Abstract
Mitochondria are dynamic organelles that undergo transport, fission and fusion. The three main functions of mitochondria are to generate ATP, buffer cytosolic calcium and generate reactive oxygen species. A large body of evidence indicates that mitochondria are either primary targets for neurological disease states and nervous system injury, or are major contributors to the ensuing pathologies. However, the roles of mitochondria in the development and regeneration of axons have just begun to be elucidated. Advances in the understanding of the functional roles of mitochondria in neurons had been largely impeded by insufficient knowledge regarding the molecular mechanisms that regulate mitochondrial transport, stalling, fission/fusion and a paucity of approaches to image and analyze mitochondria in living axons at the level of the single mitochondrion. However, technical advances in the imaging and analysis of mitochondria in living neurons and significant insights into the mechanisms that regulate mitochondrial dynamics have allowed the field to advance. Mitochondria have now been attributed important roles in the mechanism of axon extension, regeneration and axon branching. The availability of new experimental tools is expected to rapidly increase our understanding of the functions of axonal mitochondria during both development and later regenerative attempts.
Keywords: organelle, mitochondrion, growth cone
INTRODUCTION
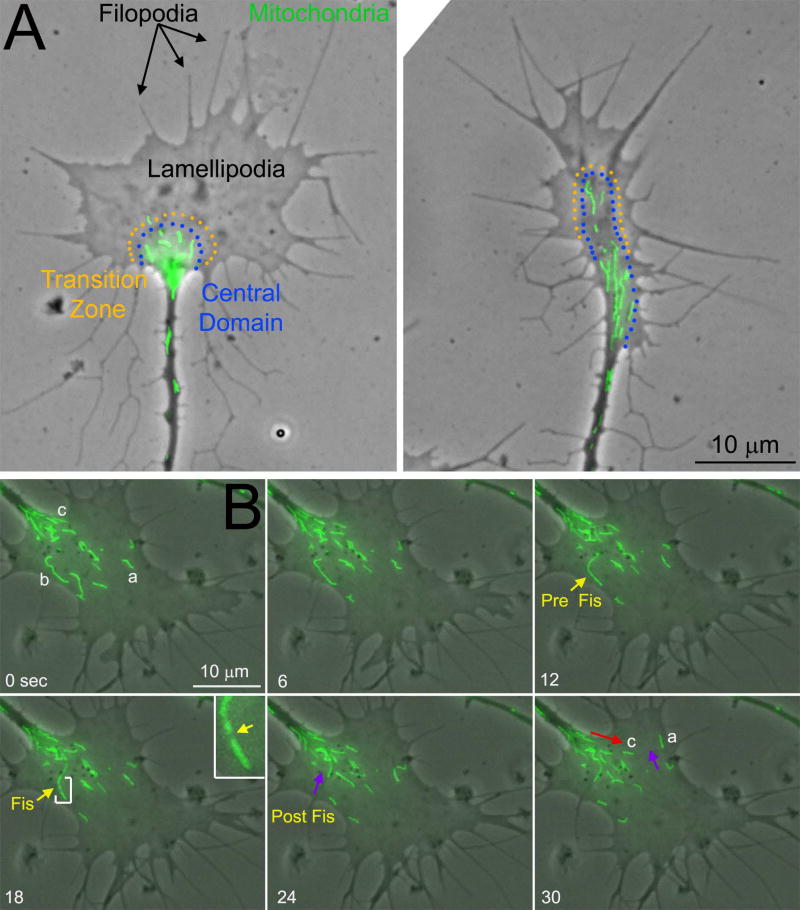
The cytoskeletal and signaling aspects of the mechanism of axon extension and regeneration are fairly well understood (reviewed in: Dent and Gertler, 2003; Menon and Gupton, 2016). Axon extension and regeneration are considered to be processes occurring at the distal tip of the axon. During development the distal tip often exhibits a clearly defined growth cone. Growth cones are described as consisting of two main domains, the “peripheral” (P) and “central” (C) domains, respectively separated by a “transition” zone (Figure 1A). The P-domain is dominated by actin filaments that support the protrusion and dynamics of lamellipodia and filopodia, veil-like and finger-like cellular protrusions respectively (Figure 1A). Lamellipodia and filopodia are necessary components of the mechanism of growth cone guidance and actin filaments contribute to the rate of axon advance. The C-domain contains lower densities of actin filaments and it is generally considered to be involved in substratum attachment. The plus tips of microtubules emanating from the axon shaft predominate in the C-domain. Microtubule plus tips are highly dynamic undergoing repeated bouts of polymerization and depolymerization allowing the tips to probe the intracellular environment of the C- and P-domains. The polymerization of microtubules in the growth cone, and to some extent the transport of short microtubules through the axon, are required for axon extension. Interactions between microtubules and actin filaments mediate aspects of axon extension and guidance, and extracellular signals regulate the growth cone cytoskeleton through the regulation of multiple signaling pathways.
Figure 1.
Growth cone morphology and mitochondria. (A) The two panels show examples of the growth cones of cultured chicken sensory neurons (phase contrast and Mitotracker Green labeled mitochondria). The approximate location of the central (C) domain and transition zone of the growth cone are denoted by the lines of orange and blue dots, respectively. In these examples, the C-domain and transition zone are approximated for didactic presentation using these phase contrast images, as direct determination of the extent of these two components of the growth cones requires imaging of the cytoskeleton. The peripheral (P) domain of the growth cone extends distal to the C-domain and consists of lamellipodia and filopodia. The mitochondria reside in the C-domain and are not usually observed to penetrate the P-domain. (B) Examples of the dynamics of mitochondria in growth cones. Phase contrast and Mitotracker Green labeled mitochondria are shown in these panels for a time lapse imaging sequence. Three mitochondria labeled a-c are used to show the behaviors of mitochondria in growth cones and referred to as Ma, Mb, and Mc (M for mitochondria). Ma can be noted to undergo changes in morphology from bent (0–6 sec) to a more linear appearance as it eventually undergoes “retrograde” transport away from the leading edge of the growth cone (purple arrow at 30 sec). Mb undergoes fission between 12–24 sec and the more proximal emergent mitochondria then undergoes retrograde transport (purple arrow at 24 sec). The inset at 18 sec shows a 3× magnified view of Mb (region bracketed by white lines) and the yellow arrow in the inset points to the site of fission. Mc undergoes “anterograde” transport from its initial position at the base of the growth cone toward the leading edge (red arrow).
In contrast to the role of the cytoskeleton and signaling pathways, the role of cellular organelles in axon extension and growth cone dynamics is poorly understood. Growth cones have long been known to contain multiple classes of vesicles, endoplasmic reticulum and mitochondria in vivo and in vitro (Yamada et al., 1971; Tennyson, 1970). Mitochondria are found throughout the axon and in the C-domain of the growth cone of developing axons (Figure 1A). Mitochondria arrive at the growth cone through anterograde transport, undergo movement and shape changes within the growth cone, exhibit fission and fusion, and can be retrogradely evacuated from the growth cone (Figure 1B shows examples of mitochondria behaviors in a growth cone). Pioneering studies of the localization of mitochondria to growth cones indicate that when the axon is prevented from undergoing forward extension mitochondria are transported away from the distal axon into the more proximal axon (Morris and Hollenbeck, 1995). This review will focus on recent emerging evidence for fundamental roles of mitochondria in regulating axon extension, regeneration and branching (also see the recent review by Sheng (2017)).
The main function of mitochondria addressed by this review is the generation of ATP through oxidative phosphorylation (i.e., aerobic mitochondrial respiration). The generation of ATP by mitochondria is likely to be critical for the actin filament dynamics that characterize growth cones. Actin filaments are formed from the nucleation and subsequent polymerization of actin monomers into filaments (for an overview of actin biology see Gallo, 2017). Actin must be ATP loaded in order to undergo polymerization and nucleation. Following incorporation into a filament the ATPase activity of actin hydrolyzes ATP to ADP, and most actin monomers in the main shaft of filament are ADP bound. Actin filaments undergo rapid turnover as the actin subunits are released from filaments for subsequent reutilization in nucleation and polymerization. In growth cones this process is considered to predominate in the transition zone. Once released in ADP-bound form the actin monomer is rapidly reloaded with ATP. Actin filaments in the P-domain undergo polymerization in the vicinity of the leading edge of lamellipodia and filopodia but are then moved by myosin-driven retrograde flow toward the C-domain, wherein the filaments are depolymerized. Estimates of the role of actin filament turnover in developing neurons in vitro indicate that up to 50% of cellular ATP is utilized by the turnover of actin filaments (Bernstein and Bamburg, 2003). The source of ATP utilized by actin filament turnover (i.e., oxidative phosphorylation or glycolysis) is not known. Due to their positioning within growth cones, mitochondria are ideally suited to regulate actin filament turnover by generating the ATP required to reload actin monomers that are being recycled.
