Abstract
The association between stress and mental illness has been well documented, but the molecular consequences of repeated exposure to stress have not been completely identified. The present study sought to elucidate the combinatorial effects of early life maternal separation stress and adult social defeat stress on alterations in signal transduction and gene expression that have been previously implicated in susceptibility to psychosocial stress. Molecular analyses were performed in the prelimbic/infralimbic cortex, amygdala, and nucleus accumbens, three brain regions that have been suggested to play critical roles in determining stress responses. The current data reveal that both maternal separation and social defeat significantly impact the expression of genes involved in histone methylation and the β-catenin-, endogenous opioid-, neurotrophin-, and glucocorticoid signaling pathways. Although the effects of maternal separation and social defeat were largely non-overlapping, a subset of genes in each brain region were governed by additive, opposing, or other types of interactions between these stress paradigms, thus highlighting potential molecular mechanisms through which these stressors might coordinately regulate brain function and behavior.
Keywords: maternal separation, social defeat stress, amygdala, nucleus accumbens, frontal cortex
Introduction
Exposure to stress has been associated with numerous negative consequences, including impaired cognitive performance and the development of neuropsychiatric disorders (Heim and Nemeroff, 2001; Bale et al., 2010). However, the reasons why some individuals remain resilient in the face of stress while others experience significant dysfunction remain largely unknown. Two and three-hit models of stress susceptibility suggest that vulnerability to stress results from a variety of genetic and psychological factors, including prior stress experience (Daskalakis et al., 2013; Bateson et al., 2014; Pena et al., 2017). The duration and developmental timing of early life stress exposure are thought to be critical in determining subsequent stress susceptibility (Lupien et al., 2009; Pena et al., 2017), and gene by environment interactions play key roles as well (Caspi et al., 2003). However, whether various genetic and environmental risk factors for stress susceptibility confer vulnerability to stress through shared or distinct pathways has not been established. The current work seeks to examine the molecular alterations that are associated with and could contribute to the combined effects of early life stress and adult psychosocial stress. This was accomplished using the maternal separation stress (MSS) model of early life stress and the social defeat stress (SDS) model of adult psychosocial stress, which is one of the most commonly used animal models to examine the mechanisms leading to stress vulnerability vs. resilience (Golden et al., 2011). Prior work using the SDS model has shown that exposure to psychosocial stress in adolescence increases vulnerability to SDS in adult Syrian hamsters (Rosenhauer et al., 2017), and published studies have shown that MSS can increase susceptibility to anhedonia in rats (Der-Avakian and Markou, 2010) and social avoidance in c57BL6/J mice (Pena et al., 2017) induced by repeated exposure to SDS. In keeping with these findings, unpublished work from our group has shown that the MSS paradigm employed here also increases susceptibility to social avoidance induced by SDS in adult c57BL6/J mice (B.D. Sachs, unpublished observations).
There are many potential cellular and molecular mechanisms through which MSS could lead to altered susceptibility to SDS in adulthood. For example, the MSS-induced increase in SDS susceptibility in c57BL6/J mice has recently been shown to involve transcriptional alterations in the ventral tegmental area (VTA) (Pena et al., 2017), but the transcriptional changes that occur outside of the VTA in response to combined MSS+SDS have not been reported. In addition to the VTA, the nucleus accumbens (NAc) has also been prominently implicated in the regulation of SDS responses (Berton et al., 2006; Krishnan et al., 2007), and neural circuits outside of mesolimbic reward pathway, including the prelimbic cortex (PLC) and the amygdala (Amyg), have also been shown to play a role in SDS responses (Covington et al., 2010; Kumar et al., 2014) and to exhibit transcriptional alterations in response to SDS (Covington et al., 2010; Bagot et al., 2016;). The dysregulation of multiple signaling pathways has been suggested to influence stress responses, including the Wnt-β-catenin (Wilkinson et al., 2011; Dias et al., 2014; Sachs et al., 2015), glucocorticoid (Wagner et al., 2012; Jacobson, 2014; Arloth et al., 2015; Jochems et al., 2015;), endogenous opioid (Wiedenmayer et al., 2002; Nikulina et al., 2005; McLaughlin et al., 2006; Nocjar et al., 2012; Berube et al., 2013; Donahue et al., 2015;), and brain derived neurotrophic factor (BDNF)-cAMP-response element binding protein (CREB) (Berton et al., 2006; Krishnan et al., 2007) pathways, as well as transcriptional programming pathways involving the orthodenticle homeobox 2 (Otx2) transcription factor (Pena et al., 2017) and histone methylation enzymes (Murgatroyd et al., 2009; Covington et al., 2011; Saunderson et al., 2016). Here, we evaluated the combinatorial effects of MSS and SDS on the expression of a subset of genes from each of these pathways in the PLC, Amyg, and NAc.
In addition to the many potential transcriptional markers of stress susceptibility that have been identified, several specific protein-level alterations have been reported as being important determinants of SDS responses. For example, c57BL/6J mice that display social avoidance behavior following SDS have been shown to exhibit increased phosphorylation of ERK and Akt in the NAc compared to animals that remain sociable following SDS (Krishnan et al., 2007). In addition, animals that remain sociable following SDS have also been observed to exhibit an increase in the protein levels of β-catenin in the NAc compared to avoidant animals (Sachs et al., 2015). Given that overexpression of β-catenin in the NAc has been shown to promote sociability in mice following SDS, alterations in the NAc levels of β-catenin have been hypothesized to be functionally important in determining resilience to stress (Dias et al., 2014). However, whether any of the protein alterations in the NAc that were found to differentiate socially avoidant from sociable mice following exposure to SDS alone (Krishnan et al., 2007; Sachs et al., 2015) also occur in MSS-exposed vs. control populations following SDS has not been established. Overall, the present study aimed to provide new insight into the molecular adaptations that occur in response to repeated stress exposure, which could have important implications for our understanding of susceptibility vs. resilience to stress.
Experimental Procedures
Subjects
This study used c57BL6 mice that were initially obtained from Jackson laboratories and bred in the Caron lab at Duke University. Mice were housed on a 12 h light-dark cycle and provided food and water ad libitum. Male mice were eight-ten weeks of age at the start of SDS. All experiments were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee and were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Maternal Separation and Social Defeat Stress
MSS was performed as described previously (Sachs et al., 2013b). Briefly, the experimental pups were separated from their dams for three hours each day during the light phase (starting between 1–3 hours after the start of the light phase) on PNDs 1–14, and control pups were reared under standard conditions. During the separation period, the pups were placed on a heating pad and remained in contact with their littermates. All animals were weaned on day 21, at which point they were all housed in groups of five per cage. At the time of weaning, both standard facility reared controls and MSS-exposed animals were randomly divided into two additional groups (SDS and control) that were subjected to SDS (or not for controls) starting at 8-10 weeks of age. For the SDS group (both for MSS and non-MSS animals), the SDS paradigm was performed as described previously (Sachs et al., 2015) and consisted of introducing each experimental mouse into the home cage of a singly housed resident CD1 male for five minutes a day for ten days during the light phase of the light-dark cycle (between 1-3 hours prior to the start of the dark phase). Following each defeat session, each experimental mouse was singly housed for 24 hours. The experimental mice were introduced into the home cage of a new CD1 male mouse each day. Control animals were group housed throughout the experiment and had no exposure to CD1 animals until social interaction testing. On day 12 (~48 hours after the final defeat episode), experimental animals were sacrificed for molecular analyses.
