absent in chemoexosomes and 62 exclusively present in chemoexosomes compared to control exosomes. Gene ontology enrichment analysis of proteins exclusively present in chemoexosomes revealed that chemoexosomes are enriched in proteins that regulate mitosis and the cell cycle (Fig. 5B and Table 1). There was also an enrichment in nucleotide biosynthetic pathway-related proteins in chemoexosomes. These data demonstrate that treatment of the myeloma cells with bortezomib not only enhances exosome secretion, it substantially impacts the composition of protein cargo loaded into the chemoexosomes, possibly influencing regulation of the cell cycle and nucleotide biosynthesis in target cells.
Fig. 5.
(A) Proteomic analysis of exosomes. Proteins identified in exosomes secreted by cells not treated (control exosomes) or treated with bortezomib (chemoexosomes) were classified based on “normalized total spectra” [NTS] values. Protein with NTS value of >=1 in chemoexosomes and 0 in control exosomes were referred to be exclusively present, while those with 0 NTS value in chemoexosome and >=1 in control exosomes were considered as exclusively absent. In addition, proteins with non-zero NTS values in both chemoexosome and control exosome were used to identify up-regulated and down-regulated proteins (fold change of 2 or more). (B) Gene ontology enrichment analysis was performed using DAVID (Database for Annotation, Visualization and Integrated Discovery) v6.8 [43] to understand the biological role of proteins that were found to be exclusively present in chemoexosomes compared to control exosomes. The functional annotation chart was derived with default parameters (gene count of 2 and EASE of 0.1). Statistically enriched GO biological processes specific to gene set were identified (p-value <0.05).
Table 1.
Biological processes associated with proteins exclusively expressed in chemoexosomes
| Biological process term | Gene count | P-value | Gene names of proteins identified |
|---|---|---|---|
| Cell cycle phase | 9 | 1.25E-04 | CCNB1, NUMA1, NOLC1, H2AFX, CLASP2, RCC1, SMC1A, TDRD1, DNM2 |
| M phase | 8 | 1.92E-04 | CCNB1, NUMA1, NOLC1, H2AFX, CLASP2, RCC1, SMC1A, TDRD1, DNM2 |
| Cell cycle process | 9 | 0.001004 | CCNB1, NUMA1, NOLC1, H2AFX, CLASP2, RCC1, SMC1A, TDRD1, DNM2 |
| Nuclear division | 6 | 0.00127 | CCNB1, NUMA1, NOLC1, CLASP2, RCC1, SMC1A |
| Mitosis | 6 | 0.00127 | CCNB1, NUMA1, NOLC1, CLASP2, RCC1, SMC1A |
| M phase of mitotic cell cycle | 6 | 0.001375 | CCNB1, NUMA1, NOLC1, CLASP2, RCC1, SMC1A |
| Organelle fission | 6 | 0.001516 | CCNB1, NUMA1, NOLC1, CLASP2, RCC1, SMC1A |
| Mitotic cell cycle | 7 | 0.002313 | CCNB1, NUMA1, NOLC1, CLASP2, RCC1, SMC1A, DNM2 |
| DNA metabolic process | 8 | 0.002433 | DKC1, PPIA, PCNA, H2AFX, TOP2B, SMC1A, FEN1, TDRD1 |
| Meiotic cell cycle | 4 | 0.005973 | NUMA1, H2AFX, SMC1A, TDRD1 |
| Cell cycle | 9 | 0.007122 | CCNB1, NUMA1, NOLC1, H2AFX, CLASP2, RCC1, SMC1A, TDRD1, DNM2 |
| IMP biosynthetic process | 2 | 0.021976 | PFAS, GART |
| ‘de novo’ IMP biosynthetic process | 2 | 0.021976 | PFAS, GART |
| IMP metabolic process | 2 | 0.021976 | PFAS, GART |
| Chromosome organization | 6 | 0.032722 | DKC1, HP1BP3, RCOR1, H2AFX, IRF4, SMC1A |
| Regulation of cell cycle | 5 | 0.033572 | CCNB1, H2AFX, ASNS, RCC1, SMC1A |
Discussion
Although it is clear that exosomes secreted by tumor cells play an important role in mediating tumor-tumor and tumor-host cell crosstalk [19], less is known about the impact that anti-tumor therapy has on exosome secretion and function. In the present work, using myeloma cells as a model, we demonstrate that drugs commonly used to treat multiple myeloma patients substantially enhance exosome secretion. This included proteasome inhibitors, a relatively new class of targeted chemotherapeutic drugs, and melphalan, a genotoxic compound widely utilized in traditional chemotherapy. The secreted exosomes, which we refer to as chemoexosomes, also differ in their protein composition compared to exosomes secreted by the same cells not treated with drug. Chemoexosomes were shown to have important regulatory functions when exposed to cells that had not been treated with drug. This included enhancing cellular heparanase activity, activating signaling pathways and enhancing syndecan-1 shedding by myeloma cells. In macrophages, chemoexosomes induced macrophage migration and stimulated secretion of TNF-α.