Imaging mitochondria
Mitochondria were initially identified in growth cones through transmission electron microscopy (TEM) and live cell imaging using video enhanced contrast differential interference microscopy (VEC-DIC; Tennyson, 1970; Bunge, 1973; Goldberg and Burmeister, 1986; Burmeister et al., 1988). While TEM has the advantage of providing ultrastructural information it only provides snapshots in time. In contrast, VEC-DIC allows for live imaging but the interpretation that what is being observed is a mitochondrion relies on definitional parameters. Direct imaging of mitochondria is now possible through the use of fluorescent labeling approaches. There are a number of fluorescent dyes that are cell permeable and target specifically to mitochondria (Wang and Schwarz, 2009; Kilgore et al., 2013). These dyes are easy to use and when used at the appropriate concentrations are very specific for mitochondria. The identification of sequences in mitochondrial membrane proteins that target the proteins into the outer or inner mitochondrial membrane have also provided a platform for the generation of genetically encoded fluorescent proteins and biosensors that specifically target to mitochondria (De Michele et al., 2014). These genetic tools have been particularly useful in labeling mitochondria in living animals. Both of these fluorescence-based approaches are now standard in the field.
The healthy mitochondrion has a highly hyperpolarized membrane potential relative to the plasma membrane and other organelles. Rhodamine based positively charged dyes (e.g., TMRE, tetramethylrhodamine ester) are specifically incorporated into mitochondria due to charge-based interactions driven by the strong hyperpolarization of the mitochondrial membrane. Mitochondria with depolarized potential uptake less TMRE per unit time than those with hyperpolarized potential. TMRE is thus an indirect indicator of the relative potential of the mitochondrial membrane and decreases and increases in dye uptake report on membrane depolarization and hyperpolarization, respectively. JC-1 is another dye that targets to the mitochondrion are is used as a reporter for membrane potential. This dye incorporates into mitochondria and exhibits a shift in fluorescence as a function of membrane potential that drives the aggregation of the dye. Ratiometric measurement of the two fluorescence intensities is then used as a metric of relative membrane potential. Both of these dyes are well suited to live imaging and report on change in membrane potential as a function of experimental manipulation. Similarly, the relative levels of reactive oxygen species generated by mitochondria can be monitored with mitochondrially targeted dyes that undergo changes in fluorescence upon oxidation. MitoSOX is a dye that specifically reacts with superoxide and has been widely used along the lines discussed above. Rhod2 is a dye that preferentially labels mitochondria and upon calcium binding exhibits strong increases in fluorescence. However, the dye is not ideal for specifically addressing mitochondrial calcium in neurons. Although mitochondrial calcium signals can be extracted from images, the dye also shows cytoplasmic staining (e.g., Peng et al., 1998; Peng and Greenamyre, 1998; Urushitani et al., 2001), particularly upon cytosolic calcium elevations, and the dye may also report on mitochondrial zinc levels (Sensi et al., 2000). However, genetically encoded calcium and ROS sensors that specifically target to mitochondria have been developed and these tools are applicable to neurons. Killer red is an engineered fluorescent protein that upon illumination generates large amounts of ROS (Sano et al., 2014). This protein has been targeted to mitochondria and used to locally ablate mitochondria in axons (Spillane et al., 2013; Ashrafi et al., 2014), providing a method for subcellular analysis of mitochondrial function. In conclusion, the development of both dye-based and genetically encoded sensors has opened the gateway to detailed analysis of mitochondrial dynamics, morphology and function in living neurons. For additional information on approaches to imaging mitochondria the reader is directed to the following review on the topic (Wang and Schwarz, 2009).
The dynamics of the axonal transport of mitochondria
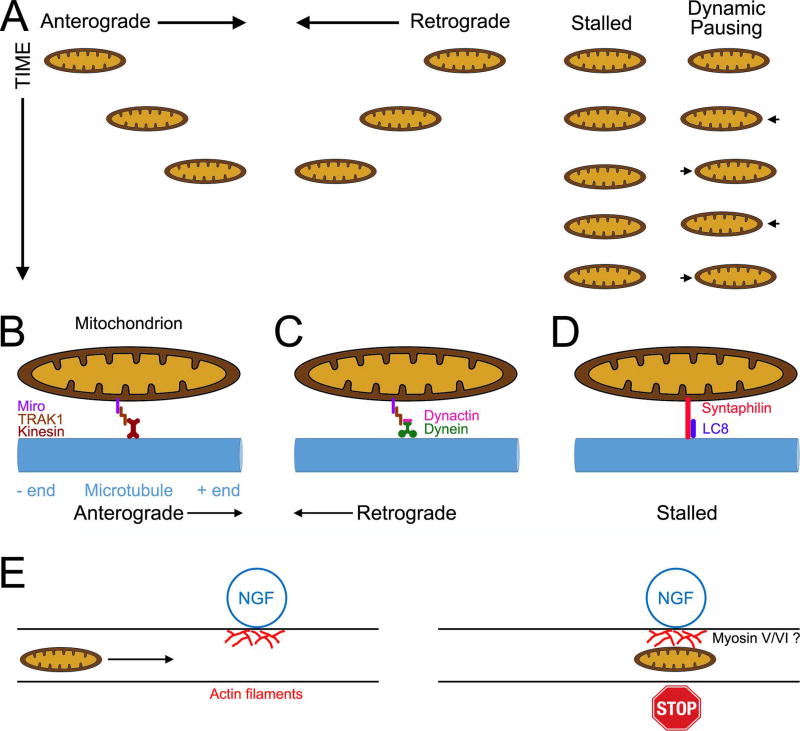
The transport of mitochondria into axons was initially studied in vivo using the same approach as used to track other forms of axonal transport (i.e., determining the distribution of radioactively labeled proteins as they move from the cell body into the axons using biochemical methods). These early studies revealed that mitochondrial proteins, as reporters for mitochondria, moved into axons and underwent net transport at a rate representative of the upper limit of the rates of slow transport but still much lower than the rates of fast transport (e.g., Jeffrey et al., 1972). The availability of the reagents discussed in the prior section was the technical leap forward that allowed direct live imaging of mitochondria transport in living axons in vitro and in vivo, thereby providing a new understanding of how mitochondria undergo transport in axons. As it has now also been established for classic slow transport cargoes (reviewed in Black, 2016), the rate of mitochondrial transport detected in vivo using the classical approaches is likely due to the intermittent transport of mitochondria that when moving however move at fast transport rates. Only a subpopulation of mitochondria (∼30–40%) in axons is motile at any given time in vivo and in vitro (see Chen et al., 2016 for the most detailed analysis of axonal mitochondrial dynamics to date). Motile mitochondria undergo directed runs of varying duration and distance in either the anterograde or retrograde directions (Figure 2A shows a summary of the types of mitochondrial motility), and mitochondria can undergo switches in the directionality of transport while moving. Another form of mitochondrial behavior within the axon has been described as “dynamic pausing” (Chen et al., 2016), reflecting repeated cycles of minor anterograde and retrograde displacements in the position of the mitochondrion without net movement. Mitochondria in the dynamic pausing state have a greater propensity to subsequently engage in long duration directed movement (Chen et al., 2016).
Figure 2.
Overview of the mechanism of the axonal transport and stalling of mitochondria. (A) Schematic summary of the types of mitochondrial behaviors in axons. Mitochondria can undergo runs in both the anterograde and retrograde directions, remained stalled in place with no detectable movement or exhibit dynamic pausing wherein the mitochondrion undergoes “wobbling” between short movements in opposite directions. (B) Machinery of anterograde transport. The mitochondrial outer membrane protein Miro scaffolds Milton/TRACK proteins to the mitochondrial surface. Milton/TRACK in turn engage the kinesin motor proteins responsible for the anterograde microtubule + end directed transport. (C) Machinery of retrograde transport. Similar to its role in anterograde transport, the Miro-Milton/TRAK complex binds the retrograde motor protein complex consisting of dynain and dynactin. (D) Machinery underlying microtubule based stalling of mitochondria. The mitochondrial outer membrane protein syntaphilin directly links mitochondria to microtubules through a microtubule binding domain. LC8 promotes the interaction of syntaphilin with microtubules. (E) Localized signals that locally generate accumulations of actin filaments trap and stall en passant mitochondria in a filament dependent manner. Although not directly addressed, myosin V and VI likely mediate this interaction (see main text).
In vivo and in vitro 60–70% of axonal mitochondria are stalled and do not undergo movement for long time periods (Figure 2A). Analysis of the position of stalled mitochondria in the axons of cultured sensory neurons indicates that the population of stalled mitochondria can undergo very slow but synchronous anterograde movement during axon extension (Miller and Sheetz, 2006). Similar observations were also obtained in vivo during Drosophila development (Roossien et al., 2013). These observations have been interpreted as reflective of the mitochondria being tethered to the axonal microtubule cytoskeleton that may in turn undergo slow anterograde transport, but additional mechanisms have not been ruled out. A recent study tracked mitochondria movement and stalling in axons over a period of up to 16 hours in cultured hippocampal neurons (Niescier et al., 2016). Interestingly, few mitochondria were observed to leave the cell body and reach the distal axon terminals (1.5 mm or more from the cell body) through anterograde transport. Similarly, few mitochondria were noted to undergo retrograde transport away from the terminals and reach the cell body. In both cases mitochondria exhibited a tendency to stall along the axon. In vivo imaging of dye labeled mitochondria in the adult mouse saphenous peripheral nerve also revealed that a majority of axonal mitochondria are stalled (1.5 hr imaging period) and few are noted to undergo transport (Sajic et al., 2013). Similar observations were obtained through analysis of axonal mitochondria in the intercostal nerves of mice engineered to express mitochondrially targeted fluorescent proteins (Misgeld et al., 2007). In vivo imaging of mitochondria in the axons of developing Zebrafish sensory or dopaminergic central nervous system neurons expressing a mitochondrially targeted fluorescent protein also revealed a large proportion of stalled mitochondria and a smaller population of bidirectionally transported mitochondria (Dukes et al., 2016). Observations in live motor neuron axons in Drosophila larvae expressing mitochondrially targeted fluorescent proteins similarly showed a large proportion of stalled mitochondria (Louie et al., 2008). Furthermore, imaging of mitochondria dynamics in the proximal axons of retinal ganglion cells within the nerve fiber layer of the retina of mice in vivo also revealed a large proportion of stalled mitochondria which increased with the age of the animal (Takihara et al., 2015). Whether similar changes also occur in the distal axons located in the optic nerve remains to be determined. Similarly, analysis of mitochondria transport in cortical neurons in vivo and in vitro also revealed age dependent declines in mitochondrial transport within axons resulting in increased numbers of stalled mitochondria (Lewis et al., 2016). Collectively, these studies indicate that in vivo and in vitro a large proportion of mitochondria are stalled along axons while a smaller population undergoes active transport, and the stalling of mitochondria becomes more pronounced during aging.
