Abstract
After traumatic spinal cord injury (SCI), a scar may form with a fibrotic core (fibrotic scar) and surrounding reactive astrocytes (glial scar) at the lesion site. The scar tissue is considered a major obstacle preventing regeneration both as a physical barrier and as a source for secretion of inhibitors of axonal regeneration. Understanding the mechanism of scar formation and how to control it may lead to effective SCI therapies. Using a compression-SCI model on adult transgenic mice, we demonstrate that the canonical Wnt/β-catenin signaling reporter TOPgal (TCF/Lef1-lacZ) positive cells appeared at the lesion site by 5 days, peaked on 7 days, and diminished by 14 days post injury. Using various representative cell lineage markers, we demonstrate that, these transiently TOPgal positive cells are a group of Fibronectin(+);GFAP(−) fibroblast-like cells in the core scar region. Some of them are proliferative. These results indicate that Wnt/β-catenin signaling may play a key role in fibrotic scar formation after traumatic spinal cord injury.
Keywords: Traumatic spinal cord injury (SCI), spinal cord compression, fibrotic scar, Wnt/β-catenin signaling, TOPgal, transgenic mice
Introduction
Wnt signaling is activated in neural stem cells and progenitors as well as in glial cells, and is critical for neurogenesis in the embryonic and adult CNS [1–6]. Wnt signaling also plays an indispensable role in neurite outgrowth and axon guidance during neural development and regeneration [7–11]. In the adult spinal cord, the expression of various Wnt signaling molecules is normally low, but is induced by spinal cord injury (SCI) in rodents or zebrafish [12–15]. Reinduction of repulsive Wnt/Ryk signaling molecules in the adult spinal cord has been shown to suppress axonal regeneration after SCI [16, 17]. Conversely, Wnt3a administration enhances axonal regeneration and functional recovery after SCI in rats [18, 19], while overexpression of an inhibitory ligand Dkk inhibits axon regeneration in zebrafish [15]. Nevertheless, the identities of the Wnt responsive cells in the injured spinal cord and the cellular mechanisms by which Wnt signaling influences recovery from SCI remain poorly understood.
The potential of axons to regenerate is likely conserved in the adult spinal cord, but it is blocked by various inhibitory factors generated at the lesion site [20–22]. Immediately after SCI, oligodendrocyte/myelin-derived axon growth inhibitors, such as Nogo, MAG, and OMG are produced [23–25]. Subsequently, a scar forms at the lesion site that becomes a physical barrier and also generates chemical inhibitors, such as chondroitin sulfate proteoglycans (CSPGs), further limiting the regenerative potential of the adult CNS axons [26–28]. The core scar region consists of dominantly fibroblasts that secrete extracellular matrix molecules, including Fibronectin, Laminin, and collagen type IV (Col IV) [29, 30], but the cellular and signaling mechanisms responsible for fibrotic scar formation are not well understood.
In this study, we determined that the injury-induced Wnt responsive cells are neither neuronal nor glial lineages, but a group of fibroblasts at the lesion site. Many of them are proliferative. These results provide new insights into the function of Wnt/β-catenin signaling in fibrotic scar formation after adult neural injury and suggest Wnt signaling as a novel therapeutic target for control of scar formation and enhanced regeneration after SCI.
Materials and methods
Animals
Wnt signaling reporter TOPgal transgenic mice (Tg(Fos-lacZ)34Efu/J) [31] were obtained through the Jackson Laboratory and expanded/housed in the UC Davis vivarium. Two- to four-month-old adult mice were used in this study. All animal procedures were approved by the Institutional Animal Care and Use Committee at UC Davis and conformed to NIH guidelines.
