Abstract
Treatments for cognitive and functional impairments associated with severe mental illnesses are urgently needed. We tested a 12-week, manualized, Compensatory Cognitive Training (CCT) intervention targeting prospective memory, attention, learning/memory, and executive functioning in the context of supported employment for people with severe mental illnesses who were seeking work. 153 unemployed, work-seeking outpatients with schizophrenia/schizoaffective disorder (n=58), bipolar disorder (n=37), or major depression (n=58) were randomized to receive supported employment plus CCT or enhanced supported employment, a robust control group. Assessments of neuropsychological performance, functional capacity, psychiatric symptom severity, and self-reported functioning and quality of life were administered at baseline and multiple follow-up assessments over two years; work outcomes were collected for two years. Forty-seven percent of the participants obtained competitive work, but there were no differences in work attainment, weeks worked, or wages earned between the CCT and the enhanced supported employment group. ANCOVAs assessing immediate post-treatment effects demonstrated significant, medium to large, CCT-associated improvements on measures of working memory (p=.038), depressive symptom severity (p=.023), and quality of life (p=.003). Longer-term results revealed no statistically significant CCT-associated improvements, but a trend (p=.058) toward a small to medium CCT-associated improvement in learning. Diagnostic group (schizophrenia-spectrum vs. mood disorder) did not affect outcomes. We conclude that CCT has the potential to improve cognitive performance, psychiatric symptom severity, and quality of life in people with severe mental illnesses. Receiving CCT did not result in better work outcomes, suggesting that supported employment can result in competitive work regardless of cognitive status.
Keywords: schizophrenia, bipolar disorder, major depressive disorder, cognition, functioning, rehabilitation
1. Introduction
Cognitive impairment is common, persistent, and associated with impaired functioning in people with severe mental illnesses (SMI; Millan et al., 2012). Impairments may occur in multiple cognitive domains, including processing speed, attention/vigilance, working memory, learning, memory, and executive functioning, and are known to occur in schizophrenia (Fioravanti et al., 2012, Mesholam-Gately et al., 2009), bipolar disorder (Lee et al., 2013, Bora & Pantelis, 2015; Sanches et al., 2015), and major depression (Rock et al., 2014; Porter et al., 2014). These impairments affect vocational outcomes and other aspects of everyday functioning (Green, Kern, & Heaton, 2004; Bowie et al., 2008; Mora et al., 2013; Depp et al., 2012; Jaeger et al., 2006; Baune et al., 2010). For example, cognitive difficulties with attention, learning, problem-solving, and pacing may affect individuals’ ability to find and keep competitive work (McGurk & Wykes, 2008). Increased understanding of the relationships between cognition and work have sparked interest in cognitive training interventions to improve cognition, functioning, and work outcomes over the last several years (Anaya et al., 2012; Bowie et al., 2013, 2014; Fisher et al., 2014; Lee et al., 2013; McGurk et al., 2009, 2015, 2016; Bell et al., 2005, 2008).
Meta-analyses of cognitive training in schizophrenia samples have found moderate, but durable, training effects in cognition as well as functioning (McGurk et al., 2007; Wykes et al., 2011). In terms of work outcomes, cognitive training techniques have been used to enhance employment outcomes in the context of employment interventions such as supported employment (Bell et al., 2005, 2008; McGurk et al., 2009, but also see Au et al., 2015). Cognitive training has been applied both during and separate from employment interventions, to both all supported employment participants and to supported employment non-responders (McGurk et al., 2015; McGurk et al., 2016). Multiple types of cognitive training interventions exist, ranging from computer-based, drill-and-practice oriented training of specific cognitive domains (e.g., Fisher et al., 2014) to compensatory-strategy-based approaches (Twamley et al., 2012; Mendella et al., 2015; Wykes et al., 2007). The bulk of cognitive training studies have been conducted with individuals having schizophrenia-spectrum disorders (Wykes et al., 2011), but there have been several recent studies of cognitive interventions for individuals with mood disorders (see meta-analysis by Motter et al., 2016). Most of the interventions studied in participants with mood disorders have been computerized (e.g., Bowie et al., 2013); some have been compensatory strategy-based (e.g., Priyamvada et al., 2015; Venza et al., 2016; Deckersbach et al., 2010), but there have been few controlled studies of compensatory strategy-based interventions, and no known trials of purely compensatory strategy-based interventions combined with employment interventions.
The present study used Compensatory Cognitive Training (CCT; Twamley et al., 2012), a 12-session compensatory strategy-based intervention which included four modules of training to address: 1) prospective memory (i.e., remembering to do things in the future), 2) conversational and task vigilance, 3) learning and memory, and 4) cognitive flexibility and problem-solving (i.e., executive functioning). CCT has previously been shown to improve attention, verbal memory, functional capacity, subjective quality of life, and negative symptom severity in people with primary psychotic disorders (Twamley et al., 2012; Mendella et al., 2015), but has not previously been studied in individuals with mood disorders or in the context of employment interventions. The employment intervention used in our study was Individual Placement and Support (IPS), also known as evidence-based supported employment. IPS emphasizes rapid, individualized searching for competitive work, integrated mental health and employment services, and time-unlimited follow-along support, and has a competitive work attainment rate of 61% across 11 randomized controlled trials (Bond, Drake, & Becker, 2008). Across international trials, IPS resulted in competitive work 2.4 times more often than control condition programs (Modini et al., 2016). We hypothesized that CCT, delivered individually by an employment specialist over the first 12 weeks of IPS, compared to a control condition involving extra supported employment sessions, would result in improved work outcomes, cognition, functioning, and symptomatology.
2. Materials and Methods
2.1 Study Participants and Procedures
This study included 153 outpatients with SMI (58 with schizophrenia or schizoaffective disorder and 95 with a mood disorder [37 with bipolar disorder, 58 with major depressive disorder]). Inclusion criteria included: (1) DSM-IV diagnosis confirmed by Structured Clinical Interview for DSM-IV (First et al., 2002) or Mini International Neuropsychiatric Interview (Sheehan et al., 1997); (2) unemployed for at least one month and stating a goal of work; (3) 18 years old or older; and (4) literate and fluent in English. Participants were excluded if they had dementia or an intellectual disability. Table 1 shows the participant demographics, clinical characteristics, and baseline assessment scores for the overall sample, by intervention group, and by diagnosis group. Data from 77 subjects were used in a previous publication examining age as a moderator of CCT effects (Thomas et al., 2017), and data from 40 subjects were used in a previous publication examining age and cognitive change as predictors of employment outcomes in the CCT group (Puig et al., 2016). Analyses including the control condition of this trial have not been published previously.
