Abstract
Neurotransmitter release occurs at active zones, which are specialized regions of the presynaptic membrane. A dense collection of proteins at the active zone provides a platform for molecular interactions that promote recruitment, docking, and priming of synaptic vesicles. At mammalian neuromuscular junctions (NMJs), muscle-derived laminin β2 interacts with presynaptic voltage-gated calcium channels to organize active zones. The molecular architecture of presynaptic active zones has been revealed using super-resolution microscopy techniques that combine nanoscale resolution and multiple molecular identification. Interestingly, the active zones of adult NMJs are not stable structures and thus become impaired during aging due to the selective degeneration of specific active zone proteins. This review will discuss recent progress in the understanding of active zone nanoarchitecture and the mechanisms underlying active zone organization in mammalian NMJs. Furthermore, we will summarize the age-related degeneration of active zones at NMJs, and the role of exercise in maintaining active zones.
Keywords: active zone, exercise, neuromuscular junctions, aging, STED, super resolution microscopy
1. Introduction
A neuromuscular junction (NMJ) is a chemical synapse formed between a presynaptic motor neuron and a postsynaptic muscle cell. These synapses ensure efficient signaling between the two cells through the regulated release of neurotransmitters. The exocytotic release of neurotransmitters occurs at specific sites on the presynaptic membrane that are termed active zones. At the ultrastructural level, active zones appear as electron-dense projections on the presynaptic membrane where synaptic vesicles fuse for subsequent exocytosis (Couteaux and Pecot-Dechavassine, 1970; Tsuji, 2006). Electron microscope tomography has revealed that active zones in frog NMJs are elongated structures, and the way synaptic vesicles attach to the active zone makes them appear as “pearls on a string” (Harlow et al., 2001). Active zones in mammalian NMJs are much smaller than those of frog NMJs and are distributed in a discrete and scattered pattern within each presynaptic terminal (Nagwaney et al., 2009; Rowley et al., 2007). Presynaptic active zones perform the following functions involved in accurate and efficient neurotransmitter release: (1) dock and prime synaptic vesicles; (2) recruit voltage-gated calcium channels (VGCCs); (3) contribute to the precise, exactly opposite locations of pre- and postsynaptic specializations; and (4) mediate presynaptic plasticity (Südhof, 2012). The first three concepts are relevant for the active zones of NMJs. This review will focus on recent findings regarding active zones at mammalian NMJs. We will briefly describe what has been shown at the NMJs of other species and at other synapses. Presynaptic active zones of the central nervous system (CNS) have been reviewed extensively elsewhere (Fejtova and Gundelfinger, 2006; Gundelfinger et al., 2016; Südhof, 2012). Our goal is also to highlight recent insights into the distribution patterns of active zone proteins in mammalian NMJs using findings from super-resolution microscopy and the selective degeneration of active zone proteins in aged animals. Analysis of active zones using super-resolution microscopy at Drosophila NMJs will be described in a separate review article by Robert Kittel in this special issue.
2. Analysis of active zone nanoarchitecture and ultrastructure
Active zones were first described using transmission electron microscopy. They were characterized as electron-dense projections to which synaptic vesicles attach (Couteaux and Pecot-Dechavassine, 1970). Electron micrographs of rodents and frogs revealed that NMJ active zones appear as triangular electron-dense projections that extend from the presynaptic membrane into the cytosol (Couteaux and Pecot-Dechavassine, 1970; Hirokawa and Heuser, 1982; Nishimune et al., 2004; Rowley et al., 2007). Three-dimensional reconstructions of serial electron micrographs of rat NMJs revealed the location of synaptic vesicle pools that were clustered in close proximity to the active zones (Rowley et al., 2007). Transmission electron microscopy of Drosophila NMJs also showed electron-dense projections at the presynaptic membranes, which appear as T-shaped structures. These projections, named T-bars, also recruit vesicles to the active zones (Kittel et al., 2006). Electron micrographs of CNS synapses from an en face view show active zones as hexagonal grids of dense projections with a web-like pattern (Gray, 1963). These projections found in CNS synapses are formed by an array of electron-dense, cone-shaped particles that project into the cytoplasm from the presynaptic membranes (Gray, 1963; Limbach et al., 2011; Pfenninger et al., 1972). The authors suggest that these dense projections represent an underlying molecular organization of the active zone material and are not artificially induced by fixation (Limbach et al., 2011; Pfenninger et al., 1972). However, whether the exact ultrastructural appearance and quantitative parameters are influenced by specimen processing remains unknown (Limbach et al., 2011).
Subsequent freeze-fracture electron microscopy studies revealed the membrane-embedded structure of active zones at a macromolecular resolution in an en face view orientation. In human, mouse, rat, and lizard NMJs, parallel rows of large intramembranous particles (10 – 12 nm in diameter) were seen on the cytosolic half of the plasma membrane (the P-face of freeze-fracture electron micrographs) (Ellisman et al., 1976; Fukunaga et al., 1982; Fukuoka et al., 1987b; Walrond and Reese, 1985). Based on these data, an active zone unit of mammalian NMJs has been proposed as consisting of a parallel array of 20 intramembranous particles arranged in four rows (Ellisman et al., 1976). These active zone units were observed in large numbers and were distributed in discrete locations within the presynaptic membrane of NMJs. However, the organization of the large intramembranous particles on the P-face was different at frog NMJs (Heuser et al., 1979; Heuser et al., 1974). At frog NMJs, an active zone was identified as a continuum of the intramembranous particles that nearly spanned the width of a nerve terminal branch (Ko, 1985). The existence of these active zone units defined by these intramembranous particles has been further supported by the tomography analyses described below.
Electron microscope tomography revealed the first three-dimensional structure of the cytoskeletal matrix of the active zones (CAZ) of frog NMJs (Harlow et al., 2001). The CAZ is a collection of proteins that constitute an active zone (see “NMJ active zone assembly and organization” for details) (Dresbach et al., 2001). The macromolecule components of the active zones identified by electron microscope tomography were named “beams,” “ribs,” and “pegs,” and they were connected to each other and arranged along the midline of the presynaptic ridge (see Figure 5 of reference (Harlow et al., 2001)). Based on the frequency and distribution of the pegs, the authors suggested that pegs are putative VGCCs embedded in the presynaptic membrane. A recent electron microscope tomography analysis of frog NMJs further revealed that an active zone has three sublayers: the superficial layer and the intermediate layer, both of which are approximately 15 nm thick, and the deepest layer, which is up to 45 nm thick (Szule et al., 2012). The superficial layer contains the previously described beam-rib-peg assembly and is followed by the intermediate layer, which contains macromolecules named steps and spars. The deepest layer includes macromolecules termed masts, booms and topmasts (Szule et al., 2012). These macromolecules are arranged in a highly ordered ultrastructure that interacts with synaptic vesicles at multiple points on an active zone (see Figure 2 of reference (Szule et al., 2012)).
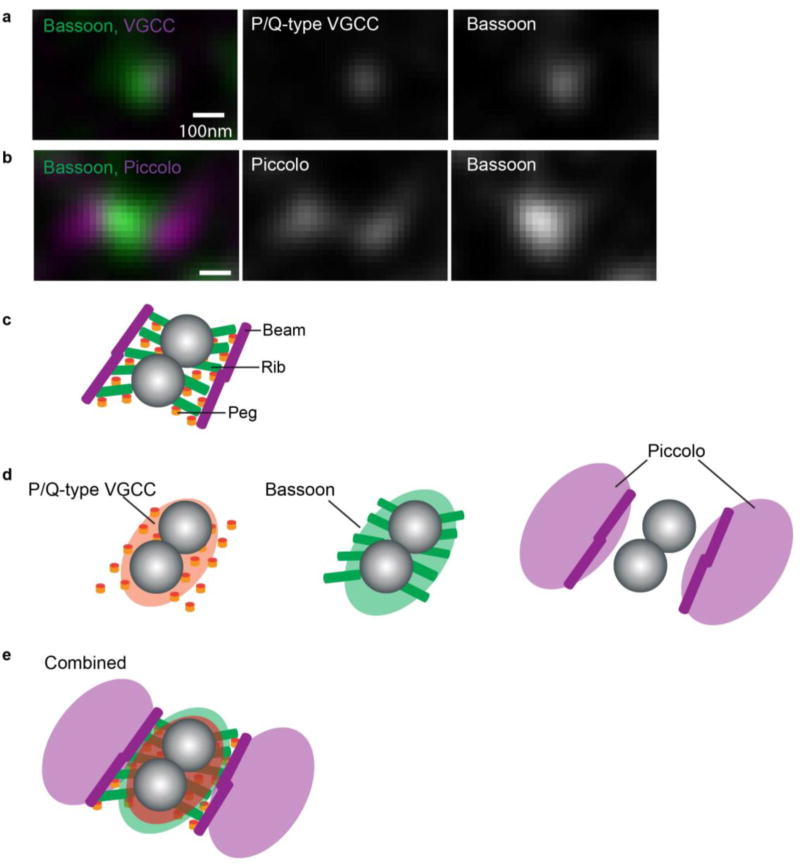
An electron microscope tomography analysis of mouse NMJs identified a similar highly ordered ultrastructure with the beam-rib-peg assemblies and two docked synaptic vesicles (Nagwaney et al., 2009). The macromolecules of mouse active zones showed a bilateral arrangement relative to the two docked synaptic vesicles (Figure 1). The numbers and distribution patterns of the pegs were very similar to the parallel rows of the large intramembranous particles that were identified as an active zone unit by freeze-fracture electron microscopy analysis of mouse NMJs. The macromolecules of mouse active zones show a bilateral arrangement, whereas the macromolecules of frog active zones show a unilateral arrangement relative to the docked vesicles (see Figure 8 of reference (Nagwaney et al., 2009) for a comparison of the arrangement of macromolecular connections in the active zones of mouse versus frog NMJs). This bilateral arrangement in mice may favor more efficient vesicle docking and/or fusion (Nagwaney et al., 2009). The authors observed only two primary docked vesicles per active zone and further predicted that there are approximately 450 primary docked vesicles per mouse NMJ. The beam-rib-peg complexes of frog and mouse NMJs are significantly different from that of CNS synapses.