Molecular mechanisms of the axonal transport of mitochondria
Mitochondria undergo bidirectional transport along microtubules in axons in both the anterograde and retrograde directions. The transport of mitochondria is powered by ATP generated through mitochondrial respiration and not axonal glycolysis (Zala et al., 2013; Spillane et al., 2013; Chen et al., 2016), unlike the transport of vesicles that is powered by on board glycolysis and not mitochondrial respiration (Zala et al., 2013; Hinckelmann et al., 2016). The kinesin KIF5 and dynein have been identified as the motor proteins responsible for anterograde and retrograde transport, respectively (Lin and Sheng, 2015) (Figure 2B,C). The interaction of KIF5 with the outer mitochondrial outer membrane is mediated by proteins called Miro (1 and 2, atypical Rho-like GTPases) and Milton/TRAK (TRAK 1 and 2 in mammals) (Figure 2B). Miro is a mitochondrial outer membrane protein that interacts with Milton/TRAK1 (Birsa et al., 2013). TRAK1 in turn binds the heavy chain of KIF5, but can also bind dynein, and TRAK2 exhibits preference for binding dynein (Van Spronsen et al., 2013). These interactions can be regulated at the level of Miro post-translational modifications (Chung et al., 2016; Shlevkov et al., 2016). Furthermore, Miro has two calcium sensing EF domains and the binding of calcium results in Miro binding directly to the motor domain of KIF5 preventing motor activity (Wang and Schwarz, 2009b). In cultured rat hippocampal neurons TRAK1 mediates mitochondrial transport into axons and is preferentially targeted to axons (Spronsen et al., 2013; Loss and Stephenson, 2015). However, evidence for additional kinesins and potential adaptors or regulators has also emerged, but these are less understood in neurons (Course and Wang, 2016). The dynein/dynactin complex can also bind the Miro/TRACK complex (Van Spronsen et al., 2013)(Figure 2C), and deletion of Miro was found to partially impair both anterograde and retrograde transport of mitochondria in Drosophila (Russo et al., 2009). Finally, the actin filament associated motor protein myosin 19 has also been determined to associate with mitochondria and may regulate their movement along actin filaments. In a neuron-like cell line (CAD cells), expression of the myosin 19 tail domain, generally considered to be a dominant negative approach for myosin function, decreased the lengths that mitochondria moved during a bout of movement but it did not affect the instantaneous rates of movement (Quintero et al., 2009). This observation suggests that myosin 19 may contribute to the movement of mitochondria along axons in an actin filament dependent manner, but the issue will require additional thorough analysis in primary neurons.
Mechanisms underlying the stalling of axonal mitochondria
As noted above, 60–70% of axonal mitochondria are stalled in place along the axon for prolonged time periods. While the stalling of mitochondria could reflect the inactivity or lack of the association of motor proteins with mitochondria, or the lack of attachment to the cytoskeleton, the identification of molecules that physically tether mitochondria to the cytoskeleton demonstrates that stalling is a direct and regulated process. Syntaphilin is a mitochondrial outer membrane protein that also contains a microtubule binding domain (Lin and Sheng, 2015)(Figure 2D). In syntaphilin knock outs of hippocampal neurons, the proportion of stalled mitochondria is decreased significantly and approximately 75% of axonal mitochondria undergo transport (Kang et al., 2008), compared to 30% in the wild type. The binding of the dynein light chain 8 to syntaphilin enhances mitochondria stalling (Chen et al., 2009). Interestingly, in the context of calcium signaling syntaphilin can also bind kinesin I and sequester the motor protein thereby further contributing to the stalling of mitochondria (Chen and Sheng, 2013). Another calcium dependent mitochondria stalling mechanism involves Miro, which has two EF calcium binding domains. As noted above, the binding of calcium to Miro results in the inhibition of Kinesin I function. Thus, calcium signaling along axons has the potential to regulate the stalling of mitochondria through both syntaphilin and Miro.
Microtubule associated proteins (MAPs) have also been determined to be regulators of the axonal transport of mitochondria. Overexpression of Tau in neuroblastoma N2a neuron-like cells results in the impairment of anterograde mitochondrial movements (Ebneth et al., 1998; Dubey et al., 2008; Stoothoff et al., 2009), an effect attributed to the impairment of kinesin binding to microtubules highly decorated with Tau (Trinczek et al., 1999). Drosophila mutants of the MAP1B homolog were found to exhibit decreased mitochondrial transport with an increase in the number of stalled mitochondria (Bettencourt et al., 2005). In hippocampal mouse MAP1B knock out neurons, the rate of retrograde mitochondrial transport was found to be elevated (Jiménez-Mateos et al., 2006). These studies indicate that continued analysis of the roles of MAPs in the regulation of mitochondrial transport is likely to yield novel observations.
Actin filament based mechanism have also been described as contributing to the regulation of mitochondria transport and stalling of along the axon. Treatment of cultured chicken sympathetic neurons with cytochalasin E, a drug that results in actin filament depolymerization through a direct action on filaments, increased the rates of mitochondria transport in both the anterograde and retrograde direction while also decreasing the proportion of mitochondria undergoing anterograde transport (Morris and Hollenbeck, 1995). Manipulation of the actin filament content of the axons of cultured hippocampal neurons with two different actin targeting drugs also provided evidence for a role of actin filaments in the transport of axonal mitochondria (Ligon and Steward, 2000). This study used cytochalasin D and latrunuclin B. Positive controls revealed that cytochalasin D resulted in decreased actin filament content in axons. However, axons contained numerous filament aggregates along their length, an effect well characterized for intermediate doses of cytochalasins (for mechanistic insights into the effects of cytochalasins see Verkhovsky et al., 1999). In contrast, latrunuclin B, which prevents filament polymerization by sequestering actin monomers, abolished actin filaments in axons. Cytochalasin D but not latrunculin B increased the number of stalled mitochondria along axons. This observation was interpreted as suggesting that actin filament aggregates induced by cytochalasin D treatment may somehow block the transport of mitochondria. This notion is consistent with a subsequent study showing that when nerve growth factor (NGF) is locally applied along axons, by using beads coated with covalently attached NGF, mitochondria undergoing transport are captured at the site of bead contact with the axon in an actin filament dependent manner (Chada and Hollenbeck, 2004)(Figure 2E). Consistent with this observation, NGF-beads locally induce actin filament polymerization (Gallo and Letourneau, 1998). Similarly, beads coated with the synaptogenic signal HB-GAM induced localized clustering of mitochondria at sites of contact along the axons of cultured Xenopus spinal neurons (Lee and Peng, 2006). As with the NGF-beads, HB-GAM coated beads also induced localized increases in actin filaments along the axon and depolymerization of actin filaments greatly impaired the localization of mitochondria at sites of axon-bead contact. Furthermore, treatment of cultured hippocampal neurons with brain derived neurotrophic factor (BDNF) increases the percent of stalled mitochondria through a mechanism involving phosphoinositide 3-kinase (PI3K)(Su et al., 2014), similar to the NGF-bead induced PI3K and actin filament dependent stalling (Chada and Hollenbeck, 2004). The BDNF induced stalling was also found to be dependent on calcium and miro. Collectively these studies indicate that although the normal distribution and level of actin filaments along axons has a modest contribution to the regulation of mitochondrial transport, localized accumulations of filaments along axons are involved in the stalling and capture of mitochondria. The mechanisms that could link mitochondria to actin filament-based stalling are not well understood. However, myosin V and myosin VI have been shown to antagonize mitochondria transport in axons in cultured Drosophila neurons (Pathak et al., 2010). Thus, these myosins may serve to tether mitochondria to sites of the axon exhibiting a high content of actin filaments (Figure 2E), but this issue will require further experimental analysis.
