Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) is a class of ligand-activated nuclear transcription factors, which is a member of type II nuclear receptor superfamily. Previous studies demonstrate that PPARγ is expressed in a variety of tumor tissues and is closely associated with the proliferation and prognosis of digestive system tumors by its roles in mediation of cell differentiation, induction of cell apoptosis, and inhibition of cell proliferation.
1. Introduction
As a class of ligand-activated cytokine of steroid hormone receptor, peroxisome proliferator-activated receptor (PPAR) is activated by binding to peroxisome proliferator, which in turn initiates a series of regulatory effects of transcription, thereby participating in adipogenesis, lipid metabolism, metabolic homeostasis, inflammation, tumorigenesis, and its progression [1, 2]. To date, three receptor subtypes of PPAR (α, β/δ, and γ) have been found, among which PPARγ is the most widely studied and is expressed in adipose tissues, esophagus, gastrointestinal, liver, spleen, pancreas, and so on. Recently, it has been discovered that PPARγ is closely linked to digestive system tumors, as it is overexpressed in esophageal cancer, gastrointestinal cancer, liver cancer, pancreatic cancer, and para-carcinoma tissues [3–5]. In this paper, we introduce the structure, organization, distribution, ligands, and function of PPARγ briefly and review the correlation between PPARγ receptor and digestive system tumor.
2. Overview of PPARγ
2.1. Structure of PPARγ
PPARγ gene is located in human's 3p25 chromosome, and according to promoter, exon, and splicing type, PPARγ mRNA has 4 distinct spliceosomes, namely, PPARγ1, PPARγ2, PPARγ3, and PPARγ4. The protein-coding sequence of PPARγ2 mRNA is different from that of the other three spliceosomes, as PPARγ2 has another 30 amino acid residues at the amino terminus. PPARγ can translate into two different proteins, PPARγ1 and PPARγ2, and their biological roles and ways of expression are not the same. There are four functional domains in the structure of PPARγ molecule: the first one is a non-ligand-dependent transcriptional activation domain for the amino terminus, which is a regulatory region with phosphorylation binding sites. It regulates the activity of PPARγ by altering the affinity of the receptor or the ligand via its phosphorylation. The second one is a DNA binding domain (DBD) consisting of two zinc finger structures, which is a binding domain initiating and regulating the gene transcription after binding to the peroxisome proliferator response element. The third one is a regulatory domain with transcriptional activity, and a number of factors in the nucleus regulate the activity of PPARγ via binding to this domain; the fourth one is a ligand binding domain (LBD) at the carboxy-terminus of PPARγ, and PPARγ exhibits its regulation on target gene expression and downstream effects on the binding of the ligand and this structural domain [6, 7].
2.2. Tissue Distribution of PPARγ
PPARγ is widely distributed in adipose tissues, esophagus, gastrointestinal, liver, and pancreas, and it is also expressed in various tissues of the immune system. The distribution and range of different types of PPARγ spliceosomes vary in different body tissue cells [8] with high tissue specificity: PPARγ1 is widely distributed throughout the body with different degrees of expression; PPARγ2 is mainly distributed in adipose tissues and liver tissues, with the former expressing more; PPARγ3 is mainly distributed in adipocytes, macrophages, and colonic epithelial cells, where it is also expressed. The distribution of PPARγ4 is not yet clear and remains to be explored.
2.3. PPARγ Ligands
The current studies show that the ligand treatment of PPARγ inhibits the proliferation of tumor cells and induces tumor cell apoptosis, which underlines its role in tumor targeted therapy [9, 10]. Currently, a series of PPARγ agonists (also known as PPARγ ligands) have been found or synthesized, which may be further divided into natural and synthetic ligands on the basis of their sources [11]. The natural ligand consists of a group of endogenously secreted molecules, whose activity is often not high. Natural ligands include all kinds of unsaturated fatty acids and their metabolic derivatives, such as linoleic acid, linolenic acid, and eicosapentaenoic acid. Certain prostaglandins and their metabolic derivatives also belong to natural ligands, and currently 15-deoxygenated prostaglandin is known to possess the strongest metabolic activity [12]. On the other hand, the synthetic ligand is mainly composed of thiazolidinedione compounds (troglitazone, rosiglitazone, pioglitazone, etc.). With stronger metabolic activity than that of the natural ligand, the synthetic ligand is widely used in diabetes management. Moreover, a growing body of research has discovered that the synthetic ligand has an antitumor effect either used independently or combined with other medications, and the mechanism underlying this is a research hotspot at present [13]. Certain nonsteroidal drugs, such as indomethacin and ibuprofen, are reported to possess an antineoplastic effect, although their metabolic activity is extremely low [14]. Additionally, some receptor antagonists, such as leukotrienes, also belong to PPARγ agonists.