Western Blotting
Mice were killed by cervical dislocation and decapitation, after which their heads were immediately cooled by immersion in liquid nitrogen for five seconds. The brains were rapidly dissected on an ice-cold surface and 1.5 mm tissue punches from the NAc (stereotactic coordinates provided below) were collected and snap frozen in liquid nitrogen. Bilateral punches from the same animal were pooled and considered a single sample. Samples were processed for Western blotting using standard methods that we have described previously (Sachs et al., 2015). Membranes were incubated overnight at 4°C with the following primary antibodies: mouse anti-GAPDH (MAB374, Millipore; 1:1000), mouse anti- β-catenin (#2698, Cell Signaling, 1:300), rabbit anti-phospho-p44/p42 MAPK (Erk1/2; #9101, Tyr202/204, Cell Signaling; 1:500); mouse anti-total ERK (#9107, Cell Signaling, 1:500), rabbit antiphospho-Akt (Ser473, #9271, Cell Signaling, 1:500), and rabbit anti-total Akt (#9272, Cell Signaling, 1:1000). The appropriate AlexaFluor 680 or IRDye 800- conjugated secondary antibodies were incubated at room temperature for ~90 minutes (Molecular Probes and Li-COR; 1:10,000 dilution), and blots were developed using an Odyssey LI-COR system (LI-COR Biosciences, USA). Quantification was performed using densitometry with the Image J program (National Institutes of Health). The relative levels of β-catenin were normalized against GAPDH and the levels of phosphorylated kinase (Akt or ERK) were normalized against total kinase (Akt or ERK) as a loading control.
Real-Time PCR
Tissue samples were obtained as described above for the Western blotting analysis, except that tissue was isolated from the PLC, Amyg, and NAc. The tissue punches were centered at approximately the following stereotactic coordinates: PLC: A/P: +2.1 mm anterior to Bregma, D/V: −2.2 mm, M/L: at the midline; NAc: A/P +1.1; D/V: −4.5 mm; M/L +/− 0.8 mm; Amyg: A/P: −1.0, D/V: −5.0 mm, M/L: +/− 2.3 mm. A single punch containing PLC from both hemispheres was obtained, but bilateral punches were obtained from NAc and Amyg. RNA was isolated from these tissue punches using RNEasy minikits according to the manufacturer’s protocol, and reverse transcription was performed using iScript First Strand Synthesis kits according to the manufacturer’s protocol and as described previously (Sachs et al., 2013a). Real-time PCR was performed using a StepOne Plus Real Time PCR System (Applied Biosystems by ThermoFisher Scientific, Waltham, MA, USA) using PowerUp Sybr Green master mix (ThermoFisher Scientific, Waltham, MA, USA). A table describing the primers used is provided in the Appendix.
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Axin 1 | CCACCACCATGTTCACCATA | TGGCATGACCATGTGTTTCT |
| Axin 2 | AAC CTA TGC CCG TTT CCT CTA | GAG TGT AAA GAC TTG GTC CAC C |
| BDNF | CAA TGC CGA ACT ACC CAA | AAC ATA AAT CCA CTA TCT TCC CC |
| beta catenin | TATTGACGGGCAGTATGCAA | CCCTCATCTAGCGTCTCAGG |
| CREB | AGC CGG GTA CTA CCA TTC TAC | GCA GCT TGA ACA ACA ACT TGG |
| CRH | CCT CAG CCG GTT CTG ATC C | GCG GAA AAA GTT AGC CGC AG |
| Delta Opioid Receptor 1 | GCTGGTGGACATCAATCGG | GCGTAGAGAACCGGGTTGAG |
| Dicer | GCCAAGAAAATACCAGGTTGAGC | GCGATGAACGTCTTCCCTGAG |
| DVL1 | AGT GGA GCC TCA GAT CAG GA | GGT CCT GGG TAC TGG TAG GG |
| DVL2 | TGA CAA TGA CGG TTC CAG TG | GCG CTG GAT ACT GGT AGG AG |
| DVL3 | CTA CAC GCA GCA GTC TGA GG | CAT AGC TTG GGT GTG TGT GG |
| Ehmt 1 | TAAAACAGAGGACGGTGATTGAG | AGGGCACTATCATCTAAGGCTT |
| FKBP5 | TTTGAAGATTCAGGCGTTATCCG | GGTGGACTTTTACCGTTGCTC |
| Ehmt 2 (G9a) | CGAGCCCGGAAAACCATGT | TCATGCGGAAATGCTGGACTT |
| GAPDH | CAT GTT CCA GTA TGA CTC CAC TC | GGC CTC ACC CCA TTT GAT GT |
| GR | AGC TCC CCC TGG TAG AGA C | GGT GAA GAC GCA GAA ACC TTG |
| GSK3b | ACAGGCCACAGGAGGTCAGT | GATGGCAACCAGTTCTCCAG |
| Kappa Opioid Receptor 1 | GAATCCGACAGTAATGGCAGTG | GACAGCGGTGATGATAACAGG |
| MR | GAA AGG CGC TGG AGT CAA GT | TGT TCG GAG TAG CAC CGG AA |
| Mu opioid receptor 1 | CCAGGGAACATCAGCGACTG | CATGGGTCGGACTGGTTGC |
| Otx2 | TATCTAAAGCAACCGCCTTACG | AAGTCCATACCCGAAGTGGTC |
| POMC | ATGCCGAGATTCTGCTACAGT | CCACACATCTATGGAGGTCTGAA |
| Prodynorphin | AGGTTGCTTTGGAAGAAGGCT | GACGCTGGTAAGGAGTCGG |
| Proenkephalin | GAGAGCACCAACAATGACGAA | TCTTCTGGTAGTCCATCCACC |
| SGK1 | TCCGCCAAGTCCCTCTCAACAAAT | TGCCTAGCCAGAAGAACCTTTCCA |
Statistical analyses
Data were analyzed using two-way ANOVAs with MSS and SDS as between-subjects factors. Shapiro-Wilk W tests were used to verify that data were normally distributed, and Obrien’s tests for homogeneity of variance were used to ensure equal variance. When Shapiro-Wilk or Obrien’s tests indicated that data were not normally distributed or had unequal variance, data were transformed (log10 or squared) to achieve normalcy and equal variance prior to performing two-way ANOVAs. When significant interactions were observed, Tukey’s post-hoc tests, which correct for multiple comparisons, were performed to evaluate potential group differences.
Results
Signaling effects of MSS-SDS
β-catenin signaling in the NAc has been suggested to be a major regulator of susceptibility vs. resilience to SDS (Dias et al., 2014). Given that MSS has been shown to increase SDS susceptibility (Pena et al., 2017), we sought to determine whether MSS might alter levels of β-catenin in the NAc in response to SDS. Our results demonstrate that after ten days of SDS, both control and MSS-exposed mice subjected to SDS exhibit increased β-catenin in the NAc [F(3, 36) = 5.62, p = 0.023, Fig. 1a], thus suggesting that MSS does not influence the alterations in the protein levels of β-catenin in the NAc induced by SDS.
Figure 1.
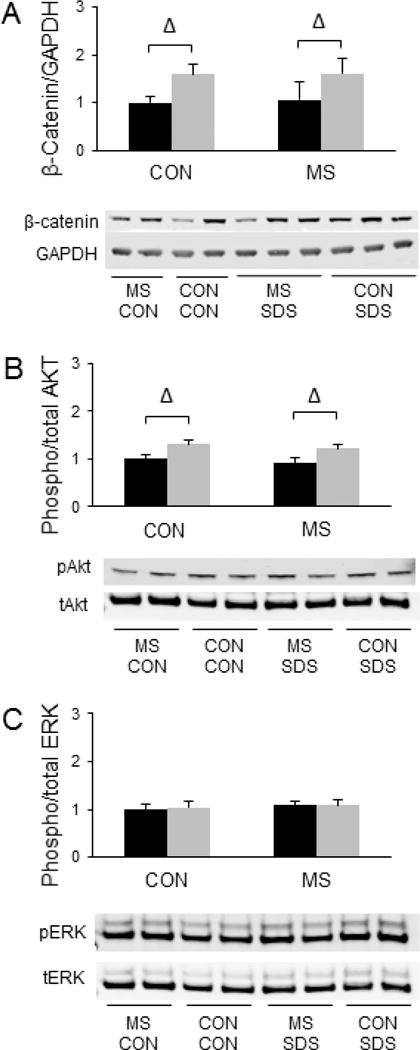
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on β-catenin levels in the nucleus accumbens compared to control (Con) animals. (A) Quantification and representative images for Western blot analysis of β-catenin/GAPDH levels in the nucleus accumbens. (B) Quantification and representative images for Western blot analysis of phosphorylated Akt/total Akt levels in the nucleus accumbens. (C) Quantification and representative images for Western blot analysis of phosphorylated ERK2/total ERK2 levels in the nucleus accumbens. Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, p < 0.05. N = 10 per group.