Table 1.
Mean (SD) or percentage for demographic, clinical, and baseline assessment characteristics
| Total Sample (N=153) | CCT (N=77) | ESE (N=76) | t or χ2 | p | Mood (N=95) | SS (N=58) | t or χ2 | p | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 43.70 (11.69) | 44.43 (11.17) | 42.96 (12.14) | −.78 | .439 | 44.95 (11.48) | 41.66 (11.84) | 1.70 | .091 |
| Education, years | 13.44 (2.77) | 13.25 (2.70) | 13.64 (2.85) | .89 | .377 | 13.96 (2.63) | 12.60 (2.83) | 3.01 | .003 |
| Female, % | 43.1% | 44.2% | 42.1% | χ2=.07 | .798 | 51.6% | 29.3% | χ2=7.21 | .007 |
| Racial/ethnic minority, % | 37.9% | 40.3% | 35.5% | χ2=.04 | .546 | 26.3% | 56.9% | χ2=14.31 | <.001 |
| Mood disorder diagnosis, % | 62.1% | 59.7% | 64.5% | χ2=.36 | .546 | - | - | - | - |
| Duration of illness, years | 24.37 (14.16) | 23.68 (12.96) | 25.07 (15.34) | .61 | .546 | 26.40 (14.31) | 21.03 (13.38) | 2.31 | .022 |
| Months worked in the past 5 years | 26.50 (19.26) | 25.13 (19.62) | 27.92 (18.90) | .89 | .377 | 29.72 (18.86) | 21.30 (18.92) | 2.65 | .009 |
| Premorbid IQ estimate | 103.10 (9.61) | 104.43 (8.10) | 101.73 (10.84) | 1.73 | .085 | 105.36 (8.22) | 99.33 (10.61) | 3.68 | <.001 |
| Intervention sessions | 10.05 (6.58) | 12.27 (7.20) | 7.78 (5.00) | −4.42 | <.001 | 10.92 (6.34) | 8.67 (6.78) | 2.05 | .042 |
| Cognitive | |||||||||
| Processing Speed Composite | −.09 (.78) | −.09 (.75) | −.08 (.81) | .13 | .894 | .12 (.72) | −.42 (.78) | 4.32 | <.001 |
| Working Memory Composite | −.07 (.88) | −.16 (.84) | .02 (.89) | 1.25 | .212 | .12 (.86) | −.38 (.84) | 3.56 | .001 |
| Learning/Memory Composite | −.21 (.83) | −.26 (.82) | −.15 (.84) | .80 | .425 | −.03 (.83) | −.50 (.75) | 3.56 | <.001 |
| Executive Functioning Composite | −.07 (.73) | −.04 (.72) | −.09 (.74) | .38 | .704 | .07 (.69) | −.29 (.73) | 3.10 | .002 |
| CPT-IP Mean | 2.51 (.85) | 2.61 (.81) | 2.41 (.89) | −1.46 | .146 | 2.73 (.76) | 2.18 (.89) | 3.99 | <.001 |
| MIST | 34.82 (9.80) | 35.57 (8.27) | 34.07 (11.14) | −.95 | .345 | 36.79 (9.25) | 31.60 (9.88) | 3.28 | .002 |
| Functioning/Quality of life | |||||||||
| UPSA-Brief Total | 78.43 (10.73) | 79.16 (10.44) | 77.69 (11.04) | −.85 | .398 | 82.78 (7.93) | 71.31 (10.96) | 6.94 | <.001 |
| UPSA-Brief Financial | 43.14 (5.97) | 44.10 (4.61) | 42.17 (6.99) | −2.02 | .046 | 45.12 (3.99) | 39.89 (7.18) | 5.09 | <.001 |
| UPSA-Brief Communication | 35.29 (7.55) | 35.06 (7.77) | 35.53 (7.37) | .38 | .707 | 37.66 (6.59) | 31.42 (7.47) | 5.40 | <.001 |
| SSPA | 4.17 (.65) | 4.14 (.63) | 4.20 (.68) | .58 | .566 | 4.34 (.53) | 3.90 (.74) | 3.91 | <.001 |
| ILSS | .78 (.08) | .78 (.07) | .78 (.09) | .02 | .981 | .79 (.08) | .77 (.08) | 1.45 | .149 |
| QOLI General Life Satisfaction | 4.04 (1.43) | 4.02 (1.49) | 4.07 (1.38) | .20 | .841 | 3.63 (1.42) | 4.70 (1.19) | −4.75 | <.001 |
| Symptom Severity | |||||||||
| PANSS Positivea | 12.53 (5.03) | 12.29 (4.89) | 12.78 (5.03) | .60 | .548 | 11.54 (4.17) | 14.16 (5.86) | −2.98 | .004 |
| PANSS Negativea | 13.20 (5.00) | 13.45 (5.11) | 12.93 (4.89) | −.64 | .521 | 12.00 (4.13) | 15.16 (5.67) | −3.68 | <.001 |
| HAM-D a | 12.94 (6.82) | 12.86 (6.94) | 13.03 (6.76) | .15 | .879 | 14.36 (6.95) | 10.52 (5.89) | 3.47 | .001 |
Denotes measures in which lower scores are better. Bold font denotes p<.05.
SS=Schizophrenia-spectrum disorders; CPT-IP=Continuous Performance Test—Identical Pairs; MIST=Memory for Intentions Screening Test; UPSA-Brief=University of California, San Diego Performance-Based Skills Assessment-Brief; SSPA=Social Skills Performance Assessment; ILSS=Independent Living Skills Survey; QOLI=Quality of Life Interview; HAM-D=Hamilton Depression Rating Scale; PANSS=Positive and Negative Syndrome Scale; Intervention sessions=total CCT or supported employment sessions during the first 12 weeks of the study.