3. Function of PPARγ
PPARγ is widely present in various tissue cells and has a broad array of biological functions. It is involved in the regulation of carbohydrate metabolism and adipogenesis in cells and also participates in the inflammatory response as well as the differentiation and apoptosis of tumor cells [15]. Researchers has found that after being activated by ligand, PPARγ can induce tumor cell differentiation, repress their proliferation, promote their apoptosis, and concomitantly reduce neoplastic angiogenesis, which eventually halts the tumor growth, proliferation, infiltration, and metastasis [16, 17]. Its most important function is mediation of gene transcription and subsequent regulation on its activation after combining with its ligands. The process is briefed as follows: the ligand-activated PPARγ forms a heterodimer complex with 9-cis-retinoid X receptor, retinoid X receptor (RXR), or glucocorticoid receptor, which subsequently forms a receptor-coregulatory factor-DNA or a protein-DNA complex after combining with peroxisome proliferator response element (PPRE, the upstream target gene promoter of PPARγ). As such, a series of PPARγ-mediated molecular events activates the target gene and plays a role in its transcription and regulation. In the course of this process, a series of auxiliary activators or inhibitors can affect the function of PPARγ [18]. PPARγ can also affect gene transcription and regulation by influencing the signal pathways of other specific transcription factors.
4. PPARγ and Digestive Tumors
Tumorigenesis involves cell proliferation dysregulation, abnormal differentiation, and apoptosis imbalance. PPARγ is highly expressed in multiple gastrointestinal cancers and is linked to the development, growth, and proliferation of them [19, 20], which again plays an important biological role in halting their invasion and metastasis [21]. Recent studies have shown that PPARγ receptor agonists inhibit the proliferation of various types of tumor cells in vitro and vivo, has a synergistic effect with other antitumor chemotherapeutics [22, 23], and also enhances radiotherapy sensitivity [24]. Currently, PPARγ is used as a potential diagnostic and prognostic biomarker of digestive tract cancers and has become the research focus as a promising therapeutic target for gastrointestinal cancers.
4.1. PPARγ and Esophageal Cancer
Esophageal cancer often originates from esophageal squamous intraepithelial neoplasia. PPARγ is expressed in normal esophageal squamous epithelium, esophageal squamous intraepithelial neoplasia, and esophageal squamous cell carcinoma. It is found [25] that the expressions of PPARγ mRNA and protein in the esophageal squamous intraepithelial neoplasia and esophageal squamous cell carcinoma are significantly lower than those of the normal esophageal squamous epithelium and are correlated with the cellular differentiation levels. In esophageal squamous cell carcinoma with various differentiation levels, the expression of PPARγ mRNA and protein is also different, and it has proved that the degree of differentiation of tumor cells is inversely correlated to PPARγ mRNA and protein expression. Taken together, the gene expression of PPARγ is associated with the development and prognosis of esophageal cancer. Since PPARγ is highly expressed in the esophageal cells, PPARγ agonist drugs, such as rosiglitazone, are found to decrease the growth, proliferation, invasion, and metastasis of the cell lines of the esophageal squamous cell carcinoma and act in a certain dose-dependent manner [26]. Such an inhibitory effect is also inversely correlated to the differentiation level of the esophageal squamous cell carcinoma [26]. Besides, some results suggest that efatutazone, but not the conventional PPAR-γ agonist troglitazone, alone or in combination with cetuximab, may offer therapeutic effects [27]. At present, it is assumed that after ligand activation, PPARγ inhibits the proliferation and growth of tumor cells in a dose-dependent manner, which is also associated with the differentiation level of tumor cells, suggesting that PPARγ may be an early diagnostic factor for esophageal cancer, which provides clinicians with a new interventive target for esophageal cancer.
4.2. PPARγ and Gastric Cancer
Recent studies [28] on PPARγ and gastric cancer show that the expression rate of PPARγ is closely related to the dysplasia of gastric mucosal tissue: the expression level of PPARγ is low in normal gastric mucosa, and the expression of PPARγ in gastric mucosal tissue is significantly lower in patients with chronic atrophic and chronic nonatrophic gastritis than that in patients with atypical hyperplasia of gastric mucosa, while there is a significant high expression in the gastric cancer tissue, indicating that the expression rate of PPARγ in the gastric mucosa is positively correlated to the degree of dysplasia. Helicobacter pylori infection is one of the most common carcinogenic factors, and elimination of Helicobacter pylori has also reduced the expression of PPARγ in the gastric mucosa, suggesting that PPARγ has been involved in the carcinogenic process of Helicobacter pylori [29].
