Abstract
Objective
We investigated factors associated with distant recurrence, disease-free survival (DFS), and overall survival (OS) following R0 lobectomy for pathologic node-negative (pN0) lung adenocarcinoma.
Methods
We performed a retrospective analysis of a prospectively maintained database of patients with pT1-3N0M0 non-small cell lung cancer. Exclusion criteria included: metachronous lung cancer, sublobar/incomplete resection, non-adenocarcinoma histology, or induction/adjuvant therapy. The primary outcome was distant recurrence; secondary outcomes were DFS/OS. Associations between variables and outcomes were assessed by Fine and Gray competing risk regression for distant recurrence and Cox proportional hazard models for DFS/OS.
Results
Of 2392 patients identified with pT1-3N0M0 lung adenocarcinoma, 893 met the inclusion criteria. Median follow-up was 35.0 months (range, 0.1–202 months). Thirteen percent of patients developed recurrence (n=115); 86% (n=99) were distant. The 5-year cumulative incidence of distant recurrence was 14% (95% confidence interval [CI], 11%–17%). On multivariable analysis, pT2a (HR, 2.84; 95% CI 1.56–5.16, P=0.001) and pT2b/3 (HR, 6.53; 95% CI, 3.17–13.5; P<0.001) were associated with distant recurrence. Recent surgery was associated with decreased distant recurrence (HR 0.43, 95% CI 0.20–0.91, P=0.028), and lymphovascular invasion (LVI) was strongly associated with distant recurrence (HR, 1.62; 95% CI, 1.00–2.63; P=0.05). DFS was independently associated with pT stage (P<0.001) and lymphovascular invasion (P=0.004).
Conclusion
In patients undergoing R0 lobectomy with pN0 lung adenocarcinoma, pT stage and lymphovascular invasion were associated with distant recurrence and decreased DFS. These observations support the inclusion of these patients in future clinical trials investigating adjuvant targeted and immunotherapies.
INTRODUCTION
Five-year overall survival (OS) is 66% to 82% for patients with surgically resected stage I non-small cell lung cancer (NSCLC) and 47% to 52% for patients with stage II NSCLC.1 The primary factor that contributes to decreased survival is recurrence—3-year disease-free survival (DFS) is 77% to 86% for all stages of NSCLC.2 It is widely accepted that complete resection (R0) offers improved survival over R1 or R2 resection. In patients with stage I NSCLC, 5-year OS increases from 37% for patients with R1 resection to 62% for those with R0 resection.3 For patients with stage II NSCLC, OS increases from 29% for patients with R1 resection to 41% for those with R0 resection.3 In addition, the results of the Lung Cancer Study Group trial support lobectomy over sublobar resection as the preferred extent of resection, even for peripheral T1N0 lesions.4 In that study, local recurrence rates were 3-fold higher for patients who underwent sublobar resection, although distant recurrence rates were the same.5, 6
There is a surprising lack of information regarding the incidence of distant recurrence—the primary driver of both DFS and OS—following R0 lobectomy for pathologic node-negative (pN0) disease. Moreover, evidence-based predictors of development of distant recurrence may support the inclusion of patients with these characteristics in future randomized trials designed to evaluate the role of adjuvant therapies in the treatment of early-stage node-negative lung cancer. To address this knowledge gap, we analyzed clinicopathologic and tumor genomic features associated with distant recurrence, any recurrence, DFS, and OS in a highly curated group of patients who underwent R0 lobectomy for pN0 lung adenocarcinoma.
METHODS
Patient Characteristics
After approval from our Institutional Review Board, we performed a retrospective analysis of our institution’s prospectively maintained database for patients diagnosed with pT1-3N0M0 NSCLC who underwent surgical resection between January 2000 and March 2016. All patients underwent surgery with curative intent. Exclusion criteria included sublobar resection, non-adenocarcinoma histologic subtype, R1 or R2 resection, treatment with any induction or adjuvant therapy, synchronous lung primaries that recurred or were treated non-surgically, or metachronous lung cancer. Metachronous lung cancers were distinguished from recurrent lesions using criteria established by Martini & Melamed and molecular testing, when available.7
Patient demographic characteristics, forced expiratory volume in 1 second (FEV1), diffusion capacity of the lung for carbon monoxide (DLCO), primary tumor maximum standardized uptake value (SUVmax), tumor pathology (American Joint Committee on Cancer 7th edition), and tumor genomic data (EGFR, KRAS) were recorded. A positive EGFR mutation was classified as an activating EGFR mutation (i.e., exon 19 deletion or exon 21 L858R mutation). KRAS mutations were defined as present or absent.
Follow-up
Information on patient follow-up was extracted from the medical record and included medical history, physical examination, and chest CT every 6 months for 2 to 3 years and then annually, in accordance with the National Comprehensive Cancer Network (NCCN) Guidelines.8 The date of the last follow-up was noted, as well as the date of death, if applicable.
For patients who experienced recurrence, the first site of recurrence was classified as either distant, locoregional, or both. Locoregional recurrence (LR) was defined as a relapse in the surgical margin or bronchus staple line or involvement of the ipsilateral mediastinal and/or ipsilateral hilar lymph nodes.9, 10 Distant recurrence included all locations outside of locoregional recurrence and included supraclavicular nodes, contralateral mediastinal or hilar nodes, another lobe in the ipsilateral lung, or any tissue or organ outside of the locoregional recurrence zone. Simultaneous locoregional and distant recurrence was classified as distant recurrence. Recurrence was identified radiographically (PET or CT) and/or by pathologic confirmation.