We do not know the mechanism of drug-induced increase in exosome secretion. However, we previously demonstrated that upregulation of heparanase enhances exosome secretion and this requires enzymatically active heparanase [17]. We also demonstrated that exposure of myeloma cells to cytotoxic drugs enhances heparanase expression and release from cells [9]. Thus, it is likely that the burst in exosome secretion following exposure of cells to drugs is related, at least in part, to the drug-induced increase in heparanase expression. In addition, genotoxic drugs may enhance exosome secretion as a response to cell stress [27]. Hepatocellular carcinoma cells when exposed to anti-cancer drugs were shown to enhance secretion of exosomes having high levels of stress-induced heat shock proteins [28]. Although the burst in exosome secretion following exposure of cells to cytotoxic drugs is likely short-lived (because many of the tumor cells will undergo apoptosis and rapidly die) the impact of the chemoexosomes could be substantial and lasting. Chemoexosomes may become trapped within the tumor microenvironment where they could modify the ECM or be stored and later interact with surviving tumor or host cells. In addition, chemoexosomes may escape the tumor microenvironment, lodge in distal tissues and contribute to formation of pre-metastatic niches thereby aiding in tumor metastasis [11–13]. In contrast, chemoexosomes could have immediate and profound effects on neighboring tumor cells by enhancing signaling pathways (e.g., ERK, P38) that are known to enhance chemoresistance. Our proteomics data demonstrate that chemoexosomes have a protein signature distinct from control exosomes (Fig. 5). This includes a number of proteins exclusively absent or present in chemoexosomes which may influence the behavior of tumor and/or host cells. Notable was the clearly enhanced level of cell cycle regulatory proteins and nucleotide biosynthesis pathway proteins making it reasonable to speculate that chemoexosomes could deliver these regulatory proteins to other tumor cells and perhaps endow them with an enhanced aggressive growth phenotype. Moreover, the finding that the chemoexosome protein profile differs significantly from that of the exosomes from untreated tumor cells, and that chemoexosomes may negatively affect patient outcome, underscores the need to closely examine the impact of anti-cancer drugs on exosome secretion, composition and function.
At least some of the functions of chemoexosomes we examined are directly due to the high level of heparanase present on chemoexosomes. Heparanase was readily transferred to both tumor cells and macrophages resulting in the cells bearing high levels of the enzymatically active enzyme. We have previously demonstrated that upregulation of heparanase expression or delivery of recombinant heparanase to myeloma tumor cells upregulates multiple genes associated with tumor progression including VEGF, HGF, and RANKL. In addition, heparanase increases ERK signaling leading to enhanced MMP-9 expression thereby stimulating shedding of syndecan-1 from the myeloma cell surface, an event that further contributes to tumor progression. Together these events drive myeloma growth, metastasis, osteolysis and angiogenesis [7, 29]. Our finding now that heparanase delivered by chemoexosomes can lead to enhanced ERK signaling and syndecan-1 shedding is consistent with the known role for heparanase in these cells and suggests that exposure of myeloma cells to drug could, via released exosomes, contribute to aggressive tumor cell behavior.