Figure 1.
STED microscopy has revealed the distribution patterns of active zone proteins in mouse neuromuscular junctions (NMJs). This Figure (a–e) is reproduced with modifications from Nishimune et al., 2016. (a) Dual-color STED micrographs showing Bassoon (green) and P/Q-type VGCCs (magenta) overlapping at the NMJ. (b) Dual-color STED micrographs demonstrating the side-by-side distribution pattern of Piccolo (magenta) and Bassoon (green). (c) The active zone structure in mouse NMJs revealed by electron microscope tomography adapted from Nagwaney et al., 2009, Journal of Comparative Neurology (reprinted by permission of John Wiley & Sons, Inc., license number: 4097730642797). (d, e) A theoretical overlay of the active zone protein distribution pattern identified using STED microscopy on the active zone structure revealed by electron tomography. The “pegs” in Nagwaney’s model represent P/Q-type VGCCs, the “beams” represent Bassoon, and the “ribs” represent Piccolo.
Electron microscope tomography analyses of neocortical synapses revealed an array of polyhedral cages approximately 60 nm in diameter at active zones (Zampighi et al., 2008). Previously, filamentous proteins protruding from presynaptic membranes were observed using rapid freezing combined with freeze-etched electron microscopy (Landis et al., 1988). Synaptosomes prepared from adult rat cortices were stained with ethanolic phospho-tungstic acid (EPTA) and analyzed using electron microscopy. These micrographs clearly showed a presynaptic particle web formed by electron-dense protrusions from the membrane that were connected with thin linkers (Phillips et al., 2001). This ultrastructure of CNS active zones detected using EPTA staining was somewhat similar to the distribution pattern of polyhedral cages revealed by the tomography method (Figure 8 of reference (Phillips et al., 2001) and Figure 10 of reference (Zampighi et al., 2008)). Hair cell ribbon synapses analyzed using electron microscope tomography revealed a ribbon attached to a presynaptic density, which are located in the center of two rows of docked synaptic vesicles (Frank et al., 2010). This structure was closer to, but not the same as, the frog NMJ active zone. There seem to be ultrastructural differences between the active zones of CNS synapses, hair cell synapses, and NMJs (see Figure 1 of (Zhai and Bellen, 2004) or Figure 1 of (Ackermann et al., 2015) or Figures 6 and 7 of (Slater, 2015) for descriptions of the different active zone morphologies in various species and synapses). Despite the structural heterogeneity between these synapses and NMJs, these active zones function similarly to facilitate the docking of synaptic vesicles to the presynaptic membrane.
The freeze-fracture electron microscopy and electron microscope tomography analyses revealed the ultrastructure of active zones as described in the sections above. Furthermore, immunoelectron microscopy with the SDS-digested freeze-fracture replica labeling method has revealed the clustered distribution pattern of P/Q-type VGCCs within the intramembrane particles of the presynaptic membrane at rodent cerebellar synapses and calyx of Held synapses (Indriati et al., 2013; Nakamura et al., 2015; Ritzau-Jost et al., 2014). These results are in agreement with the observation that an active zone unit contains a cluster of 20 intramembrane particles at NMJs (Ellisman et al., 1976). Immunoelectron microscopy was used to analyze the distribution of active zone proteins at the ultrastructural level in CNS and sensory neuron synapses (Dieck et al., 1998; Limbach et al., 2011; Tao-Cheng, 2006, 2007; tom Dieck et al., 2005). However, there are only a few reports of analysis of NMJ active zones using immunoelectron microscopy (Fukuoka et al., 1987a; Juranek et al., 2006; Mizoguchi et al., 1992) and the precise ultrastructural distribution pattern of CAZ proteins within an active zone unit has not been reported using these electron microscopy techniques.
Super-resolution microscopy techniques have greatly improved the resolving power of light microscopy to under 50 nm compared to the diffraction-limited resolution of approximately 200 nm using confocal microscopy. Super resolution microscopy methods with a resolving power below 50 nm include Stimulated Emission Depletion (STED) microscopy, PhotoActivated Localization Microscopy (PALM), and STochastic Optical Reconstruction Microscopy (STORM) (Betzig et al., 2006; Hell and Wichmann, 1994; Rust et al., 2006). These techniques have been utilized to reveal the molecular architecture of presynaptic terminals (Dani et al., 2010; Ehmann et al., 2014; Kittel et al., 2006; Willig et al., 2006). These techniques are easier than electron microscopy-based methods for analyzing the relative locations of two or more proteins in a large number of synapses from multiple animals (Nishimune et al., 2016).
The first study of active zone proteins using super-resolution microscopy analyzed Drosophila NMJs (Kittel et al., 2006). STED microscopy revealed a doughnut-like distribution pattern of Bruchpilot protein at the active zones of Drosophila NMJs. Further analysis using STED microscopy demonstrated that the N-terminal epitope of Bruchpilot localized within the “doughnut hole” of the C-terminal epitope of Bruchpilot (Fouquet et al., 2009). The N- and C-terminal epitopes of Bruchpilot were separated along an axis vertical to the presynaptic membrane, with its N terminus closer to the presynaptic membrane than its C terminus. These data suggested that individual Bruchpilot proteins can adopt an elongated bouquet-like conformation, which shapes the T-bar structure (Fouquet et al., 2009). Calcium channels (Calcium channel α1 subunit, Cacophony) and Rab3-interacting molecule-binding proteins are essential for neurotransmitter release at Drosophila NMJs, and their locations relative to Bruchpilot have also been revealed using STED microscopy (Fouquet et al., 2009; Liu et al., 2011a). A detailed description of the super-resolution microscopy analysis of Drosophila active zones are discussed by Robert Kittel in a separate review in this special issue.
Active zone-specific proteins at mammalian NMJs were recently analyzed for the first time using super-resolution microscopy (Nishimune et al., 2016). Our STED microscopy analysis of mouse NMJs revealed punctate and discrete distribution patterns of two active zone proteins, Bassoon and the P/Q-type VGCC, with the two proteins co-localizing together. This is consistent with the protein interaction analysis that showed Bassoon interacting with P/Q-type VGCC β subunit (Chen et al., 2011). In addition, we identified that the active zone protein Piccolo does not co-localize with Bassoon (Figure 1). Piccolo puncta sandwiched a Bassoon punctum in a side-by-side pattern. There were many Piccolo-Bassoon-Piccolo structure units within a single NMJ, which suggested these may be functional units of an active zone (Nishimune et al., 2016). This distribution pattern of Bassoon and Piccolo at subdiffraction-limited resolution was not previously known and could not be resolved using confocal microscopy analyses of mouse NMJs. The epitopes close to the N and C termini of Bassoon colocalized and were not present in a bouquet pattern, as Bruchpilot was at Drosophila NMJs (Nishimune et al., 2016). However, the punctate distribution pattern of VGCCs remained similar between mouse and Drosophila active zones, in addition to the colocalization of VGCC puncta with active zone-specific proteins (Bassoon and Bruchpilot). On the postsynaptic side of the mouse NMJ, accumulation of acetylcholine receptors at the mouth of postsynaptic junctional folds and in front of the active zones were revealed by 3D-STORM analysis (York and Zheng, 2017).
In the CNS, super-resolution microscopy has also been used to reveal the relative location of the active zone proteins and pre- and postsynaptic proteins in the mouse hippocampus, main olfactory bulb, calyx of Held, and ribbon synapses (Chamma et al., 2016; Dani et al., 2010; Dzyubenko et al., 2016; Kempf et al., 2013; Wong et al., 2014). Analyses using 3D-STORM revealed a discrete punctate distribution pattern of Bassoon in GABAergic inhibitory interneuron synapses in the mouse hippocampus (Dudok et al., 2015). This pattern was similar to the Bassoon distribution pattern at mouse NMJs that we found using STED microscopy (Nishimune et al., 2016) and to the Bassoon distribution pattern in mouse hippocampal synapses identified using confocal microscopy (Bednarek and Caroni, 2011). Furthermore, 3D-STORM analysis of excitatory synapses of the mouse main olfactory bulb revealed similarities to the non-colocalizing distribution of Piccolo and Bassoon at mouse NMJs (Dani et al., 2010). The connection between this ultrastructure information and functional studies of the synapses will be described in the next section.