Pathologic Evaluation
Tumors were classified according to the predominant histologic subtype, in accordance with the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society guidelines, into lepidic (including adenocarcinoma in situ), acinar, papillary, micropapillary, solid, or other (including minimally invasive adenocarcinoma and colloid subtypes).11
Statistical Analyses
Patient demographic and clinical characteristics were summarized with descriptive statistics. Of the 893 patients included, 2 (0.2%) had missing pleural invasion status, 15 (1.6%) had missing lymphovascular invasion (LVI) status, 128 (14%) had missing histologic subtype, 21 (2.3%) had missing FEV1, 330 (37%) had missing EGFR mutation status, 481 (54%) had missing KRAS mutation status, and 172 (19%) had missing primary tumor SUVmax. Covariates, including outcome measures, were used in the imputation of missing data. Variables included in the multiple imputation models were age at surgery, sex, pulmonary comorbidity, cardiac comorbidity, year of surgery (divided into tertiles: 2000–2006, 2007–2013, 2014–2016), primary tumor SUVmax, FEV1, DLCO, tumor size, tumor histologic subtype, LVI, pleural invasion, and pathologic tumor (pT) stage. EGFR and KRAS were not included in the imputation procedure due to excessive missing data points. Based on the recommendation from van der Kruijk (2015), we included the log of duration from surgery to any recurrence (and death without recurrence) or last follow-up, which is available for all patients.12
Five different imputed data sets were created by predictive mean matching using the aregimpute imputation routine in R 3.1.1. (R Development Core Team, Vienna, Austria). Analyses of the survival endpoints were applied to each imputed data set, which were then combined across the data sets using the Rubin rule to obtain overall estimates of each regression coefficient and variance.13 Year of surgery (included in models as tertiles) is an adjustment factor/surrogate for potential changes in clinical practice, especially non-surgical therapies.
The primary endpoint was cumulative incidence of distant recurrence (CIR-distant). Secondary endpoints were cumulative incidence of any recurrence (CIR-any), DFS, and OS. All survival endpoints were estimated from the time of surgery and censored on the date of the last follow-up. Associations between variables and recurrence were analyzed using the competing risk method. For CIR-distant, independent locoregional recurrence and death without any recurrence were considered competing events; for CIR-any, death without any recurrence was the competing event. Differences in CIR-distant and CIR-any between groups were assessed using Gray’s test.14 Associations between variables and hazard of recurrence (distant or any) were estimated using the Fine and Gray competing risk model.15 Multivariable models were constructed by starting with variables with P<0.1 in univariable analyses, keeping age at surgery in the model. Visceral pleural invasion and tumor size were not included in the multivariable model secondary to possible collinearity with pT. All P values were two-tailed, and P<0.05 was considered statistically significant. DFS was defined from the time of surgery to the time of death or recurrence, and OS was defined from the time of surgery to the time of death; both were estimated using the Kaplan-Meier method and compared between groups using the log-rank test. The non-linear relationship between tumor size and distant recurrence (and other time-to-event endpoints) were assessed using restricted cubic splines.16 Goodness of fit was assessed based on modified weighted Schoenfeld residuals to test the proportionality of subdistribution hazards for the Fine-Gray model, appropriate for outcomes with competing risks.16 We examined various functional forms of time including linear, quadratic, and log. Analyses were conducted in R 3.1.1 (R Development Core Team, Vienna, Austria) using the cmprsk package and Stata 13 (StataCorp, College Station, TX).
RESULTS
Patient Population
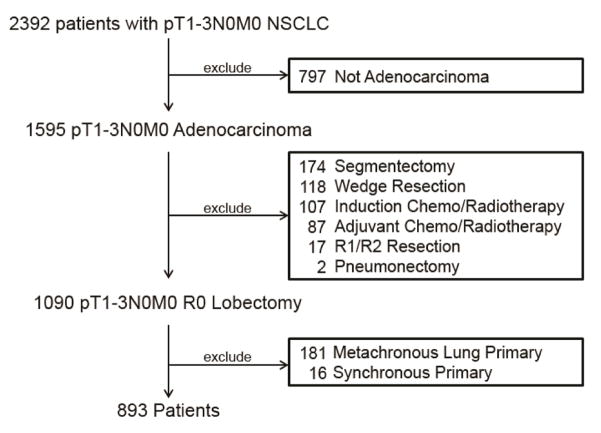
In total, 2392 patients with pT1-3N0M0 NSCLC underwent surgical resection from January 2000 to March 2016. Of these, 893 met the inclusion criteria and were included in the study (Fig. 1).
Figure 1.
CONSORT flow diagram. Original population and exclusion criteria used to determine the final cohort of patients.
Patient clinicopathologic features are summarized in Table 1. Median follow-up was 35.0 months (range, 0.1–202 months). At the conclusion of our analysis, 638 patients (71%) remained alive without recurrence (Table 2). Twenty-seven patients (3%) had no additional follow up beyond 90 days after their surgery. Among the entire cohort, 13% of patients developed recurrence (N=115)—86% of these were pathologically confirmed. In patients with isolated pulmonary recurrence, 19% (8/42) had molecular testing which supported recurrent disease. Most recurrences were distant (N=99). The most common sites of DR were lung (N=42), brain (N=22), and bone (N=19) (Table 2). The median disease-free interval for all patients who developed any recurrence was 21.3 months (range, 0.9–176 months). The median disease-free interval was 26.9 months (range, 2.5–176 months) for pT1a, 28.4 months (range, 7–130 months) for pT1b, 20.5 months (range, 0.9–82 months) for pT2a, and 10.8 months (range, 1.3–57 months) for pT2b/3 tumors. Of the 115 patients who had recurrence, 85 (74%) died (median time to death after recurrence, 18.6 months; range, 0.3–151 months). Of the 99 patients with distant recurrence, 76 (77%) died (median time to death, 15.4 months; range, 0.3–151 months). Of the 16 patients with isolated LR, 9 (56%) died (median time to death was 45 months (range 11.4–107). Of these 9 patients with isolated LR who died, the cause of death was distant disease (N=2), local disease (N=2), and unknown (N=5). Isolated locoregional recurrences were treated with radiation (n=6), concurrent chemoradiotherapy (n=4), surveillance (n=2), re-resection (n=2), or unknown (n=2).
Table 1.