We also demonstrate for the first time that heparanase can be localized to the exosome surface where it can degrade heparan sulfate present within an intact ECM. This was shown by introducing intact exosomes to an ECM assembled by cells. This is an important observation because it reveals that secreted exosomes can directly impact the ECM by degrading heparan sulfate. This action may release heparan sulfate-bound growth factors that support tumor progression and also could enhance migration of cells by removing or altering structural barriers. Although there are only sparse reports of enzymes functioning on exosome surfaces, it has been shown that MT1-MMP on intact exosomes secreted by fibrosarcoma and melanoma cells can activate pro-MMP-2 and degrade type I collagen and gelatin [30, 31]. Also a dynamic interaction between exosomes and invadopodia was found on metastatic breast cancer cells leading the authors to speculate that maturation of invadopodia and ECM degradation are dependent on exosome delivery of MT1-MMP and other proteases [32]. Similarly, exosomes promote directional cell movement by providing an ECM on their surface (e.g., fibronectin) on which tumor cells move [33]. This directional movement may also involve exosome-bound proteases and or glycosidases such as heparanase which may be particularly important as invading cells traverse heparan sulfate rich basement membranes. Interestingly, another glycosidase, the sialidase neruainidase-1, is present on the surface of extracellular vesicles secreted by microglial cells in response to inflammatory stimuli. The extracellular vesical-bound neuraminidase degrades polysialic acid on the microglial surface releasing neurotrophin [34].
It is intriguing that heparanase can be localized to the exosome surface via its binding to heparan sulfate, yet the heparan sulfate is apparently not degraded by the enzyme. There are several possible explanations for this observation. Heparanase has multiple domains that bind to heparan sulfate, some of which are not localized near the cleavage furrow of the enzyme. It is possible that when heparanase is bound at these distal sites, the enzyme is rendered in an inactive confirmation or is sterically hindered from further interaction with its heparan sulfate substrate. Once the exosome docks with a molecule in the ECM or with a cell, the enzyme may be freed and the active site once again available. Also as shown in Fig. 2D, most of the heparanase enzyme found on the exosome surface is the inactive 65 kDa form (Fig. 2D). Exosome surfaces would therefore be an ideal way to transport the inactive enzyme which could then be activated by cathepsin L present either in the ECM or encountered intracellularly after exosome uptake by a target cell. Also, because heparanase enzyme activity is optimal at pH 5.0 – 6.0, any active enzyme bound to the cell surface may exhibit only weak activity until it encounters an acidic environment. This could be particularly relevant in situations where exosomes enter hypoxic microenvironments, a situation often present in tumors and where heparanase is known to stimulate angiogenesis.
In multiple myeloma, survival of tumor cells is dependent on close interaction with the bone marrow microenvironment. Circulating monocytes are recruited to the bone marrow by chemoattractants secreted by both tumor and stromal cells where they become differentiated into mature macrophages [35]. Late stage and refractory multiple myeloma patients are marked by a high level of tumor-associated macrophage (TAM) infiltration which is associated with poor prognosis [36]. Interaction between TAMs and myeloma cells within the bone marrow activates signaling pathways that protect tumor cells from chemotherapy induced apoptosis [37]. We previously demonstrated that soluble heparanase released by tumor cells during chemotherapy induces macrophages to upregulate secretion of TNF-α, a factor known to promote the growth of malignant plasma cells [9]. We now extend this to demonstrate that chemoexosomes, having a high level of heparanase as cargo, activate macrophages to secrete TNF- α. Importantly, this effect of exosomes was specific as it did not upregulate expression of any other cytokines that were detectable using the cytokine array. Heparanase may modulate macrophage behavior via multiple mechanisms such as altering gene expression or activating signaling through ERK as has been found in myeloma cells [25]. Heparanase delivered by chemoexosomes also stimulated macrophage migration. Mechanistically it was previously shown that in myeloma and endothelial cells, heparanase enhances syndecan-1 shedding leading to activation of signaling complexes that stimulate cell migration [4]. A similar mechanism may be in play on the macrophages examined here. Importantly, chemoexosome-mediated macrophage migration was blocked by a monoclonal antibody to heparanase that blocks its enzyme activity. This antibody, H1023, has been shown in animal models to block myeloma growth [38]. Thus, it is possible that at least some of the in vivo effect of the antibody is due to interference with delivery and/or activity of exosomal heparanase.