3. Correlations between active zone ultrastructure and synaptic function
NMJs have been described as strong and reliable synapses, and these two features allow for the efficient, consistent, and finely tuned movement of body parts (Meriney and Dittrich, 2013). The NMJ is a reliable synapse because it releases neurotransmitters in excess of that which is required to excite and cause contraction of postsynaptic muscle cells (Wood and Slater, 2001). Early observations showed that the size of active zones correlates with the quantal content or the number of synaptic vesicles released by the presynaptic terminal of frog NMJs (Propst and Ko, 1987). Subsequent analyses of mouse NMJs suggested that these synapses are assembled using low probability release sites (Meriney and Dittrich, 2013; Nagwaney et al., 2009). We previously showed that adult mouse NMJs contain 600 – 800 active zones (Chen et al., 2012), and Nagwaney and others showed that each active zone has 2 docked synaptic vesicles (Nagwaney et al., 2009). Therefore, a mouse NMJ may contain approximately 1200 – 1600 docked vesicles in the readily releasable pool. However, following an action potential, only approximately 60 – 80 vesicles are released (Ruiz et al., 2011; Wang et al., 2004), suggesting that approximately 5% of active zones release synaptic vesicles (Tarr et al., 2013). Several other studies have shown that only a subpopulation of NMJ active zones are used per action potential for synaptic transmission, partly due to the low release probability (Luo et al., 2015; Luo et al., 2011; Peled and Isacoff, 2011; Ruiz et al., 2011; Wang et al., 2010; Wu and Betz, 1999; Wyatt and Balice-Gordon, 2008). Interestingly, Luo and others used a novel high-resolution calcium fluorescence imaging and single-pixel optical fluctuation analysis to estimate the opening probability of individual VGCCs at frog NMJs. They concluded that the low probability of vesicle release is due to the low probability (approximately 0.2) of opening of individual calcium channel at frog active zones (Luo et al., 2011). This conclusion is consistent with the results at mouse NMJs that used electrophysiological estimates of the readily releasable pool size in a model of sequential depletion and refilling of vesicles (Ruiz et al., 2011). Taken together, these studies led to the hypothesis that transmitter release at the NMJ is controlled by the assembly of many low-probability vesicle release sites (Tarr et al., 2013).
4. The role of synaptic activity and exercise in maintaining active zone density
The mammalian NMJ contains multiple active zones that are distributed in a discrete pattern. Active zone density at NMJs is preserved in mammals and remains relatively constant during postnatal development (Chen et al., 2012). During the first two months of mouse postnatal development, there is a 3.3-fold increase in the size of NMJs and a similar increase in the active zone number. However, the density of active zones at these NMJs remained constant at 2.3 active zones/µm2 (Chen et al., 2012). Similar maintenance of active zone density has also been shown at Drosophila NMJs during the larval stage and at rat calyx of Held synapses between postnatal day (P) 9 and P21 (Dondzillo et al., 2010; Graf et al., 2009; Meinertzhagen et al., 1998; Reiff et al., 2002; Sigrist et al., 2002). Maintaining a constant density of active zones at synapses has the potential advantage of ensuring efficient synaptic transmission because a regulated distance between active zones in the presynaptic terminal ensures their access to synaptic vesicles and calcium buffering systems (Chen et al., 2012; Llinas et al., 1992; Neher, 1998). Active zone density is an essential parameter for maintaining synaptic transmission efficiency.
Meanwhile, does synaptic activity influence the organization and density of active zones? For the initial formation of active zones, synaptic activity does not seem necessary. At embryonic mouse NMJs that lack synaptic transmission due to a null mutation of choline acetyltransferase, active zone density is unaltered compared to wild-type control NMJs (Misgeld et al., 2002).
Differences in motor neuron firing rates do not seem to alter the active zone density at NMJs during the maturation stage of NMJs, either. Muscle fibers acquire slow or fast fiber type characteristics during development (Condon et al., 1990; Narusawa et al., 1987) and the motor neurons innervating them are classified based on the contractile properties of the motor units (Burke et al., 1973; Kanning et al., 2010). Therefore, these fast and slow motor neurons have different firing rates and activities (Gardiner, 1993; Lee and Heckman, 1998). However, the NMJ active zone densities of these fast and slow motor neurons were similar in the rat diaphragm (Rowley et al., 2007), suggesting that differences in synaptic activity levels do not influence active zone density.
During aging, pre- and postsynaptic NMJ morphology is altered in humans and rodents (Arizono et al., 1984; Fahim et al., 1983; Fahim and Robbins, 1982; Li et al., 2011; Smith and Rosenheimer, 1982; Valdez et al., 2010; Valdez et al., 2012). In mice, partial denervation of NMJs begins at 12 months of age and fully denervated NMJs are present at 18 months of age. By 24 months of age, the number of fully denervated NMJs appears significantly higher compared to that of young-adult NMJs (Valdez et al., 2010). This is accompanied by a reduced number of synaptic vesicles and an altered synaptic transmission efficiency (Banker et al., 1983; Fahim, 1997; Fahim et al., 1987; Gutmann et al., 1971; Jacob and Robbins, 1990; Kelly and Robbins, 1983; Smith, 1988). Specifically, mEPP frequency decreased (Banker et al., 1983; Gutmann et al., 1971; Smith, 1988), but mEPP amplitude and input resistance increased with aging (Banker et al., 1983; Fahim, 1997). EPP amplitude increased for the first EPP in a train of stimuli, and the quantal content showed a matching increase at aged NMJs. However, EPP amplitude decreased after repetitive stimulation to the plateau level (Banker et al., 1983; Fahim, 1997). The age-related changes in NMJ synaptic transmission are described in Table 1.
Table 1.
Aging-related changes in active zones at mammalian NMJs
| Parameter | Effect of aging |
|---|---|
| Density of presynaptic structures | |
| Active zone density | Decreases (Chen et al., 2012) |
| Synaptic vesicle density | Decreased at nerve terminals (Banker et al., 1983; Fahim and Robbins, 1982) |
| Active zone-specific proteins | |
| Bassoon | Protein level per synapse area and puncta density decreases (Chen et al., 2012; Nishimune et al., 2016; Nishimune et al., 2012) |
| ELKS1 (CAST2/ERC1) | Unknown |
| Munc13 | Unknown |
| Piccolo | No significant change in protein level and puncta density per synapse area (Nishimune et al., 2016) |
| P/Q-type VGCC | Protein level per synapse area decreases with no significant change in puncta density (Nishimune et al., 2016) |
| RIM 1/2 | Unknown |
| Synaptic transmission | |
| Miniature end-plate potentials (mEPP) frequency | Decreased in mouse EDL, soleus, and EDC muscles (Banker et al., 1983), and rat levator ani muscles (Gutmann et al., 1971) |
| No change in mouse GM and diaphragm (Banker et al., 1983), mouse soleus (Jacob and Robbins, 1990), rat EDL, diaphragm, and soleus (Smith, 1988) | |
| mEPP amplitude | Increased in mouse GM muscles (Fahim, 1997) and diaphragm (Banker et al., 1983) |
| No change in EDL, soleus or GM muscle (Banker et al., 1983) | |
| End-plate potentials (EPP) | Increased amplitude for the first EPP of a train, decreased amplitude after repetitive stimulation (plateau level), in mouse EDL, soleus, and GM muscles (Banker et al., 1983; Fahim, 1997) |
| Quantal release | Increased for the first EPP of a train (Banker et al., 1983; Fahim, 1997) |
| Input resistance | Increased in mouse GM, EDL, and soleus, no change in diaphragm (Banker et al., 1983; Fahim, 1997) |
EDC= extensor digitorum communis; EDL= extensor digitorum longus; GM= gluteus maximus; VGCC= voltage-gated calcium channel
Heterogeneity of the aging process complicates the interpretation of these electrophysiology findings; however, the decrease in mEPP frequency and EPP amplitude suggest a decrease in the number or function of NMJ active zones in aged animals. Consistently, the active zone-specific protein Bassoon was under the detection limit at many aged mouse and rat NMJs (27- and 24-month-old, respectively) (Chen et al., 2012; Nishimune et al., 2012). These data indicate that NMJ active zones are not stable structures and can degenerate during aging. In addition, this loss of Bassoon occurred before the denervation of aged NMJs, suggesting that a loss of active zones may play a role in age-dependent NMJ denervation.
In aged animals, synaptic activity is necessary for the maintenance of active zone density. Interestingly, exercise has been shown to restore presynaptic proteins and ameliorate the active zone impairment of aged NMJs. In rodents, resistance exercise ameliorated the loss of Bassoon at the NMJs of 24-month-old aged rats (Nishimune et al., 2012). This is consistent with the improvements in NMJ function that were observed using electrophysiology in aged mice after endurance training (Fahim, 1997). Additionally, other beneficial effects of exercise have been demonstrated for NMJ maintenance and for reversing age-related changes at rodent NMJs (for review see (Booth et al., 2002; English et al., 2011; Handschin and Spiegelman, 2008; Houle and Cote, 2013; Nishimune et al., 2014; Panenic and Gardiner, 1998; Vandervoort, 2002; Wilson and Deschenes, 2005)). This includes a decrease in the incidence of denervation and fragmentation of postsynaptic structures as a result of exercise and caloric restriction (Valdez et al., 2010). These data suggest that synaptic activity has different roles in the organization of active zone density during different life stages of the animal.