Several papers [30, 31] have reported an antitumor effect of PPARγ agonists (such as thiazolidinedione, prostaglandin, and their metabolic derivatives) in gastric cancer tissues, where PPARγ agonists disturb the growth cycle of tumor cells, induce tumor cell differentiation [28], promote tumor cell apoptosis, repress tumor cell proliferation and metastasis [32], and reduce tumor angiogenesis, and these effects are dose-dependent. The combination of retinoid X receptor agonist (9-cis-retinoic acid) and troglitazone could induce the maximal inhibitory effects on tumor growth and apoptosis via promoting the formation of RXR/PPARγ heterodimer [33].
The research mentioned above shows that the high expression of PPARγ may be the molecular marker of chronic gastritis developing into gastric mucosal atypical hyperplasia or even gastric cancer, and it is expected to be a marker for early detection of gastric cancer and assessment of the malignancy degree. After being activated by ligands, PPARγ can not only inhibit the proliferation of gastric cancer growth, but also prevent the development and growth of gastric cancer. In conclusion, PPARγ may become a new therapeutic target for the gastric cancer treatment.
4.3. PPARγ and Colorectal Cancer
PPARγ is expressed in normal intestinal mucosa, colorectal cancer tissues, and paraneoplastic tissues, and the level of PPARγ expression is related to the tumor cell differentiation, infiltration, and metastasis [34, 35]: (1) the higher the differentiation level of colorectal cancer tissue, the higher the expression level of PPARγ protein; (2) the protein expression level of PPARγ is positively related to the range of the infiltration, lymph node metastasis, or distant metastasis. PPARγ agonists can halt the tumor cell growth cycle of the colorectal cancer, induce the differentiation of intestinal cancer cells, change the morphology of tumor cells, and promote tumor cell apoptosis to achieve the antitumor effect [36]. Currently, troglitazone is considered to enhance the apoptotic response of colon cancer cells to photodynamic therapy [37]. Rosiglitazone, as a novel radiosensitizer, suppresses radiation-induced survival signals and DNA damage response and enhances the radiation-induced apoptosis signaling cascade [38]. The combination of resveratrol with a PPARγ agonist could play a role in resveratrol-induced apoptosis of colon carcinoma cells [39]. There are studies [40, 41] indicating that several kinds of PPARγ agonists, such as thiazolidinediones, are expected to combine with chemotherapeutic agents or other target medications to work as a second-line treatment regimen, in the hope of improving the prognosis of patients with advanced colorectal cancer. Conclusively, PPARγ is closely associated with the occurrence, development, proliferation, and prognosis of colorectal cancer and may become a novel molecular biomarker for clinical staging of colorectal cancer and a promising therapeutic target for colorectal cancer.
4.4. PPARγ and Liver Cancer
PPARγ is expressed in normal hepatocytes, nonalcoholic fatty liver cells, hepatocellular carcinoma tissues, and various liver cancer cell lines. Studies [42] have shown that, compared to patients with overexpression of PPARγ, patients with low expression of PPARγ have larger primary tumors, more inflicted lymph nodes, and commoner distant metastasis and are often accompanied by vascular invasion. A series of current papers [43] indicate that, due to the differences in metabolic environment, cell types, and carcinogenic signal pathways, PPARγ may be expressed as either inhibiting or promoting the growth and proliferation of the hepatocellular carcinoma. More studies [44] have suggested that after being treated with PPARγ agonists, such as rosiglitazone, the growth of liver cancer cells in vitro can be blocked at Phase G1. Additionally, PPARγ agonists also decrease the specific enzyme for liver tumor cell differentiation (γ-glutamyl transferase) and the protein markers (alpha-fetoprotein) significantly. Furthermore, the sensitivity of chemotherapy against liver cancer has been potentiated with rosiglitazone. However, there is a study indicating that rosiglitazone has a pleiotropic anticancer effect independent of PPARγ [45]. An experimentation on animals indicate that, in PPARγ-expressing and PPARγ-deficient mouse models of hepatic carcinogenesis, PPARγ deletion in hepatocytes of did not modify hepatic carcinogenesis but increased the thiazolidinedione antitumorigenic effect in part by inhibition of nucleophosmin expression and p53 activation [46]. We hypothesize that the net effect of thiazolidinedione in cancer cells depends on the balance of PPARγ-mediated (prooncogenic) and PPARγ-independent (antioncogenic) mechanisms, by means of regulation different factors including receptor expression levels, phosphorylation status, expression of the heterodimeric partners, and the presence of endogenous ligands.
Overall, the studies mentioned above suggest that, at present, the role of PPARγ in the formation, differentiation, proliferation, and apoptosis of hepatocellular carcinoma cells is still unclear, and the regulation role of PPARγ in energy metabolism and adipogenesis as well as its relation to hepatocellular carcinoma should be further investigated.