In summary, we have demonstrated that exposure of myeloma cells to cytotoxic drugs enhances secretion of exosomes loaded with high levels of heparanase that remains functional and impacts behavior of both tumor and host cells. In addition, other changes in the composition of exosomes following drug therapy, particularly those that may enhance tumor cell proliferation, may be functionally important. All together, these findings reveal a novel mechanism whereby anti-tumor therapy releases exosomes that may enhance tumor survival and patient relapse. Therapies designed to target exosome function and overcome this negative side effect of cytotoxic drugs may improve patient outcome.
Experimental procedures
Cell lines and reagents
Human myeloma cell lines CAG, CAG-HPSE and RPMI-8226 cells were cultured in RPMI media supplemented with 10% FBS, L-Glutamine, antibiotics and antimycotics. CAG-HPSE cells were previously engineered to express human heparanase [26]. RAW 264.7 murine macrophages were cultured in DMEM medium supplemented with 10% FBS, L-Glutamate, antibiotics and antimycotics. Reagents utilized were bortezomib and carfilzomib (Sellekchem), melphalan (Sigma Aldrich), anti-clathrin (System Biosciences), affinity purified anti-human heparanase polyclonal antibody (#1453) [39] and anti-human heparanase monoclonal antibody H1023 [9].
Exosome isolation from cell culture supernatants and from serum of myeloma patients
Human myeloma cells growing in culture were washed twice with PBS and grown in serum-free medium for 16 h. Exosomes secreted into the medium by myeloma cells were isolated by differential centrifugation as described previously, characterized by surface marker expression and size, and found to be consistent with the characteristics of exosomes [17]. Particle size was measured by nanoparticle tracking analysis using a NanoSight 300 (Malvern Instruments Ltd.). For some experiments exosomes underwent an additional purification step by binding them to anti-CD63 antibody bound to magnetic beads or by ultracentrifugation over a cushion of 40% iodixanol (Optiprep).
Serum samples were obtained from incident, treatment-naïve multiple myeloma patients enrolled in the Molecular And Genetic Epidemiology (iMAGE) study of myeloma who met the revised and updated International Multiple Myeloma Working Group classification criteria for myeloma [40]. Approvals from the appropriate Institutional Review Boards were obtained prior to study initiation. Exosomes were isolated from serum using an ExoQuick isolation kit (System Biosciences). Briefly, 30 μl of ExoQuick solution was added to 100 μl of serum and incubated at 4 °C for 1 h followed by centrifugation at 1500 × g for 30 min. The pellet was suspended in PBS, and the exosomes were further purified using anti-CD63 conjugated to magnetic beads (System Biosciences), according to the manufacturer’s instructions. Particle size and number was assessed using NanoSight 300 according to manufacturer’s instructions.
Flow cytometry analysis of exosomes
Flow cytometry analysis to identify heparanase on the surface of exosomes was performed after attaching exosomes to magnetic beads bearing anti-CD63 antibody. 100 μg of purified exosomes were mixed with the anti-CD63 beads and incubated on a rotating rack at 4°C overnight. Exosomes bound to beads were suspended in 200 μl of PBS containing 1% BSA and stained with polyclonal antibody against heparanase prior to analysis with a Becton Dickinson FACSCalibur flow cytometer. Following incubation with primary antibody, beads were washed with PBS, incubated with anti-rabbit Alexa Fluor 594. Rabbit IgG (Santa Cruz) was used as the control.
Macrophage migration assay
The migration of murine macrophages was analyzed using Biocoat Migration invasion chambers (BD Biosciences) consisting of cell culture inserts containing a polyethylene membrane having 8-μm pores. RAW 264.7 murine macrophages (2 × 104) were suspended in 500 μl serum free DMEM media and seeded in the upper chamber and treated with or without chemoexosomes (introduced into the upper chamber) in presence of anti-heparanase monoclonal antibody H1023 (15 μg/ml) or anti-mouse IgG1 isotype control. 750 μl of DMEM media containing 1% FBS was added to lower chamber. The chambers were incubated at 37°C and cells allowed to migrate through the 8-μm pores for 12 h at which time the non-invading cells were removed from the upper surface of the membrane using a cotton swab and invading cells on the underside of the filter were fixed and stained with Diff-Quick solution (IMEB Inc.) and counted. Each assay was performed in triplicate.