Other signaling molecules in the NAc that have been implicated in determining SDS responses include Akt and ERK, two kinases that have been shown to be more highly phosphorylated in socially avoidant mice compared to sociable mice following SDS (Krishnan et al., 2007). We sought to evaluate whether MSS-exposed animals would also exhibit increased phosphorylation of these kinases in the NAc following SDS compared to SDS-exposed animals with no history of MSS. However, our results reveal no significant effects of MSS on the phosphorylation of Akt (Fig. 1b) or ERK (Fig. 1c). In contrast, SDS was shown to lead to a significant increase in Akt phosphorylation [F(3,36) = 12.79, p = 0.001], but MSS did not modify this effect (Fig. 1b). No significant effects of SDS on pERK levels were observed in the NAc (Fig. 1c).
MSS-SDS and Gene Expression
Nucleus Accumbens
We next sought to evaluate potential effects of combined MSS-SDS on gene expression in the NAc. We first evaluated whether the expression of β-catenin target genes was significantly impacted by MSS and/or SDS. Our results demonstrate that MSS led to a significant increase in the mRNA expression of Axin1 [F(3, 28) = 8.72, p = 0.006, (Fig. 2a)] and Dicer [F(3, 28) = 5.71, p = 0.024, (Fig. 2c)]. MSS also led to a significant increase in the expression of several genes known to regulate β-catenin that have been previously implicated in SDS responses (Wilkinson et al., 2011), including GSK3β [F(3, 28) = 12.71, p = 0.001, (Fig 2d)] and disheveled-1 (DVL-1) [F(3, 28) = 5.84, p = 0.022, (Fig 2e)]. Like MSS, SDS also induced a significant increase in GSK3β mRNA expression [F(3, 28) = 14.13, p = 0.0008,(Fig. 2d)], thus suggesting that additive effects of SDS and MSS on GSK3β expression in the NAc could represent one mechanism through which MSS and SDS might coordinately regulate behavior. However, no significant effects of MSS or SDS on the expression of Axin 2 (Fig. 2b), DVL-2 (Fig. 2f), DVL-3 (Fig 2g), or β-catenin (Fig. 2h) were observed in the NAc.
Figure 2.
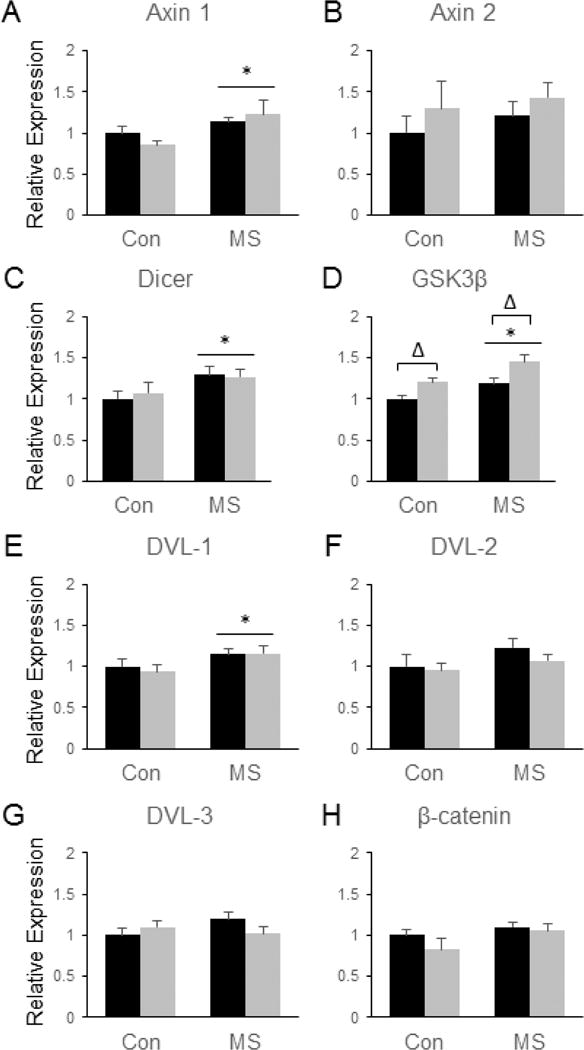
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of β-catenin-related genes in the nucleus accumbens compared to control (Con) animals. Quantification of real-time PCR data using primers specific for Axin 1 (A), Axin 2 (B), Dicer (C), GSK3β (D), DVL-1 (E), DVL-2 (F), DVL-3 (G), and β-catenin (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS. N = 8 mice per group.
As expected, genes involved in glucocorticoid signaling and histone methylation were significantly impacted by MSS and/or SDS in the NAc. Specifically, SDS led to an up-regulation of the glucocorticoid receptor (GR) [F(3, 28) = 5.37, p = 0.028, (Fig. 3a)] and the mineralocorticoid receptor (MR) [F(3, 28) = 8.34, p = 0.0074, (Fig. 3b)] and a significant down-regulation of FK506 Binding Protein 5 (FKBP5) [F(3, 28) = 5.30, p = 0.029, (Fig. 3d)], which is a known regulator of GR (Binder, 2009). In addition, MSS led to increased expression of serum/glucocorticoid-regulated kinase 1 (SGK1) [F(3, 28) = 12.02, p = 0.002, (Fig. 3c)] in the NAc. A trend towards an MSS by SDS interaction was observed for the mRNA expression of corticotropin-releasing hormone (CRH) following stress (p = 0.056), but none of the individual group comparisons reached statistical significance (Fig. 3e). In addition, MSS increased the expression of the histone methyl transferase, EHMT1, in the NAc [F(3, 28) = 5.54, p = 0.026, (Fig. 3f)], demonstrating that MSS does influence epigenetic processes, but no significant effects of SDS on EHMT1 or EHMT2 expression were observed in the NAc (Fig. 3g). No significant main or interactive effects of SDS or MSS were observed on the expression of Otx2 in the NAc, although there was a slight trend towards reduced Otx2 expression following MSS (p = 0.09, Fig. 3h).
Figure 3.
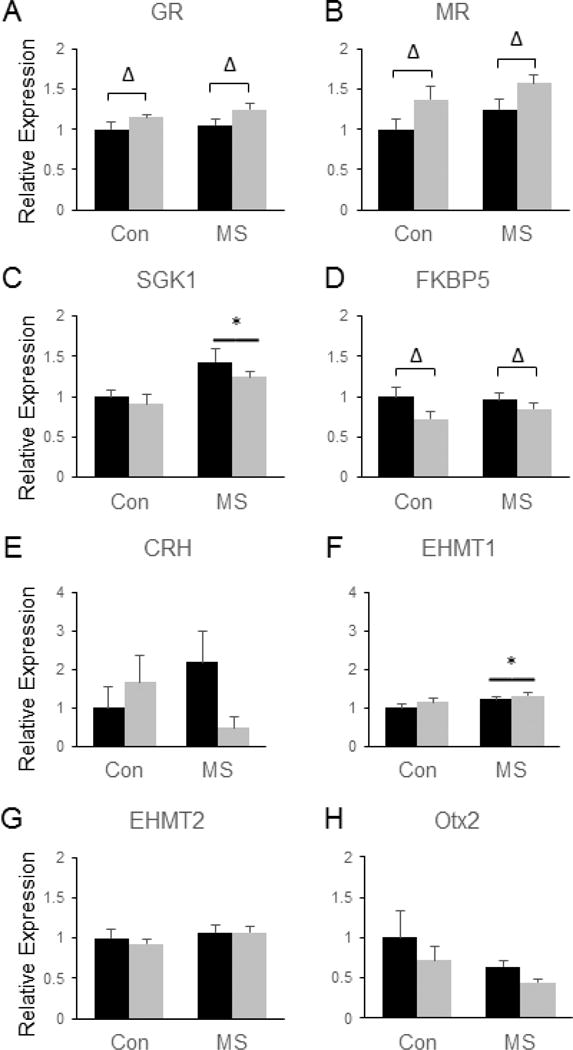
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of genes involved in glucocorticoid signaling and histone methylation in the nucleus accumbens compared to control (Con) animals. Quantification of real-time PCR data using primers specific for GR (A), MR (B), SGK1 (C), FKBP5 (D), CRH (E), EHMT1 (F), EHMT2 (G) and Otx2 (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS by two way ANOVA. N = 8 mice per group.