Surgical procedures
All surgeries were performed under aseptic conditions. Spinal cord injury was carried out using the compression or crush method as described previously with minor modifications [32–34]. Animals were anesthetized with Ketamine/Xylazine (100/10 mg/kg) administered intraperitoneally (i.p.). Depth of anesthesia was assured by monitoring lack of response to a foot pinch prior to the surgery. The back of the mouse was shaved and disinfected with povidone-iodine (Betadine) and 70% alcohol. A skin incision was made above the lower thoracic vertebrae. Paravertebral muscles on both sides of the T10-T12 vertebrae level were cut, and the vertebrae were exposed. A laminectomy was then performed between T10-T12, and the spinal cord was compressed by inserting a sterilized No.5 Dumont forceps (Fine Science Tools) with or without 0.5 mm spacer [32] for 30 seconds at the T11 level. After the compression injury, muscles and skin were sutured, and animals were placed on a heat pad until ambulatory. Each animal was given systematic postoperative analgesia (Buprenrphine, 0.05mg/kg i.p.) to prevent pain. Animals were examined daily to record their body weight. After surgery, the mouse bladder was manually expressed twice daily until the recovery of spontaneous bladder function, which generally occurred between 7 and 14 days post-injury (dpi).
X-gal staining and immunohistochemistry
For X-gal staining to detect TOPgal activity, the injured mice were perfused transcardially with phosphate-buffered saline (PBS, pH7.4). The spinal cord was dissected out and immersed into X-gal (5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside) substrate solution (with 1 mg/ml X-gal in 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, and 5 mM MgCl2) for 12–24 hours in the dark to detect β-galactosidase enzymatic activity. The stained sample was washed three times with PBS and postfixed with 4% paraformaldehyde (PFA), then processed for histological analysis. For co-labeling of X-gal and immunohistochemistry, the X-gal-stained and fixed tissue was washed with PBS and incubated in 30% sucrose at 4°C and embedded in O.C.T. compound (Sakura Finetek). Fourteen-micrometer thick longitudinal frozen sections were prepared. Bright-field immunohistochemistry was performed using an HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc.) and color-developed with diaminobenzidine (DAB) (Sigma). The injury site and adjacent spinal cord tissue (2.5 mm caudal and rostral from the epicenter, 5 mm total length) was dissected out and post-fixed in 4% PFA. Primary antibodies including the Goat anti-Nestin (1:100, Santa Cruz), mouse anti-BrdU (1:100, Dako), rat anti-MBP (Dako, 1:200), rabbit anti-NG2 (1:200, Millipore), rabbit anti-Fibronectin (1:1000, Millipore), rabbit anti-GFAP (1:1000, Dako), mouse anti-GFAP (1:500, Covance), rabbit anti-Laminin (1:1000, Millipore), rabbit anti-p75 (1:500, Millipore), rabbit anti-PDGFRβ (1:100, Upstate), and rabbit anti-Vimentin (1:1000, kindly provided by P. Fitzgerald at UC Davis) were used in this study. Stained sections were examined and photographed using Olympus BX61 microscope for bright fields.
Results
Spinal cord injury induced transient activation of the Wnt signaling reporter TOPgal
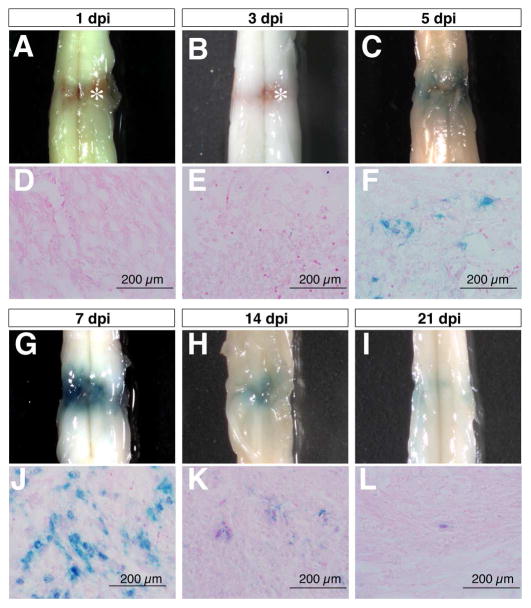
In order to investigate the effects of SCI on canonical Wnt/β-catenin signaling, we performed complete compression injury at the lower thoracic level to induce mechanical and ischemic injuries in adult TOPgal reporter mice. Wnt signaling reporter TOPgal activity in the injured spinal cord was detected by X-gal staining, and showed a dynamic change within the first two weeks after SCI (Fig. 1). X-gal positive cells began to appear at the lesion site by five days post injury (5 dpi, Fig. 1C,F), reached peak numbers by 7 dpi (Fig. 1G,J), were substantially diminished by 14 dpi (Fig. 1H,K), and had almost disappeared by 21 dpi (Fig. 1I,L). These observations indicated that Wnt/β-catenin signaling is transiently induced by SCI in a group of cells at the lesion site.