Activity-dependent homeostatic synaptic plasticity can also occur in response to altered pre- or postsynaptic function in order to maintain constant synaptic transmission (Davis and Muller, 2015). In mammalian NMJs, an example of this homeostatic process has been observed in studies of the neuromuscular disease myasthenia gravis, which is characterized by a loss or impairment of postsynaptic acetylcholine receptors. The associated reduction in postsynaptic sensitivity results in a compensatory increase in quantal content to restore the amplitude of excitatory postsynaptic potentials and to maintain muscle excitation (Cull-Candy et al., 1980; Plomp et al., 1995; Plomp et al., 1992). Modification of active zone function may underlie this homeostatic plasticity. At the Drosophila NMJ, recent progress has been made towards identifying molecules that play a role in homeostatic plasticity. Studies linking active zone ultrastructure with postsynaptic function at Drosophila NMJs have been reviewed elsewhere (Davis and Muller, 2015; Frank, 2014; Kittel and Heckmann, 2016).
5. NMJ active zone assembly and organization
What are the molecular mechanisms that organize active zones at NMJs? Laminin β2 is an extracellular matrix protein that organizes the active zone facing the postsynaptic specialization at mouse NMJs (Nishimune et al., 2004). Laminin β2 is secreted by muscles and becomes concentrated in the synaptic cleft of NMJs (Hunter et al., 1989; Sanes and Hall, 1979). The important role of laminin β2 in organizing NMJ active zones was demonstrated by the active zone loss phenotype observed in laminin β2 knockout mice (Noakes et al., 1995). In addition, these knockout mice showed impaired presynaptic differentiation, reduced miniature end plate potentials (mEPPs), and decreased quantal content at NMJs (Knight et al., 2003; Noakes et al., 1995). The specific receptors for laminin β2 were identified as P/Q- and N-type VGCCs on the presynaptic terminals of NMJs (Nishimune et al., 2004). The binding of laminin β2 and VGCCs induced presynaptic differentiation by the clustering of VGCCs and presynaptic proteins (Nishimune et al., 2004). The functional role of this interaction was confirmed in vivo through blocking the interaction between VGCC and laminin β2 in wild-type mice by infusing an inhibitor of the interaction. This infusion experiment showed a reduction in the number of active zones at NMJs (Nishimune et al., 2004). At the developing rat NMJ (P0 – 4), electrophysiological studies showed that both P/Q- and N-type VGCCs are used for synaptic transmission, whereas only P/Q-type VGCCs are utilized in more mature (P5 – 11) rats and adult mice (Rosato Siri and Uchitel, 1999; Uchitel et al., 1992). P/Q- or N-type VGCC single or double knockout mice showed reduced numbers of both active zones and docked synaptic vesicles, which was demonstrated by quantitative analyses of electron micrographs (Chen et al., 2011; Nishimune et al., 2004). Laminin β2 was not detected at CNS synapses (Yin et al., 2003), which indicates that this mechanism is specific to NMJs and photoreceptor synapses in the retina, where laminin β2 also accumulates (Hunter et al., 1992).
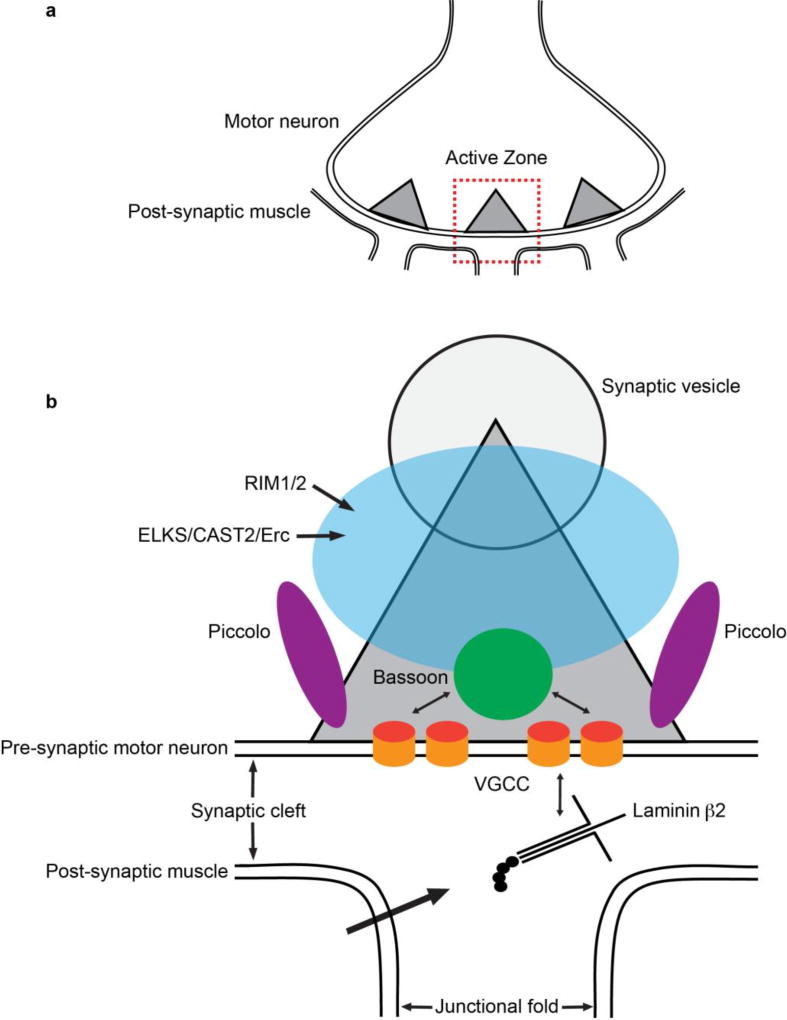
At NMJs, laminin β2 can anchor P/Q-type VGCCs from the extracellular side, and the P/Q-type VGCCs can function as a scaffolding protein for cytosolic proteins that make up the CAZ. The CAZ-specific proteins include Bassoon, ELKS/CAST2/Erc1, Munc13, Piccolo, and Rab3 interacting proteins 1 and 2 (RIM1/2). These proteins play roles in synaptic vesicle accumulation and release following calcium influx through VGCCs (details will be explained below). These proteins and their molecular interactions, including their interaction with VGCCs (Figure 2), will be discussed in the following paragraphs. In addition, the role of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins in synaptic vesicle docking and exocytosis at the presynaptic membrane will be described later.
Figure 2.
Schematic diagram of the mammalian neuromuscular junction. (a) A motor neuron with active zones shown as triangles that represent the electron-dense material detected by electron microscopy and (b) a close-up of one active zone. Laminin β2 is secreted from the postsynaptic muscle fibers and interacts extracellularly with P/Q-type voltage-gated calcium channels (VGCCs) to organize the active zone. Active zone proteins, Bassoon (green), ELKS/CAST2/Erc1 (blue), Piccolo (magenta), and Rim1/2 (blue), interact intracellularly with VGCCs (orange). The active zone Figure depicts the theoretical localization of Bassoon, Piccolo, and VGCC identified with STED super-resolution microscopy in Nishimune et al., 2016. The localization of ELKS/CAST2/Erc1 and RIM1/2 within the active zone has only been resolved at the level of confocal microscopy. The sizes of the proteins and the synaptic cleft are not to scale.
Bassoon and Piccolo are the two largest active zone proteins, with molecular weights of 420 kDa and 530 kDa, respectively (Cases-Langhoff et al., 1996; Dieck et al., 1998). Structurally, both proteins are closely related; each consists of two zinc finger domains and three coiled coil (CC) domains (Gundelfinger et al., 2016). Therefore, these proteins were thought to co-localize and share functional roles at active zones of mouse NMJs (Chen et al., 2012) and CNS synapses (Dick et al., 2001; Dondzillo et al., 2010; Fenster et al., 2000). Synaptic vesicle clusters are disrupted in synapses of cultured hippocampal neurons, cortical neurons, and ribbon synapses with a loss of functional bassoon or piccolo or both bassoon and piccolo (Altrock et al., 2003; Dick et al., 2003; Mukherjee et al., 2010; Waites et al., 2013). These results indicated a role for these proteins in vesicle clustering.
In these structural studies, hippocampal synapses of Bassoon knockout mice showed a normal ultrastructure (Altrock et al., 2003). Interestingly, a lack of Bassoon in the retina impaired the formation of functional synaptic ribbons, which are active zone structures in photoreceptor neuron synapses that have been identified in electron micrographs. Synaptic ribbons in the Bassoon mutant mice did not attach to the presynaptic membrane but rather remained floating in the cytoplasm (tom Dieck et al., 2005). These results suggested the role of Bassoon in the structural organization of active zones in some synapses. The consequences of Bassoon and Piccolo deletion from NMJ active zones remain unknown. However, an experimental manipulation to decrease active zone organizer signaling at mouse NMJs resulted in a reduced number of active zones and a reduced level of Bassoon in NMJs (Nishimune et al., 2004).