In the NAc, there were no significant main effects of MSS on the expression of any of the components of the endogenous opioid system that we examined. No significant effects of SDS were observed on the expression of the mu opioid receptor (MOR1) (Fig. 4a) or the delta opioid receptor (DOR1) (Fig. 4c), but SDS significantly reduced the levels of kappa opioid receptor (KOR1) [F(3, 28) = 6.64, p = 0.016, (Fig. 4b)], proenkephalin (PENK) [F(3, 28) = 23.35, p < 0.0001 (Fig. 4d)], prodynorphin (PDYN) [F(3, 28) = 13.7, p = 0.0009, (Fig. 4e)], and pro-opiomelanocortin (POMC) [F(3, 28) = 4.53, p = 0.042, (Fig. 4f)]. In addition, significant interactions between MSS and SDS were observed for KOR [F(3, 28) = 5.46, p = 0.027, (Fig. 4b)] and PDYN [F(3, 28) = 6.33, p = 0.018, (Fig. 4e)], with SDS only leading to a significant reduction in PDYN and KOR levels in control mice, not in MSS-exposed animals (Fig. 4b and e, p < 0.05 by Tukey’s post hoc tests). No significant group differences in BDNF levels were observed in the NAc (Fig. 4g), but SDS significantly increased the mRNA levels of CREB in this brain region [F(3, 28) = 11.64, p = 0.002, (Fig. 4h)].
Figure 4.
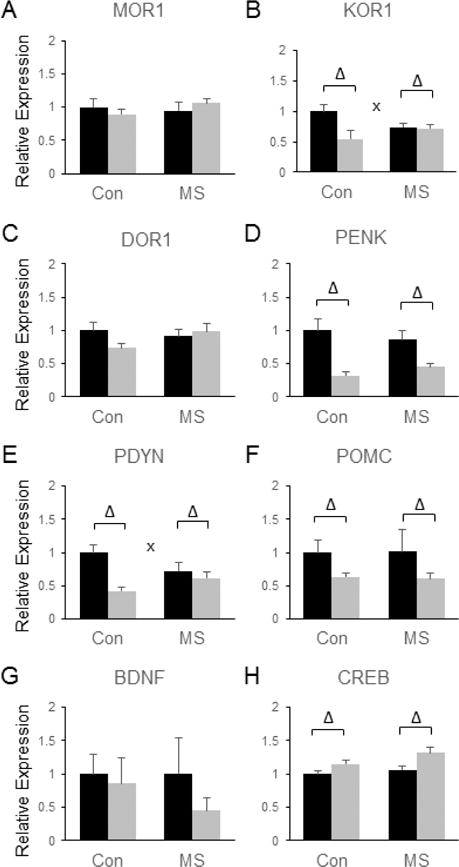
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of genes involved in endorphin and neurotrophin signaling in the nucleus accumbens compared to control (Con) animals. Quantification of real-time PCR data using primers specific for MOR1 (A), KOR1 (B), DOR1 (C), PENK (D), PDYN (E), POMC (F), BDNF (G), and CREB (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS, ‘x’ denotes significant MS by SDS interaction by two way ANOVA. N = 8 mice per group.
Prelimbic/Infralimbic Cortex
Like the NAc, the PLC was highly responsive to both MSS- and SDS-induced changes in gene expression, although the transcriptional alterations observed were largely distinct between the two brain regions. SDS significantly reduced the expression of Axin1 [F(3, 28) = 4.93, p = 0.035,(Fig. 5a)], Dicer [F(3, 28) = 11.27, p = 0.002, (Fig. 5c)] and DVL-3 [F(3, 28) = 19.8, p = 0.0001, (Fig. 5g)] in the FC. In addition, MSS led to significant increases in the mRNA expression of the β-catenin-related genes Axin2 [F(3, 28) = 4.52, p = 0.042, (Fig. 5b)] and DVL3 [F(3, 28) = 4.38, p = 0.046, (Fig. 5g)]. No significant alterations in the expression of GSK3β (Fig, 5d), DVL-1 (Fig. 5e), DVL-2 (Fig. 5f), or β-catenin itself (Fig. 5h) were observed in response to SDS or MSS in the PLC.
Figure 5.
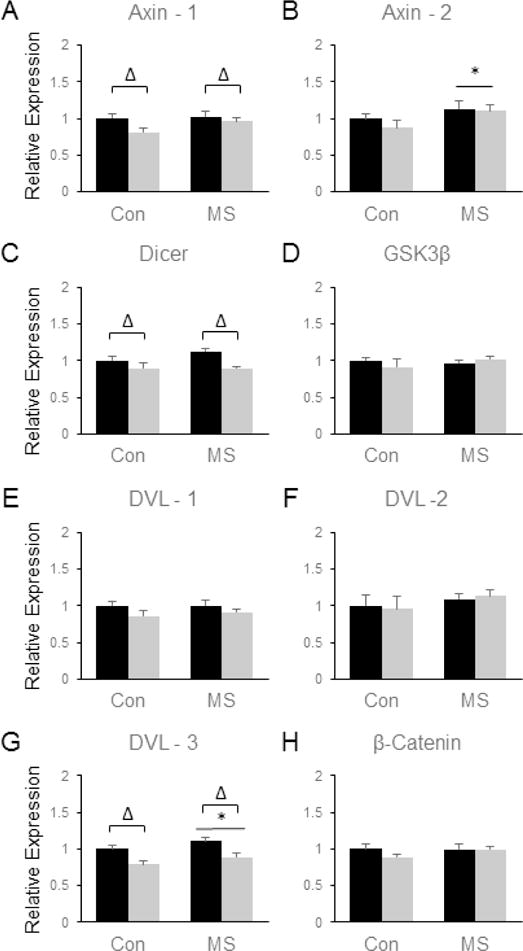
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of β-catenin-related genes in the prelimbic/infralimbic cortex compared to control (Con) animals. Quantification of real-time PCR data using primers specific for Axin 1 (A), Axin 2 (B), Dicer (C), GSK3β (D), DVL-1 (E), DVL-2 (F), DVL-3 (G), and β-catenin (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS, ‘x’ denotes significant MS by SDS interaction by two way ANOVA. N = 8 mice per group.
As expected, genes related to glucocorticoid signaling were significantly impacted by both MSS and SDS in the PLC. Specifically, MSS significantly increased the levels of GR [F(3, 28) = 5.09, p = 0.032, (Fig. 6a)], MR [F(3, 28) = 5.03, p = 0.033, (Fig. 6b)], and SGK1 [F(3, 28) = 17.79, p = 0.0002, (Fig. 6c)], and MSS led to a trend towards increased expression of FKBP5 in the PLC (p = 0.056, Fig. 6d). SDS led to a significant reduction in CRH [F(3, 28) = 38.88, p < 0.0001, (Fig. 6e)] and SGK1 [F(3, 28) = 9.75, p = 0.004, (Fig. 6c)] in this brain region. Similar to the NAc, MSS significantly upregulated EHMT1 mRNA in the PLC [F(3, 28) = 6.33, p = 0.018, (Fig. 6f)]. EHMT2 expression in the PLC was also dependent on stress exposure, as a significant SDS by MSS interaction was observed in which SDS tended to decrease EHMT2 levels in control animals and increase them in MSS-exposed mice [F(3, 28) = 9.91, p = 0.004, (Fig. 6g)]. Tukey’s post-hoc tests revealed that SDS-MSS-exposed mice had significantly more EHMT2 mRNA in the PLC compared to mice exposed to SDS alone (p < 0.05, Fig. 6g). No significant group differences were observed for the PLC expression of Otx2 (Fig. 6h).