Syndecan-1 ELISA
The level of shed syndecan-1 present in the medium after treating CAG myeloma cells with or without chemoexosomes (75 μg) for 12 hours was assessed by an ELISA using an Eli-pair kit specific for human syndecan-1 core protein (Cell Sciences). The standard curve was linear between 8 and 256 ng/ml, and all samples were diluted to concentrations within that range. All samples were run in triplicate.
Western blot
Purified exosomes were incubated in RIPA buffer containing protease inhibitors for 10 minutes on ice. Proteins were separated utilizing 4–20% gradient SDS-PAGE, electroblotted to a nitrocellulose membrane and probed with polyclonal anti-heparanase antibody or antibody to clathrin, ERK or phospho-ERK (R&D Systems). Bound antibodies were detected using HRP-conjugated secondary antibody and visualized by chemiluminescence.
Cytokine array
A membrane based antibody array (R&D systems) that determines the relative levels of 43 different cytokines was used according to manufacturer’s instructions. Briefly, 3 × 106 murine macrophages (RAW 264.7) were incubated overnight without or with 100 μg of chemoexosomes, washed and the cells extracted using RIPA cell lysis buffer. Cell extracts were incubated overnight with cytokine array membrane, washed to remove unbound proteins and then incubated with a cocktail of biotinylated anti-cytokine detection antibodies. Streptavidin-HRP and chemiluminescent detection reagents were applied to visualize the signal produced at each capture spot corresponding to the amount of cytokine bound.
Heparan sulfate degradation assay
This assay has been described in detail previously [41, 42]. Briefly, intact exosomes or cell extracts were incubated for 18 h at 37 °C, pH 6.2–6.6, with a 35S-labeled ECM that had been deposited by bovine corneal endothelial cells growing on 35 mm tissue culture plates. The incubation medium containing 35S-labeled fragments of heparan sulfate released by the heparanase was centrifuged and the supernatant was analyzed by gel filtration on a Sepharose 6B column (0.9 × 30 cm).
RNA isolation and PCR
After different treatments, cells were washed once with PBS and RNA was extracted and isolated using RNeasy columns (Qiagen). 2 μg of total RNA was reverse transcribed using Applied Biosystems High Capacity cDNA synthesis kit for RT-qPCR (Thermo Scientific).
1 μl of cDNA was used to perform RT-qPCR using Applied Biosystems gene expression analysis kit using Taqman assays (Thermo Scientific) according to the manufacturer’s protocol. The primers used were HPSE (HSs00935036_m1), TNF-α (Mm00443258_m1), GAPDH (Hs02786624_g1), 18S (Hs99999901_s1). The cycle parameters and analyses of real-time PCR have been performed according to the manufacturer’s instructions.
Exosome protein analysis by mass spectrometry (MS/MS)
Exosomes excluded by an iodixanol cushion were solubilized in 1X LDS sample buffer (NuPAGE, Life Technologies) followed by membrane disruption for 10 min in an ultrasonic bath (Thermo Fisher), and heat denaturation as per manufacturer’s instructions for the LDS buffer. Protein extracts were then quantified using the BCA Protein Assay Kit (Pierce, Life Technologies). An aliquot containing 20 μg of protein was reduced, denatured, and loaded onto a 10% Bis-tris gel (NuPAGE reagents, Life Technologies) and separated as a short stack run (~1 cm). The gel was stained with Colloidal Blue Staining Kit (NuPAGE, Life Technologies), de-stained and visualized. The upper gel section containing protein for each sample was cut out and digested using Trypsin Gold (Promega), followed by peptide extraction as per manufacturers instructions, and the volumes were reduced using a Savant SpinVac Concentrator (Thermo Fisher). One microgram of peptide extract (diluted to ~1 μg/10 ul in 0.1% formic acid) was loaded onto a 100 μm × 13 cm capillary column, packed in-house with C18 Monitor 100 A-spherical silica beads, and eluted over a 90 min gradient (0%–30% acetonitrile in 0.1% TFA). Liquid Chromatography Mass spectrometric analysis was performed in duplicate using an LTQ Velos Pro Orbitrap (Thermo Fisher).