Meanwhile, Bassoon and Piccolo also show some differences. Piccolo contains a unique PDZ domain and two C2 domains (C2A and C2B) in its C terminus (Dieck et al., 1998; Fenster et al., 2000). Piccolo knockdown in rat primary hippocampal neuron cultures showed that Piccolo negatively regulates vesicle exocytosis through Synapsin1 modulation (Leal-Ortiz et al., 2008). This function of synaptic vesicle recruitment from the reserve pool to the active zone was unique to Piccolo and not shared by Bassoon (Leal-Ortiz et al., 2008). At the mouse NMJ, Bassoon and Piccolo exhibited non-overlapping side-by-side distribution patterns, as revealed by super-resolution microscopy STED (Nishimune et al., 2016). As described earlier, this side-by-side distribution pattern is consistent with the electron tomography data. The functional significance of this Bassoon and Piccolo distribution pattern is unknown, but some hints may be obtained from the study of aged NMJs (see the last section).
Multiple interacting proteins were identified for Bassoon and Piccolo, including CAST, Munc13, and Rim in the mouse brain (Wang et al., 2009). Both proteins interacted with ELKS (Takao-Rikitsu et al., 2004) and with two members of the C-terminal binding protein family, CtBP1 and CtBP2 (tom Dieck et al., 2005). In addition, Piccolo interacted with multiple other proteins, such as the L-type VGCC (Shibasaki et al., 2004), GIT1 (Kim et al., 2003), profilin, and PRA-1 (Fenster et al., 2000). Piccolo also interacted with Abp1(Fenster et al., 2003), a protein implicated in actin binding and endocytosis, which demonstrated a potentially unique role for Piccolo at active zones. The active zone proteins Bassoon and ELKS2/CAST/Erc2 directly interacted with the VGCC β subunits and were significantly reduced in the NMJs of P/Q-type or N-type VGCC (α subunit) knockouts or in P/Q- and N-type VGCC double knockout mice (Chen et al., 2011; Nishimune et al., 2004). Bassoon indirectly interacted with P/Q-type VGCCs via an interaction with RIM-binding proteins, and this interaction positioned VGCCs near synaptic vesicle release sites in cultured hippocampal neurons (Davydova et al., 2014).
ELKS proteins are scaffolding proteins, whose name is derived from their high content of the following amino acids: glutamate (E), leucine (L), lysine (K), and serine (S) (Nakata et al., 1999). ELKS was identified separately as an active zone protein and named CAST (Ohtsuka et al., 2002) or ERC (Wang et al., 2002). Mammals contain two ELKS genes: ELKS1 (CAST2/ERC1), which is present at mouse NMJs, and ELKS2, which is not detected at mouse NMJs (Tokoro et al., 2007). At NMJs, the physiological role of ELKS remains unclear. One hint about its importance for active zone function at NMJs came from the analysis of Bruchpilot, which shares significant N-terminal sequence homology with ELKS (Kittel et al., 2006; Wagh et al., 2006). Bruchpilot Drosophila mutants showed a loss of T-bars, impaired clustering of calcium channels, and a reduction in evoked synaptic transmission, confirming its role in active zone organization. The ELKS proteins will be reviewed in a separate article by Toshihisa Ohtsuka in this special issue.
The Munc13 protein family consists of three highly homologous members in mammals, including Munc13-1/2/3 with the splice variants bMunc13-2/ubMunc13-2 for Munc13-2 (Brose et al., 2000). Of these, Munc13-1 and ubMunc13-2, but not bMunc13-2 and Munc13-3, were detected at mouse NMJs (Varoqueaux et al., 2005). In CNS synapses, the Munc13 proteins are essential for synaptic vesicle priming and evoked synaptic transmission (Aravamudan et al., 1999; Augustin et al., 1999; Richmond et al., 1999; Varoqueaux et al., 2002). However, analysis of NMJs in Munc13-1/2 double knockout mice revealed that vesicle priming is partly independent of Munc13 at this synapse (Varoqueaux et al., 2005). Munc13-1/2 double knockout mice exhibited an increase in the frequency of mEPPs, but a significant reduction in the end plate potential (EPP) amplitude and EPP quantal content at NMJs (Varoqueaux et al., 2005). NMJ electron micrographs of the Munc13-1/2 double knockout mice revealed well-formed synapses containing active zones with docked synaptic vesicles at the presynaptic membrane (Varoqueaux et al., 2005). These results suggest that Munc13-1 and 2 can play a role in synaptic transmission but are not essential for active zone assembly at NMJs.
RIMs are multidomain proteins that are central components of the CAZ. In vertebrates, the RIM protein family is composed of 7 members (RIM1α, 1β, 2α, 2β, 2γ, 3γ and 4γ) formed by four different genes (RIM1 – 4) (Wang and Südhof, 2003). At NMJs, RIM plays an important role in synaptic transmission. RIM1α and RIM2α double knockout mice exhibited no ultrastructural defects at NMJs according to a qualitative level analysis (Schoch et al., 2006). However, they were unable to mediate normal calcium-triggered neurotransmitter release. The double knockout mice had a reduced frequency of mEPPs and reduced EPP amplitudes compared to control NMJs (Schoch et al., 2006). At Drosophila NMJs, RIM promoted VGCC clustering (Graf et al., 2012). In vertebrate CNS synapses, RIM bound to P/Q- and N-type, but not L-type, VGCCs (Kaeser et al., 2011). These interactions were essential for recruiting calcium channels to active zones in cultured hippocampal neurons and at calyx of Held synapses (Han et al., 2011; Kaeser et al., 2011). A RIM1/2 double conditional knockout mouse model demonstrated that RIMs are important for synaptic vesicle docking and priming at the calyx of Held (Han et al., 2011). RIM bound to SNAP 25 in a calcium-dependent manner in vitro (Coppola et al., 2001). This interaction could potentially link RIM directly to the SNARE complex for vesicle exocytosis (see next paragraph). Taken together, the interactions between RIMs and VGCCs target VGCCs to the presynaptic terminals of NMJs and promote synaptic transmission.
SNAREs represent a large family of proteins, characterized by a 70-residue sequence known as the SNARE motif (Rizo and Südhof, 2002), which brings the plasma membrane and a synaptic vesicle close together (Rizo and Rosenmund, 2008). They are not enriched in active zones; however, they form the core fusion proteins necessary for synaptic vesicle release. SNAREs play essential roles in synaptic transmission and neurotransmitter release through the formation of the SNARE complex during calcium-triggered vesicle exocytosis (Jahn and Scheller, 2006; Südhof and Rothman, 2009). During docking, the SNARE complex is formed between syntaxin-1(Bennett et al., 1992; Inoue et al., 1992) and synaptic vesicle–associated protein of 25 kDa (SNAP 25) (Oyler et al., 1989) on the presynaptic terminal membrane, and synaptobrevin (Ferro-Novick and Jahn, 1994) on the synaptic vesicle membrane. Genetic experiments and the application of neurotoxins have shown that SNARE formation is necessary for fast synaptic calcium-triggered exocytosis (Bronk et al., 2007; Deák et al., 2004; Sørensen et al., 2003). A spontaneous null mutation in synaptobrevin 1 significantly impaired the function of mouse NMJs by reducing both mEPP frequency and EPP amplitude compared to control mice (Liu et al., 2011b). Short-term synaptic plasticity was also altered at NMJs of the synaptobrevin 1-null mice. This mutation did not alter the NMJ ultrastructure, and there was no change in the size of the readily releasable pool. Thus, these impairments were attributed to a reduced sensitivity to calcium due to the absence of synaptobrevin 1. The alignment between VGCCs and the synaptic vesicle release machinery in the rat brain was supported by the direct interactions between presynaptic P/Q- and N-type VGCCs and syntaxin, SNAP-25, and synaptotagmin proteins (Martin-Moutot et al., 1996; Rettig et al., 1996; Sheng et al., 1996; Sheng et al., 1994; Sheng et al., 1997).
In summary, the postsynaptically secreted laminin β2 organizes active zones at NMJs by anchoring its presynaptic receptor VGCCs and by the cytosolic interactions between VGCCs and the active zone-specific proteins: Bassoon, ELKS, Munc13, Piccolo, and RIMs. These VGCCs and CAZ-specific proteins likely make up the electron-dense material in active zones shown in electron micrographs of NMJs. This estimation is supported by the loss of electron-dense materials at the NMJs of VGCC knockout mice (Chen et al., 2011; Nishimune et al., 2004). The regulated release of neurotransmitters also requires an interplay between VGCCs, CAZ proteins, and the SNARE complexes to achieve the tethering, docking, priming, and fusion of synaptic vesicles with the plasma membrane (Südhof and Rizo, 2011). Additional research into the molecular mechanisms of active zone organization is needed to understand the necessary interactions for maintaining the NMJ active zones. The following sections will describe how active zones at NMJs can be affected during aging and other neurodegenerative diseases.
6. Selective degeneration of active zone proteins during aging
Aging leads to a reduction in active zone protein levels and in active zone number (Chen et al., 2012; Nishimune et al., 2016), as explained in section “4. The role of synaptic activity and exercise in maintaining active zone density.” Interestingly, selective degeneration of active zone proteins occurred at NMJs during aging (Nishimune et al., 2016). At the aged NMJs of 29-month-old mice, the level of P/Q-type VGCCs and Bassoon protein decreased significantly compared to adult NMJs of 8-month-old mice. However, the protein level of another active zone-specific protein, Piccolo, at these aged NMJs remained similar to that of adult NMJs. These findings suggested that NMJ active zone proteins decrease selectively, but not as a uniform degeneration, prior to NMJ denervation during aging. In addition, Bassoon puncta density was significantly decreased in aged NMJs, but P/Q-type VGCC puncta and Piccolo puncta density in aged NMJs remained similar to the adult NMJ level. These results indicated that a reduced amount of P/Q-type VGCC protein remained in the active zones, even though the protein level decreased in most of the active zones of the aged NMJs. In our previous electrophysiology study, the lack of Bassoon enhanced the inactivation property of P/Q-type VGCC (Nishimune et al., 2012). Together, these results imply that the active zones of aged mouse NMJs function less efficiently due to the reduced protein level and function of P/Q-type VGCCs.