The fission and fusion of axonal mitochondria
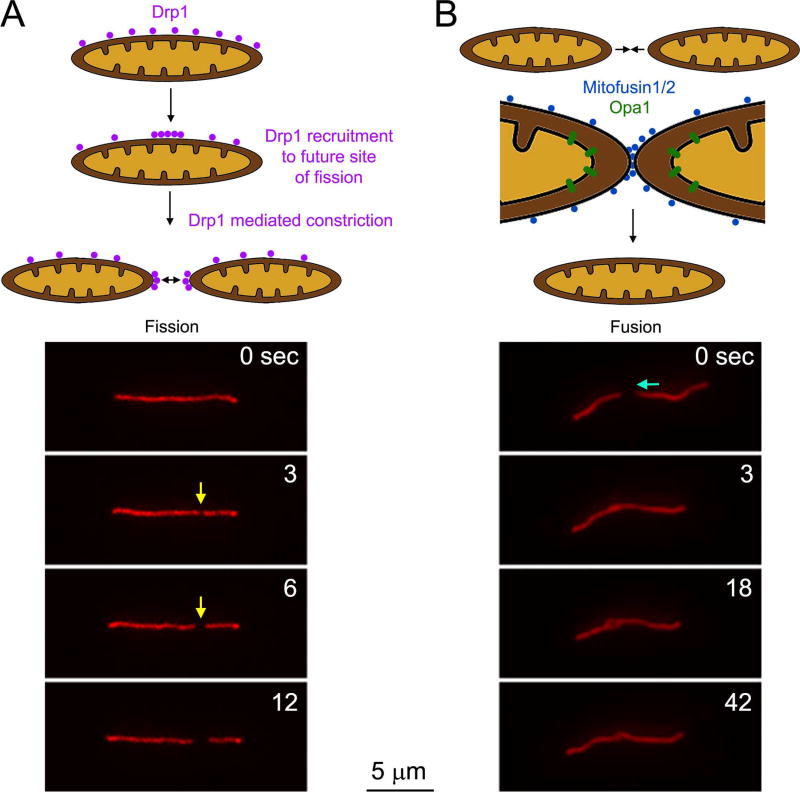
Mitochondria are dynamic organelles that normally undergo fission and fusion in order to maintain steady state mitochondrial lengths and densities, and regulate mitochondrial quality control (i.e., the removal of dysfunctional fragmented mitochondria)(Figure 3). The fission mechanism, resulting in the splitting of a mitochondrion into two, involves the Drp1 GTPase that is considered to form a contractile ring around the mitochondrion resulting in the fission of the mitochondrion at the site of Drp1 accumulation (reviewed in Otera et al., 2013). Drp1 is recruited to the mitochondrion by mitochondrial outer membrane proteins and the mechanism of fission also involves actin filaments, myosin II and contact sites with the endoplasmic reticulum (for a recent review on the mechanisms of fission and fusion see Lee and Yoon, 2016; Figure 3A). Drp1 is generally considered to be the canonical mechanism for regulating mitochondrial fission. However, it is relevant to note that there are reported instances of Drp1 independent mitochondrial fragmentation and changes in length and number (Rival et al., 2011; Stavru et al., 2013) indicating the existence of alternative mechanism for the regulation of mitochondria length and number through fission. Fusion of two mitochondria is mediated by a separate set of proteins that promote the interaction and fusion of the outer mitochondrial membrane (mitofusins) and subsequently the inner membrane (Opa1) (Lee and Yoon, 2016; Figure 3B). Both fission and fusion have been shown to occur along axons and axonal mitochondria are associated with the relevant molecular machinery (Amiri and Hollenbeck, 2008; Yu et al., 2016).
Figure 3.
Overview of the mechanisms of mitochondria fission and fusion. (A) Fission. The Drp1 GTPase associates with the outer membrane of mitochondria through a variety of adaptors (e.g., mitochondrial fission factor, MiD49/51; Lee and Yoon, 2016). Drp1 dynamically redistributes along the mitochondrial surface resulting in localized accumulations that predict the future site of fission. Drp1 oligomers form and ring around the mitochondrion that upon constriction fissions the mitochondrion. An example of fission along the axon of a cultured embryonic chicken sensory neuron expressing mitochondrially targeted DsRed. The yellow arrow denotes the site of fission. At 3 sec a constriction along the mitochondrion is noted which by 6 sec has resulted in fission. (B) Fusion. Fusion is initiate by the close apposition of mitochondrial outer membranes resulting in the engagement of mitofusins from the two contacting surfaces and accumulation and the site of contact. Mitofusins promote the fusion of the outer membranes. Following outer membrane fusion, contact of the inner membranes of the two mitochondria results in engagement of Opa1 proteins that drive the fusion of the inner membrane. As in panel B an example of fusion is shown below the schematic. The blue arrow denotes that the mitochondrion on the right moves toward the one on the left between 0–3 sec. The ends of the two mitochondria then spatially overlap and undergo apparent full fusion by 42 sec. (The time lapse sequences of mitochondrial fission/fusion in the axons of chicken sensory axons were provided by L. Armijo-Wingart in the laboratory of G. Gallo).
The importance of the balance between fission and fusion in the regulation of axonal mitochondria is underscored by the observation that the transport of axonal mitochondria is inversely proportional to the length of the mitochondrion in vitro and in vivo (Misgeld et al., 2007; Narayanareddy et al., 2014). Genetic deletion of Drp1 results in abnormally long mitochondria that have greatly decreased probability of transport within the axon and also decreased rates of movement when moving (Berthet et al., 2014). Inhibition of mitochondria fission prior to axon formation results in abnormally long mitochondria that fail to populate the axon (Steketee et al., 2012; Spillane et al., 2013). However, axons can still form and extend with a greatly reduced, but not abolished, mitochondrial content. When Drp1 function is inhibited using the drug mDivi-1 (Cassidy-Stone et al., 2008) rat retinal ganglion cell form and extend axons of normal lengths (Steketee et al., 2012). Culturing chicken sensory neurons in the continuous presence of mDivi-1, at the same doses as the study addressing retinal ganglion cells, results in axons with lengths approximately half that of control axons (Spillane et al., 2013). Thus, it appears that the extension of axons by different neuron types may have differential sensitivity to the reduction of mitochondrial axonal content resulting from inhibition of mitochondrial fission.
The role of mitochondria in growth cone dynamics
Distal axons contain higher densities of mitochondria that correlate with the state of axon extension, and mitochondria localized at the growth cone are more hyperpolarized than along the axon shaft (Morris and Hollenbeck, 1993; Dedov et al., 2000; Verburg and Hollenbeck, 2008; Ketschek and gallo, 2010). When axon extension is blocked by physical constraints or depolymerization of actin filaments mitochondria undergo redistribution away from the distal axon into the more proximal axon (Morris and Hollenbeck, 1993). Analysis of mitochondrial distribution and transport in cultured rat hippocampal neurons undergoing the initial stages of axon specification revealed that mitochondria exhibit preferential transport into the nascent axon after the axon has elongated beyond the length of the other processes emanating from the cell body (Ruthel and Hollenbeck, 2003), which are destined to mature into dendrites. The same study also found that axonal mitochondria were preferentially targeted into axon branches in a manner that positively correlated with whether the branch was currently undergoing extension. Collectively, these studies indicate that mitochondria are targeted into axons and branches in a manner correlating with the growth state of the process. The mechanism underlying the preferential targeting of mitochondria into actively extending processes remains to be uncovered, but may involve AMP-activated protein kinase (Tao et al., 2014; Cunniff et al, 2016).
The growth cones of extending axons are highly dynamic and characterized by frequent cycles of extension and retraction of filopodia and lamellipodia, protrusive structures strictly dependent on actin filament polymerization and turnover. To our knowledge, there have been no detailed studies using high spatio-temporal resolution to address the relationship between mitochondria positioning and/or function within growth cones and growth cone dynamics. However, studies of the motility and lamellipodia of non-neuronal cells provide observations to consider as the field addressing growth cones moves forward. The role of mitochondria targeting in the context of cell motility has recently received attention in the context of metastatic mechanisms. Increased expression and activation of Drp1 in metastatic breast cancer cells results in smaller more motile mitochondria that, as evidenced by interfering with Drp1 function, promote the cell’s metastatic potential (Zhao et al., 2013). NF-κB-inducing kinase has similarly been found to activate Drp1 and thereby promote mitochondria redistribution to the leading edges of migrating cancer cells (Jung et al., 2016). Invadopodia are protrusive structures used by migrating cancer cells to penetrate tissues and establish new sites of tumor growth (Seano and Primo, 2015). Invadopodia share structural and molecular similarities with neuronal growth cones (Santiago-Medina et al., 2015). The targeting of mitochondria to invadopodia has been described and attributed a necessary function in the invasive potential of cancer cells (Arismendi-Morillo et al., 2012; Cunniff et al., 2016). A recent study presented evidence that syntaphilin is downregulated in a variety of cancer cell types resulting in the promotion of mitochondria targeting to the leading edges of migrating cells and promoting their migration and invasiveness (Caino et al., 2016). Thus, emerging evidence from the analysis of cancer cells highlights a role for mitochondrial positioning in regulating cell migration and invasiveness.