4.5. PPARγ and Pancreatic Cancer
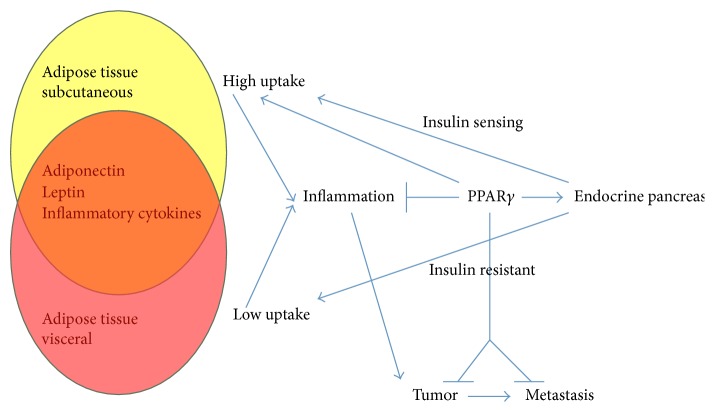
PPARγ is expressed in normal pancreas, pancreatic cancer, and para-carcinoma tissues and is highly expressed in pancreatic cancer tissue cells. Insulin resistance, obesity, oxidative stress, and inflammatory response all play important roles in pancreatic cancer (Figure 1). PPARγ indirectly regulates the insulin transcription mediated by pancreatic and duodenal homeobox 1 (PDX-1), who is a master regulator of pancreas development and differentiation and regulates the expression of insulin gene in β-cells [47]. Through the regulation of the cholesterol transporter ATP-binding cassette transporter A1, PPARγ regulates insulin secretion regulating the expression of G-protein-coupled transmembrane receptor [48]. By means of increasing the uptake of glucose in the skeletal and adipose tissue by regulation of glucose transporters, also promoting catabolic events to produce ATP by increasing insulin signaling modulating AMP-activated protein kinase (AMPK), PPARγ increases the insulin sensitivity in the peripheral tissues [49]. PPARγ is a master regulator of adipocytes differentiation. By the Wnt/β-catenin pathway that recruits the Single Molecule, Real-Time (SMRT) corepressor complex to repress PPARγ1 and PPARγ2 gene, preadipocytes are maintained in undifferentiated state [50]. In preadipocytes, activation of PPARγ leads to the novo differentiation of subcutaneous adipocytes and the apoptosis of older visceral adipocytes [51]. Moreover, in the new adipose tissue, PPARγ reduces leptin expression indirectly by antagonism on leptin promoter with CCAAT/enhancer binding protein (C/EBP), whereas the nuclear receptor transcription factors control adiponectin directly which also induces the secretion of HMW adiponectin from adipocytes [52]. In adipocytes, PPARγ activation has been associated with the upregulation of insulin receptor substrate- (IRS-) 2 and cytapheresis components of insulin pathway and hence with increased insulin sensitivity [53]. PPARγ inhibits resistin synthesis, which is an adipokine associated with inflammation and diabetes type 2, which is the main source of macrophages [54]. Depending on the stimuli of the environment, macrophages can acquire distinct phenotypes: the M1 is an inflammatory phenotype, whereas the M2 is anti-inflammatory. In obese and diabetes type 2 patients, adipose tissue is particularly rich in M1 macrophages. PPARγ primes primary human monocytes into M2 differentiation and keeps M2 marker expression in resting state or M1 macrophages [55]. In adipose tissue, PPARγ reduces the production of the proinflammatory cytokines tumor necrosis factor- (TNF-) α, interleukin- (IL-) 6, and plasminogen activator inhibitor- (PAI-) 1. Suppression of these cytokines is associated with the repression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling cascade. PPARγ transrepresses NF-κb acting as a transcriptional corepressor of NF-κB-target genes or by direct binding with NF-κB; the nuclear receptor may reduce NF-κB activation and induce its degradation [56, 57]. In immune cells, the inhibition of NF-κB signaling by PPARγ results in a substantial anti-inflammatory response [57].
Figure 1.
Effects of peroxisome proliferator-activated receptor-γ on diabetes, obesity, and pancreatic ductal adenocarcinoma [5].