Summary.
We find that anti-myeloma chemotherapy dramatically stimulates secretion of exosomes and alters exosome composition. Exosomes secreted during therapy contain high levels of heparanase on their surface that can degrade ECM and also can be transferred to both tumor and host cells, altering their behavior in ways that may enhance tumor survival and progression.
Highlights.
Anti-myeloma drugs enhance secretion of exosomes by tumor cells
Exosomes secreted in response to chemotherapy (chemoexosomes) contain a high level of heparanase
Heparanase is present on the chemoexosome surface and can degrade heparan sulfate embedded within an extracellular matrix
Chemoexosomes regulate signaling of tumor cells and the migration and cytokine expression of macrophages
Acknowledgments
Funding information
This work was supported by grants from the National Institutes of Health CA138340 and CA211752 (to RDS), CA186646 (EEB) and the United States – Israel Binational Science Foundation (jointly to RDS and IV). Israel Vlodavsky is a Research Professor of the Israel Cancer Research Fund (ICRF). GJB was supported by NIH Medical Scientist Training Program T32GM008361. Support was also provided by NIH CA13148 for the UAB Comprehensive Cancer Center Shared Facilities (Mass Spectrometry/Proteomics Shared Facility and High-Resolution Imaging Shared Facility) and NIH AR048311 and AI27667 for the UAB Comprehensive Flow Cytometry Core. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used
- ECM
Extracellular matrix
- ERK
extracellular signal-related kinase
- HPSE
heparanase
- MMP
matrix metalloproteinase
- RANKL
receptor activator of nuclear factor-B ligand
- TAM
tumor associated macrophage
- TNF-α
tumor necrosis factor alpha
Footnotes
Declarations of interest
The authors declare no competing financial interests.
Author Contributions
S.K.B, A.P., V.C.R. and R.D.S. designed the project. S.K.B., A.P., V.C.R. and G.J.B. performed experiments. D.S.C. and S.V. analyzed the proteomic data. J.A.M. performed the proteomic studies. Y.Z. produced monoclonal antibody H1023. E.E.B. provided serum from myeloma patients. I.V. performed and analyzed the heparanase matrix degrading assay. S.K.B and R.D.S. wrote the manuscript. All authors reviewed and edited the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramani VC, Yang Y, Ren Y, Nan L, Sanderson RD. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J Biol Chem. 2011;286:6490–9. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Ren Y, Ramani VC, Nan L, Suva LJ, Sanderson RD. Heparanase enhances local and systemic osteolysis in multiple myeloma by upregulating the expression and secretion of RANKL. Cancer Res. 2010;70:8329–38. doi: 10.1158/0008-5472.CAN-10-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Macleod V, Bendre M, Huang Y, Theus AM, Miao HQ, Kussie P, Yaccoby S, Epstein J, Suva LJ, Kelly T, Sanderson RD. Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood. 2005;105:1303–1309. doi: 10.1182/blood-2004-06-2141. [DOI] [PubMed] [Google Scholar]
- 4.Jung O, Trapp-Stamborski V, Purushothaman A, Jin H, Wang H, Sanderson RD, Rapraeger AC. Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogenesis. 2016;5:e202. doi: 10.1038/oncsis.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–57. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly T, Miao HQ, Yang Y, Navarro E, Kussie P, Huang Y, MacLeod V, Casciano J, Joseph L, Zhan F, Zangari M, Barlogie B, Shaughnessy J, Sanderson RD. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63:8749–56. [PubMed] [Google Scholar]
- 7.Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J. 2017;284:42–55. doi: 10.1111/febs.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Heparanase: From basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75. doi: 10.1016/j.drup.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramani VC, Vlodavsky I, Ng M, Zhang Y, Barbieri P, Noseda A, Sanderson RD. Chemotherapy induces expression and release of heparanase leading to changes associated with an aggressive tumor phenotype. Matrix Biol. 2016;55:22–34. doi: 10.1016/j.matbio.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramani VC, Zhan F, He J, Barbieri P, Noseda A, Tricot G, Sanderson RD. Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma. Oncotarget. 2016;7:1598–1607. doi: 10.18632/oncotarget.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Raimondi L, De Luca A, Amodio N, Manno M, Raccosta S, Taverna S, Bellavia D, Naselli F, Fontana S, Schillaci O, Giardino R, Fini M, Tassone P, Santoro A, De Leo G, Giavaresi G, Alessandro R. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget. 2015;6:13772–89. doi: 10.18632/oncotarget.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–57. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, De Veirman K, Faict S, Frassanito MA, Ribatti D, Vacca A, Menu E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239:162–73. doi: 10.1002/path.4712. [DOI] [PubMed] [Google Scholar]