Fig. 1.
Compression spinal cord injury activates the Wnt signaling reporter TOPgal in a time-dependent manner. The wholemount spinal cords (A-C,G-I) and respective transverse sections (D-F,J-L) show the X-gal staining results for the TOPgal activities in the adult TOPgal mice at 1, 3, 5, 7, 14, and 21 days post injury (dpi). Note that the blue X-gal staining appears at 5 dpi (C,F), peaks at 7 dpi (G,J), and diminishes dramatically at 14 (H,K) and 21 dpi (I,L). Asterisks (A,B) indicate the reddish-brown substance caused by hemorrhage in the lesion site at 1 and 3 dpi. The sections were counterstained with Eosin solution.
The SCI-induced Wnt responsive cells were proliferative non-neural stem cells
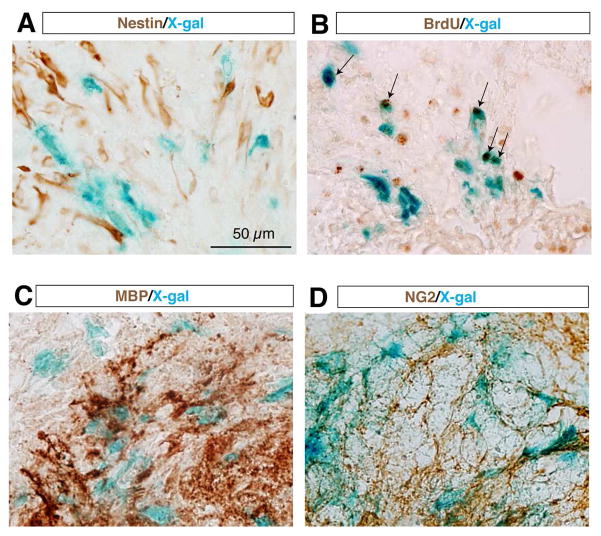
To determine the cell identity of the SCI-induced TOPgal(+) cells at the lesion site, we performed immunohistochemistry with antibodies for representative cell lineage marker proteins on the spinal cord sections after X-gal staining (Fig. 2). As Wnt/β-catenin signaling is crucial in the proliferation and differentiation of neural stem cells that may be responsive to SCI, we used anti-Nestin antibodies to mark the possible neural stem cell population. We found no overlapped labeling of the Nestin(+) cells and X-gal stained cells (Fig. 2A), indicating that these SCI-induced TOPgal(+) cells are not neural stem cells. BrdU incorporation and immunolabeling assay demonstrated that one half of the BrdU(+) proliferating cells were X-gal stained Wnt responsive cells (Fig. 2B). Many X-gal stained cells were intermingled with the crushed MBP(+) myelinated axonal fibers and oligodendrocytes or NG2 positive polydendrocytes around the lesion site (Fig. 2C,D).
Fig. 2.
The SCI-induced TOPgal positive cells are proliferative and non-neural stem cells. The injured spinal cord at 7 dpi were stained with X-gal solution, and then immunolabeled with antibodies against the neural stem cell marker Nestin (A), incorporated BrdU (B), myelin basic protein MBP (C), and polydendrocyte marker NG2 (D). Arrows in B indicate the cells co-labeled by X-gal staining (blue) and BrdU immunoreactivities (brown).