Since PPARγ is involved in carbohydrate metabolism, adipogenesis, and inflammation, PPARγ agonists have been widely used in the management of diabetes and oxidative stress-related diseases, and its antineoplastic effect against pancreatic cancer has been further confirmed in a series of studies [5]. Some studies propose that a triple-agent regime, including metformin, pioglitazone, and lithium, may provide a metabolic adjuvant therapy for pancreatic cancer [58]. In the development and progression of pancreatic cancer, the activated PPARγ inhibits tumor cell proliferation and growth and promotes tumor cell apoptosis by inducing cell differentiation, regulating cell cycle and mediating the expression of target genes including apoptotic genes and proapoptotic genes. Altogether, PPARγ has the antitumor effect through a series of complicate biological functions. This combination of interferon-β and the PPAR-γ agonist troglitazone induced a synergistic effect on the growth inhibition of BxPC-3, a pancreatic cancer cell line, through the counteraction of the interferon-β-induced activation of signal transducer and activator of transcription- (STAT-) 3, the mitogen-activated protein kinase (MAPK), and AKT and the increase in the binding of both STAT-1 related complexes and PPAR-γ with specific DNA responsive elements [59]. Some papers have suggested [58, 60] that prostaglandin and PPARγ activated by its metabolic derivatives inhibit the growth and proliferation of pancreatic cancer cells in a certain dose-dependent manner; rosiglitazone and metformin, either used alone or combined, both inhibit the growth and proliferation of pancreatic cancer in vitro and promote the tumor cell apoptosis, and it has been found that the potency of their combination is stronger than that of either single drug. Consequently, it is indicated that PPARγ may provide a new molecular biological marker for the early diagnosis of pancreatic cancer and also offer new ways to reinforce the therapy effect of pancreatic cancer and improve its prognosis.
5. Conclusions and Prospects
At present, the incidence and mortality rate of gastrointestinal cancer in China are relatively high, whose pathophysiological mechanisms are also relatively complex. The development and progress are a sophisticate multistep, multistage process involving a variety of genes. By the time of treatment, cancer invasion and metastasis have occurred in the majority of patients with gastrointestinal cancer, which makes the efficacy of surgery, radiotherapy, and chemotherapy poor. Therefore, it will be of great significance to look for a new molecular marker of digestive tumors in order to assist their diagnosis and to explore new targets for the targeted intervention.
In summary, PPARγ is closely related to digestive system tumors. After being activated by the ligand, it can inhibit the development and proliferation of tumor cells through a series of molecular biological effects, such as induction of tumor cell differentiation, promotion of tumor cell apoptosis, inhibition of tumor cell proliferation, and the invasion as well as metastasis, while its detailed molecular biological mechanism still needs an in-depth study. With regard to the diagnosis and treatment of gastrointestinal cancer, PPARγ has now become a molecular biology marker for the detection of gastrointestinal tumors as well as a molecular star in target therapy for gastrointestinal cancer, which may prove a breakthrough in the diagnosis and management of digestive system cancers.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Tyagi S., Gupta P., Saini A. S., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. Journal of Advanced Pharmaceutical Technology & Research. 2011;2(4):236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanale D., Amodeo V., Caruso S. The Interplay between Metabolism, PPAR Signaling Pathway, and Cancer. PPAR Research. 2017;2017:1–2. doi: 10.1155/2017/1830626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai X. H., Zheng X., Tang X., et al. Induction of apoptosis of human gastric carcinoma SGC-7901 cell line by 5, 7-dihydroxy-8-nitrochrysin in vitro. World Journal of Gastroenterology. 2007;13(28):3824–3828. doi: 10.3748/wjg.v13.i28.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen B., Chu E. S. H., Zhao G., et al. PPARgamma inhibits hepatocellular carcinoma metastases in vitro and in mice. British Journal of Cancer. 2012;106(9):1486–1494. doi: 10.1038/bjc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polvani S. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World Journal of Gastroenterology. 2016;22(8):2441–2459. doi: 10.3748/wjg.v22.i8.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazumder M., Ponnan P., Das U., et al. Investigations on Binding Pattern of Kinase Inhibitors with PPAR. PPAR Research. 2017;2017:1–11. doi: 10.1155/2017/6397836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zieleniak A., Wójcik M., Woźniak L. A. Structure and physiological functions of the human peroxisome proliferator-activated receptor γ . Archivum Immunologiae et Therapia Experimentalis. 2008;56(5):331–345. doi: 10.1007/s00005-008-0037-y. [DOI] [PubMed] [Google Scholar]