While similar studies addressing the role of mitochondrial dynamics in growth cone motility are overall lacking, Steketee et al (2012) addressed the role of mitochondria fission in the protrusive dynamics of lamellipodia and filopodia at the growth cones of cultured rat retinal ganglion cell axons. The main approach was to acutely inhibit Drp1 function, thereby inhibiting fission, using mDivi-1 (see prior section on fission). Treatment with mDivi-1 resulted in elongation of mitochondria within 20 min of treatment, indicating that fission had been impaired. By 20 min of treatment with mDivi-1 the rate of axon extension was decreased. Interestingly, mDivi-1 treatment decreased the rates of lamellipodial protrusion, but not filopodial protrusion. All observed effects were reversible by washout of mDivi-1. These data indicate that maintenance of the balance of fission and fusion is required for normal growth cone dynamics and axon extension on a relatively short time scale (i.e., 20 min). Furthermore, disruption of the balance between fission and fusion by inhibiting Drp1 or overexpression of mitofusin-2 also resulted in guidance errors when the axons were challenged with choices between substrata. However, this study did not address how mitochondrial behavior at the growth cone was changed by acute inhibition of fission (e.g., localization of mitochondria in the growth cone and their dynamic redistribution within the growth cone), or how mitochondria fission/fusion/redistribute within the growth cone during guidance decisions. Addressing these question could yield insights into how fission may specifically contribute to lamellipodial but not filopodial protrusion, and how it may contribute to guidance decisions made by growth cones.
Enhancing mitochondria bioenergentics and/or transport facilitates axon regeneration
Axonal regeneration is an energy intensive process requiring not only the resealing of the cut membrane, formation of a new growth cone and mobility, it also requires the activation of a growth program that increases synthesis of new proteins, transport, and assembly to support continued cytoskeleton reorganization to promote axon extension and sprouting. This process is thought to recapitulate many of the active growth processes involved in axon growth during development, with the caveat that regeneration in the adult is less efficient in providing both intrinsic as well as extrinsic growth responses. Likewise, as neurons mature they show a progressive decrease in mitochondrial mobility (Milde et al., 2015; Lewis et al., 2016). In non-injured peripheral nerves mitochondria are mostly stationary showing some but little anterograde or retrograde transport, with the majority of mobile mitochondria being smaller than the stationary one (Misgeld et al., 2007). In regenerating axons after nerve injury, anterograde and retrograde transport of mitochondria increased within hours and was sustained for more than 21 days (Misgeld et al., 2007). These transport rates also showed an anterograde bias, with mitochondria extending into the growth cone as previously observed with growing axons in vitro. Lesions to non-regenerating sensory axons within the spinal cord do not result in similar increases in regeneration and at early time points axonal transport of mitochondria remained unchanged or slightly decreased (Mar et al., 2014). All sensory axons have both a peripheral and central branch, in which pre-injury to the peripheral branch, but not the central branch, increases regeneration of both (Wujek and Lasek 1983). This is known as the conditioning lesion response and it is well known to increase the rate of general axonal transport (Wujek and Lasek 1983; Oblinger and Lasek, 1988). Similarly this conditioning response increases the transport of mitochondria both within the regenerating central and peripheral branches, initially with an anterograde bias followed after a slight delay with increased retrograde transport (Mar et al., 2014).
A recent study in C. elegans showed that regenerating axons have a higher density of mitochondria within the growth region than non-regenerating axons (Han et al., 2016). Manipulation of mitochondrial density within these injuried axons greatly influenced their regenerative ability, in which increasing mitochondrial density within the axons promoted regeneration, whereas reducing their density inhibited axonal regeneration. In rodent models, the knockout of the mitochondria stop signaling protein syntaphilin (Zhou et al., 2016) was shown to enhance regeneration of sciatic nerve axons; where as, over-expression of the Armadillo Repeat Containing X-Linked 1 (Armcx1) protein (Cartoni et al., 2016), which is thought to aid tethering of the mitochondrial membrane with the Miro1 adaptor protein, enhanced optic nerve regeneration after injury. In the latter paper, overexpression of Armcx1 was show to promote axonal regeneration alone as well as further enhanced the general regenerative ability of neurons after knockdown of PTEN, a regeneration inhibitory signaling protein, indicating an important role of mitochondria in the regenerative growth process within the CNS. Whether or not knockout of syntaphilin will promote regeneration within the injured CNS needs to be examined.
One of the primary roles of mitochondria is to produce ATP and altering mitochondria respiration and ATP production slightly increased peripheral nerve regenerative ability (Han et al., 2016; Zhou et al., 2016). Reducing mitochondrial respiration reduced axonal regeneration in C. elegans (Han et al., 2016); whereas, syntaphilin knockout not only enhanced mitochondrial migration into the distal lesioned sciatic nerve axons but was also associated with increased ATP production. Indeed, increasing mitochondrial respiration by overexpressing a mutant form of STAT3 missing the DNA binding domain and containing a mitochondrial location signal peptide showed increased ATP production, membrane potential and complex IV interaction. Overexpression of this mutant STAT3 failed to promote regeneration alone, but enhanced retinal ganglion axon regeneration after optic nerve crush in combination with constitutively active STAT3 (Luo et al., 2016). Together these studies support the need for increased energy requirements as being an essential component for successful axonal regeneration.
The role of mitochondria in axon branching
While the role of mitochondria in the regulation of growth cone dynamics remains a largely unexplored venue, multiple recent studies have shed insight into the role of axonal mitochondria in the regulation of axonal protrusive dynamics and the emergence of axon collateral branches. Axon collateral branches arise de novo from the main axon shaft. They are initiated by localized protrusive activity in the form of filopodia, or sometimes lamellipodia, that subsequently become invaded by microtubules and mature into a branch with its own independent growth cone (reviewed in Kalil and Dent, 2014; Pacheco and Gallo, 2016). The localization of the emergence of axonal filopodia along axons is largely dependent on the positioning and respiration of stalled mitochondria (Spillane et al., 2013; Tao et al., 2014; Sainath et al., 2016). Axonal filopodia arise from transient precursor structures termed axonal actin patches that represent small meshworks of filaments from which the filopodium arises (Spillane et al., 2011). The formation of axonal actin patches requires mitochondrial respiration and patches that form preferentially at axonal sites populated by mitochondria exhibit longer durations when associated with mitochondria (Ketschek and Gallo, 2010; Sainath et al., 2016). Live imaging of axon branch formation and mitochondria distribution along cultured sensory and dentate granule cell axons revealed that branches arise from sites populated by stalled mitochondria (Spillane et al., 2013; Tao et al., 2014). Also, localized application of NGF to sites of the axon that do not contain mitochondria results in the trapping of mitochondria undergoing transport into these sites (Chada and Hollenbeck, 2004) and the same application of NGF also promotes axon branching (Gallo and Letourneau, 1998). Importantly, decreasing the density of axonal mitochondria or minimizing their stalling by knocking down syntaphilin impaired branching in cultured sensory and cortical neurons respectively (Courchet et al., 2013; Spillane et al., 2013). In vivo knock down of syntaphilin also impaired the branching of cortical axons (Courchet et al., 2013). The mechanism of mitochondria stalling along cortical axons involves signaling by LKB1-NUAK1 kinases through still to be defined downstream targets (Courchet et al., 2013).
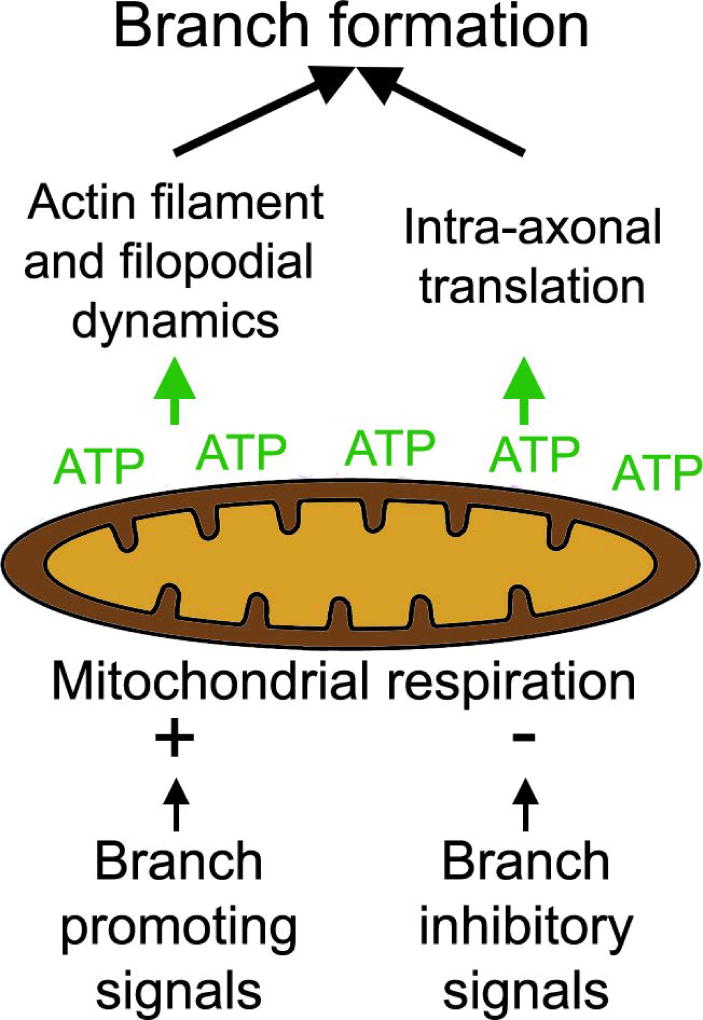
NGF-induced branching along sensory axons requires the intra-axonal translation of actin regulatory proteins (Spillane et al., 2012, 2013; Donnelly et al., 2013). An additional role for stalled mitochondria in NGF-induced branching is to define sites of the axon of preferential intra-axonal protein synthesis in a manner dependent on mitochondrial respiration (Spillane et al., 2013). Interestingly, NGF treatment also results in hyperpolarization of the membrane potential of axonal mitochondria (Verburg and Hollenbeck, 2008), and similarly BDNF increases mitochondrial respiration in synaptosomal preparations (Markham et al., 2012). The regulation of mitochondrial respiration is also involved in the suppression of axon branching by chondroitin sulfate proteoglycans (CSPGs), extracellular matrix components that inhibit axon branching and regeneration. CSPGs do not affect mitochondrial density and transport within the axon but depolarize the mitochondrial membrane potential (Sainath et al., 2016). This effect of CSPGs on mitochondrial respiration suppresses the formation of axonal actin patches and the probability that a patch will give rise to a filopodium, as evidenced by restoration of these parameters by driving mitochondrial respiration using acetyl-L-carnitine. Similarly, the intra-axonal translation of the branching promoting actin regulatory protein cortactin is suppressed on CSPGs in a manner reversible by acetyl-L-carnitine treatment. A recent study of dopaminergic substantia nigra neurons revealed a positive correlation between the density of axonal mitochondria and their level of oxidative phosphorylation with their degree of branching (Pacelli et al., 2015). This same study also reports that semaphorin 7A, which suppresses axon branching, decreases mitochondria respiration as also observed for CSPGs. Similarly, another semaphorin that inhibits axon branching (semaphorin 3A; Dent et al., 2004) was found to depolarize mitochondria in the axons of cultured sensory neurons when applied globally (Verburg and Hollenbeck, 2008). Surprisingly, when NGF and semaphorin 3A were applied locally along axons using covalently coated beads both resulted in hyperpolarization of axonal mitochondria in the vicinity (<10 µm) of the source of signal (Verburg and Hollenbeck, 2008). Overall these studies provide evidence that the respiratory function of mitochondria is subject to regulation by extracellular signals that either promote or inhibit axon branching (Figure 4).