The SCI-induced Wnt responsive cells were Fibronectin(+);GFAP(−) fibroblast-like cells in the core scar region
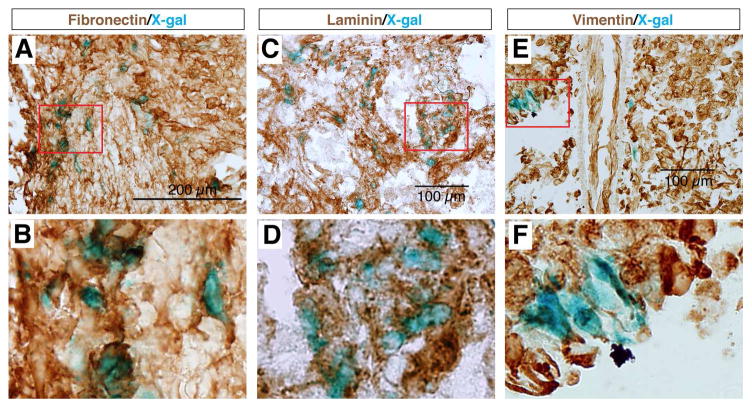
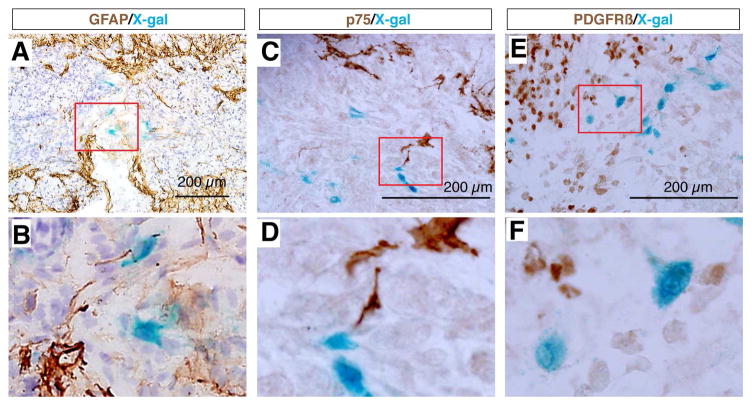
We found that most X-gal stained cells at the epicenter of the lesion site were co-immunolabeled with antibodies against the fibroblastic markers Fibronectin, Laminin, and Vimentin (Fig. 3), but did not express GFAP (Fig. 4), whereas cells surrounding this core were X-gal negative but GFAP positive (Fig. 4A,B). These results indicated that the Wnt signaling reporter TOPgal was activated in the fibroblast-like cells that formed the core fibrotic scar after SCI. Moreover, we demonstrated that the core fibroblast-like cells were not co-immunolabeled for p75, a protein normally expressed in Schwann cells and induced in oligodendrocytes by SCI [35] (Fig. 4C,D), nor for PDGFRβ, a marker for pericytes [36, 37] (Fig. 4E,F). These observations suggest a lineage-specific activation of Wnt/β-catenin signaling in fibroblast-like cells at the epicenter of the lesion site.
Fig. 3.
The SCI-induced TOPgal positive cells are fibroblast-like cells. The X-gal stained cells are co-immunolabeled with the fibroblastic marker antibodies against Fibronectin (A,B), Laminin (C,D), and Vimentin (E,F). Red rectangles in the upper panels indicate the area enlarged in the respective lower panels.
Fig. 4.
The SCI-induced TOPgal positive cells neither glia nor pericytes. X-gal stained cells do not express the astrocyte marker GFAP (A,B), injured oligodendrocyte marker p75 (C,D), and pericyte marker PDGFRβ (E,F). Red rectangles in the upper panels indicate the area enlarged in the respective lower panels.