- 8.Takeyama K., Kodera Y., Suzawa M., Kato S. Peroxisome proliferator-activated receptor(PPAR)-structure, function, tissue distribution, gene expression. Nippon rinsho. Japanese journal of clinical medicine. 2000;58(2):357–363. [PubMed] [Google Scholar]
- 9.Apostoli A. J., Roche J. M., Schneider M. M., et al. Opposing roles for mammary epithelial-specific PPARγ signaling and activation during breast tumour progression. Molecular Cancer. 2015:1–14. doi: 10.1186/s12943-015-0347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu L., Huang R., Wu S., et al. PPARγ; and Its Ligands: Potential Antitumor Agents in the Digestive System. Current Stem Cell Research & Therapy. 2016;11(3):274–281. doi: 10.2174/1574888X10666150630111618. [DOI] [PubMed] [Google Scholar]
- 11.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutrition Journal . 2014;13, article 17 doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He P., Li Y., Ding X., et al. Expression of 15-Hydroxyprostaglandin Dehydrogenase in Human Chorion Is Associated with Peroxisome Proliferator-Activated Receptor Isoform Expression in Term Labor. The American Journal of Pathology. 2015;185(7, article no. 2042):1981–1990. doi: 10.1016/j.ajpath.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Chiu M., Mcbeth L., Sindhwani P., Hinds T. D. Deciphering the Roles of Thiazolidinediones and PPAR γ in Bladder Cancer. PPAR Research. 2017;2017 doi: 10.1155/2017/4810672.4810672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puhl A. C., Milton F. A., Cvoro A., et al. Mechanisms of peroxisome proliferator activated receptor ? regulation by non-steroidal anti-inflammatory drugs. Nuclear Receptor Signaling. 2015;5(13):p. e004. doi: 10.1621/nrs.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egerod F. L., Brünner N., Svendsen J. E., Oleksiewicz M. B. PPARα and PPARγ are co-expressed, functional and show positive interactions in the rat urinary bladder urothelium. Journal of Applied Toxicology. 2010;30(2):151–162. doi: 10.1002/jat.1481. [DOI] [PubMed] [Google Scholar]
- 16.Ying S., Xiao X., Chen T., Lou J. PPAR ligands function as suppressors that target biological actions of HMGB1. PPAR Research. 2016;2016:10. doi: 10.1155/2016/2612743.2612743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bojkova B., Kajo K., Garajova M., et al. Rosiglitazone shows partial oncostatic effect in rat mammary carcinogenesis. Neoplasma. 2013;60(1):46–55. doi: 10.4149/neo_2013_007. [DOI] [PubMed] [Google Scholar]
- 18.Viswakarma N., Jia Y., Bai L., et al. Coactivators in PPAR-regulated gene expression. PPAR Research. 2010;2010:21. doi: 10.1155/2010/250126.250126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbins G. T., Nie D. PPAR gamma, bioactive lipids, and cancer progression. Frontiers in Bioscience. 2012;17(5):1816–1834. doi: 10.2741/4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukahara T., Haniu H. Peroxisome proliferator-activated receptor gamma overexpression suppresses proliferation of human colon cancer cells. Biochemical and Biophysical Research Communications. 2012;424(3):524–529. doi: 10.1016/j.bbrc.2012.06.149. [DOI] [PubMed] [Google Scholar]
- 21.Reka A. K., Kurapati H., Narala V. R., et al. Peroxisome proliferator-activated receptor-γ activation inhibits tumor metastasis by antagonizing smad3-mediated epithelial-mesenchymal transition. Molecular Cancer Therapeutics. 2010;9(12):3221–3232. doi: 10.1158/1535-7163.mct-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skelhorne-Gross G., Nicol C. J. B. The key to unlocking the chemotherapeutic potential of PPAR ligands: Having the right combination. PPAR Research. 2012 doi: 10.1155/2012/946943.946943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youssef J., Badr M. Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. British Journal of Pharmacology. 2011;164(1):68–82. doi: 10.1111/j.1476-5381.2011.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu G. L., Wang H., Xie R. J. Radiosensitization of selective cyclooxygenase-2 inhibitor, celecoxib in breast cancer and its mechanism. Journal of Chinese Oncology. 2011;7(3):172–176. [Google Scholar]
- 25.Wang W., Wang R., Zhang Z., Li D., Yu Y. Enhanced PPAR-γ expression may correlate with the development of Barrett's esophagus and esophageal adenocarcinoma. Oncology Research : Featuring Preclinical and Clinical Cancer Therapeutics. 2011;19(3-4):141–147. doi: 10.3727/096504011X12935427587849. [DOI] [PubMed] [Google Scholar]