Figure 4.
Overview of the role of stalled mitochondria in axon branching. Stalled mitochondria locally regulate actin filament and filopodial dynamics through ATP production. Signals that promote or inhibit branching increase and decrease mitochondrial respiration, respectively. Mitochondrial respiration also promotes mitochondria-associated intra-axonal translation of actin regulatory proteins involved in branching. Both the mitochondria-dependent regulation of actin dynamics and intra-axonal translation are in part regulated by modulation of mitochondrial respiration by signals that control axon branching.
The respiration of mitochondria locally promotes actin filament dynamics and when relevant the intra-axonal translation of actin regulatory proteins required for signal-induced branching. In contrast, inhibition of mitochondrial respiration was found to have no effect on the promotion of microtubule polymerization and targeting of microtubules into axonal filopodia by NGF (Spillane et al., 2013), indicating that at least during the early stages of NGF-mediated induced axons branching mitochondrial respiration does not regulate the effects of NGF on the microtubule cytoskeleton. If and how mitochondrial respiration may contribute to the regulation of the axonal microtubule cytoskeleton in other contexts remains to be determined.
Collectively, these studies determine that the positioning of stalled mitochondria along axons is a component of the mechanism of axon branching. However, it is important to consider that the role of the mitochondrion does not appear to be instructive in determining where axon branches arise. If this where the case axons would generate a number of branches approximating the number of stalled mitochondria, which is certainly not the case as the latter greatly outnumbers the former. Mitochondria are thus best considered to have a permissive role by determining where along the axon branches can form, but ultimately the specific site of branch formation is likely driven by the spatio-temporal convergence of the many molecular mechanisms required to establish a branch (i.e., regulation of microtubule dynamics and stabilization, directed traffic, regulation of actin filament dynamics, etc; Gallo, 2015; Armijo-Weingart and Gallo, 2017).
Concluding statement
Mitochondria are now recognized as fundamental components of axon extension/regeneration and branching. However, our understanding of mitochondrial biology in developing and regenerating axons and their growth cones is just beginning to be elucidated. The field largely remains a frontier and multiple aspects of how mitochondria relate to axon development and regeneration are wide open for investigation. Even basic studies on the specific relationship between mitochondria positioning and movement within growth cones, and how these dynamics impact the localized protrusive function of growth cones, remain to be considered. The literature reviewed herein addressing this issue in non-neuronal cell motility indicates that this is likely to be a fruitful venue of investigation. Furthermore, specific analysis of mitochondrial function in growth cones will require subcellular regulation. The advent of microfluidic chambers that allow specific delivery of reagents to distal axons and growth cones, localized microperfusion and approaches for the localized ablation of mitochondria will be crucial as the field advances. Similarly, although mitochondria have been shown to be under regulation by extracellular signals, how this level of regulation contributes to the extension/regeneration and guidance of axons remains to be elucidated. Mitochondria buffer increases in cytosolic calcium levels by the uptake of calcium into the mitochondrial intermembrane space and the matrix (Bravo-Sagua et al., 2017) and calcium signaling is of great importance to axon extension and guidance (Gomez and Zheng, 2006). However, to our knowledge, there have not been any studies addressing the role of mitochondria in the regulation of calcium dynamics and signaling in growth cones, although the issue has received considerable attention in the context of synaptic function (Raefsky and Mattson, 2017; Todorova and Blokland, 2017). In addition, mitochondria and the endoplasmic reticulum form a functional dyad (Filadi et al., 2017). The endoplasmic reticulum is found throughout axons and at the growth cone, and accumulates at branch points (Spillane et al., 2013). Analysis of the interplay between these organelles in growth cones and during axon branching will likely be a fruitful venue of investigation. Furthermore, considering the broader perspective of the bioenergetics of axon extension/regeneration, studies of the role of glycolysis will also be required to complement the emerging role of mitochondria. Evidence indicates developmental differences in the relative proportions of ATP generated by mitochondrial respiration and glycolysis in hippocampal neurons at different developmental stages (Surin et al., 2013). The contributions of ATP generating systems to cellular processes are likely to exhibit subcellular regional specificity as evidenced by the observations that vesicle-associated glycolysis drives the transport of axonal vesicles while mitochondrial respiration drives the transport of mitochondria, as previously described. Ultimately, consideration of the localization and dynamics of ATP generating systems in growth cones will need to be considered and experimental analysis will be required to detail which cellular processes depend specifically on mitochondrial respiration or glycolysis, or both. Finally, transcriptomic analysis of the mRNAs present in axons revealed that a number of nuclear genome encoded mitochondria proteins have axonal mRNAs (Gumy et al., 2011; Aschrafi et al., 2016; Gale et al., 2017). Inhibition of intra-axonal translation impairs mitochondrial function (Hillefors et al., 2007) and axon specific inhibition of the intra-axonal translation of ATP synthase subunit 9 impacts mitochondrial function and decreases axon extension (Natera-Naranjo et al., 2012). Continued analysis of the localized translation of nuclear genome encoded mitochondrial proteins and their role in mitochondrial function and axon extension is expected to yield novel insights. In conclusion, the understanding of mitochondrial biology in the mechanism of axon development and regeneration remains a relatively unexplored frontier.
Acknowledgments
This work was supported by NIH awards to GG (NS095471, NS078030) and grants from Shriners Hospitals for Pediatric Research and DOD (SC140089) to GMS.
Footnotes
The authors do not have any conflicts of interests to disclose.