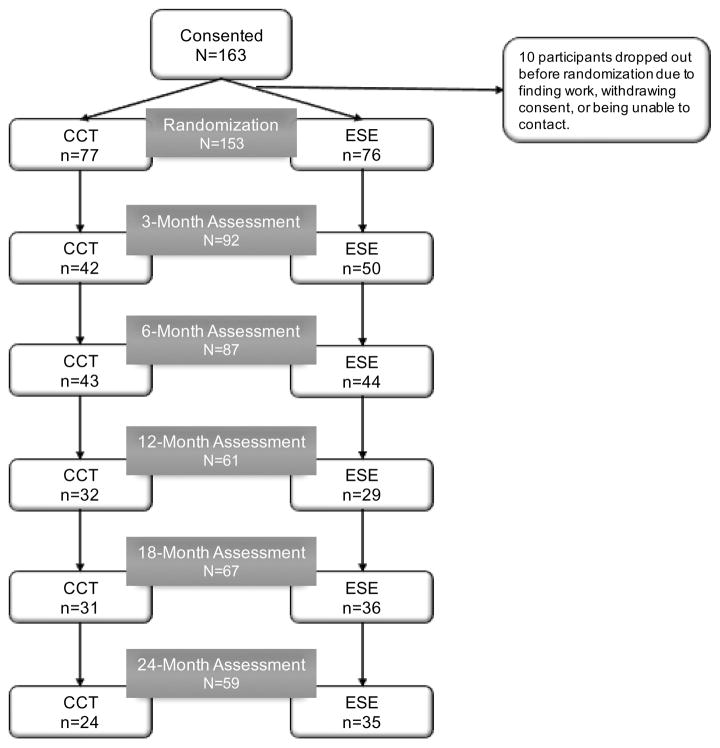
The study was approved by the University of California, San Diego Institutional Review Board and all participants provided informed, written consent prior to enrolling in the study. The study was registered as NCT00895258, and the primary outcome was number of weeks worked during the two-year trial. Participants were randomized to either the Compensatory Cognitive Training (CCT; n=77) condition that received 12-weeks of CCT in the context of supported employment or an active control condition that received Enhanced Supported Employment (ESE; n=76). Fidelity to supported employment was rated as “fair” during the study period. Figure 1 shows a CONSORT diagram of the number of participants from each intervention group that completed each assessment visit. Not all participants who were missing assessments dropped out of the study; some returned for later assessments, and some declined the assessments but provided employment data. There were no differences in the proportion of mood vs. schizophrenia-spectrum disorders who were randomized to each intervention group. Also, there were no significant differences in dropout by intervention group or diagnostic group at 12-months or 24-months (all ps>.05).
Figure 1.
CONSORT diagram showing number of assessments at each occasion. CCT=compensatory cognitive training; ESE=enhanced supported employment.
Compensatory Cognitive Training (CCT)
CCT is a 12-week, manualized intervention designed to target cognitive domains of prospective memory, attention/vigilance, learning and memory, and executive functioning/cognitive flexibility. CCT teaches skills and strategies for implementing the skills to compensate for cognitive difficulties commonly observed in SMI (Twamley et al., 2012). CCT was delivered individually to participants by a master’s-level employment specialist during the first 12 weeks of the study; each of the 12 CCT sessions was approximately 1 hour. CCT sessions were audio-recorded and a random 20% of the sessions were coded for fidelity each month. Our fidelity criteria were met, in that >80% of sessions were rated at ≥80% fidelity; most sessions were rated at 90–100% fidelity to the CCT manual. The CCT condition participants also received supported employment services as indicated by the need of the individual participant for the duration of the study (24 months). Participants in the CCT condition completed a mean (SD) of 8.23 (4.88) CCT sessions (median=12.00; range 0–12) and a mean (SD) of 4.04 (3.02) supported employment sessions (median = 4.00; range 0–11) during the first 12 weeks of the study.
Enhanced Supported Employment (ESE)
The ESE group, like the CCT participants, received individualized supported employment services for the entire study. ESE participants received supported employment from a different master’s level employment specialist to prevent treatment contamination between groups. During the first 12 weeks of the study, they were assigned to receive one extra supported employment session per week to match the CCT group’s contact time. Participants in the ESE condition completed a mean (SD) of 7.78 (5.00) sessions of supported employment (median=8.00; range=0–21) in the first 12 weeks.
2.2 Measures
Work Outcomes
Number of weeks worked over the two-year study was the primary outcome. Work outcomes (job attainment, hours worked, and wages earned) were ascertained weekly from each participant throughout the study by the employment specialist (if engaged in weekly contact with the participant) or a blinded rater; paystubs were used to corroborate work participation and earnings. Competitive work was defined as employment not set aside for a person with a disability, paying at least minimum wage. Participants who dropped out of the study were assumed not to work for the entire duration of the study.
Secondary Outcomes
Participants completed assessments at baseline, 3-month (after initial intervention), 6-month, 12-month, 18-month, and 24-month follow-up visits. Cognitive measures and the University of California, San Diego Performance-Based Skills Assessment-Brief (UPSA-Brief; Mausbach, Harvey, Goldman, Jeste, & Patterson, 2007) and Social Skills Performance Assessment (SSPA; Patterson et al., 2001) were completed at baseline through 12-month visits. Symptom severity measures (Hamilton Depression Rating Scale [HAM-D; Hamilton, 1967]; Positive and Negative Syndrome Scale [PANSS; Kay, Fiszbein, & Opler, 1987]), the Independent Living Skills Survey (ILSS; Wallace et al., 2000), and the Quality of Life Interview (QOLI; Lehman, 1988) were completed at all visits. Raters were blind to participants’ treatment assignment and were not involved in their treatment.