Figure 6.
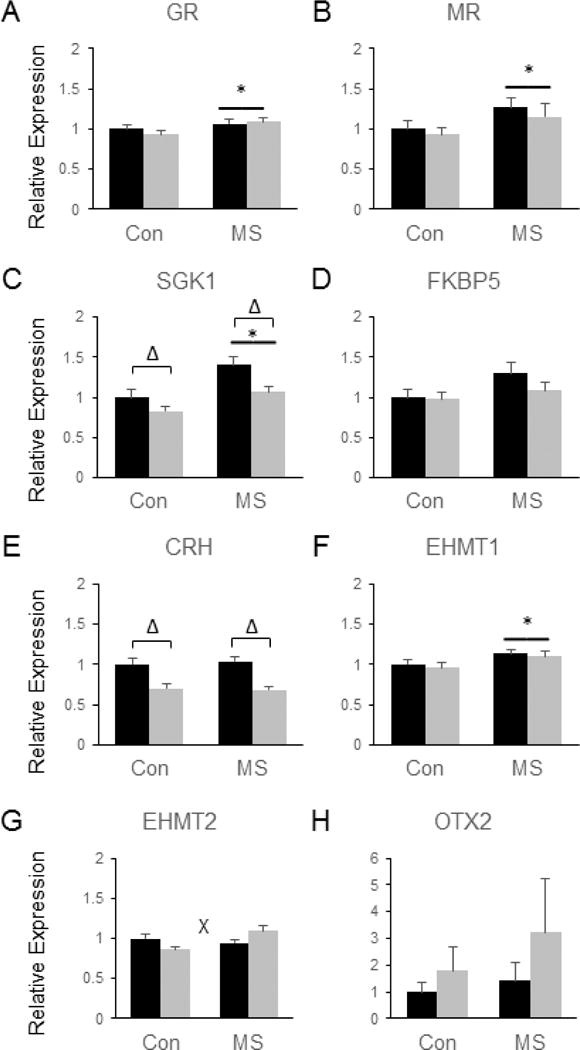
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of genes involved in glucocorticoid signaling and histone methylation in the prelimbic/infralimbic cortex compared to control (Con) animals. Quantification of real-time PCR data using primers specific for GR (A), MR (B), SGK1 (C), FKBP5 (D), CRH (E), EHMT1 (F), EHMT2 (G), and Otx2 (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS, ‘x’ denotes significant MS by SDS interaction by two way ANOVA. N = 8 mice per group.
Several endogenous opioid-related genes were also differentially regulated by SDS and MSS in the PLC. Although no significant group differences were observed in the expression of MOR1 (Fig. 7a) or DOR1 (Fig. 7c), MSS significantly increased FC POMC mRNA [F(3, 28) = 4.8, p = 0.037, (Fig. 7f)]. However, a significant MSS by SDS interaction was also observed for POMC expression [F(3, 28) = 8.11, p = 0.008], and post-hoc tests revealed that all three stress-exposed groups displayed elevated levels of POMC mRNA compared to control animals (p <0.05 by Tukey’s, Fig. 7f). Although PENK expression was not affected by MSS or SDS (Fig. 7d), MSS led to a significant up-regulation of PDYN in the PLC [F(3, 28) = 7.13, p = 0.013, (Fig. 7e)]. In addition, SDS led to a significant increase in KOR1 [F(3, 28) = 4.89, p = 0.035, (Fig. 7b)] and significant reductions in BDNF [F(3, 28) = 6.47, p = 0.017, (Fig. 7g)] and CREB mRNA [F(3, 28) = 8.72, p = 0.006, (Fig. 7h)] in the PLC, but these effects were not modified by MSS.
Figure 7.
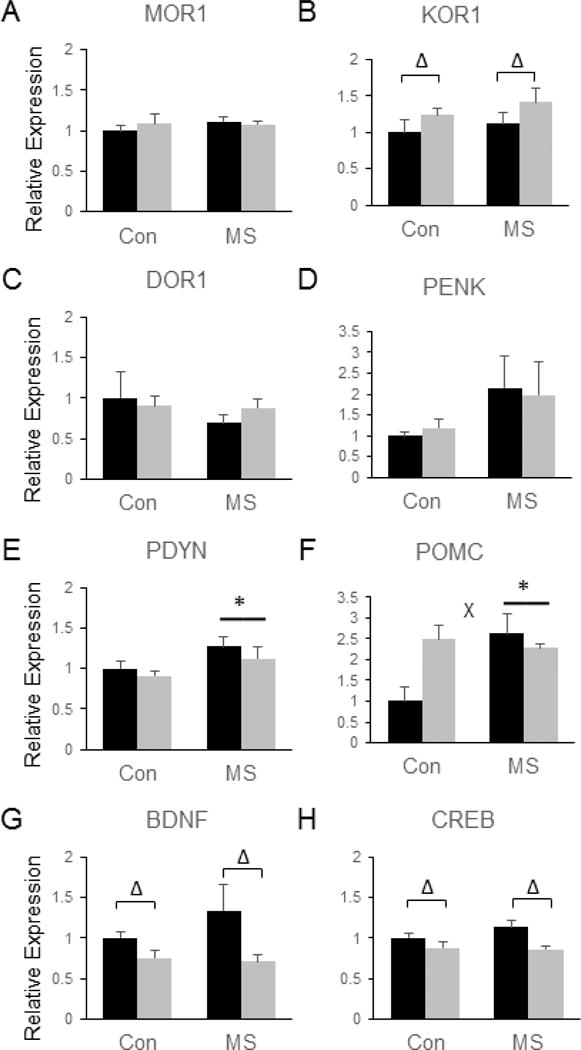
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of genes involved in endorphin and neurotrophin signaling in the prelimbic/infralimbic cortex compared to control (Con) animals. Quantification of real-time PCR data using primers specific for MOR1 (A), KOR1 (B), DOR1 (C), PENK (D), PDYN (E), POMC (F), BDNF (G), and CREB (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS, ‘x’ denotes significant MS by SDS interaction by two way ANOVA. N = 8 mice per group.
Amygdala
The stress-induced changes in gene expression in the Amyg were again distinct from those observed in other brain regions. SDS led to several significant main effects on the expression of β-catenin-related genes in the Amyg. For example, SDS significantly increased the levels of Axin2 [F(3, 28) = 8.38, p = 0.007, (Fig. 8b)] and GSK3β [F(3, 28) = 5.73, p = 0.02, (Fig. 8d)]. SDS also led to a significant decrease in the expression of DVL1 in the Amyg [F(3, 28) = 4.53, p = 0.042, (Fig. 8e)]. However, no significant effects of SDS were observed for Axin-1 (Fig. 8a), DVL-2 (Fig. 8f), DVL-3 (Fig. 8g) or β-catenin itself (Fig. 8h). The only β-catenin-related gene for which a significant main effect of MSS was observed in the Amyg was DVL1, the expression of which was reduced by MSS exposure [F(3, 28) = 6.7, p = 0.015, (Fig. 8 e)].
Figure 8.