Clinicopathologic Characteristics of pT1-3N0M0 Lung Adenocarcinoma Cohort (N=893)
| Characteristic | Median (Range) or N (%) |
|---|---|
| Age | 72 (36 – 92) |
| Gender | |
| Female | 559 (63%) |
| Male | 334 (37%) |
| Comorbidities | |
| Pulmonary | 220 (25%) |
| Cardiac | 492 (55%) |
| Pulmonary Function | |
| FEV1 (%) (N=872) | 94 (26 – 408) |
| Diffusion (%) (N=799) | 84.0 (24 – 165) |
| Time of Surgery (Tertile) | |
| 2000–2006 | 313 (35%) |
| 2007–2013 | 306 (34%) |
| 2014–2016 | 274 (31%) |
| Tumor Characteristics | |
| Tumor SUV (N=721) | 3.4 (0.5 – 9.8) |
| Tumor Size (cm) | 2.2 (0.2 – 9.0) |
| Predominant Histologic Subtype (N=765) | |
| Lepidic | 113 (15%) |
| Acinar | 361 (47%) |
| Papillary | 144 (19%) |
| Micropapillary | 27 (3.5%) |
| Solid | 109 (14%) |
| Other | 11 (1.4%) |
| Invasion | |
| Visceral Pleural Invasion (N=891) | 118 (13%) |
| Vascular Invasion (N=878) | 273 (31%) |
| Pathologic Tumor Stage (pT) | |
| 1a | 367 (41%) |
| 1b | 231 (26%) |
| 2a | 236 (26%) |
| 2b/3 | 52 (6.1%) |
| Driver Mutation | |
| EGFR (N=563) | 108 (19%) |
| KRAS (N=412) | 140 (34%) |
FEV1, forced expiratory volume in 1 second; pT, pathologic tumor; Tumor SUVmax, maximum standardized uptake value.
Table 2.
Sites of Recurrence (N=893)
| Factor | All Patient (N = 893) N (%) |
Patients with Recurrence (N=115) N (%) |
|---|---|---|
| Recurrence Site | ||
| None | 638 (71%) | - |
| Death without Recurrence | 140 (16%) | - |
| Locoregional Only | 16 (1.8%) | 16 (14%) |
| Distant | 99 (11%) | 99 (86%) |
| Brain | 22 (2.5%) | 22 (19%) |
| Lung or Pleura | 42 (4.7%) | 42 (37%) |
| Bone | 19 (2.1%) | 19 (17%) |
| Adrenal | 6 (0.7%) | 6 (5.2%) |
| Liver | 4 (0.4%) | 4 (3.5%) |
| Other | 6 (0.7%) | 6 (5.2%) |
| Multi-Site Recurrence | ||
| No | 90 (10%) | 90 (78%) |
| Yes | 25 (2.8%) | 25 (22%) |
Distant Recurrence
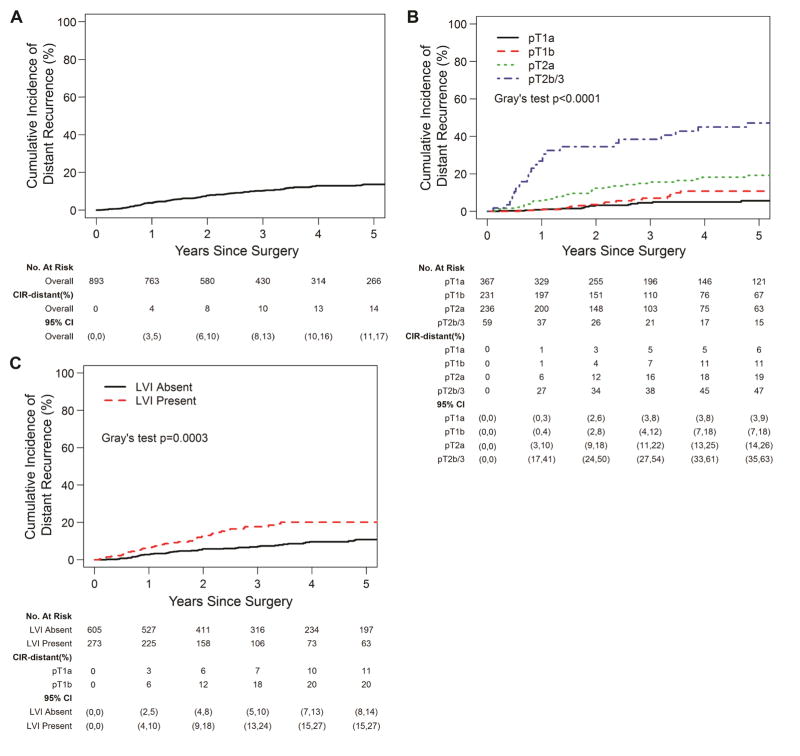
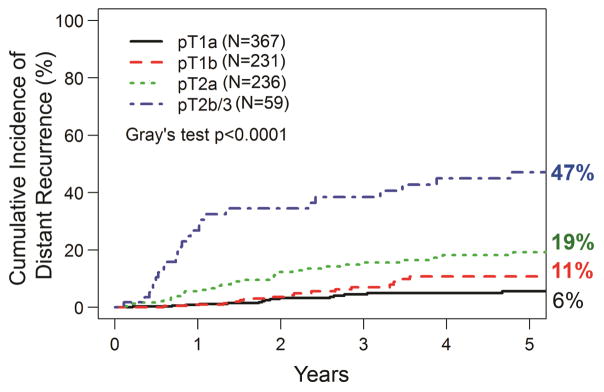
The 5-year CIR-distant for the entire cohort was 14% (95% CI, 11%–17%) (Fig. 2A). When stratified by pT stage, the 5-year CIR-distant was 6% (95% CI, 3%–9%) for pT1a, 11% (95% CI, 7%–18%) for pT1b, 19% (95% CI, 14%–26%) for pT2a, and 47% (95% CI, 35%–63%) for pT2b/3 tumors (P<0.001, Fig. 2B). The 5-year CIR-distant was 11% (95% CI, 8%–14%) for patients without LVI versus 20% (95% CI, 15%–27%) for those with LVI (Fig. 2C). To determine factors associated with distant recurrence, we performed an initial univariable analysis using competing risk regression (Table 3). As expected, several tumor features (SUVmax, tumor size, pT stage, histologic subtype, and LVI) were significantly associated with the development of distant recurrence. Surgery in the more recent two tertiles was also associated with decreased distant recurrence (2007–2013: HR: 0.64; 95% CI: 0.41–0.99, P=0.045/2014–2016: 0.43; 0.20–0.91, P=0.028). Neither EGFR nor KRAS mutation were associated with distant recurrence. There was no evidence of non-linear relationship between age of surgery (p=0.095) and tumor size (p=0.093) with respect to distant recurrence. On multivariable analysis, pT2a, pT2b/3 (more likely) and tertile of surgery (2014–2016; less likely) were associated with distant recurrence. Lymphovascular invasion had a strong correlation with distant recurrence (P=0.05). Primary tumor SUVmax and histologic subtype, including solid and micropapillary subtypes, were not significant in the multivariable model. Based on the proposed method by Zhou et al (2013), the goodness-of-fit test did not detect significant violation of the proportionality assumption among the variables in the final multivariable competing risk regression models for distant recurrence 16, 17. Multivariable competing risk regression model based on complete-cases was also performed. Characteristics of the complete case population are reported in Appendix Table 1. Results from complete-case multivariable analysis of recurrence are reported in Appendix Table 2 for reference, though estimates should be interpreted with caution due to smaller sample size and lower event number.