Cognitive Measures
Premorbid intellectual functioning was measured by the Wide Range Achievement Test-III Reading subtest (Wilkinson, 1993). Cognitive functioning was measured by the MATRICS Consensus Cognitive Battery (Nuechterlein et al., 2008) with supplemental neuropsychological measures (Trail Making Test, Part B [Heaton et al., 2004]; Wisconsin Card Sorting Test 64-item version [WCST-64; Kongs et al., 2000]; Letter Fluency using the letters F, A, and S [Heaton et al., 2004]; Memory for Intentions Screening Test [MIST; Raskin, 2004]). Cognitive composite scores were used for the purpose of data reduction. They were created based on a priori categories and included composite scores in the domains of processing speed, working memory, learning/memory, and executive functioning. The processing speed measures in which a lower score was better (e.g., Trail Making Test, Part A), were reversed prior to creating the composite. Therefore, for all composites, higher scores reflect better performance. The cognitive composite scores were created by first converting all individual measure raw scores to z-scores, then calculating the mean of the z-scores within each domain.
The Processing Speed composite score included: Trail Making Test (TMT) Part A, Brief Assessment of Cognition in Schizophrenia (BACS) Symbol-Coding, and Category Fluency. The Working Memory composite score included: WMS-III Spatial Span and University of Maryland (UM) Letter-Number Span. The Learning/Memory composite included: Hopkins Verbal Learning Test—Revised (HVLT-R) and Brief Visual Memory Test—Revised (BVMT-R) total immediate recall. The Executive Functioning composite included: Neuropsychological Assessment Battery (NAB) Mazes, Wisconsin Card Sorting Test 64-item version, TMT Part B, and Letter Fluency (FAS). The Continuous Performance Test—Identical Pairs (CPT-IP), a measure of sustained attention, and the Memory for Intentions Screening Test (MIST; Raskin, 2004), a measure of prospective memory, were also examined.
Functioning and Qualify of Life
The UPSA-Brief (Mausbach et al., 2007) was used to measure performance-based everyday functional skills in the domains of financial management and communication. The SSPA (Patterson et al., 2001) is a performance-based measure that used role-plays to capture social skills of neutral and adversarial situations. The ILSS (Wallace et al., 2000) measured self-reported independence in daily activities (e.g., finances, hygiene, social interactions) and community integration. The QOLI (Lehman, 1998) assessed objective indicators and subjective ratings of quality of life in the domains of finances, employment, living situation, daily activities, family and social relationships, health, and safety.
Symptom Severity
The PANSS (Kay, Fiszbein, & Opler, 1987) measured the severity of positive and negative symptoms of psychosis. Depressive symptom severity was measured using the HAM-D (17-item version; Hamilton, 1967).
2.3 Statistical Analyses
Work outcome analyses
Differences in competitive work attainment, weeks worked, and dollars earned during the 24-month study were analyzed using chi-square and independent-samples t-tests.
Baseline group differences
Baseline differences by intervention group as well as baseline difference by diagnostic group (mood vs. schizophrenia-spectrum disorder) were examined using independent samples t-tests for continuous variables and chi-squared tests for categorical variables (see Table 1). Of note, there were no significant demographic differences between the CCT and ESE groups. There were group differences in total number of intervention sessions in the first 12-weeks of the study, with the CCT group receiving more face-to-face sessions than the ESE group (p<.05). There were significant baseline demographic differences by diagnostic group in education, gender, and racial/ethnic minority status (p<.05) and a trend for age (p<0.1). Therefore, age, education, gender, minority status, and total sessions were included as covariates in subsequent analyses.
Immediate training effects
Analyses of covariance (ANCOVAs) were used to examine the effects of group (CCT or ESE), diagnosis (mood or schizophrenia-spectrum), and the interaction (group × diagnosis) from baseline to 3-months on cognitive, functional, and symptom severity measures. The 3-month post-intervention score was included as the dependent variable and the baseline score, intervention group membership, diagnosis, and intervention group × diagnosis variables were included as independent variables. As described above, given intervention group differences in total sessions and diagnostic group differences in age, education, gender, and minority status, these variables were included as covariates. Effect sizes are reported as partial eta-squared (small=0.01; medium=0.06; large=0.14; Cohen, 1988).
Long-term effects analyses
Hierarchical linear models (HLM) were conducted to examine the long-term outcomes of CCT plus supported employment. Full information maximum likelihood estimation was used to account for missing data, allowing for all available data to be used for parameter estimates (Schafer & Graham, 2002; Singer & Willet, 2003). Prior to analyses, the data were transformed into a z-score metric so the resulting effect estimates are comparable. The random effect of intercept for individuals was included in all models. Visit (baseline, 3-month, 6-month, 12-month, 18-month, and 24-month visits) was modeled as a continuous parameter. Consistent with the ANCOVAs and so that the models would be considered nested if follow up analyses were conducted, age, education, gender, minority status, and total sessions were included as covariates. The predictor variables included in the model included group (CCT and ESE), diagnosis (mood and schizophrenia-spectrum), visit, and the group × visit interaction. Group and diagnosis were dummy coded, with ESE and schizophrenia-spectrum as the reference groups. Follow up HLMs were conducted to examine whether diagnosis moderated the effect of the intervention over time (group × visit) and included the diagnosis × visit, diagnosis × group, and three-way group × visit × diagnosis interactions. Changes in −2 log likelihood (−2LL), Akaike Information Criterion (AIC), and Schwarz’s Bayesian Criterion (BIC) were used to determine whether the inclusion of these additional interactions improved model fit. Estimate effect sizes are reported as r-values (small=0.10; medium=0.30; large=0.50; Cohen, 1992).
3. Results
3.1 Baseline group differences
Table 1 shows the baseline demographic, clinical, and assessment characteristics. When comparing the CCT and ESE groups, there were no significant differences in any of these variables, except for the UPSA-Brief financial subscale, such that the CCT group performed higher at baseline. Overall, the CCT group had significantly more intervention sessions in the first 12 weeks than did the ESE group. When comparing the mood and schizophrenia-spectrum diagnostic groups, the mood disorder group had more education, were more likely to be female, were more likely to be White/non-Latino, had a higher premorbid IQ, had a longer duration of illness, worked more months in the last 5 years (ps<.05), and showed a trend toward being older (p<.10). Across all of the cognitive variables, the mood disorders group performed significantly better than did the schizophrenia-spectrum disorder group. The mood disorders group also performed better on the performance-based measures of functional capacity (UPSA-Brief) and social skills (SSPA), but did not significantly differ on a self-report measure of independence in daily functioning (ILSS). The mood disorders group endorsed a lower quality of life (QOLI general life satisfaction) and more depressive symptoms (HAM-D), but endorsed fewer positive and negative symptoms of psychosis (PANSS) compared to the schizophrenia-spectrum disorders group (all ps<.05).