- 26.Park S. Y., Sohn U. D. Inhibitory effect of rosiglitazone on the acid-induced intracellular generation of hydrogen peroxide in cultured feline esophageal epithelial cells. Naunyn-Schmiedeberg's Archives of Pharmacology. 2011;383(2):191–201. doi: 10.1007/s00210-010-0594-6. [DOI] [PubMed] [Google Scholar]
- 27.Sawayama H., Ishimoto T., Watanabe M., et al. Small molecule agonists of PPAR-γ exert therapeutic effects in esophageal cancer. Cancer Research. 2014;74(2):575–585. doi: 10.1158/0008-5472.CAN-13-1836. [DOI] [PubMed] [Google Scholar]
- 28.Min B.-H., Hwang J., Kim N. K. D., et al. Dysregulated Wnt signalling and recurrent mutations of the tumour suppressor RNF43 in early gastric carcinogenesis. The Journal of Pathology. 2016;240(3):304–314. doi: 10.1002/path.4777. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel-Nunes P., Gonçalves N., Boal-Carvalho I., et al. Helicobacter pylori induces increased expression of toll-like receptors and decreased toll-interacting protein in gastric mucosa that persists throughout gastric carcinogenesis. Helicobacter. 2013;18(1):22–32. doi: 10.1111/hel.12008. [DOI] [PubMed] [Google Scholar]
- 30.Vella V., Nicolosi M. L., Giuliano S., Bellomo M., Belfiore A., Malaguarnera R. PPAR-γ agonists as antineoplastic agents in cancers with dysregulated IGF axis. Frontiers in Endocrinology. 2017;8, article no. 31 doi: 10.3389/fendo.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fröhlich E., Wahl R. Chemotherapy and chemoprevention by thiazolidinediones. BioMed Research International. 2015;2015:14. doi: 10.1155/2015/845340.845340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo F., Ren X., Dong Y., et al. Constitutive expression of PPARγ inhibits proliferation and migration of gastric cancer cells and down-regulates Wnt/β-catenin signaling pathway downstream target genes TERT and ENAH. Gene. 2016;584(1):31–37. doi: 10.1016/j.gene.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Zhu Z.-A., Zhang S.-N., et al. Combinational effect of PPARγ agonist and RXR agonist on the growth of SGC7901 gastric carcinoma cells in vitro. Tumor Biology. 2013;34(4):2409–2418. doi: 10.1007/s13277-013-0791-2. [DOI] [PubMed] [Google Scholar]
- 34.Lin M. S., Huang J. X., Chen W. C., et al. Expression of PPARγ and PTEN in human colorectal cancer: An immunohistochemical study using tissue microarray methodology. Oncology Letters. 2011;2(6):1219–1224. doi: 10.3892/ol.2011.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brabletz T., Hlubek F., Spaderna S., et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and β-catenin. Cells Tissues Organs. 2005;179(1-2):56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 36.Pellerito O., Notaro A., Sabella S., et al. WIN induces apoptotic cell death in human colon cancer cells through a block of autophagic flux dependent on PPARγ down-regulation. Apoptosis. 2014;19(6):1029–1042. doi: 10.1007/s10495-014-0985-0. [DOI] [PubMed] [Google Scholar]
- 37.Park H., Ko S.-H., Lee J. M., Park J. H., Choi Y.-H. Troglitazone enhances the apoptotic response of DLD-1 colon cancer cells to photodynamic therapy. Yonsei Medical Journal. 2016;57(6):1494–1499. doi: 10.3349/ymj.2016.57.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu S.-J., Hsaio C.-H., Tseng H.-H., et al. Rosiglitazone enhances the radiosensitivity of p53-mutant HT-29 human colorectal cancer cells. Biochemical and Biophysical Research Communications. 2010;394(3):774–779. doi: 10.1016/j.bbrc.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 39.Aires V., Brassart B., Carlier A., et al. A role for peroxisome proliferator-activated receptor gamma in resveratrol-induced colon cancer cell apoptosis. Molecular Nutrition & Food Research. 2014;58(9):1785–1794. doi: 10.1002/mnfr.201300962. [DOI] [PubMed] [Google Scholar]
- 40.Komatsu Y., Yoshino T., Yamazaki K., et al. Phase 1 study of efatutazone, a novel oral peroxisome proliferator- activated receptor gamma agonist, in combination with FOLFIRI as second-line therapy in patients with metastatic colorectal cancer. Investigational New Drugs. 2014;32(3):473–480. doi: 10.1007/s10637-013-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecarpentier Y., Claes V., Vallée A., Hébert J. Interactions between PPAR Gamma and the Canonical Wnt/Beta-Catenin Pathway in Type 2 Diabetes and Colon Cancer. PPAR Research. 2017;2017:1–9. doi: 10.1155/2017/5879090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu H.-T., Sung M.-T., Lee C.-C., et al. Peroxisome proliferator-activated receptor γ expression is inversely associated with macroscopic vascular invasion in human hepatocellular carcinoma. International Journal of Molecular Sciences. 