REFERENCED LITERATURE
- Arismendi-Morillo G, Hoa NT, Ge L, Jadus MR. Mitochondrial network in glioma's invadopodia displays an activated state both in situ and in vitro: potential functional implications. Ultrastruct Pathol. 2012;36:409–14. doi: 10.3109/01913123.2012.694582. [DOI] [PubMed] [Google Scholar]
- Armijo-Weingart L, Gallo G. It takes a village to raise a branch: Cellular mechanisms of the initiation of axon collateral branches. Mol Cell Neurosci. 2016;S1044–7431(16):30209–3. doi: 10.1016/j.mcn.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–70. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A, Kar AN, Gale JR, Elkahloun AG, Vargas JN, Sales N, Wilson G, Tompkins M, Gioio AE, Kaplan BB. A heterogeneous population of nuclear-encoded mitochondrial mRNAs is present in the axons of primary sympathetic neurons. Mitochondrion. 2016;30:18–23. doi: 10.1016/j.mito.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS, Ahmad J, Edwards RH, Sesaki H, Huang EJ, Nakamura K. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci. 2014;34:14304–17. doi: 10.1523/JNEUROSCI.0930-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt da Cruz A, Schwärzel M, Schulze S, Niyyati M, Heisenberg M, Kretzschmar D. Disruption of the MAP1B-related protein FUTSCH leads to changes in the neuronal cytoskeleton, axonal transport defects, and progressive neurodegeneration in Drosophila. Mol Biol Cell. 2005;16:2433–42. doi: 10.1091/mbc.E04-11-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsa N, Norkett R, Higgs N, Lopez-Domenech G, Kittler JT. Mitochondrial trafficking in neurons and the role of the Miro family of GTPase proteins. Biochem Soc Trans. 2013;41:1525–31. doi: 10.1042/BST20130234. [DOI] [PubMed] [Google Scholar]
- Black MM. Axonal transport: The orderly motion of axonal structures. Methods Cell Biol. 2016;131:1–19. doi: 10.1016/bs.mcb.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Bravo-Sagua R, Parra V, López-Crisosto C, Díaz P, Quest AF, Lavandero S. Calcium Transport and Signaling in Mitochondria. Compr Physiol. 2017;7:623–634. doi: 10.1002/cphy.c160013. [DOI] [PubMed] [Google Scholar]
- Bunge MB. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J Cell Biol. 1973;56:713–35. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister DW, Chen M, Bailey CH, Goldberg DJ. The distribution and movement of organelles in maturing growth cones: correlated video-enhanced and electron microscopic studies. J Neurocytol. 1988;17:783–95. doi: 10.1007/BF01216706. [DOI] [PubMed] [Google Scholar]
- Caino MC, Seo JH, Aguinaldo A, Wait E, Bryant KG, Kossenkov AV, Hayden JE, Vaira V, Morotti A, Ferrero S, Bosari S, Gabrilovich DI, Languino LR, Cohen AR, Altieri DC. A neuronal network of mitochondrial dynamics regulates metastasis. Nat Commun. 2016;7:13730. doi: 10.1038/ncomms13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R, Norsworthy MW, Bei F, Wang C, Li S, Zhang Y, Gabel CV, Schwarz TL, He Z. The Mammalian-Specific Protein Armcx1 Regulates Mitochondrial Transport during Axon Regeneration. Neuron. 2016;92:1294–1307. doi: 10.1016/j.neuron.2016.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–6. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Chen YM, Gerwin C, Sheng ZH. Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J Neurosci. 2009;29:9429–38. doi: 10.1523/JNEUROSCI.1472-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sheng ZH. Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol. 2013;202:351–64. doi: 10.1083/jcb.201302040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Li Y, Yang M, Chen X, Chen Y, Yang F, Lu S, Yao S, Zhou T, Liu J, Zhu L, Du S, Wu JY. A new method for quantifying mitochondrial axonal transport. Protein Cell. 2016;7:804–819. doi: 10.1007/s13238-016-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Steen JA, Schwarz TL. Phosphorylation-Induced Motor Shedding Is Required at Mitosis for Proper Distribution and Passive Inheritance of Mitochondria. Cell Rep. 2016;16:2142–55. doi: 10.1016/j.celrep.2016.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–25. doi: 10.1016/j.cell.2013.05.021. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Course MM, Wang X. Transporting mitochondria in neurons. F1000Res. 2016:5. doi: 10.12688/f1000research.7864.1. pii: F1000 Faculty Rev-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniff B, McKenzie AJ, Heintz NH, Howe AK. AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. Mol Biol Cell. 2016;27:2662–74. doi: 10.1091/mbc.E16-05-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele R, Carimi F, Frommer WB. Mitochondrial biosensors. Int J Biochem Cell Biol. 2014;48:39–44. doi: 10.1016/j.biocel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Dedov VN, Armati PJ, Roufogalis BD. Three-dimensional organisation of mitochondrial clusters in regenerating dorsal root ganglion (DRG) neurons from neonatal rats: evidence for mobile mitochondrial pools. J Peripher Nerv Syst. 2000;5:3–10. doi: 10.1046/j.1529-8027.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–27. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–12. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CJ, Park M, Spillane M, Yoo S, Pacheco A, Gomes C, Vuppalanchi D, McDonald M, Kim HH, Merianda TT, Gallo G, Twiss JL. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J Neurosci. 2013;33:3311–22. doi: 10.1523/JNEUROSCI.1722-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey M, Chaudhury P, Kabiru H, Shea TB. Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell Motil Cytoskeleton. 2008;65:89–99. doi: 10.1002/cm.20243. [DOI] [PubMed] [Google Scholar]
- Dukes AA, Bai Q, Van Laar VS, Zhou Y, Ilin V, David CN, Agim ZS, Bonkowsky JL, Cannon JR, Watkins SC, Croix CM, Burton EA, Berman SB. Live imaging of mitochondrial dynamics in CNS dopaminergic neurons in vivo demonstrates early reversal of mitochondrial transport following MPP(+) exposure. Neurobiol Dis. 2016;95:238–49. doi: 10.1016/j.nbd.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998;143:777–94. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filadi R, Theurey P, Pizzo P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium. 2017;62:1–15. doi: 10.1016/j.ceca.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Gale JR, Aschrafi A, Gioio AE, Kaplan BB. Nuclear-Encoded Mitochondrial mRNAs: A Powerful Force in Axonal Growth and Development. Neuroscientist. 2017 doi: 10.1177/1073858417714225. In press. [DOI] [PubMed] [Google Scholar]
- Gallo G. Localized regulation of the axon shaft during the emergence of collateral branches. Neural Regen Res. 2015;10:1206–8. doi: 10.4103/1673-5374.162694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. ACT. In: Choi S, editor. Encyclopedia of Signaling Molecules. 2. Springer International Publishing AG; 2017. In press. [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–14. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DJ, Burmeister DW. Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol. 1986;103:1921–31. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–25. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Gumy LF, Yeo GS, Tung YC, Zivraj KH, Willis D, Coppola G, Lam BY, Twiss JL, Holt CE, Fawcett JW. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SM, Baig HS, Hammarlund M. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron. 2016;92:1308–1323. doi: 10.1016/j.neuron.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillefors M, Gioio AE, Mameza MG, Kaplan BB. Axon viability and mitochondrial function are dependent on local protein synthesis in sympathetic neurons. Cell Mol Neurobiol. 2007;27:701–16. doi: 10.1007/s10571-007-9148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckelmann MV, Virlogeux A, Niehage C, Poujol C, Choquet D, Hoflack B, Zala D, Saudou F. Self-propelling vesicles define glycolysis as the minimal energy machinery for neuronal transport. Nat Commun. 2016;7:13233. doi: 10.1038/ncomms13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey PL, James KA, Kidman AD, Richards AM, Austin L. The flow of mitochondria in chicken sciatic nerve. J Neurobiol. 1972;3:199–208. doi: 10.1002/neu.480030303. [DOI] [PubMed] [Google Scholar]
- Jiménez-Mateos EM, González-Billault C, Dawson HN, Vitek MP, Avila J. Role of MAP1B in axonal retrograde transport of mitochondria. Biochem J. 2006;397:53–9. doi: 10.1042/BJ20060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JU, Ravi S, Lee DW, McFadden K, Kamradt ML, Toussaint LG, Sitcheran R. NIK/MAP3K14 Regulates Mitochondrial Dynamics and Trafficking to Promote Cell Invasion. Curr Biol. 2016;26:3288–3302. doi: 10.1016/j.cub.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, Dent EW. Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat Rev Neurosci. 2014;15:7–18. doi: 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–48. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30:12185–97. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore JA, Dolman NJ, Davidson MW2013. A review of reagents for fluorescence microscopy of cellular compartments and structures, Part II: reagents for non-vesicular organelles. Curr Protoc Cytom. 2013;66 doi: 10.1002/0471142956.cy1231s66. Unit 12.31. [DOI] [PubMed] [Google Scholar]
- Lee CW, Peng HB. Mitochondrial clustering at the vertebrate neuromuscular junction during presynaptic differentiation. J Neurobiol. 2006;66:522–36. doi: 10.1002/neu.20245. [DOI] [PubMed] [Google Scholar]
- Lee H, Yoon Y. Mitochondrial fission and fusion. Biochem Soc Trans. 2016;44:1725–1735. doi: 10.1042/BST20160129. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Jr, Turi GF, Kwon SK, Losonczy A, Polleux F. Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Curr Biol. 2016;26:2602–2608. doi: 10.1016/j.cub.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:351–61. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lin MY, Sheng ZH. Regulation of mitochondrial transport in neurons. Exp Cell Res. 2015;334:35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loss O, Stephenson FA. Localization of the kinesin adaptor proteins trafficking kinesin proteins 1 and 2 in primary cultures of hippocampal pyramidal and cortical neurons. J Neurosci Res. 2015;93:1056–66. doi: 10.1002/jnr.23549. [DOI] [PubMed] [Google Scholar]
- Louie K, Russo GJ, Salkoff DB, Wellington A, Zinsmaier KE. Effects of imaging conditions on mitochondrial transport and length in larval motor axons of Drosophila. Comp Biochem Physiol A Mol Integr Physiol. 2008;151:159–72. doi: 10.1016/j.cbpa.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Ribeiro M, Bray ER, Lee DH, Yungher BJ, Mehta ST, Thakor KA, Diaz F, Lee JK, Moraes CT, Bixby JL, Lemmon VP, Park KK. Enhanced Transcriptional Activity and Mitochondrial Localization of STAT3 Co-induce Axon Regrowth in the Adult Central Nervous System. Cell Rep. 2016;15:398–410. doi: 10.1016/j.celrep.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar FM, Simões AR, Leite S, Morgado MM, Santos TE, Rodrigo IS, Teixeira CA, Misgeld T, Sousa MM. CNS axons globally increase axonal transport after peripheral conditioning. J Neurosci. 2014;34:5965–70. doi: 10.1523/JNEUROSCI.4680-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A, Cameron I, Bains R, Franklin P, Kiss JP, Schwendimann L, Gressens P, Spedding M. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci. 2012;35:366–74. doi: 10.1111/j.1460-9568.2011.07965.x. [DOI] [PubMed] [Google Scholar]