Figure 2.
Cumulative incidence of distant recurrence (CIR-distant). A, The CIR-distant for the entire cohort was 14% (95% confidence interval [CI], 11%–17%). B, Five-year CIR-distant by pathologic tumor (pT) stage and C, lymphovascular invasion (LVI).
Table 3.
Variables Associated with Distant Recurrence (N=893)
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.01 (0.99–1.03) | 0.234 | 1.01 (0.98–1.03) | 0.611 |
| Tumor SUV | 1.10 (1.06–1.14) | <0.001 | 1.04 (0.99–1.08) | 0.118 |
| FEV1 | 1.00 (0.99–1.01) | 0.454 | - | - |
| DLCO | 0.99 (0.98–1.00) | 0.246 | - | - |
| Time of Surgery (Tertile) | ||||
| 2000–2006 | 1.00 | - | 1.00 | - |
| 2007–2013 | 0.64 (0.41–0.99) | 0.045 | 0.67 (0.41–1.10) | 0.113 |
| 2014–2016 | 0.45 (0.23–0.90) | 0.023 | 0.43 (0.20–0.91) | 0.028 |
| Tumor Size (cm) | 1.40 (1.30–1.51) | <0.001 | - | - |
| Gender | ||||
| Female | 1.00 | - | - | - |
| Male | 1.37 (0.92–2.03) | 0.121 | - | - |
| Pulmonary Comorbidity | ||||
| No | 1.00 | - | - | - |
| Yes | 0.87 (0.54–1.39) | 0.554 | - | - |
| Cardiac Comorbidity | ||||
| No | 1.00 | - | - | - |
| Yes | 1.51 (1.00–2.26) | 0.049 | - | - |
| Histologic Subtype | ||||
| Lepidic | 1.00 | - | 1.00 | - |
| Acinar | 2.58 (0.82–8.09) | 0.104 | 1.82 (0.58–5.66) | 0.301 |
| Papillary | 2.59 (0.63–10.5) | 0.185 | 1.67 (0.45–6.20) | 0.444 |
| Micropapillary | 6.12 (1.63–23.0) | 0.007 | 3.16 (0.81–12.3) | 0.099 |
| Solid | 4.92 (1.58–15.3) | 0.006 | 2.10 (0.62–7.09) | 0.234 |
| Other | 7.40 (1.25–43.7) | 0.027 | 3.74 (0.65–21.4) | 0.139 |
| Visceral Pleural Invasion | ||||
| Absent | 1.00 | - | - | - |
| Present | 3.03 (1.95–4.69) | <0.001 | - | - |
| Lymphovascular Invasion | ||||
| Absent | 1.00 | - | 1.00 | - |
| Present | 2.11 (1.41–3.15) | <0.001 | 1.62 (1.00–2.63) | 0.050 |
| pT stage | ||||
| 1a | 1.00 | - | 1.00 | - |
| 1b | 1.86 (0.97–3.55) | 0.060 | 1.72 (0.89–3.34) | 0.107 |
| 2a | 3.71 (2.10–6.54) | <0.001 | 2.84 (1.56–5.16) | 0.001 |
| 2b/3 | 11.0 (5.80–20.8) | <0.001 | 6.53 (3.17–13.5) | <0.001 |
| EGFR Mutation | ||||
| No | 1.00 | - | - | - |
| Yes | 0.61 (0.26–1.44) | 0.257 | - | - |
| KRAS Mutation | ||||
| No | 1.00 | - | - | |
| Yes | 0.94 (0.46–1.91) | 0.861 | - | - |
DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; SUVmax, maximum standardized uptake value.
Appendix Table 1.
Clinicopathologic Characteristics of Complete Case Cohort (N=622)
| Characteristic | Median (Range) or N (%) |
|---|---|
| Age | 72 (36 – 92) |
| Gender | |
| Female | 398 (64%) |
| Male | 224 (36%) |
| Time of Surgery (Tertile) | |
| 2000–2006 | 156 (25%) |
| 2007–2013 | 240 (39%) |
| 2014–2016 | 226 (36%) |
| Tumor Characteristics | |
| Tumor SUV (N=721) | 3.4 (0.5 – 9.8) |
| Tumor Size (cm) | 2.2 (0.6 – 9.0) |
| Predominant Histologic Subtype (N=765) | |
| Lepidic | 89 (14%) |
| Acinar | 294 (47%) |
| Papillary | 113 (18%) |
| Micropapillary | 23 (3.7%) |
| Solid | 92 (15%) |
| Other | 11 (1.8%) |
| Lymphovascular Invasion (N=878) | 217 (35%) |
| pT stage | |
| 1a | 242 (39%) |
| 1b | 158 (25%) |
| 2a | 180 (29%) |
| 2b/3 | 42 (6.8%) |
Appendix Table 2.