3.2 Effects of treatment group on work outcomes
Using intent-to-treat analyses of all 153 randomized participants, regardless of their level of participation in CCT or supported employment sessions, we found that 72 participants (i.e., 47%) obtained competitive work during the two-year study. Receiving CCT was not associated with competitive work attainment, weeks worked, or wages earned (see Table 2).
Table 2.
Work outcomes across intervention groups (n=153)
| CCT (n=77) M (SD) or % |
ESE (n=76) M (SD) or % |
t or X2 | df | p | |
|---|---|---|---|---|---|
| Attained competitive work, % | 40.3% | 53.9% | 2.88 | 1 | .090 |
| Weeks worked in two years | 15.8 (28.5) | 22.6 (32.5) | 1.39 | 151 | .167 |
| Wages earned in two years | $5790.5 (14,315.0) | $7810.2 (14,380.3) | 0.87 | 151 | .385 |
3.3 Immediate training effects
The significant findings are discussed below, but all data are included in the tables. Table 3 shows the adjusted means, F-statistics, p-values, and partial η2 of the ANCOVAs examining baseline-to-3-month changes by group, diagnosis, and group × diagnosis. Compared to those in the ESE condition, after 3 months, the CCT group showed a medium improvement in working memory, a medium improvement in depressive symptomatology on the HAM-D, and a medium-to-large improvement on QOLI general life satisfaction. Across intervention groups, participants with a diagnosis of a mood disorder improved more on a measure of attention (CPT-IP mean), social skills (SSPA), and symptoms of psychosis (PANSS positive and negative). There was not a moderating effect of diagnosis on intervention group for any of the immediate training effects (all ps>.05)
Table 3.
Immediate post-intervention effects of intervention group, diagnosis, and intervention group x diagnosis.
| Measure | Intervention Group | Diagnosis | Group × Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| CCT Mean (SE) |
ESE Mean (SE) |
F- statistic |
p | partial η2 |
Mood Mean (SE) |
SS Mean (SE) |
F- statistic |
p | partial η2 |
F- statistic |
p | partial η2 |
|
|
|
|||||||||||||
| Cognitive | |||||||||||||
| Processing Speed Composite | 0.03 (0.10) | −0.13 (0.10) | 0.93 | .337 | .011 | 0.06 (0.07) | −0.16 (0.10) | 2.77 | .100 | .033 | 1.04 | .311 | .013 |
| Working Memory Composite | 0.11 (0.09) | −0.19 (0.09) | 4.44 | .038 | .051 | −0.04 (0.07) | −0.04 (0.09) | 0.00 | .984 | .000 | 0.59 | .444 | .007 |
| Learning/Memory Composite | 0.15 (0.11) | −0.04 (0.11) | 1.21 | .274 | .015 | 0.14 (0.08) | −0.02 (0.11) | 1.26 | .265 | .015 | 2.82 | .097 | .033 |
| Executive Functioning Composite | −0.02(0.07) | −0.10 (0.07) | 0.47 | .495 | .006 | 0.03 (0.05) | −0.14 (0.07) | 3.24 | .076 | .038 | 0.06 | .812 | .001 |
| CPT-IP Mean | 2.66 (0.01) | 2.53 (0.10) | 0.74 | .392 | .010 | 2.74 (0.08) | 2.45 (0.10) | 5.24 | .025 | .064 | 0.41 | .522 | .005 |
| MIST | 35.19 (1.35) | 37.36 (1.38) | 1.01 | .318 | .012 | 37.08 (1.01) | 35.47 (1.39) | 0.82 | .368 | .010 | 0.03 | .865 | .000 |
| Functioning/Quality of life | |||||||||||||
| UPSA-Brief Total | 82.20 (1.64) | 77.88 (1.72) | 2.63 | .108 | .031 | 80.27 (1.26) | 79.81 (1.79) | 0.04 | .841 | .000 | 3.33 | .072 | .039 |
| UPSA-Brief Financial | 44.64 (0.78) | 42.51 (0.82) | 2.71 | .103 | .032 | 43.48 (0.59) | 43.67 (0.81) | 0.03 | .858 | .000 | 1.38 | .244 | .017 |
| UPSA-Brief Communication | 37.20 (1.24) | 35.15 (1.27) | 1.08 | .303 | .013 | 36.96 (0.93) | 35.40 (1.30) | 0.90 | .345 | .011 | 2.61 | .110 | .032 |
| SSPA | 3.93 (0.11) | 4.02 (0.11) | 0.23 | .636 | .003 | 4.18 (0.09) | 3.77 (0.12) | 7.43 | .008 | .083 | 0.06 | .812 | .001 |
| ILSS | 0.80 (0.01) | 0.79 (0.01) | 0.15 | .700 | .002 | 0.79 (0.01) | 0.80 (0.01) | 0.65 | .424 | .008 | 0.70 | .406 | .009 |
| QOLI General Life Satisfaction | 4.76 (0.19) | 3.83 (0.19) | 9.67 | .003 | .109 | 4.42 (0.14) | 4.16 (0.19) | 1.13 | .291 | .014 | 0.14 | .709 | .002 |
| Symptom Severity | |||||||||||||
| PANSS Positivea | 12.21 (0.75) | 12.87 (0.77) | 0.30 | .584 | .004 | 11.46 (0.57) | 13.61 (0.78) | 4.67 | .034 | .054 | 0.81 | .372 | .010 |
| PANSS Negativea | 14.58 (0.93) | 14.00 (0.95) | 0.16 | .693 | .002 | 12.94 (0.71) | 15.64 (0.97) | 4.67 | .034 | .054 | 1.09 | .299 | .013 |
| HAM-Da | 10.40 (1.22) | 14.92 (1.26) | 5.36 | .023 | .063 | 12.19 (0.92) | 13.13 (1.28) | 0.34 | .560 | .004 | 1.23 | .271 | .015 |
Denotes measures in which lower scores are better. Bold font denotes p<.05 and corresponding effect size.