In the CNS, examples of selective degeneration of presynaptic proteins were reported in aged patients with Alzheimer's disease (Sze et al., 2000). Synaptobrevin, synaptotagmin, and Rab3a were significantly decreased in the hippocampus and entorhinal cortex of Alzheimer's disease patients compared to controls. In contrast, the levels of two synaptic vesicle proteins, synapsin I and SV2, and two presynaptic SNARE proteins, syntaxin and SNAP-25, remained comparable to the controls. Of the tested proteins, synaptobrevin was the only protein that was reduced in Alzheimer's disease patients in all four of the examined brain regions, including the hippocampus, entorhinal cortex, occipital cortex, and the caudate nucleus (Sze et al., 2000). The levels of active zone-specific proteins were not examined in this study.
These results suggest that pathological mechanisms leading to synaptic impairments may involve a selective degeneration of active zone proteins prior to the entire loss of active zones. Interestingly, cultured hippocampal neurons that lack Bassoon and Piccolo exhibited degeneration of glutamatergic synapses (Waites et al., 2013), which was associated with the induction of presynaptic autophagy (Okerlund et al., 2017). Bassoon played a role in the local regulation of presynaptic autophagy through its interaction with Atg5, an E3-like ubiquitin ligase essential for autophagy (Okerlund et al., 2017). A gain of function of Bassoon led to the suppression of presynaptic autophagy, and loss of Bassoon function enhanced autophagy (Okerlund et al., 2017). These results indicate a potential consequence of selective active zone protein loss and a downstream mechanism that results in active zone degeneration. Alterations in selective protein–protein interactions that are important for the maintenance of NMJ active zones and regulation of neurotransmission can also play a role in aging and the pathogenesis of other neurodegenerative diseases. Therefore, it is necessary to identify higher resolution distribution patterns of active zone proteins and investigate how they are affected during aging and in neurodegenerative diseases to better understand the mechanisms of neuromuscular function loss. The beneficial effect of exercise in restoring Bassoon levels at aged NMJs has been described (Nishimune et al., 2012). Whether exercise or increasing synaptic activity can ameliorate the selective degradation of other active zone proteins will be an interesting avenue to pursue.
Highlights.
Nanoarchitecture of active zones was revealed using super resolution microscopy.
Active zone ultrastructure and synaptic function show correlations at NMJs.
Synaptic activity and exercise play roles in maintaining the active zone density.
Mechanisms of assembly and organization of NMJ active zones will be detailed.
Active zone proteins show selective degeneration during aging.
Acknowledgments
This work was supported by grants from NIH, R01 NS078214 and R01 AG051470 (H.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- Ackermann F, Waites CL, Garner CC. Presynaptic active zones in invertebrates and vertebrates. EMBO Rep. 2015;16:923–938. doi: 10.15252/embr.201540434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altrock WD, Tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fässler R, Richter K, Boeckers TM, Potschka H. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. doi: 10.1016/s0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nature neuroscience. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Arizono N, Koreto O, Iwai Y, Hidaka T, Takeoka O. Morphometric analysis of human neuromuscular junction in different ages. Pathology International. 1984;34:1243–1249. doi: 10.1111/j.1440-1827.1984.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Banker BQ, Kelly S, Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. The Journal of physiology. 1983;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek E, Caroni P. beta-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69:1132–1146. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Bronk P, Deák F, Wilson MC, Liu X, Südhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+-dependent and Ca2+-independent neurotransmission. Journal of neurophysiology. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Current opinion in neurobiology. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- Burke R, Levine D, Tsairis P, Zajac Iii F. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. The Journal of physiology. 1973;234:723. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases-Langhoff C, Voss B, Garner A, Appeltauer U, Takei K, Kindler S, Veh R, De Camilli P, Gundelfinger E, Garner C. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. European journal of cell biology. 1996;69:214–223. [PubMed] [Google Scholar]
- Chamma I, Letellier M, Butler C, Tessier B, Lim K-H, Gauthereau I, Choquet D, Sibarita J-B, Park S, Sainlos M. Mapping the dynamics and nanoscale organization of synaptic adhesion proteins using monomeric streptavidin. Nature communications. 2016;7 doi: 10.1038/ncomms10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. Journal of Neuroscience. 2011;31:512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. Journal of Comparative Neurology. 2012;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon K, Silberstein L, Blau HM, Thompson WJ. Development of muscle fiber types in the prenatal rat hindlimb. Developmental biology. 1990;138:256–274. doi: 10.1016/0012-1606(90)90196-p. [DOI] [PubMed] [Google Scholar]
- Coppola T, Magnin-Lüthi S, Perret-Menoud V, Gattesco S, Schiavo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. Journal of Biological Chemistry. 2001;276:32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- Couteaux R, Pecot-Dechavassine M. Synaptic vesicles and pouches at the level of "active zones" of the neuromuscular junction. Comptes rendus hebdomadaires des seances de l'Academie des sciences. Serie D: Sciences naturelles. 1970;271:2346–2349. [PubMed] [Google Scholar]
- Cull-Candy SG, Miledi R, Trautmann A, Uchitel OD. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Muller M. Homeostatic control of presynaptic neurotransmitter release. Annu Rev Physiol. 2015;77:251–270. doi: 10.1146/annurev-physiol-021014-071740. [DOI] [PubMed] [Google Scholar]
- Davydova D, Marini C, King C, Klueva J, Bischof F, Romorini S, Montenegro-Venegas C, Heine M, Schneider R, Schroder MS, Altrock WD, Henneberger C, Rusakov DA, Gundelfinger ED, Fejtova A. Bassoon specifically controls presynaptic P/Q-type Ca(2+) channels via RIM-binding protein. Neuron. 2014;82:181–194. doi: 10.1016/j.neuron.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Deák F, Schoch S, Liu X, Südhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nature cell biology. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Dick O, Hack I, Altrock WD, Garner CC, Gundelfinger ED, Brandstätter JH. Localization of the presynaptic cytomatrix protein Piccolo at ribbon and conventional synapses in the rat retina: comparison with Bassoon. Journal of Comparative Neurology. 2001;439:224–234. doi: 10.1002/cne.1344. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermüller J, Weiler R, Garner CC, Gundelfinger ED, Brandstätter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Dieck S, Sanmartí-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla K-H, Kämpf U, Fränzer J-T. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. The Journal of cell biology. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondzillo A, Saetzler K, Horstmann H, Altrock WD, Gundelfinger ED, Kuner T. Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins Bassoon and Piccolo in the rat calyx of Held before and after the onset of hearing. Journal of Comparative Neurology. 2010;518:1008–1029. doi: 10.1002/cne.22260. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Qualmann B, Kessels M, Garner C, Gundelfinger* E. The presynaptic cytomatrix of brain synapses. Cellular and Molecular Life Sciences. 2001;58:94–116. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B, Woodhams SG, Henstridge CM, Balla GY, Nyilas R. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nature neuroscience. 2015;18:75–86. doi: 10.1038/nn.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzyubenko E, Rozenberg A, Hermann DM, Faissner A. Colocalization of synapse marker proteins evaluated by STED-microscopy reveals patterns of neuronal synapse distribution in vitro. J Neurosci Methods. 2016;273:149–159. doi: 10.1016/j.jneumeth.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Ehmann N, Van De Linde S, Alon A, Ljaschenko D, Keung XZ, Holm T, Rings A, DiAntonio A, Hallermann S, Ashery U. Quantitative super-resolution imaging of Bruchpilot distinguishes active zone states. Nature communications. 2014;5:4650. doi: 10.1038/ncomms5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman MH, Rash JE, Staehelin LA, Porter KR. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. The Journal of cell biology. 1976;68:752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat. 2011;193:354–361. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M, Holley J, Robbins N. Scanning and light microscopic study of age changes at a neuromuscular junction in the mouse. Journal of neurocytology. 1983;12:13–25. doi: 10.1007/BF01148085. [DOI] [PubMed] [Google Scholar]
- Fahim M, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. Journal of neurocytology. 1982;11:641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. Journal of applied physiology. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- Fahim MA, Robbins N, Price R. Fixation effects on synaptic vesicle density in neuromuscular junctions of young and old mice. Neurobiology of aging. 1987;8:71–75. doi: 10.1016/0197-4580(87)90061-3. [DOI] [PubMed] [Google Scholar]
- Fejtova A, Gundelfinger ED. Molecular organization and assembly of the presynaptic active zone of neurotransmitter release, Cell Communication in Nervous and Immune System. Springer; 2006. pp. 49–68. [DOI] [PubMed] [Google Scholar]
- Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25:203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Fenster SD, Kessels MM, Qualmann B, Chung WJ, Nash J, Gundelfinger ED, Garner CC. Interactions between Piccolo and the actin/dynamin-binding protein Abp1 link vesicle endocytosis to presynaptic active zones. Journal of Biological Chemistry. 2003;278:20268–20277. doi: 10.1074/jbc.M210792200. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. The Journal of cell biology. 2009;186:129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA. Homeostatic plasticity at the Drosophila neuromuscular junction. Neuropharmacology. 2014;78:63–74. doi: 10.1016/j.neuropharm.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, Fejtova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H, Engel AG, Osame M, Lambert EH. Paucity and disorganization of presynaptic membrane active zones in the lambert-eaton myasthenic syndrome. Muscle & Nerve. 1982;5:686–697. [Google Scholar]
- Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Vincent A. Lambert-Eaton myasthenic syndrome: II. Immunoelectron microscopy localization of IgG at the mouse motor end-plate. Ann Neurol. 1987a;22:200–211. doi: 10.1002/ana.410220204. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Prior C, W-Wray D. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Annals of neurology. 1987b;22:193–199. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. Journal of neurophysiology. 1993;69:1160–1170. doi: 10.1152/jn.1993.69.4.1160. [DOI] [PubMed] [Google Scholar]
- Graf ER, Daniels RW, Burgess RW, Schwarz TL, DiAntonio A. Rab3 dynamically controls protein composition at active zones. Neuron. 2009;64:663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Valakh V, Wright CM, Wu C, Liu Z, Zhang YQ, DiAntonio A. RIM promotes calcium channel accumulation at active zones of the Drosophila neuromuscular junction. Journal of Neuroscience. 2012;32:16586–16596. doi: 10.1523/JNEUROSCI.0965-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E. Electron microscopy of presynaptic organelles of the spinal cord. Journal of Anatomy. 1963;97:101. [PMC free article] [PubMed] [Google Scholar]
- Gundelfinger ED, Reissner C, Garner CC. Role of Bassoon and Piccolo in assembly and molecular organization of the active zone. Frontiers in synaptic neuroscience. 2016;7:19. doi: 10.3389/fnsyn.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E, Hanzlikova V, Vyskočil F. Age changes in cross striated muscle of the rat. The Journal of physiology. 1971;216:331. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca 2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog's neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Optics letters. 1994;19:780–782. doi: 10.1364/ol.19.000780. [DOI] [PubMed] [Google Scholar]
- Heuser J, Reese T, Dennis M, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J, Reese T, Landis D. Functional changes in frog neuromuscular junctions studied with freeze-fracture. Journal of neurocytology. 1974;3:109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Heuser JE. Internal and external differentiations of the postsynaptic membrane at the neuromuscular junction. Journal of neurocytology. 1982;11:487–510. doi: 10.1007/BF01257990. [DOI] [PubMed] [Google Scholar]
- Houle JD, Cote MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci. 2013;1279:154–163. doi: 10.1111/nyas.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Murphy MD, Olsson CV, Brunken WJ. S-laminin expression in adult and developing retinae: a potential cue for photoreceptor morphogenesis. Neuron. 1992;8:399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Indriati DW, Kamasawa N, Matsui K, Meredith AL, Watanabe M, Shigemoto R. Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. J Neurosci. 2013;33:3668–3678. doi: 10.1523/JNEUROSCI.2921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Obata K, Akagawa K. Cloning and sequence analysis of cDNA for a neuronal cell membrane antigen, HPC-1. Journal of Biological Chemistry. 1992;267:10613–10619. [PubMed] [Google Scholar]
- Jacob JM, Robbins N. Differential effects of age on neuromuscular transmission in partially denervated mouse muscle. J Neurosci. 1990;10:1522–1529. doi: 10.1523/JNEUROSCI.10-05-01522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nature reviews Molecular cell biology. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Juranek J, Mukherjee K, Rickmann M, Martens H, Calka J, Südhof TC, Jahn R. Differential expression of active zone proteins in neuromuscular junctions suggests functional diversification. European Journal of Neuroscience. 2006;24:3043–3052. doi: 10.1111/j.1460-9568.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca 2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annual review of neuroscience. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Kelly S, Robbins N. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. The Journal of physiology. 1983;343:375. doi: 10.1113/jphysiol.1983.sp014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf C, Staudt T, Bingen P, Horstmann H, Engelhardt J, Hell SW, Kuner T. Tissue multicolor STED nanoscopy of presynaptic proteins in the calyx of held. PloS one. 2013;8:e62893. doi: 10.1371/journal.pone.0062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ko J, Shin H, Lee J-R, Lim C, Han J-H, Altrock WD, Garner CC, Gundelfinger ED, Premont RT. The GIT family of proteins forms multimers and associates with the presynaptic cytomatrix protein Piccolo. Journal of Biological Chemistry. 2003;278:6291–6300. doi: 10.1074/jbc.M212287200. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Heckmann M. Synaptic Vesicle Proteins and Active Zone Plasticity. Front Synaptic Neurosci. 2016;8:8. doi: 10.3389/fnsyn.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Knight D, Tolley LK, Kim DK, Lavidis NA, Noakes PG. Functional analysis of neurotransmission at β2-laminin deficient terminals. The Journal of physiology. 2003;546:789–800. doi: 10.1113/jphysiol.2002.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C-P. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. Journal of neurocytology. 1985;14:487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1:201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Leal-Ortiz S, Waites CL, Terry-Lorenzo R, Zamorano P, Gundelfinger ED, Garner CC. Piccolo modulation of Synapsin1a dynamics regulates synaptic vesicle exocytosis. The Journal of cell biology. 2008;181:831–846. doi: 10.1083/jcb.200711167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Heckman C. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. Journal of neurophysiology. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Li Y, Lee YI, Thompson WJ. Changes in Aging Mouse Neuromuscular Junctions Are Explained by Degeneration and Regeneration of Muscle Fiber Segments at the Synapse. Journal of Neuroscience. 2011;31:14910–14919. doi: 10.1523/JNEUROSCI.3590-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach C, Laue MM, Wang X, Hu B, Thiede N, Hultqvist G, Kilimann MW. Molecular in situ topology of Aczonin/Piccolo and associated proteins at the mammalian neurotransmitter release site. Proceedings of the National Academy of Sciences. 2011;108:E392–E401. doi: 10.1073/pnas.1101707108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettker C. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011a;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sugiura Y, Lin W. The role of Synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. The Journal of physiology. 2011b;589:1603–1618. doi: 10.1113/jphysiol.2010.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Silver R. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Luo F, Dittrich M, Cho S, Stiles JR, Meriney SD. Transmitter release is evoked with low probability predominately by calcium flux through single channel openings at the frog neuromuscular junction. Journal of neurophysiology. 2015;113:2480–2489. doi: 10.1152/jn.00879.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. Journal of Neuroscience. 2011;31:11268–11281. doi: 10.1523/JNEUROSCI.1394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T-i, Kozaki S, Takahashi M, Seagar M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. Journal of Biological Chemistry. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen I, Govind C, Stewart B, Carter J, Atwood H. Regulated spacing of synapses and presynaptic active zones at larval neuromuscular junctions in different genotypes of the flies Drosophila and Sarcophaga. Journal of Comparative Neurology. 1998;393:482–492. doi: 10.1002/(sici)1096-9861(19980420)393:4<482::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Dittrich M. Organization and function of transmitter release sites at the neuromuscular junction. The Journal of physiology. 2013;591:3159–3165. doi: 10.1113/jphysiol.2012.248625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Arakawa M, Masutani M, Tamekane A, Yamaguchi H, Minami N, Takai Y, Ide C. Localization of smg p25A/rab3A p25, a small GTP-binding protein, at the active zone of the rat neuromuscular junction. Biochem Biophys Res Commun. 1992;186:1345–1352. doi: 10.1016/s0006-291x(05)81554-2. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Yang X, Gerber SH, Kwon H-B, Ho A, Castillo PE, Liu X, Südhof TC. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proceedings of the National Academy of Sciences. 2010;107:6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. Journal of Comparative Neurology. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS, Shigemoto R, Silver RA, DiGregorio DA, Takahashi T. Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron. 2015;85:145–158. doi: 10.1016/j.neuron.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Kitamura Y, Shimizu K, Tanaka S, Fujimori M, Yokoyama S, Ito K, Emi M. Fusion of a novel gene, ELKS, to RET due to translocation t (10; 12)(q11; p13) in a papillary thyroid carcinoma. Genes, Chromosomes and Cancer. 1999;25:97–103. doi: 10.1002/(sici)1098-2264(199906)25:2<97::aid-gcc4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Narusawa M, Fitzsimons R, Izumo S, Nadal-Ginard B, Rubinstein N, Kelly A. Slow myosin in developing rat skeletal muscle. The Journal of cell biology. 1987;104:447–459. doi: 10.1083/jcb.104.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca 2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Badawi Y, Mori S, Shigemoto K. Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Scientific reports. 2016;6:27935. doi: 10.1038/srep27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H, Numata T, Chen J, Aoki Y, Wang Y, Starr MP, Mori Y, Stanford JA. Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PLoS One. 2012;7:e38029. doi: 10.1371/journal.pone.0038029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin–calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle & nerve. 2014;49:315–324. doi: 10.1002/mus.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta2. Nature. 1995;374:258. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, Deguchi-Tawarada M, Satoh K, Morimoto K, Nakanishi H. Cast a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and Munc13-1. The Journal of cell biology. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okerlund ND, Schneider K, Leal-Ortiz S, Montenegro-Venegas C, Kim SA, Garner LC, Gundelfinger ED, Reimer RJ, Garner CC. Bassoon Controls Presynaptic Autophagy through Atg5. Neuron. 2017;93:897–913. e897. doi: 10.1016/j.neuron.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. The Journal of Cell Biology. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panenic R, Gardiner PF. The case for adaptability of the neuromuscular junction to endurance exercise training. Can J Appl Physiol. 1998;23:339–360. doi: 10.1139/h98-019. [DOI] [PubMed] [Google Scholar]