Multivariable Complete Case Analysis (N=622)
| Variable | Distant Recurrence | Any Recurrence | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.01 (0.99–1.04) | 0.326 | 1.02 (0.99–1.04) | 0.215 |
| Tumor SUV | 1.04 (0.99–1.10) | 0.107 | 1.04 (0.98–1.09) | 0.177 |
| Time of Surgery (Tertile) | ||||
| 2000–2006 | 1.00 | - | 1.00 | - |
| 2007–2013 | 0.68 (0.38–1.24) | 0.212 | 0.71 (0.40–1.25) | 0.235 |
| 2014–2016 | 0.46 (0.19–1.08) | 0.074 | 0.46 (0.20–1.05) | 0.065 |
| Histologic Subtype | ||||
| Lepidic | 1.00 | - | 1.00 | - |
| Acinar | 2.11 (0.63–7.07) | 0.228 | 1.48 (0.53–4.15) | 0.453 |
| Papillary | 1.95 (0.53–7.25) | 0.317 | 1.76 (0.58–5.31) | 0.317 |
| Micropapillary | 4.48 (1.06–19.0) | 0.042 | 3.38 (0.97–11.7) | 0.056 |
| Solid | 3.20 (0.88–11.6) | 0.078 | 2.18 (0.70–6.84) | 0.180 |
| Other | 4.70 (0.84–26.4) | 0.079 | 2.91 (0.57–14.9) | 0.201 |
| Lymphovascular Invasion | ||||
| Absent | 1.00 | - | 1.00 | - |
| Present | 1.26 (0.71–2.27) | 0.432 | 1.49 (0.86–2.58) | 0.154 |
| pT stage | ||||
| 1a | 1.00 | - | 1.00 | - |
| 1b | 2.07 (0.91–4.72) | 0.084 | 2.13 (0.98–4.61) | 0.055 |
| 2a | 3.25 (1.55–6.83) | 0.002 | 3.25 (1.60–6.58) | 0.001 |
| 2b/3 | 6.43 (2.40–17.3) | <0.001 | 7.86 (3.09–20.0) | <0.001 |
Other Recurrence
Of the 893 patients included in the study, 16 developed isolated locoregional recurrence (1.8% of the entire cohort). Of the 367 patients with pT1a tumors, 3 (0.8%) developed isolated locoregional recurrence; in comparison, 4 of 231 (1.7%) with pT1b tumors, 6 of 236 (2.5%) with pT2a tumors, and 3 of 59 (5.1%) with pT2b/3 tumors developed isolated locoregional recurrence. The CIR-any for the entire cohort was 16% (95% CI, 13%–19%). The CIR-any was 6% (95% CI, 4%–10%) for pT1a, 14% (95% CI, 9%–21%) for pT1b, 24% (95% CI, 18%–31%) for pT2a, and 53% (95% CI, 41%–69%) for pT2b/3 tumors. As expected, variables associated with any recurrence on univariable analysis were largely the same as those associated with distant recurrence; these included primary tumor SUVmax, tumor size, pT1b, pT2a, pT2b/3, surgery in more recent tertiles, histologic subtype, visceral pleural invasion, and LVI (Supplemental Table 1). There was no evidence of non-linear relationship between tertile of surgery (p=0.286) and tumor size (p=0.070) with respect to any recurrence. On multivariable analysis, the same variables associated with distant recurrence remained associated with any recurrence (surgery in the most recent tertile (decreased risk), and lymphovascular invasion, pT2a, and pT2b/3 (increased risk) (Supplemental Table 1). The goodness-of-fit test did not detect significant violation of the proportionality assumption among the variables in the final multivariable competing risk regression models for any recurrence.
DFS and OS
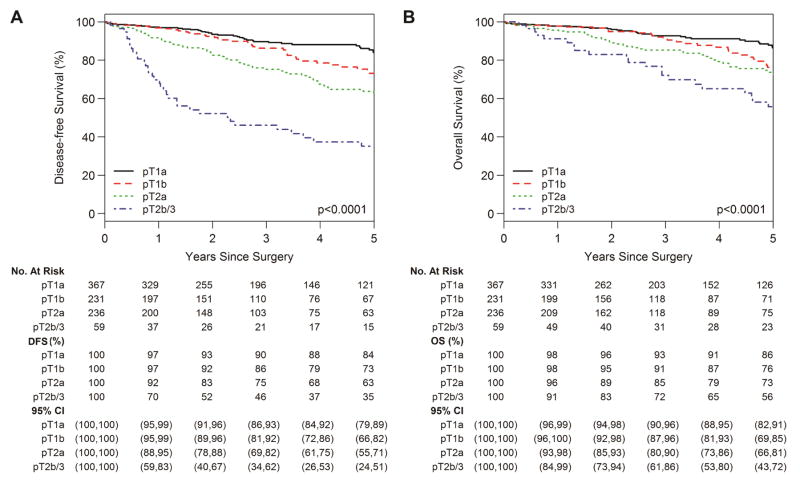
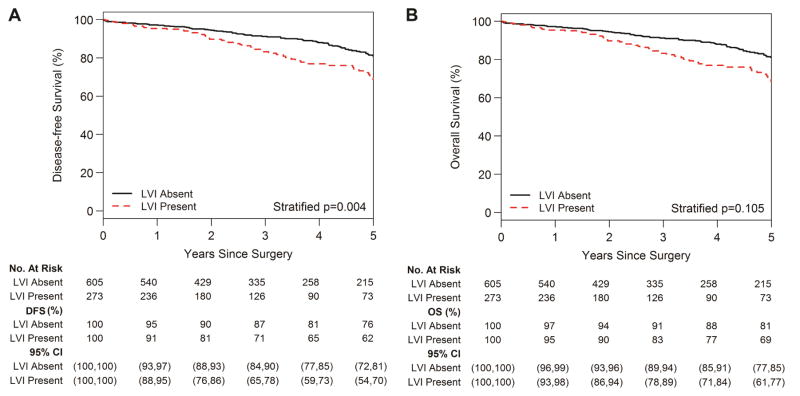
The 5-year DFS for the entire cohort was 72% (95% CI, 68%–76%) and 5-year OS for the entire cohort was 78% (95% CI, 74%–82%). The 5-year DFS was 84% (95% CI, 79%–89%) for pT1a, 73% (95% CI, 66%–82%) for pT1b, 63% (95% CI, 55%–71%) for pT2a, and 35% (95% CI, 24%–51%) for pT2b/3 tumors (Fig. 3A; P<0.001). The 5-year OS was 86% (95% CI, 82%–91%) for pT1a, 76% (95% CI, 69%–85%) for pT1b, 73% (95% CI, 66%–81%) for pT2a, and 56% (95% CI, 43%–72%) for pT2b/3 tumors (Fig. 3B; P<0.001). The 5-year DFS was 62% (95% CI, 54%–70%) for patients with LVI versus 76% (95% CI, 72%–81%) for patients without LVI (Fig. 4A; P=0.004). The 5-year OS was 69% (95% CI, 61%–77%) for patients with LVI versus 81% (95% CI, 77%–85%) for patients without LVI (Fig. 4B; P=0.105).