- 17.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288:10093–9. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem. 2016;291:1652–63. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–15. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, Ji MH, Hu Q, Luo Z, Wu JZ, Tang JH. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9:e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin J, Yan X, Yao X, Zhang Y, Shan Y, Mao N, Yang Y, Pan L. Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. J Cell Mol Med. 2012;16:337–48. doi: 10.1111/j.1582-4934.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–92. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Purushothaman A, Babitz SK, Sanderson RD. Heparanase enhances the insulin receptor signaling pathway to activate extracellular signal-regulated kinase in multiple myeloma. J Biol Chem. 2012;287:41288–96. doi: 10.1074/jbc.M112.391417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008;283:32628–36. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–33. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- 27.Vulpis E, Cecere F, Molfetta R, Soriani A, Fionda C, Peruzzi G, Caracciolo G, Palchetti S, Masuelli L, Simonelli L, D’Oro U, Abruzzese MP, Petrucci MT, Ricciardi MR, Paolini R, Cippitelli M, Santoni A, Zingoni A. Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: Role of HSP70/TLR2/NF-kB axis. Oncoimmunology. 2017;6:e1279372. doi: 10.1080/2162402X.2017.1279372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874–85. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, Sanderson RD. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J. 2013;280:2294–306. doi: 10.1111/febs.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105:1211–8. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- 31.Shay G, Lynch CC, Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44–46:200–6. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–68. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumida M, Hane M, Yabe U, Shimoda Y, Pearce OM, Kiso M, Miyagi T, Sawada M, Varki A, Kitajima K, Sato C. Rapid trimming of cell surface polysialic acid (PolySia) by exovesicular sialidase triggers release of preexisting surface neurotrophin. J Biol Chem. 2015;290:13202–14. doi: 10.1074/jbc.M115.638759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribatti D, Moschetta M, Vacca A. Macrophages in multiple myeloma. Immunol Lett. 2014;161:241–4. doi: 10.1016/j.imlet.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Beider K, Bitner H, Leiba M, Gutwein O, Koren-Michowitz M, Ostrovsky O, Abraham M, Wald H, Galun E, Peled A, Nagler A. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget. 2014;5:11283–96. doi: 10.18632/oncotarget.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–8. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissmann M, Arvatz G, Horowitz N, Feld S, Naroditsky I, Zhang Y, Ng M, Hammond E, Nevo E, Vlodavsky I, Ilan N. Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis. Proc Natl Acad Sci U S A. 2016;113:704–09. doi: 10.1073/pnas.1519453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gingis-Velitski S, Zetser A, Kaplan V, Ben-Zaken O, Cohen E, Levy-Adam F, Bashenko Y, Flugelman MY, Vlodavsky I, Ilan N. Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J Biol Chem. 2004;279:44084–92. doi: 10.1074/jbc.M402131200. [DOI] [PubMed] [Google Scholar]
- 40.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 41.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 42.Bar-Ner M, Kramer MD, Schirrmacher V, Ishai-Michaeli R, Fuks Z, Vlodavsky I. Sequential degradation of heparan sulfate in the subendothelial extracellular matrix by highly metastatic lymphoma cells. Int J Cancer. 1985;35:483–91. doi: 10.1002/ijc.2910350411. [DOI] [PubMed] [Google Scholar]
- 43.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–75. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]