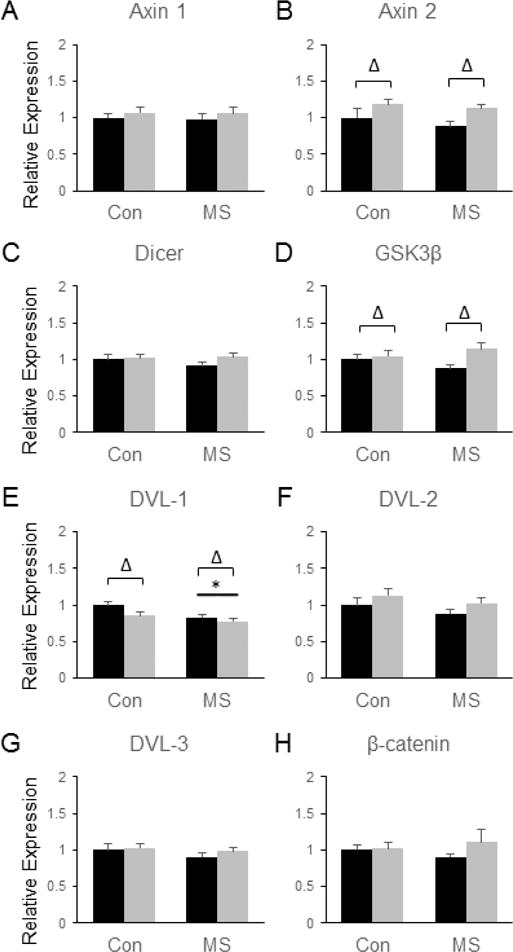
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of β-catenin-related genes in the amygdala compared to control (Con) animals. Quantification of real-time PCR data using primers specific for Axin 1 (A), Axin 2 (B), Dicer (C), GSK3β (D), DVL-1 (E), DVL-2 (F), DVL-3 (G), and β-catenin (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS, ‘x’ denotes significant MS by SDS interaction by two way ANOVA. N = 8 mice per group.
No significant effects of MSS or SDS on GR (Fig. 9a), MR (Fig. 9b), SGK1 (Fig 9c), CRH (Fig. 9e), or Otx2 (Fig. 9h) were observed in the Amyg, but a trend towards increased GR levels following SDS was observed (p = 0.052) (Fig. 9a). SDS was shown to significantly increase the expression of FKBP5 in the Amyg [F(3, 28) = 22.23, p < 0.0001], but MSS did not influence this effect (Fig. 9d). While no significant effects on EHMT1 were observed (Fig. 9f), a main effect of SDS [F(3, 28) = 8.69, p = 0.0064] and a significant MSS by SDS interaction were observed for the expression of EHMT2 in the Amyg [F(3, 28) =4.71, p = 0.039]. Specifically, the EHMT2-increasing effects of SDS were potentiated in MSS-exposed animals (~35% increase) compared to controls (~5% increase, see Fig. 9g).
Figure 9.
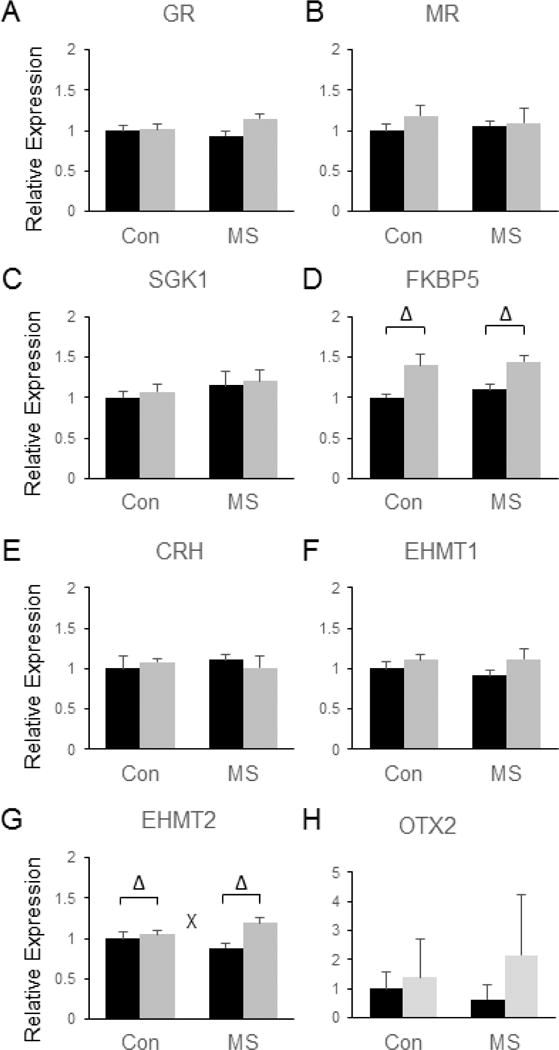
Combined effects of maternal separation (MS) stress and social defeat stress (SDS) on the transcription of genes involved in glucocorticoid signaling and histone methylation in the amygdala compared to control (Con) animals. Quantification of real-time PCR data using primers specific for GR (A), MR (B), SGK1 (C), FKBP5 (D), CRH (E), EHMT1 (F), EHMT2 (G), and Otx2 (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MS, ‘x’ denotes significant MS by SDS interaction by two way ANOVA. N = 8 mice per group.
The endogenous opioid system in the Amyg was quite responsive to SDS, with SDS leading to significant increases in the expression of MOR1 [F(3, 28) = 41.36, p < 0.0001, (Fig. 10a)], KOR1 [F(3, 28) = 51.4, p < 0.0001, (Fig. 10b)], DOR1 [F(3, 26) = 64.97, p < 0.0001, (Fig. 10c)], PENK [F(3, 28) = 16.6, p = 0.0003, (Fig. 10d)], and POMC [F(3, 28) = 8.04, p = 0.008, (Fig. 10f)]. However, no significant effects of SDS were observed on the expression of PDYN (Fig. 10e), BDNF (Fig. 10g), or CREB (Fig. 10h) in the Amyg. In addition, no significant main effects of MSS and no significant MSS by SDS interactions were observed for CREB, BDNF, POMC, PENK, or PDYN genes (Fig. 10).
Figure 10.
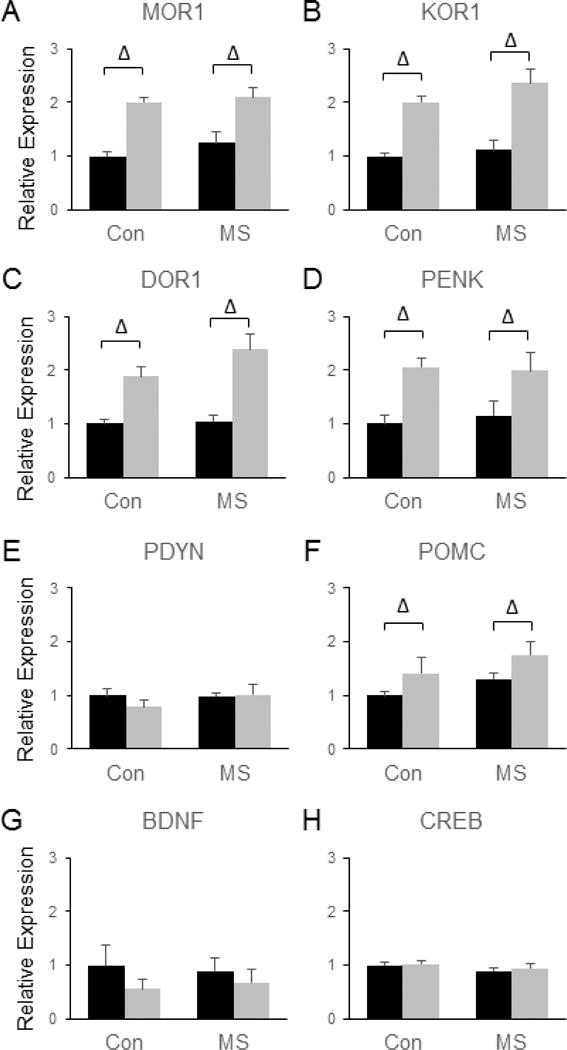
Combined effects of maternal separation stress and social defeat stress on the transcription of genes involved in endorphin and neurotrophin signaling in the amygdala. Quantification of real-time PCR data using primers specific for MOR1 (A), KOR1 (B), DOR1 (C), PENK (D), PDYN (E), POMC (F), BDNF (G), and CREB (H). Black bars show data from control mice not subjected to SDS; grey bars show data from mice exposed to SDS. Δ indicates significant main effect of SDS, * indicates a significant main effect of MSS, ‘x’ denotes significant MSS by SDS interaction by two way ANOVA. N = 8 mice per group.