Figure 3.
Disease-free survival (DFS) (A) and overall survival (OS) (B) by pathologic tumor stage (pT).
Figure 4.
Disease-free survival (DFS) (A) and overall survival (OS) (B) by presence versus absence of lymphovascular invasion (LVI).
DISCUSSION
There is a paucity of data on predictors of distant recurrence—the major driver of decreased DFS and OS—for early-stage lung cancer. Our study is unique in that it focuses on patients with lung adenocarcinoma who have had optimal surgical treatment (lobectomy and R0 resection) and node-negative disease (pN0) and should therefore have a relatively low risk of developing distant recurrence. Interestingly, even in this select cohort, the overall 5-year CIR-distant was 14%, which increased to 47% for T2b/3 tumors. We found that pT stage was associated with both distant recurrence and decreased DFS and LVI strongly correlated with pT stage and was associated with decreased DFS. This suggests that, to mitigate the risk of distant recurrence and to improve survival, adjuvant therapy should be considered for appropriately selected patients, although the type and duration of such systemic treatment is not currently known.
In this study, pT stage, which is largely defined by tumor size, was associated with distant recurrence. Wu et al. found that tumor size was a predictor of all recurrence in patients with stage I NSCLC.18 That study did not directly evaluate distant recurrence but instead created a scoring system for various factors to evaluate the risk of distant recurrence. Large tumors (3–5 cm) were given higher weight in the scoring system, and, overall, patients with high or intermediate risk scores had a higher risk of distant metastases than patients with low risk scores (P=0.016).18 The role of adjuvant therapy for larger, node-negative tumors remains unclear. The Cancer and Leukemia Group B (CALGB) 9633 trial evaluated the benefit of adjuvant paclitaxel and carboplatin versus placebo after resection of pT2N0M0 NSCLC.19 The trial included all tumor histologic subtypes and found no difference in DFS or OS between groups. However, post hoc analysis suggested that patients with tumors > 4 cm treated with adjuvant chemotherapy experienced improved DFS and OS versus placebo.19 We found an association between both pT2a (>3 cm) and pT2b/3 (>5 cm) tumors and distant recurrence. Collectively, the findings of the CALGB 9633 study and our findings support that higher pT stage node-negative tumors are associated with a high incidence of distant recurrence. The current NCCN guidelines suggest that adjuvant chemotherapy should be considered for patients with high-risk factors, including tumor size > 4 cm (level 2A evidence).8
We also found a strong correlation between LVI and distant recurrence, an association between presence of LVI and any recurrence, and decreased DFS in patients with LVI. However, in our complete case analysis, LVI was not associated with increased risk of recurrence. This discrepancy in findings may be due to the smaller sample size in the complete cohort, an overwhelming significance of pT stage which overshadows less dramatic associations, or a lack of true significance. Previous studies have demonstrated a relationship between LVI and recurrence. Kiankhooey et al. found that LVI was a predictor of any recurrence and multisite recurrence in 532 patients with stage IA–IIA NSCLC.20 In addition, a meta-analysis by Mollberg et al. of patients with stage I NSCLC found that LVI was significantly associated with worse DFS (P=0.036) and OS (P=0.006).21 For tumors > 4 cm, the NCCN guidelines cite LVI as a “high-risk factor” that may be used to select patients for adjuvant therapy; however, the presence of isolated LVI is currently not an indication for adjuvant therapy (level 2A evidence).8
Our group has led an effort to find associations between histologic subtype and outcomes in patients with lung adenocarcinoma.22, 23 In the present study, we found no association between micropapillary histologic subtype and recurrence on multivariable analysis (Table 3, Supplemental Table 1). Previous studies have demonstrated that micropapillary subtype is associated with poor outcomes, including increased overall recurrence, decreased DFS, and decreased OS; however, very few studies have focused on the relationship between micropapillary subtype and distant recurrence.24, 25 We have previously shown that patients with small (<2 cm) node-negative adenocarcinomas with micropapillary-predominant histologic subtype who underwent sublobar resection had increased overall recurrence, particularly local recurrence. However, distant recurrence was not specifically evaluated, and micropapillary histologic subtype had no effect on recurrence in the lobectomy arm of the analysis.23 Additionally, we found no association between solid histologic subtype and distant recurrence on multivariable analysis. We have previously reported that, in patients with surgically resected small (< 2 cm) lung adenocarcinoma, solid histologic subtype was associated with earlier, extrathoracic, and multisite recurrence particularly in sublobar resections.22, 23 Our cohort differs in that >50% of patients had tumors >2 cm and all patients underwent lobectomy; nearly one-third of the patients in the aforementioned report underwent sublobar resection.
Although we found an association between tumor SUVmax and distant recurrence on univariable analysis, it did not remain significant on multivariable analysis. A review of the literature reveals mixed results on whether there is an association between tumor SUVmax and recurrence.20, 26–28 Possible explanations for this lack of consensus include differences in the inclusion criteria for each study, varied tumor SUVmax classification systems (categorical vs. continuous variable), and variability of PET/CT scans across institutions.
In addition, we found that year of surgery, as expressed by tertile, was associated with distant recurrence on multivariable analysis—meaning that patients included in the early years of the study had a higher risk of recurrence than patients who underwent surgery more recently. Previous studies have demonstrated a 0.5% to 2% recurrence rate per year after 5 years, even in patients with stage IA NSCLC.29 This association in our study is likely due to both longer cumulative times to develop recurrence for patients in the earlier years of the study, as well as changes in clinical practice throughout the duration of our study.