SS=Schizophrenia-spectrum disorders; CPT-IP=Continuous Performance Test—Identical Pairs; MIST=Memory for Intentions Screening Test; UPSA-Brief=University of California, San Diego Performance-Based Skills Assessment-Brief; SSPA=Social Skills Performance Assessment; ILSS=Independent Living Skills Survey; QOLI=Quality of Life Interview; HAM-D=Hamilton Depression Rating Scale; PANSS=Positive and Negative Syndrome Scale. In addition to the independent variables of group, diagnosis, and group × diagnosis, the following covariates were included: age, education, sex, minority status, and number of intervention sessions. Effect size interpretation for partial η2: small=0.01; medium=0.06; large=0.14 (Cohen, 1988).
3.4 Long-term training effects
Table 4 presents the parameter estimates, p-values, and effect sizes for the main effects of group, visit, and the group × visit interaction for all HLM outcome measures over 12- (for cognitive measures, UPSA-Brief, and SSPA) and 24 months (ILSS, QOLI general life satisfaction, PANSS, and HAM-D). Across both intervention groups, there were significant main effects of visit for processing speed, working memory, learning/memory, executive functioning, prospective memory (MIST), UPSA-Brief, and depressive symptoms in the direction of improvement over time. Across measures, there were no significant group × visit interactions, suggesting that the immediate training effects of CCT were not maintained over the long term (through 12 or 24 months). There was a trend toward a small-to-medium effect favoring the CCT group on the learning composite (r=.202, p=.058).
Table 4.
Estimates for effects of visit, intervention group, and visit × group interaction
| Measure | Visit | Group | Visit × Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Estimate (SE) | p | r | Estimate (SE) | p | r | Estimate (SE) | p | r | |
|
|
|||||||||
| Cognitive | |||||||||
| Processing Speed Composite | .194 (.057) | .001 | .322 | −.054 (.081) | .507 | −.046 | −.044 (.056) | .433 | −.078 |
| Working Memory Composite | .115 (.047) | .015 | .252 | −.059 (.081) | .470 | −.053 | .009 (.046) | .854 | .019 |
| Learning/Memory Composite | .249 (.048) | <.001 | .484 | −.014 (.078) | .854 | −.013 | .092 (.048) | .058 | .202 |
| Executive Functioning Composite | .213 (.042) | <.001 | .416 | −.023 (.080) | .776 | −.021 | −.023 (.042) | .584 | −.050 |
| CPT-IP Mean | .089 (.051) | .085 | .195 | .130 (.085) | .128 | .118 | −.033 (.050) | .521 | −.073 |
| MIST | .260 (.069) | <.001 | .318 | −.047 (.076) | .541 | −.044 | −.124 (.069) | .074 | −.160 |
| Functioning/Quality of life | |||||||||
| UPSA-Brief Total | .278 (.070) | <.001 | .351 | .051 (.079) | .522 | .049 | −.110 (.070) | .119 | −.148 |
| UPSA-Brief Financial | .174 (.066) | .009 | .257 | .176 (.078) | .025 | .178 | −.094 (.065) | .150 | −.144 |
| UPSA-Brief Communication | .254 (.082) | .003 | .268 | −.050 (.079) | .531 | −.048 | −.102 (.081) | .212 | −.112 |
| SSPA | −.055 (.063) | .386 | −.083 | .048 (.078) | .539 | .044 | .035 (.062) | .572 | .054 |
| ILSS | −.028 (.042) | .513 | −.061 | .004 (.079) | .962 | .004 | −.022 (.042) | .605 | −.048 |
| QOLI General Life Satisfaction | −.006 (.017) | .748 | −.039 | .054 (.040) | .175 | .116 | .003 (.017) | .875 | .019 |
| Symptom Severity | |||||||||
| PANSS Positivea | −.047 (.035) | .174 | −.136 | −.039 (.078) | .618 | −.038 | .015 (.035) | .674 | .042 |
| PANSS Negativea | −.001 (.039) | .985 | −.001 | .026 (.074) | .727 | .027 | .009 (.039) | .810 | .022 |
| HAM-D a | −.096 (.043) | .027 | −.196 | −.086 (.077) | .267 | −.085 | .007 (.043) | .875 | .014 |
Denotes measures in which lower scores are better. Bold font denotes p<.05 and corresponding effect size.
CPT-IP=Continuous Performance Test—Identical Pairs; MIST=Memory for Intentions Screening Test; UPSA=University of California, San Diego Performance-Based Skills Assessment; SSPA=Social Skills Performance Assessment; ILSS=Independent Living Skills Survey; QOLI=Quality of Life Interview; PANSS=Positive and Negative Syndrome Scale; HAM-D=Hamilton Depression Rating Scale. In addition to the independent variables of visit, group, and visit × group, the following covariates were included: age, education, sex, minority minority status, diagnosis, and intervention sessions. Effect size interpretation for r: small=0.10; medium=0.30; large=0.50 (Cohen, 1992).
The follow up HLMs that included the additional diagnosis × visit, diagnosis × group, and three-way group × visit × diagnosis interactions to determine whether the diagnosis group moderated the intervention effects showed that the results were largely not moderated by diagnosis. There was, however, one significant group × visit × diagnosis interaction for prospective memory (MIST; t(131.091)=-2.20, p=.029, r=−.189), such that schizophrenia-spectrum participants in the ESE group improved at the fastest rate over 12 months. This was an unexpected result that was no longer significant when an outlier (participant with a MIST score=0 at 12-month follow-up) was removed. Furthermore, the addition of these follow up interactions did not improve model fit for the MIST as determined by the non-significant change in −2LL (χ2 =4.82, df=3, p=.185) and increase in AIC and BIC relative to the previous model.