- Peled ES, Isacoff EY. Optical quantal analysis of synaptic transmission in wild-type and rab3-mutant Drosophila motor axons. Nature neuroscience. 2011;14:519–526. doi: 10.1038/nn.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger K, Akert K, Moor H, Sandri C. The fine structure of freeze-fractured presynaptic membranes. Journal of neurocytology. 1972;1:129–149. doi: 10.1007/BF01099180. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, Van Kempen GT, De Baets MB, Graus YM, Kuks JB, Molenaar PC. Acetylcholine release in myasthenia gravis: regulation at single end-plate level. Ann Neurol. 1995;37:627–636. doi: 10.1002/ana.410370513. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst JW, Ko C-P. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. Journal of Neuroscience. 1987;7:3654–3664. doi: 10.1523/JNEUROSCI.07-11-03654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff DF, Thiel PR, Schuster CM. Differential regulation of active zone density during long-term strengthening of Drosophila neuromuscular junctions. Journal of Neuroscience. 2002;22:9399–9409. doi: 10.1523/JNEUROSCI.22-21-09399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng Z-H, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proceedings of the National Academy of Sciences. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzau-Jost A, Delvendahl I, Rings A, Byczkowicz N, Harada H, Shigemoto R, Hirrlinger J, Eilers J, Hallermann S. Ultrafast action potentials mediate kilohertz signaling at a central synapse. Neuron. 2014;84:152–163. doi: 10.1016/j.neuron.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nature structural & molecular biology. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Südhof TC. Snares and Munc18 in synaptic vesicle fusion. Nature Reviews Neuroscience. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- Rosato Siri MD, Uchitel OD. Calcium channels coupled to neurotransmitter release at neonatal rat neuromuscular junctions. J Physiol. 1999;514(Pt 2):533–540. doi: 10.1111/j.1469-7793.1999.533ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. Journal of neurophysiology. 2007;98:478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- Ruiz R, Cano R, Casañas JJ, Gaffield MA, Betz WJ, Tabares L. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. Journal of Neuroscience. 2011;31:2000–2008. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nature methods. 2006;3:793–796. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Hall ZW. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. The Journal of cell biology. 1979;83:357–370. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, Castillo PE, Hammer RE, Han W, Schmitz F. Redundant functions of RIM1α and RIM2α in Ca2+-triggered neurotransmitter release. The EMBO Journal. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379:451. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proceedings of the National Academy of Sciences. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. Journal of Biological Chemistry. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. Journal of Neuroscience. 2002;22:7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater CR. The functional organization of motor nerve terminals. Prog Neurobiol. 2015;134:55–103. doi: 10.1016/j.pneurobio.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Smith D, Rosenheimer J. Decreased sprouting and degeneration of nerve terminals of active muscles in aged rats. Journal of neurophysiology. 1982;48:100–109. doi: 10.1152/jn.1982.48.1.100. [DOI] [PubMed] [Google Scholar]
- Smith DO. Muscle-specific decrease in presynaptic calcium dependence and clearance during neuromuscular transmission in aged rats. J Neurophysiol. 1988;59:1069–1082. doi: 10.1152/jn.1988.59.4.1069. [DOI] [PubMed] [Google Scholar]
- Sørensen JB, Fernández-Chacón R, Südhof TC, Neher E. Examining synaptotagmin 1 function in dense core vesicle exocytosis under direct control of Ca2+ The Journal of general physiology. 2003;122:265–276. doi: 10.1085/jgp.200308855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harbor perspectives in biology. 2011;3:a005637. doi: 10.1101/cshperspect.a005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze C-I, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. Journal of the neurological sciences. 2000;175:81–90. doi: 10.1016/s0022-510x(00)00285-9. [DOI] [PubMed] [Google Scholar]
- Szule JA, Harlow ML, Jung JH, De-Miguel FF, Marshall RM, McMahan UJ. Regulation of synaptic vesicle docking by different classes of macromolecules in active zone material. PLoS One. 2012;7:e33333. doi: 10.1371/journal.pone.0033333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, Takai Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. The Journal of cell biology. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH. Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate. Neuroscience. 2007;150:575–584. doi: 10.1016/j.neuroscience.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr TB, Dittrich M, Meriney SD. Are unreliable release mechanisms conserved from NMJ to CNS? Trends in neurosciences. 2013;36:14–22. doi: 10.1016/j.tins.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoro T, Higa S, Deguchi-Tawarada M, Inoue E, Kitajima I, Ohtsuka T. Localization of the active zone proteins CAST, ELKS, and Piccolo at neuromuscular junctions. Neuroreport. 2007;18:313–316. doi: 10.1097/WNR.0b013e3280287abe. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtová A, Bracko O, Gundelfinger ED, Brandstätter JH. Molecular dissection of the photoreceptor ribbon synapse physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. The Journal of cell biology. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S. Rene Couteaux (1909–1999) and the morphological identification of synapses. Biol Cell. 2006;98:503–509. doi: 10.1042/BC20050036. [DOI] [PubMed] [Google Scholar]
- Uchitel O, Protti D, Sanchez V, Cherksey B, Sugimori M, Llinas R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proceedings of the National Academy of Sciences. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Gage F, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proceedings of the National Academy of Sciences. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PloS one. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle & nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Plomp JJ, Brose N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Molecular and Cellular Biology. 2005;25:5973–5984. doi: 10.1128/MCB.25.14.5973-5984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle M-C, Schmidt M, Qin G. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Waites CL, Leal-Ortiz SA, Okerlund N, Dalke H, Fejtova A, Altrock WD, Gundelfinger ED, Garner CC. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. The EMBO journal. 2013;32:954–969. doi: 10.1038/emboj.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrond J, Reese T. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. Journal of Neuroscience. 1985;5:1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Engisch KL, Li Y, Pinter MJ, Cope TC, Rich MM. Decreased synaptic activity shifts the calcium dependence of release at the mammalian neuromuscular junction in vivo. Journal of Neuroscience. 2004;24:10687–10692. doi: 10.1523/JNEUROSCI.2755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu B, Zieba A, Neumann NG, Kasper-Sonnenberg M, Honsbein A, Hultqvist G, Conze T, Witt W, Limbach C. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. Journal of Neuroscience. 2009;29:12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pinter MJ, Rich MM. Ca2+ dependence of the binomial parameters p and n at the mouse neuromuscular junction. Journal of neurophysiology. 2010;103:659–666. doi: 10.1152/jn.00708.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu X, Biederer T, Südhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proceedings of the National Academy of Sciences. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Südhof TC. Genomic definition of RIM proteins: evolutionary amplification of a family of synaptic regulatory proteins. Genomics. 2003;81:126–137. doi: 10.1016/s0888-7543(02)00024-1. [DOI] [PubMed] [Google Scholar]
- Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Deschenes MR. The neuromuscular junction: anatomical features and adaptations to various forms of increased, or decreased neuromuscular activity. The International journal of neuroscience. 2005;115:803–828. doi: 10.1080/00207450590882172. [DOI] [PubMed] [Google Scholar]
- Wong AB, Rutherford MA, Gabrielaitis M, Pangršič T, Göttfert F, Frank T, Michanski S, Hell S, Wolf F, Wichmann C. Developmental refinement of hair cell synapses tightens the coupling of Ca2+ influx to exocytosis. The EMBO journal. 2014;33:247–264. doi: 10.1002/embj.201387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Progress in neurobiology. 2001;64:393–429. doi: 10.1016/s0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Wu L-G, Betz W. Spatial variability in release at the frog neuromuscular junction measured with FM1–43. Canadian journal of physiology and pharmacology. 1999;77:672–678. [PubMed] [Google Scholar]
- Wyatt RM, Balice-Gordon RJ. Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin. Journal of Neuroscience. 2008;28:325–335. doi: 10.1523/JNEUROSCI.3544-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Kikkawa Y, Mudd JL, Skarnes WC, Sanes JR, Miner JH. Expression of laminin chains by central neurons: analysis with gene and protein trapping techniques. Genesis. 2003;36:114–127. doi: 10.1002/gene.10206. [DOI] [PubMed] [Google Scholar]
- York AL, Zheng JQ. Super-Resolution Microscopy Reveals a Nanoscale Organization of Acetylcholine Receptors for Trans-Synaptic Alignment at Neuromuscular Synapses. eNeuro. 2017;4 doi: 10.1523/ENEURO.0232-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Fain N, Zampighi LM, Cantele F, Lanzavecchia S, Wright EM. Conical electron tomography of a chemical synapse: polyhedral cages dock vesicles to the active zone. Journal of Neuroscience. 2008;28:4151–4160. doi: 10.1523/JNEUROSCI.4639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology. 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]