Molecular genetics play an increasingly important role of pathological examination of tumors. The NCCN guidelines recommend routine molecular testing for EGFR (category 1), ALK (category 1), ROS1 (category 2A), BRAF (category 2A), and PD-L1 (category 2A) to help guide therapies.8 At our institution, we also perform Integrated Mutation Profiling of Actionable Cancer Targets (IMPACT) testing on nearly all of our primary NSCLC to profile molecular aberrations and discover potential new molecular drivers.30 Here, we evaluated two clinically relevant mutated genes in NSCLC (KRAS and EGFR). We found no significant associations between EGFR activating or KRAS mutations and recurrence in our cohort. Izar et al. reported on 307 patients with resected stage IA lung adenocarcinoma (30% sublobar resections) and found a lower recurrence rate for patients with an activating EGFR-mutation (9.7%) versus EGFR wild-type (21.6%) tumors (P=0.03).31 In contrast, Kobayashi et al. examined 127 patients with p-stage IA lung adenocarcinoma who underwent lobectomy and found no association between EGFR mutation status and recurrence.32 Our group found no association between recurrence and EGFR mutation; however, we eliminated all patients who underwent sublobar resection and included much larger tumors than either of the aforementioned studies. In addition, EGFR mutation data (n=541) were not available for all patients: 37% of patients lacked these data and this missing data may have impacted our findings.
We also found no association between KRAS mutation and recurrence in our highly selected patient population. A review of the literature on the prognostic value of KRAS in early-stage, node-negative lung cancer offers no clear answers. The study by Izar et al. demonstrated an association between KRAS mutation and worse survival in patients with stage I disease.33 However, a meta-analysis of four adjuvant chemotherapy trials (n=1543) found no association between KRAS mutation and OS or DFS.34 We recently examined the prognostic value of KRAS mutation in patients with node-negative, p-stage I or II lung adenocarcinoma and found no association between KRAS mutation and risk of recurrence (P=0.29).35 A subset analysis by histologic subtype revealed an increased risk of overall recurrence for solid-predominant KRAS-mutant tumors (12% of entire cohort) but not for other histologic subtypes.35
The utility of targeted therapies for patients with pN0 lung adenocarcinoma who are at high risk of distant recurrence has been inadequately studied. The Randomized Double-Blind Trial in Adjuvant NSCLC with Tarceva (RADIANT) evaluated adjuvant erlotinib therapy in patients with surgically resected stage IB–IIIA NSCLC whose tumors expressed EGFR, using immunohistochemical analysis (≥1% staining) or fluorescence in situ hybridization.10 DNA sequencing of the tumor to discern the presence of activating mutations was not performed.10 Unsurprisingly, there was no difference in DFS or OS between the placebo and erlotinib arms.10 More recently, the Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (ALCHEMIST) began accruing patients with stage IB–IIIA NSCLC and stratifies patients to receive adjuvant erlotinib (EGFR-activating mutations), crizotinib (ALK-EML4 rearrangements), or nivolumab (neither EGFR-activating nor ALK-EML4 rearrangement).36 The results of this study will help determine whether highly selected patient populations may benefit from adjuvant targeted therapies or immunotherapies, including those with earlier stage lung cancers.
The limitations of this study include its retrospective, single-institution design, which may limit the generalizability of our findings. In addition, variances in preoperative staging approaches during the study period may have affected our results. For example, 7% (N=63) of patients did not have a preoperative PET scan to rule out metastatic disease. However, only 2 of those patients had a distant recurrence, neither of which occurred before 24 months. This suggests that none of these patients had metastatic disease at the time of surgery. Another potential limitation is that this study extends across 16 years. While our long-term follow-up is a strength, it may be a limitation in that treatment algorithms have changed over time. For example adjuvant chemotherapy has been increasingly used for larger, node-negative tumors. Therefore, more of these patients in the later years of our analysis may have been excluded, leading to an unintended selection bias. Finally, lack of a prospective, standardized intraoperative lymph node assessment protocol may have led to pathologic nodal understaging. However, the high OS of 78% in our entire cohort and the relatively similar pstage-specific results in the recent International Association for the Study of Lung Cancer studies suggest this is unlikely.37
Conclusions
Our study demonstrates that pT stage and LVI are associated with distant recurrence in patients undergoing complete (R0) lobectomy for pN0 lung adenocarcinoma, without induction or adjuvant therapies. Distant recurrence rates remain very high (19%–47%) for larger T-stage tumors, despite the use of an optimal surgical approach and node-negative disease. This study emphasizes the importance of actively accruing patients to current and future adjuvant therapy trials that examine the effect of targeted therapies and immunotherapies on decreasing distant recurrence.
Supplementary Material
Figure 5. Central Picture.
Cumulative incidence of distant recurrence by pathologic T (pT) stage (N=893).
Central message.
In patients with pN0 lung adenocarcinoma treated with R0 lobectomy, pathologic T stage (pT) and lymphovascular invasion are associated with distant recurrence and decreased survival.
Perspective statement.
Factors associated with distant recurrence following R0 lobectomy for node-negative lung adenocarcinoma have not been well-studied. We sought to identify factors associated with distant recurrence and disease-free survival in patients with early-stage lung adenocarcinoma to better identify patients who may benefit from adjuvant targeted or immunotherapies.
Acknowledgments
Funding: This work was supported by NIH grants R01 CA136705 (to D.R.J.) and T32 CA009501 (to W.S.B.). This work was also supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Glossary of abbreviations
- CALGB
Cancer and Leukemia Group B
- CI
confidence interval
- CIR-any
cumulative incidence of any recurrence
- CIR-distant
cumulative incidence of distant recurrence
- DFS
disease-free survival
- DLCO
diffusion capacity of the lung for carbon monoxide
- FEV1
forced expiratory volume in 1 second
- HR
hazard ratio
- LVI
lymphovascular invasion
- NCCN
National Comprehensive Cancer Network
- NSCLC
non-small cell lung cancer
- OS
overall survival
- pN0
pathologic node-negative
- pT
pathologic tumor
- SUVmax
maximum standardized uptake value
Footnotes
Conflicts of interest: All authors declare no conflicts of interest.