Discussion
The current study employs two ethologically and translationally relevant behavioral stress paradigms to investigate the long-term molecular consequences of chronic stress exposure. Given that early life stress has been shown to increase vulnerability to SDS (Der-Avakian and Markou, 2010; Pena et al., 2017; Rosenhauer et al., 2017), this study also provides some insight into the molecular correlates of stress susceptibility. Our results reveal that the molecular alterations induced by combined exposure to MSS+SDS are largely distinct from the ‘hallmark’ molecular adaptations that have been described in other populations of susceptible mice. For example, our data demonstrate that the alterations in protein phosphorylation in the NAc that have been reported to differentiate susceptible from resilient c57BL6/J mice following SDS (Krishnan et al., 2007) do not differentiate MSS+SDS-exposed c57BL6/J mice from animals experiencing SDS alone. Similarly, many of the molecular alterations in β-catenin signaling that have been previously shown to distinguish resilient from vulnerable populations (Wilkinson et al., 2011; Dias et al., 2014; Sachs et al., 2015) were not observed in MSS-exposed animals compared to controls following SDS. Given the numerous procedural differences between these various studies, we do not view the current results as failing to replicate prior findings; rather, we interpret the current results to indicate that the specific molecular adaptations that have been previously associated with stress susceptibility or resilience are simply not generalizable across a wide range of environmental and genetic contexts.
Most of the published studies examining the molecular alterations associated with stress susceptibility have employed a single stress paradigm, SDS, in a single strain of mice, most frequently c57BL6/J. Consequently, many of the ‘hallmark’ gene expression and signal transduction changes that have been implicated in SDS susceptibility have been derived from studies comparing genetically identical animals that differ only with respect to phenotype (Berton et al., 2006; Krishnan et al., 2007). Whether putative genetic and environmental risk factors for psychiatric disease lead to similar molecular alterations has not been established. The current study provided some insight into this question by evaluating the effects of early life stress on several SDS-induced changes in gene expression and signal transduction. Incorporating a second stress paradigm during a different developmental stage predictably led to multiple changes in gene expression that were not observed in animals exposed to SDS alone, and thus, it is not entirely surprising that the molecular alterations observed comparing SDS-exposed mice to MSS+SDS-exposed mice are unique from those observed comparing socially avoidant mice exposed to SDS alone to mice who remain sociable following SDS. Going forward, it will be important to evaluate genetic influences on MSS and SDS responses by evaluating whether the molecular alterations observed here also occur in other inbred strains of mice or in genetically modified animals that display altered vulnerability to stress.
In addition to the inclusion of MSS, there are other procedural differences that could account for the differing molecular alterations observed in various populations of ‘SDS susceptible’ animals. For example, the SDS paradigm employed here is distinct from the version that is used by several other groups in that the current paradigm does not involve pair-housing defeated animals in a divided cage with their aggressors. Instead, defeated mice are singly housed between defeat episodes. While single housing is stressful to mice and has been reported to lead to some depression- or anxiety-like phenotypes (Martin and Brown, 2010; Okada et al., 2015; Ieraci et al., 2016), social isolation is likely less stressful than living in continuous sensory contact with aggressors. In addition, the controls in the current study were group housed, whereas some groups report housing their control animals in pairs that are separated by a divider (Golden et al., 2011). Future research will be required to determine which aspects of the stress paradigm (i.e., single housing vs. aggression and social subordination) lead to which molecular and behavioral responses. In addition to differences in housing conditions and the stress paradigm itself, there is also between-lab variability with respect to the timing of behavioral testing. For example, some studies have reported performing SDS and/or behavioral testing during the dark phase (Laman-Maharg et al., 2017), but the current study and many others have performed SDS during the light phase (Krishnan et al., 2007; Sachs et al., 2015). This and other differences in experimental details (e.g., time point of tissue isolation, differences in anatomical location of isolated samples) could also contribute to variability in data between labs.
While the current findings suggest that many of the specific molecular ‘hallmarks’ of stress susceptibility are not universal, our findings did identify significant dysregulation of many of these previously identified signaling pathways following stress. Overall, of the 72 gene expression analyses that we performed (24 candidate genes in each of three brain regions), 39 yielded statistically significant effects, thus suggesting that the major signaling pathways we chose to investigate on the basis of prior work did in fact become dysregulated following MSS and/or SDS. Future studies aimed at determining the functional significance of these signaling alterations will be required to identify which of these pathways might be effective targets for therapeutic intervention. It will also be important to evaluate which environmental or genetic insults actually lead to dysregulation of these pathways to gain a more comprehensive understanding of the situations in which targeting these pathways might be beneficial. In addition, it will be critical to continue to evaluate molecular changes in multiple brain regions. Indeed, a recent study examining SDS-induced transcriptional changes in multiple brain regions has shown that many SDS-induced alterations in gene expression are region-specific (Bagot et al., 2016). In keeping with this, the spatial distribution of mRNA changes observed following MSS and SDS in the current study was not uniform across brain regions. For example, while both SDS and MSS significantly impacted the expression of endogenous opioid system genes, SDS primarily impacted these genes in the Amyg and NAc, not the PLC, whereas MSS affected endogenous opioid system genes in the PLC but not the Amyg or NAc. It is also important to note that stress led to opposing effects on the expression of some genes depending on the brain region. For example, SDS increased POMC expression in the Amyg but decreased its expression in the NAc, a finding that could potentially have important implications for how this system could be targeted for therapeutic purposes.
In addition to examining multiple pathways in multiple brain regions following a variety of different stressors, it will also be critical to evaluate different time points following stress exposure. The current data examined only a single time point following MSS and/or SDS exposure, but it is likely that different results would be obtained depending on the age of the animals and how recently they experienced stress. Indeed, prior research has suggested that Otx2-dependent alterations in transcription in the VTA underlie MSS-induced increases in SDS susceptibility (Pena et al., 2017). However, Pena and colleagues reported no significant effects of MSS on the expression of Otx2 in the VTA of adult mice (Pena et al., 2017). Rather, the Otx2-dependent long-term transcriptional alterations induced by MSS were shown to stem from transient MSS-induced down-regulation of Otx2 that recovers by adulthood (Pena et al., 2017). Future research will be required to determine whether transient alterations in the expression of other genes in response to MSS in the NAc, Amyg, or PLC play an important role in long-term changes in behavior following stress.
Of all the significant effects of MSS and SDS that were observed in the current study, only two (MSS- and SDS-induced decreases in DVL-1 in the Amyg and MSS- and SDS-induced increases in GSK3β in the NAc) were additive in the sense that both stressors exhibited qualitatively similar significant main effects on the same gene in the same brain region. Similarly, MSS and SDS had directly opposing effects on gene expression for only two of the genes examined (DVL-3 and SGK1 in the PLC). In contrast to our initial expectations, no synergistic effects were observed. Of course, it is possible that synergistic effects do occur for genes other than those examined here, but future research will be required to identify which genes, if any, exhibit supra-additive responses to the combination of these stressors. Finally, a total of four significant MSS by SDS interactions were observed (KOR1 and PDYN in the NAc, POMC in the PLC, and EHMT2 in the Amyg) in which MSS significantly impacted transcriptional responses to SDS. Taken together, our results suggest that the influence of MSS on SDS responses is likely mediated through complex mechanisms that involve directly additive, antagonistic and interactive effects on gene expression. In addition to these overlapping effects on gene expression, the independent targeting of different genes within the same pathways (or independent targeting of entirely different pathways) by MSS and SDS could provide another mechanism through which these stressors could coordinately affect brain function and behavior.