2016;17(8, article no. 1226) doi: 10.3390/ijms17081226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mello T., Materozzi M., Galli A. PPARs and mitochondrial metabolism: From NAFLD to HCC. PPAR Research. 2016;2016 doi: 10.1155/2016/7403230.7403230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bo Q.-F., Sun X.-M., Liu J., Sui X.-M., Li G.-X. Antitumor action of the peroxisome proliferator-activated receptor-γ agonist rosiglitazone in hepatocellular carcinoma. Oncology Letters. 2015;10(4):1979–1984. doi: 10.3892/ol.2015.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laganà A., Vitale S., Nigro A., et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in dysregulated metabolic homeostasis, inflammation and cancer: current evidence and future perspectives. International Journal of Molecular Sciences. 2016;17(7):p. 999. doi: 10.3390/ijms17070999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galli A., Ceni E., Mello T., et al. Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis B virus-transgenic mice by peroxisome proliferator-activated receptor γ-independent regulation of nucleophosmin. Hepatology. 2010;52(2):493–505. doi: 10.1002/hep.23669. [DOI] [PubMed] [Google Scholar]
- 47.Gupta D., Kono T., Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: Therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2010;12(12):1036–1047. doi: 10.1111/j.1463-1326.2010.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou Y., Gao J., Xu H., et al. PPARγ E3 ubiquitin ligase regulates MUC1-C oncoprotein stability. Oncogene. 2014;33:5619–5625. doi: 10.1038/onc.2013.504. [DOI] [PubMed] [Google Scholar]
- 49.Ruderman N. B., Carling D., Prentki M., Cacicedo J. M. AMPK, insulin resistance, and the metabolic syndrome. The Journal of Clinical Investigation. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamura M., Kudo H., Wakabayashi K.-I., et al. COUP-TFII acts downstream of Wnt/β-catenin signal to silence PPARγ gene expression and repress adipogenesis. Proceedings of the National Acadamy of Sciences of the United States of America. 2009;106(14):5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajita K., Mori I., Kitada Y., et al. Small proliferative adipocytes: Identification of proliferative cells expressing adipocyte markers. Endocrine Journal. 2013;60(8):931–939. doi: 10.1507/endocrj.EJ13-0141. [DOI] [PubMed] [Google Scholar]
- 52.Hollenberg A. N., Susulic V. S., Madura J. P., et al. Functional antagonism between CCAAT/enhancer binding protein-α and peroxisome proliferator-activated receptor-γ on the leptin promoter. The Journal of Biological Chemistry. 1997;272(8):5283–5290. doi: 10.1074/jbc.272.8.5283. [DOI] [PubMed] [Google Scholar]
- 53.Smith U., Gogg S., Johansson A., Olausson T., Rotter V., Svalstedt B. Thiazolidinediones (PPARγ agonists) but not PPARα agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. The FASEB Journal. 2001;15(1):215–220. doi: 10.1096/fj.00-0020com. [DOI] [PubMed] [Google Scholar]
- 54.Patel L., Buckels A. C., Kinghorn I. J., et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochemical and Biophysical Research Communications. 2003;300(2):472–476. doi: 10.1016/S0006-291X(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 55.Bouhlel M. A., Derudas B., Rigamonti E., et al. PPARγ activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Hou Y., Moreau F., Chadee K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nature Communications. 2012;3, article 1300 doi: 10.1038/ncomms2270. [DOI] [PubMed] [Google Scholar]
- 57.Polvani S., Tarocchi M., Galli A. PPAR and oxidative stress: con(β) catenating NRF2 and FOXO. PPAR Research. 2012;2012:15. doi: 10.1155/2012/641087.641087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmaci İ., Altinoz M. A. A Metabolic Inhibitory Cocktail for Grave Cancers: Metformin, Pioglitazone and Lithium Combination in Treatment of Pancreatic Cancer and Glioblastoma Multiforme. Biochemical Genetics. 2016;54(5):573–618. doi: 10.1007/s10528-016-9754-9. [DOI] [PubMed] [Google Scholar]
- 59.Vitale G., Zappavigna S., Marra M., et al. The PPAR-γ agonist troglitazone antagonizes survival pathways induced by STAT-3 in recombinant interferon-β treated pancreatic cancer cells. Biotechnology Advances. 2012;30(1):169–184. doi: 10.1016/j.biotechadv.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Chang E. Y., Chang Y. C., Shun C. T., et al. Inhibition of prostaglandin reductase 2, a putative oncogene overexpressed in human pancreatic adenocarcinoma, induces oxidative stress-mediated cell death involving xCT and CTH gene expressions through 15-keto-PGE2 . PLoS ONE. 2016;11(1) doi: 10.1371/journal.pone.0147390.e0147390 [DOI] [PMC free article] [PubMed] [Google Scholar]