Date and number of IRB approval: 1/13/2015 — WA0021-15
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Paul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ. 2014;349:g5575. doi: 10.1136/bmj.g5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock JG, Rosen JE, Antonicelli A, Moreno A, Kim AW, Detterbeck FC, et al. Impact of adjuvant treatment for microscopic residual disease after non-small cell lung cancer surgery. Ann Thorac Surg. 2015;99:406–413. doi: 10.1016/j.athoracsur.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 5.Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer </= 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol. 2016;34:3175–3182. doi: 10.1200/JCO.2015.64.6729. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg. 2013;96:742–744. doi: 10.1016/j.athoracsur.2013.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 8.Network NCC. [April 25th, 2017];NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. 2017 https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 9.Kelsey CR, Higgins KA, Peterson BL, Chino JP, Marks LB, D’Amico TA, et al. Local recurrence after surgery for non-small cell lung cancer: a recursive partitioning analysis of multi-institutional data. J Thorac Cardiovasc Surg. 2013;146:768–773. e761. doi: 10.1016/j.jtcvs.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Kelly K, Altorki NK, Eberhardt WE, O’Brien ME, Spigel DR, Crino L, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2015;33:4007–4014. doi: 10.1200/JCO.2015.61.8918. [DOI] [PubMed] [Google Scholar]
- 11.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruijk Mvd. Multiple imputation with chained equation and survival outcomes: A simulation study. Department of Mathematics, Universiteit Leiden; 2015. [Google Scholar]
- 13.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons, Inc; 1987. [Google Scholar]
- 14.Fine JP. Regression modeling of competing crude failure probabilities. Biostatistics. 2001;2:85–97. doi: 10.1093/biostatistics/2.1.85. [DOI] [PubMed] [Google Scholar]
- 15.Gray JPFRJ. A Proportional Hazards Model for the Subdistribution of Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 16.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Fine J, Laird G. Goodness-of-fit test for proportional subdistribution hazards model. Stat Med. 2013;32:3804–3811. doi: 10.1002/sim.5815. [DOI] [PubMed] [Google Scholar]
- 18.Wu CF, Fu JY, Yeh CJ, Liu YH, Hsieh MJ, Wu YC, et al. Recurrence Risk Factors Analysis for Stage I Non-small Cell Lung Cancer. Medicine (Baltimore) 2015;94:e1337. doi: 10.1097/MD.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss GM, Herndon JE, 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiankhooy A, Taylor MD, LaPar DJ, Isbell JM, Lau CL, Kozower BD, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg. 2014;98:1175–1183. doi: 10.1016/j.athoracsur.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 21.Mollberg NM, Bennette C, Howell E, Backhus L, Devine B, Ferguson MK. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg. 2014;97:965–971. doi: 10.1016/j.athoracsur.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol. 2015;33:2877–2884. doi: 10.1200/JCO.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–1220. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F, Zheng W, Ying L, Wu J, Wu S, Ma S, et al. A Nomogram to Predict Brain Metastases of Resected Non-Small Cell Lung Cancer Patients. Ann Surg Oncol. 2016;23:3033–3039. doi: 10.1245/s10434-016-5206-3. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida Y, Nitadori JI, Shinozaki-Ushiku A, Sato J, Miyaji T, Yamaguchi T, et al. Micropapillary histological subtype in lung adenocarcinoma of 2 cm or less: impact on recurrence and clinical predictors. Gen Thorac Cardiovasc Surg. 2017 doi: 10.1007/s11748-017-0747-3. [DOI] [PubMed] [Google Scholar]
- 26.Taylor MD, Nagji AS, Bhamidipati CM, Theodosakis N, Kozower BD, Lau CL, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg. 2012;93:1813–1820. doi: 10.1016/j.athoracsur.2012.03.031. discussion 1820–1811. [DOI] [PubMed] [Google Scholar]
- 27.Yang HC, Kim HR, Jheon S, Kim K, Cho S, Ahn S, et al. Recurrence Risk-Scoring Model for Stage I Adenocarcinoma of the Lung. Ann Surg Oncol. 2015;22:4089–4097. doi: 10.1245/s10434-015-4411-9. [DOI] [PubMed] [Google Scholar]
- 28.Shiono S, Abiko M, Okazaki T, Chiba M, Yabuki H, Sato T. Positron emission tomography for predicting recurrence in stage I lung adenocarcinoma: standardized uptake value corrected by mean liver standardized uptake value. Eur J Cardiothorac Surg. 2011;40:1165–1169. doi: 10.1016/j.ejcts.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 29.Maeda R, Yoshida J, Hishida T, Aokage K, Nishimura M, Nishiwaki Y, et al. Late recurrence of non-small cell lung cancer more than 5 years after complete resection: incidence and clinical implications in patient follow-up. Chest. 2010;138:145–150. doi: 10.1378/chest.09-2361. [DOI] [PubMed] [Google Scholar]
- 30.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izar B, Sequist L, Lee M, Muzikansky A, Heist R, Iafrate J, et al. The impact of EGFR mutation status on outcomes in patients with resected stage I non-small cell lung cancers. Ann Thorac Surg. 2013;96:962–968. doi: 10.1016/j.athoracsur.2013.05.091. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi NTS, Ichimura K, Soh J, Yamamoto H, Matsuo K, Otani H, Jida M, Kubo T, Tsukuda K, Kiura K, Sano Y, Date H. Non-BAC Component but not Epidermal Growth Factor Receptor Gene Mutation is Associated with Poor Outcomes in Small Adenocarcinoma of the Lung. J Thorac Oncol. 2008;3:704–710. doi: 10.1097/JTO.0b013e31817c6080. [DOI] [PubMed] [Google Scholar]
- 33.Izar B, Zhou H, Heist RS, Azzoli CG, Muzikansky A, Scribner EE, et al. The prognostic impact of KRAS, its codon and amino acid specific mutations, on survival in resected stage I lung adenocarcinoma. J Thorac Oncol. 2014;9:1363–1369. doi: 10.1097/JTO.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd FA, Domerg C, Hainaut P, Janne PA, Pignon JP, Graziano S, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadota KSC, Arcila ME, Hedvat C, Kris MG, Jones DR, Adusumilli PS, Travis WD. KRAS Mutation Is a Significant Prognostic Factor in Early-stage Lung Adenocarcinoma. Am J Surg Pathol. 2016;40:1579–1590. doi: 10.1097/PAS.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Institute NNC. [April 25, 2017];The ALCHEMIST Lung Cancer Trials. 2016 https://www.cancer.gov/types/lung/research/alchemist.
- 37.Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2015;10:990–1003. doi: 10.1097/JTO.0000000000000559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.