Overall, the results from the current study are consistent with the idea that various risk factors for stress susceptibility impact brain function and stress responses through distinct molecular mechanisms. Indeed, the current examination of MSS-exposed animals revealed little evidence of most of the specific transcriptional and signaling alterations that have been previously identified in other ‘SDS-susceptible’ populations (Krishnan et al., 2007; Wilkinson et al., 2011). However, the fact that both MSS and SDS did lead to significant dysregulation of the histone methylation, endogenous opioid, BDNF-CREB, β-catenin, and glucocorticoid pathways does highlight the potential importance of these pathways in the regulation of stress responses. Continuing to elucidate the precise molecular mechanisms through which various risk factors for mental illness impact the brain may provide new insight into the etiology of mental illness and may allow for the development of rational targets for therapeutic intervention.
Highlights.
-
-
Endogenous opioid signaling is significantly altered by repeated stress exposure.
-
-
SDS and MSS both lead to dysregulation of β-catenin signaling.
-
-
SDS and MSS lead to both additive and antagonistic effects on gene expression.
-
-
Transcriptional stress responses are highly brain region specific.
Acknowledgments
We would like to thank Wendy Roberts for excellent technical assistance and our funding sources for providing the means to do this work.
Funding: This work was supported in part the National Institutes of Health (MH79201 to M.G.C.) and Villanova University. These sponsors had no part in the design, collection, analysis or interpretation of the data.
Abbreviations
- Amyg
amygdala
- BDNF
brain derived neurotrophic factor
- CREB
cAMP-response element binding protein
- CRH
corticotropin-releasing hormone
- DOR1
delta opioid receptor 1
- DVL
disheveled
- FKBP5
FK506 Binding Protein 5
- GR
glucocorticoid receptor
- KOR1
kappa opioid receptor 1
- MSS
maternal separation stress
- MR
mineralocorticoid receptor
- MOR1
mu opioid receptor 1
- NAc
nucleus accumbens
- Otx2
orthodenticle homeobox 2
- PNDs
post-natal days
- PLC
prelimbic cortex
- PENK
proenkephalin
- PDYN
prodynorphin
- POMC
pro-opiomelanocortin
- SGK1
serum/glucocorticoid-regulated kinase 1
- SDS
social defeat stress
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions:
BDS and MGC designed and supervised the study. BDS and HLT performed the stress paradigms. BDS isolated and processed tissue samples. HLT performed and analyzed the western blotting experiments. EF processed some tissue samples and performed real-time PCR experiments. BDS also performed and analyzed real-time PCR experiments. BDS, HLT, EF and MGC wrote the manuscript.
References
- Arloth J, Bogdan R, Weber P, Frishman G, Menke A, Wagner KV, Balsevich G, Schmidt MV, Karbalai N, Czamara D, Altmann A, Trümbach D, Wurst W, Mehta D, Uhr M, Klengel T, Erhardt A, Carey CE, Conley ED, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (PGC) Ruepp A, Müller-Myhsok B, Hariri AR, Binder EB. Genetic Differences in the Immediate Transcriptome Response to Stress Predict Risk-Related Brain Function and Psychiatric Disorders. Neuron. 2015;86:1189–1202. doi: 10.1016/j.neuron.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, Huang X, Schluter OM, Maze I, Pena CJ, Heller EA, Issler O, Wang M, Song WM, Stein JL, Liu X, Doyle MA, Scobie KN, Sun HS, Neve RL, Geschwind D, Dong Y, Shen L, Zhang B, Nestler EJ. Circuit-wide Transcriptional Profiling Reveals Brain Region-Specific Gene Networks Regulating Depression Susceptibility. Neuron. 2016;90:969–983. doi: 10.1016/j.neuron.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiat. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Berube P, Laforest S, Bhatnagar S, Drolet G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol Behav. 2013;122:237–245. doi: 10.1016/j.physbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrino. 2009;34(Suppl 1):S186–195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, Dietz DM, Lobo MK, Ghose S, Mouzon E, Neve RL, Tamminga CA, Nestler EJ. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71:656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrino. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R, LaBonte B, Ribeiro E, Liu X, Kennedy P, Vialou V, Ferguson D, Peña C, Calipari ES, Koo JW, Mouzon E, Ghose S, Tamminga C, Neve R, Shen L, Nestler EJ. beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA., Jr Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol. 2015;26:654–663. doi: 10.1097/FBP.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiat. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Mallei A, Popoli M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016;2016 doi: 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res. 2014;1583:109–121. doi: 10.1016/j.brainres.2014.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems J, Teegarden SL, Chen Y, Boulden J, Challis C, Ben-Dor GA, Kim SF, Berton O. Enhancement of stress resilience through histone deacetylase 6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol Psychiat. 2015;77:345–355. doi: 10.1016/j.biopsych.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hultman R, Hughes D, Michel N, Katz BM, Dzirasa K. Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nat Commun. 2014;5:4537. doi: 10.1038/ncomms5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg AR, Copeland T, Sanchez EO, Campim KL, Trainor BC. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behav Brain Res. 2017;332:299–307. doi: 10.1016/j.bbr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res. 2010;207(1):196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacol. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP., Jr Prolonged effects of repeated social defeat stress on mRNA expression and function of mu-opioid receptors in the ventral tegmental area of rats. Neuropsychopharmacol. 2005;30:1096–1103. doi: 10.1038/sj.npp.1300658. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Okada R, Fujiwara H, Mizuki D, Araki R, Yabe T, Matsumoto K. Involvement of dopaminergic and cholinergic systems in social isolation-induced deficits in social affiliation and conditional fear memory in mice. Neuroscience. 2015;299:134–45. doi: 10.1016/j.neuroscience.2015.04.064. [DOI] [PubMed] [Google Scholar]
- Pena CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, Issler O, Loh YE, Leong T, Kiraly DD, Goodman E, Neve RL, Shen L, Nestler EJ. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356:1185–1188. doi: 10.1126/science.aan4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhauer AM, McCann KE, Norvelle A, Huhman KL. An acute social defeat stressor in early puberty increases susceptibility to social defeat in adulthood. Horm Behav. 2017;93:31–38. doi: 10.1016/j.yhbeh.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiat. 2013a;3:e291. doi: 10.1038/tp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Rodriguiz RM, Siesser WB, Kenan A, Royer EL, Jacobsen JP, Wetsel WC, Caron MG. The effects of brain serotonin deficiency on behavioural disinhibition and anxiety-like behaviour following mild early life stress. Int J Neuropsychopharmacol. 2013b;18(4):1–14. doi: 10.1017/S1461145713000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Ni JR, Caron MG. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc Natl Acad Sci USA. 2015;112:2557–62. doi: 10.1073/pnas.1416866112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson EA, Spiers H, Mifsud KR, Gutierrez-Mecinas M, Trollope AF, Shaikh A, Mill J, Reul JM. Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proc Natl Acad Sci U S A. 2016;113:4830–4835. doi: 10.1073/pnas.1524857113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KV, Marinescu D, Hartmann J, Wang XD, Labermaier C, Scharf SH, Liebl C, Uhr M, Holsboer F, Muller MB, Schmidt MV. Differences in FKBP51 regulation following chronic social defeat stress correlate with individual stress sensitivity: influence of paroxetine treatment. Neuropsychopharmacol. 2012;37:2797–2808. doi: 10.1038/npp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer CP, Noailles PA, Angulo JA, Barr GA. Stress-induced preproenkephalin mRNA expression in the amygdala changes during early ontogeny in the rat. Neuroscience. 2002;114:7–11. doi: 10.1016/s0306-4522(02)00268-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, Dietz D, Covington H, 3rd, Russo S, Neve R, Ghose S, Tamminga C, Nestler EJ. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