- Menon S, Gupton SL. Building Blocks of Functioning Brain: Cytoskeletal Dynamics in Neuronal Development. Int Rev Cell Mol Biol. 2016;322:183–245. doi: 10.1016/bs.ircmb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde S, Adalbert R, Elaman MH, Coleman MP. Axonal transport declines with age in two distinct phases separated by a period of relative stability. Neurobiol Aging. 2015;36:971–81. doi: 10.1016/j.neurobiolaging.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Direct evidence for coherent low velocity axonal transport of mitochondria. J Cell Biol. 2006;173:373–81. doi: 10.1083/jcb.200510097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat Methods. 2007;4:559–61. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–27. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–26. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanareddy BR, Vartiainen S, Hariri N, O'Dowd DK, Gross SP. A biophysical analysis of mitochondrial movement: differences between transport in neuronal cell bodies versus processes. Traffic. 2014;15:762–71. doi: 10.1111/tra.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natera-Naranjo O, Kar AN, Aschrafi A, Gervasi NM, Macgibeny MA, Gioio AE, Kaplan BB. Local translation of ATP synthase subunit 9 mRNA alters ATP levels and the production of ROS in the axon. Mol Cell Neurosci. 2012;49:263–70. doi: 10.1016/j.mcn.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Niescier RF, Kwak SK, Joo SH, Chang KT, Min KT. Dynamics of Mitochondrial Transport in Axons. Front Cell Neurosci. 2016;10:123. doi: 10.3389/fncel.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. Axotomy-induced alterations in the synthesis and transport of neurofilaments and microtubules in dorsal root ganglion cells. J Neurosci. 1988;8:1747–58. doi: 10.1523/JNEUROSCI.08-05-01747.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833:1256–68. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Pacelli C, Giguère N, Bourque MJ, Lévesque M, Slack RS, Trudeau LÉ. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol. 2015;25:2349–60. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Pacheco A, Gallo G. Actin filament-microtubule interactions in axon initiation and branching. Brain Res Bull. 2016;126:300–310. doi: 10.1016/j.brainresbull.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–92. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng TI, Jou MJ, Sheu SS, Greenamyre JT. Visualization of NMDA receptor-induced mitochondrial calcium accumulation in striatal neurons. Exp Neurol. 1998;149:1–12. doi: 10.1006/exnr.1997.6599. [DOI] [PubMed] [Google Scholar]
- Peng TI, Greenamyre JT. Privileged access to mitochondria of calcium influx through N-methyl-D-aspartate receptors. Mol Pharmacol. 1998;53:974–80. [PubMed] [Google Scholar]
- Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, Lier AJ, Panaretos NS, Slater SQ, Rengarajan M, Feliu M, Cheney RE. Human Myo19 is a novel myosin that associates with mitochondria. Curr Biol. 2009;19:2008–13. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raefsky SM, Mattson MP. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic Biol Med. 2017;102:203–216. doi: 10.1016/j.freeradbiomed.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T, Macchi M, Arnauné-Pelloquin L, Poidevin M, Maillet F, Richard F, Fatmi A, Belenguer P, Royet J. Inner-membrane proteins PMI/TMEM11 regulate mitochondrial morphogenesis independently of the DRP1/MFN fission/fusion pathways. EMBO Rep. 2011;12:223–30. doi: 10.1038/embor.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossien DH, Lamoureux P, Van Vactor D, Miller KE. Drosophila growth cones advance by forward translocation of the neuronal cytoskeletal meshwork in vivo. PLoS One. 2013;8:e80136. doi: 10.1371/journal.pone.0080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci. 2009;29:5443–55. doi: 10.1523/JNEUROSCI.5417-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J Neurosci. 2003;23:8618–24. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainath R, Ketschek A, Grandi L, Gallo G. CSPGs inhibit axon branching by impairing mitochondria-dependent regulation of actin dynamics and axonal translation. Dev Neurobiol. 2017;77:454–473. doi: 10.1002/dneu.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajic M, Mastrolia V, Lee CY, Trigo D, Sadeghian M, Mosley AJ, Gregson NA, Duchen MR, Smith KJ. Impulse conduction increases mitochondrial transport in adult mammalian peripheral nerves in vivo. PLoS Biol. 2013;11:e1001754. doi: 10.1371/journal.pbio.1001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Watanabe W, Matsunaga S. Chromophore-assisted laser inactivation--towards a spatiotemporal-functional analysis of proteins, and the ablation of chromatin, organelle and cell function. J Cell Sci. 2014;127:1621–9. doi: 10.1242/jcs.144527. [DOI] [PubMed] [Google Scholar]
- Santiago-Medina M, Gregus KA, Nichol RH, O'Toole SM, Gomez TM. Regulation of ECM degradation and axon guidance by growth cone invadosomes. Development. 2015;142:486–96. doi: 10.1242/dev.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seano G, Primo L. Podosomes and invadopodia: tools to breach vascular basement membrane. Cell Cycle. 2015;14:1370–4. doi: 10.1080/15384101.2015.1026523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Yin HZ, Weiss JH. AMPA/kainate receptor-triggered Zn2+ entry into cortical neurons induces mitochondrial Zn2+ uptake and persistent mitochondrial dysfunction. Eur J Neurosci. 2000;12:3813–8. doi: 10.1046/j.1460-9568.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- Sheng ZH. The Interplay of Axonal Energy Homeostasis and Mitochondrial Trafficking and Anchoring. Trends Cell Biol. 2017;27:403–416. doi: 10.1016/j.tcb.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlevkov E, Kramer T, Schapansky J, LaVoie MJ, Schwarz TL. Miro phosphorylation sites regulate Parkin recruitment and mitochondrial motility. Proc Natl Acad Sci U S A. 2016;113:E6097–E6106. doi: 10.1073/pnas.1612283113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011;71:747–58. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci. 2012;32:17671–89. doi: 10.1523/JNEUROSCI.1079-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 2013;5:1564–75. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavru F, Palmer AE, Wang C, Youle RJ, Cossart P. Atypical mitochondrial fission upon bacterial infection. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16003–8. doi: 10.1073/pnas.1315784110. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee MB, Moysidis SN, Weinstein JE, Kreymerman A, Silva JP, Iqbal S, Goldberg JL. Mitochondrial dynamics regulate growth cone motility, guidance, and neurite growth rate in perinatal retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 2012;53:7402–11. doi: 10.1167/iovs.12-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoothoff W, Jones PB, Spires-Jones TL, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, Irimia D, Hyman BT. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem. 2009;111:417–27. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Ji YS, Sun XL, Liu XH, Chen ZY. Brain-derived neurotrophic factor (BDNF)-induced mitochondrial motility arrest and presynaptic docking contribute to BDNF-enhanced synaptic transmission. J Biol Chem. 2014;289:1213–26. doi: 10.1074/jbc.M113.526129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surin AM, Khiroug S, Gorbacheva LR, Khodorov BI, Pinelis VG, Khiroug L. Comparative analysis of cytosolic and mitochondrial ATP synthesis in embryonic and postnatal hippocampal neuronal cultures. Front Mol Neurosci. 2013;5:102. doi: 10.3389/fnmol.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takihara Y, Inatani M, Eto K, Inoue T, Kreymerman A, Miyake S, Ueno S, Nagaya M, Nakanishi A, Iwao K, Takamura Y, Sakamoto H, Satoh K, Kondo M, Sakamoto T, Goldberg JL, Nabekura J, Tanihara H. In vivo imaging of axonal transport of mitochondria in the diseased and aged mammalian CNS. Proc Natl Acad Sci U S A. 2015;112:10515–20. doi: 10.1073/pnas.1509879112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K, Matsuki N, Koyama R. AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev Neurobiol. 2014;74:557–73. doi: 10.1002/dneu.22149. [DOI] [PubMed] [Google Scholar]
- Tennyson VM. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol. 1970;44:62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorova V, Blokland A. Mitochondria and Synaptic Plasticity in the Mature and Aging Nervous System. Curr Neuropharmacol. 2017;15:166–173. doi: 10.2174/1570159X14666160414111821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112:2355–67. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- Urushitani M, Nakamizo T, Inoue R, Sawada H, Kihara T, Honda K, Akaike A, Shimohama S. N-methyl-D-aspartate receptor-mediated mitochondrial Ca(2+) overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca(2+) influx. J Neurosci Res. 2011;63:377–87. doi: 10.1002/1097-4547(20010301)63:5<377::AID-JNR1032>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, Jaarsma D, Gerritsen HC, Akhmanova A, Hoogenraad CC. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013;77:485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 2008;28:8306–15. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Polarity sorting of actin filaments in cytochalasin-treated fibroblasts. J Cell Sci. 1997;110:1693–704. doi: 10.1242/jcs.110.15.1693. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. Imaging axonal transport of mitochondria. Methods Enzymol. 2009;457:319–33. doi: 10.1016/S0076-6879(09)05018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009b;136:163–74. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wujek JR, Lasek RJ. Correlation of axonal regeneration and slow component B in two branches of a single axon. J Neurosci. 1983;3:243–51. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Spooner BS, Wessells NK. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971;49:614–35. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Lee HC, Chen KC, Suhan J, Qiu M, Ba Q, Yang G. Inner membrane fusion mediates spatial distribution of axonal mitochondria. Sci Rep. 2016;6:18981. doi: 10.1038/srep18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelières FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–91. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW, Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32:4814–24. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–19. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]