4. Discussion
This randomized controlled trial compared cognitive, functional, and clinical outcomes over two years for participants in a supported employment program receiving either Compensatory Cognitive Training or Enhanced Supported Employment as an active control. The fact that CCT participants attended more treatment sessions in the first 12 weeks of the study may reflect the dual purpose of initial meetings (i.e., receipt of CCT and supported employment rather than supported employment services alone), differential engagement in the interventions, clinician availability, or other real-world implementation factors.
CCT was not associated with any effects on competitive work attainment, weeks worked, or wages earned over the two-year study. Immediately post-treatment, we found medium to large CCT-associated improvements in working memory, depressive symptoms, and subjective ratings of life satisfaction. These effects were not moderated by diagnostic group. Longitudinal follow-up demonstrated no statistically significant effects of CCT over 12 to 24 months, although there was a trend toward a small-to-medium CCT-associated improvement in the learning composite.
The finding that CCT was not associated with work outcomes suggest that evidence-based supported employment can be effective regardless of cognitive impairment level; note, however, that we did not require cognitive impairment for study entry. Our other results suggest that providing CCT in the context of a supported employment program for people with SMI is feasible and confers an initial benefit on working memory, depressive symptoms, and subjective quality of life; it may be the case that although CCT targets numerous cognitive domains, it in fact provides more targeted benefits in the domain of working memory. Although CCT does not directly address psychiatric symptoms, it appears associated with a short-term improvement in depressive symptom severity, possibly via improved self-efficacy and activation through new skill acquisition and use, and/or social contacts with the employment specialist; these factors may also have affected the CCT-associated improvement in quality of life. These results provide additional support for the necessity of longitudinal follow-up after the initial treatment period concludes to gauge wearing off effects of initial improvements.
This study is not without limitations. Although inclusion criteria were minimized to reflect the spirit of accessibility of supported employment services, all participants were community-dwelling outpatients receiving care at a single clinic who self-selected into a research study, which may limit generalizability to other types of participants or treatment settings (e.g., intensive case management programs). Participant engagement and retention is another consideration, as participants in both conditions often did not attend the expected number of meetings with the employment specialist in the first 12 weeks. The considerable ranges suggest variability among individual participants that may have affected their willingness to continue in and receive benefit from the study interventions; future identification and investigation of treatment responders versus non-responders will be important to characterize who might benefit from these treatments and why. Further, more than half of participants in each intervention group withdrew prior to completion of the 2-year protocol, limiting the sample from which longitudinal data were collected and thereby reducing statistical power and introducing the possibility of attrition bias. However, there was no differential dropout between intervention groups and use of full information maximum likelihood estimation for the HLM allowed for all available data to be used to reduce bias that other methods may introduce (e.g., list-wise deletion). The rates appear equivalent to other longitudinal psychosocial interventions (Cohen et al., 1995; Kurtz et al., 2011); this limitation likely reflects the challenge of long-term retention in psychiatric services and research. We did not correct for multiple statistical comparisons. Finally, our lack of findings regarding an effect of CCT on work outcomes may reflect differences in effectiveness of the employment specialists assigned to deliver CCT and ESE, respectively (Corbiere et al., 2017).
Despite these limitations, to our knowledge this is the first RCT investigating supported employment combined with an exclusively compensatory strategy-based cognitive training intervention for individuals with SMI. These findings suggest immediate positive effects on working memory, depression, and quality of life. Ongoing efforts to identify individual moderators of treatment response will be critical to enhance the personalized approach to cognitive intervention to support real-world functioning and goal attainment.
Acknowledgments
Role of the Funding Source
This work was supported by grants from NIMH (R01MH080150 to E.W.T., and T32MH018399 to C.Z.B. and D.V.J.).
The authors would like to thank all of the study participants and Gabrielle Garmsen Golden, Mary Linges, and Barbara Johnson for their assistance with study data collection and management.
Footnotes
Contributors
E.W.T. designed the study, oversaw data analyses, drafted parts of the manuscript, and edited the manuscript. K.R.T conducted the primary data analyses and drafted parts of the manuscript. C.Z.B. conducted literature searches and drafted parts of the manuscript. L.V. assisted with data interpretation and edited the manuscript. D.V.J., R.K.H., and S.R.M. provided assistance with data interpretation and edited the manuscript.
Conflict of Interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anaya C, Aran AM, Ayuso-Mateos JL, Wykes T, Vieta E, Scott J. A systematic review of cognitive remediation for schizo-affective and affective disorders. J Affect Disord. 2012;142(1):13–21. doi: 10.1016/j.jad.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Au DW, Tsang HW, So WW, Bell MD, Cheung V, Yiu MG, Tam KL, Lee GTH. Effects of integrated supported employment plus cognitive remediation training for people with schizophrenia and schizoaffective disorders. Schizophr Res. 2015;166(1):297–303. doi: 10.1016/j.schres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176(2):183–189. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bell MD, Bryson GJ, Greig TC, Fiszdon JM, Wexler BE. Neurocognitive enhancement therapy with work therapy: productivity outcomes at 6-and 12-month follow-ups. J Rehabil Res Dev. 2005;42(6):829–838. doi: 10.1682/jrrd.2005.03.0061. [DOI] [PubMed] [Google Scholar]
- Bell MD, Zito W, Greig T, Wexler BE. Neurocognitive enhancement therapy with vocational services: work outcomes at two-year follow-up. Schizophr Res. 2008;105(1):18–29. doi: 10.1016/j.schres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Bond GR, Drake RE, Becker DR. An update on randomized controlled trials of evidence-based supported employment. Psychiatr Rehabil J. 2008;31(4):280. doi: 10.2975/31.4.2008.280.290. [DOI] [PubMed] [Google Scholar]
- Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr Bull. 2015;41(5):1095–1104. doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Gupta M, Holshausen K, Jokic R, Best M, Milev R. Cognitive remediation for treatment-resistant depression: effects on cognition and functioning and the role of online homework. J Nerv Ment Dis. 2013;201(8):680–685. doi: 10.1097/NMD.0b013e31829c5030. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2014;169(7):710–718. doi: 10.1176/appi.ajp.2012.11091337. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Routledge Academic; New York, NY: 1988. [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen K, Edstrom K, Smith-Papke L. Identifying early dropouts from a rehabilitation program for psychiatric outpatients. Psychiatr Serv. 1995;46(10):1076–1078. doi: 10.1176/ps.46.10.1076. [DOI] [PubMed] [Google Scholar]
- Corbière M, Lecomte T, Reinharz D, Kirsh B, Goering P, Menear M, Berbiche D, Genest K, Goldner EM. Predictors of acquisition of competitive employment for people enrolled in supported employment programs. J Nerv Ment Dis. 2017;205(4):275–282. doi: 10.1097/NMD.0000000000000612. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Nierenberg AA, Kessler R, Lund HG, Ametrano RM, Sachs G, Rauch SL, Dougherty D. Cognitive rehabilitation for bipolar disorder: an open trial for employed patients with residual depressive symptoms. CNS Neurosci Ther. 2010;16(5):298–307. doi: 10.1111/j.1755-5949.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Harmell AL, Savla GN, Bowie CR, Harvey PD, Patterson TL. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14(3):217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12(1):64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, Schlosser D, Pham L, Miskovich T, Vinogradov S. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull. 2014;41(1):250–258. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources, Inc; Lutz, Florida: 2004. [Google Scholar]
- Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39–48. doi: 10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin card sorting test-64 card version. Psychological Assessment Resources, Inc; Lutz, Florida: 2000. [Google Scholar]
- Kurtz MM, Rose J, Wexler BE. Predictors of participation in community outpatient psychosocial rehabilitation in schizophrenia. Community Ment Health J. 2011;47(6):622–627. doi: 10.1007/s10597-010-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 2013;170(3):334–341. doi: 10.1176/appi.ajp.2012.12040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Redoblado-Hodge MA, Naismith SL, Hermens DF, Porter MA, Hickie IB. Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychol Med. 2013;43(6):1161–1173. doi: 10.1017/S0033291712002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51–62. [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33(6):1364–1372. doi: 10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, DeRosa T, Wolfe R. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophr Bull. 2009;35(2):319–335. doi: 10.1093/schbul/sbn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Xie H, Feldman K, Shaya Y, Klein L, Wolfe R. Cognitive remediation for vocational rehabilitation nonresponders. Schizophr Res. 2016;175(1):48–56. doi: 10.1016/j.schres.2016.04.045. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, Xie H, Welsh J, Kaiser S, Drake RE, Becker DR, Bailey E, Fraser G, Wolfe R, McHugo GJ. Cognitive enhancement treatment for people with mental illness who do not respond to supported employment: a randomized controlled trial. Am J Psychiatry. 2015;172(9):852–861. doi: 10.1176/appi.ajp.2015.14030374. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Wykes T. Cognitive remediation and vocational rehabilitation. Psychiatr Rehabil J. 2008;31(4):350. doi: 10.2975/31.4.2008.350.359. [DOI] [PubMed] [Google Scholar]
- Mendella PD, Burton CZ, Tasca GA, Roy P, Louis LS, Twamley EW. Compensatory cognitive training for people with first-episode schizophrenia: results from a pilot randomized controlled trial. Schizophr Res. 2015;162(1):108–111. doi: 10.1016/j.schres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Modini M, Tan L, Brinchmann B, Wang M, Killackey E, Glozier N, Mykletun A, Harvey SB. Supported employment for people with severe mental illness: a systematic review and meta-analysis of the international evidence. Br J Psychiatry. 2016;209:14–22. doi: 10.1192/bjp.bp.115.165092. [DOI] [PubMed] [Google Scholar]
- Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med. 2013;43(06):1187–1196. doi: 10.1017/S0033291712001948. [DOI] [PubMed] [Google Scholar]
- Motter JN, Pimontel MA, Rindskopf D, Devanand DP, Doraiswamy PM, Sneed JR. Computerized cognitive training and functional recovery in major depressive disorder: a meta-analysis. J Affect Disord. 2016;189:184–191. doi: 10.1016/j.jad.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001;48(2):351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Douglas K, Jordan J, Bowie CR, Roiser J, Malhi GS. Psychological treatments for cognitive dysfunction in major depressive disorder: current evidence and perspectives. CNS Neurol Disord Drug Targets. 2014;13(10):1677–1692. doi: 10.2174/1871527313666141130223248. [DOI] [PubMed] [Google Scholar]
- Priyamvada R, Ranjan R, Chaudhury S. Cognitive rehabilitation of attention and memory in depression. Ind Psychiatry J. 2015;24(1):48–53. doi: 10.4103/0972-6748.160932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O, Thomas KR, Twamley EW. Age and improved attention predict work attainment in combined Compensatory Cognitive Training and supported employment for people with severe mental illness. J Nerv Ment Dis. 2016;204(11):869–872. doi: 10.1097/NMD.0000000000000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin S. Memory for Intentions Screening Test. J Int Neuropsychol Soc. 2004;10(suppl 1):110. [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Sanches M, Bauer IE, Galvez JF, Zunta-Soares GB, Soares JC. The management of cognitive impairment in bipolar disorder: current status and perspectives. Am J Ther. 2014;22(6):477–486. doi: 10.1097/MJT.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press; New York, New York: 2003. [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12(5):232–241. [Google Scholar]
- Thomas KR, Puig O, Twamley EW. Age as a moderator of change following compensatory cognitive training in individuals with severe mental illnesses. Psychiatr Rehabil J. 2017;40(1):70–78. doi: 10.1037/prj0000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK, Jeste DV. Compensatory Cognitive Training for psychosis: effects in a randomized controlled trial. J Clin Psychiatry. 2012;73(9):1212–1219. doi: 10.4088/JCP.12m07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venza EE, Chapman SB, Aslan S, Zientz JE, Tyler DL, Spence JS. Enhancing executive function and neural health in bipolar disorder through reasoning training. Front Psychol. 2016;7:1676. doi: 10.3389/fpsyg.2016.01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJ, Liberman RP, Tauber R, Wallace J. The independent living skills survey: a comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophr Bull. 2000;26(3):631–658. doi: 10.1093/oxfordjournals.schbul.a033483. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test Manual. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;68(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Landau S, Everitt B, Knapp M, Patel A, Romeo R. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry. 2005;190:421–427. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]