Discussion
Our study, which used a murine spinal cord compression model, demonstrates that Wnt signaling is activated by compression injury of spinal cord. These results are consistent with prior reports that Wnt ligands and receptors are upregulated in the injured spinal cord after hemisection or contusion lesions [12–14, 16], but adds to those studies by showing that Wnt signaling, as demonstrated with the Wnt/β-catenin signaling reporter TOPgal, is induced specifically in the fibrotic scar. Our finding of the fibrotic activation of Wnt signaling reporter TOPgal is completely different from a previous study that demonstrates induction of another Wnt signaling reporter BATgal in neurons of the dorsal horn and cells of the central canal of uninjured spinal cords after hemisection [14]. Notably, many of the SCI-induced TOPgal active cells are proliferative, suggesting a potential role of Wnt/β-catenin signaling in the expansion of fibroblasts and the formation of the fibrotic scar. SCI-induced scar formation interrupts regeneration physically and chemically [38, 39]. This study is the first to address the role of Wnt signaling in scar formation in the injured spinal cord, but is consistent with a recent publication showing that Wnt signaling is critical in the formation of fibrosis in skin, liver, and lung, and that overexpression of the inhibitory ligand Dkk1 is sufficient to reduce fibrosis in the skin [40]. These results suggest a general function of Wnt signaling in fibrosis in both injured CNS and peripheral organs/tissues.
The involvement of Wnt signaling in SCI has been previously reported at different aspects. The expression of Wnt ligands and receptors are upregulated in the injured spinal cord after hemisection or contusion lesions in rats or mice [12–14, 16]. However, the effects of this upregulated Wnt signaling with respect to myelin loss, modulation of axonal regeneration, and neural precursor differentiation are controversial, and may vary with the injury model [16, 18, 19]. Spinal cord compression injury (also referred to as crush injury) that we used in this study has been recognized as a reliable experimental approach in a wide range of SCI studies [32, 34, 41–44]. In this study, the compression injury induced a defined area of fibrosis, which will allow us to examine the effect of Wnt signaling in fibrotic scar formation in future studies.
We observed that the injury-induced Wnt signaling reporter TOPgal activity was transiently present only in a subpopulation of the fibrotic cells at the lesion site. A recent study showed that a subpopulation of pericytes is the main source of the glial-fibrotic scar after dorsal funiculus incision or hemisection [37]. These pericyte-originated cells seem to fill the core of the injury and are negative for GFAP. However, we did not detect TOPgal activation in PDGFRβ(+) pericytes at 7 dpi. The TOPgal reporter is regulated by three sets of Wnt responsive elements in the promoter region, which may visualize only the most intensively activated Wnt signaling in the cells. Therefore, only those cells with high levels of Wnt signaling may transiently become TOPgal positive, which may possibly represent intermediates between pericytes and fibroblasts. Nevertheless, the cellular origins of the TOPgal(+) cells and the role of Wnt signaling in the initiation of the potential transformation from pericytes to fibroblasts after SCI remain to be addressed in future studies.
Supplementary Material
Highlights.
The Wnt/β-catenin signaling reporter TOPgal is transiently activated at the lesion site with a peak on 7 days after compression spinal cord injury in adult mice.
The SCI-induced TOPgal positive cells are fibrotic cells in the core scar region.
Some SCI-induced TOPgal positive fibroblast-like cells are proliferative.
Acknowledgments
T.Y. received a postdoctoral stem cell training fellowship from the Californian Institute for Regenerative Medicine (C.I.R.M.). This work was partially supported by grants from the Shriners Hospitals for Children (86600 to C.Z.) and NIH (1R01DE021696 & 1R01DE026737 to C.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 2.Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells. 2011;29:528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- 5.Wang YZ, Plane JM, Jiang P, Zhou CJ, Deng W. Concise review: Quiescent and active states of endogenous adult neural stem cells: identification and characterization. Stem Cells. 2011;29:907–912. doi: 10.1002/stem.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell. 2008;15:773–780. doi: 10.1016/j.devcel.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Bovolenta P, Rodriguez J, Esteve P. Frizzled/RYK mediated signalling in axon guidance. Development. 2006;133:4399–4408. doi: 10.1242/dev.02592. [DOI] [PubMed] [Google Scholar]
- 9.Hollis ER, 2nd, Zou Y. Expression of the Wnt signaling system in central nervous system axon guidance and regeneration. Front Mol Neurosci. 2012;5:5. doi: 10.3389/fnmol.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez P, Fernandez-Martos CM, Gonzalez-Fernandez C, Arenas E, Rodriguez FJ. Spatio-temporal expression pattern of frizzled receptors after contusive spinal cord injury in adult rats. PLoS One. 2012;7:e50793. doi: 10.1371/journal.pone.0050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Fernandez C, Fernandez-Martos CM, Shields SD, Arenas E, Javier Rodriguez F. Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma. 2014;31:565–581. doi: 10.1089/neu.2013.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strand NS, Hoi KK, Phan TMT, Ray CA, Berndt JD, Moon RT. Wnt/beta-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem Biophys Res Commun. 2016;477:952–956. doi: 10.1016/j.bbrc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollis ER, 2nd, Zou Y. Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc Natl Acad Sci U S A. 2012;109:14663–14668. doi: 10.1073/pnas.1206218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin ZS, Zu B, Chang J, Zhang H. Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res. 2008;30:480–486. doi: 10.1179/174313208X284133. [DOI] [PubMed] [Google Scholar]
- 19.Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien) 2011;153:1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- 20.Bandtlow CE. Regeneration in the central nervous system. Exp Gerontol. 2003;38:79–86. doi: 10.1016/s0531-5565(02)00165-1. [DOI] [PubMed] [Google Scholar]
- 21.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 22.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu Rev Neurosci. 2004;27:341–368. doi: 10.1146/annurev.neuro.27.070203.144340. [DOI] [PubMed] [Google Scholar]
- 25.Akbik F, Cafferty WB, Strittmatter SM. Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity. Exp Neurol. 2012;235:43–52. doi: 10.1016/j.expneurol.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 28.Sharma K, Selzer ME, Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012;237:370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry M, Maxwell WL, Logan A, Mathewson A, McConnell P, Ashhurst DE, Thomas GH. Deposition of scar tissue in the central nervous system. Acta Neurochir Suppl (Wien) 1983;32:31–53. doi: 10.1007/978-3-7091-4147-2_3. [DOI] [PubMed] [Google Scholar]
- 30.Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface--a barrier to successful nerve regeneration? Cell Tissue Res. 2001;305:267–273. doi: 10.1007/s004410100384. [DOI] [PubMed] [Google Scholar]
- 31.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 32.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon PC, Gupta D, Shoichet MS, Tator CH. Clip compression model is useful for thoracic spinal cord injuries: histologic and functional correlates. Spine. 2007;32:2853–2859. doi: 10.1097/BRS.0b013e31815b7e6b. [DOI] [PubMed] [Google Scholar]
- 34.Popovich PG, Tovar CA, Wei P, Fisher L, Jakeman LB, Basso DM. A reassessment of a classic neuroprotective combination therapy for spinal cord injured rats: LPS/pregnenolone/indomethacin. Exp Neurol. 2012;233:677–685. doi: 10.1016/j.expneurol.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. Faseb J. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 37.Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 38.Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 39.David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–440. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- 40.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- 42.Zhang Z, Fujiki M, Guth L, Steward O. Genetic influences on cellular reactions to spinal cord injury: a wound-healing response present in normal mice is impaired in mice carrying a mutation (WldS) that causes delayed Wallerian degeneration. J Comp Neurol. 1996;371:485–495. doi: 10.1002/(SICI)1096-9861(19960729)371:3<485::AID-CNE10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Schultke E, Kendall E, Kamencic H, Ghong Z, Griebel RW, Juurlink BH. Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma. 2003;20:583–591. doi: 10.1089/089771503767168500. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.