Abstract
Type 2 diabetes mellitus (T2DM) is emerging as a metabolic epidemic worldwide. Pathologically, dysregulation of many biological pathways precedes hyperglycemia and the clinical diagnosis of T2DM. Changing trajectories along the process of T2DM development necessitates frequent measurement of biomarkers for early identification of at-risk individuals and successful prevention. Increase in circulating inflammatory adipokines has been suggested as predictive of T2DM. Human saliva is an easily accessible biospecimen amenable for painless frequent collection and possesses nearly 50% of serum proteome. In this study, we measured the adipokines resistin, visfatin, TNF-α, and ghrelin as markers for T2DM in unstimulated whole saliva (UWS) using specific assay kits. Resistin and visfatin concentrations were significantly higher in T2DM saliva. Although the concentration of acylated or unacylated ghrelin was lower in diabetic saliva, the decrease was not significant. Since resistin and visfatin are biomarkers integral to T2DM pathology, their salivary assessments may receive clinical acceptance.
1. Introduction
The World Health Organization estimated that globally 422 million adults were living with diabetes in 2014 [1]. Type 2 diabetes mellitus (adult-onset/noninsulin-dependent diabetes: T2DM) accounts for 90–95% of all diabetes [2]. The disease develops insidiously through periods of increased insulin secretion, insulin resistance, impaired glucose tolerance, and β-cell dysfunction [3]. Consistently, the most acceptable markers for T2DM diagnosis are based on measurements of blood glucose and glycosylated hemoglobin c (HbA1c), an indicator of average glycemic control [4]. However, research elucidating the disease pathogenesis suggests that multiple mechanisms including chronic inflammation, obesity, lipotoxicity, and oxidative stress contribute to the glucose dysregulation in T2DM [5, 6]. Hence, several hypothesis-based nonglycemic biomarkers have been assessed as risk factors for diabetes [7, 8].
Adipokines are polypeptides secreted by adipocytes, inflammatory cells, and other cells. They regulate multiple physiological functions including energy balance, insulin sensitization, appetite regulation, and inflammatory response [9]. It has been suggested that activation of the adipokine resistin in the islet cells of the pancreas inhibits cell surface glucose transporters and thereby insulin signaling [10, 11]. Visfatin, also known as pre-B-cell colony-enhancing factor (PBEF), has been described as an adipokine with a potential glucose-lowering effect due to its nicotinamide phosphoribosyltransferase (NAMPT) activity [10, 12, 13]. Ghrelin, originally identified as a growth hormone secretagogue with orexigenic and lipogenic effects, has also been shown to play significant roles in glucose regulation. While acylated ghrelin has been shown to exert hyperglycemic effects leading to insulin resistance, the unacylated ghrelin counters hyperglycemia and enhances insulin sensitivity [14, 15]. Biomarker studies showed that the circulating levels of resistin and visfatin are upregulated in T2DM [13, 16–18]. On the other hand, the plasma concentration of acylated ghrelin has been shown to be lower in T2DM individuals as well as in their healthy offspring [19–21].
Since monitoring of serological parameters typically involves invasive techniques with associated pain and distress, efforts are directed at identifying noninvasive measures for frequent monitoring of diabetes. Some of the alternative methods evaluated include assessing skin autofluorescence for accumulation of advanced glycation end-products and measuring analytes in exhaled breath, urine, or saliva [5, 22–24]. Human saliva is a rich reservoir of analytes consisting of over 3652 proteins and 12,562 peptides and shares nearly 51% of proteins and 79% of peptides with the serum proteome and peptidome, respectively [25, 26]. Alterations in the salivary flow and composition in diabetes are well documented [27, 28]. Both glucose and immunoreactive insulin are increased in saliva and are correlated with plasma levels in T2DM patients [29–32]. Circulating biomolecules are thought to reach saliva by either active (e.g., sIgA) or passive transportation (e.g., steroids) or ultrafiltration (e.g., creatinine) or from crevicular fluid [26, 33]. The objective of this study is to compare the salivary levels of two proinflammatory adipokines, namely, resistin and visfatin, and that of the anti-inflammatory adipokine ghrelin between healthy and T2DM individuals.
2. Materials and Methods
2.1. Study Population
All participants were recruited from patients attending the Indiana University School of Dentistry after obtaining informed consent in full accordance with the Indiana University Institutional Review Board. The study population consisted of twenty periodontally healthy individuals with self-reported T2DM and HbA1c values. Twenty gender-matched individuals with no known oral or systemic condition were recruited as control group. It was estimated that this sample size will be sufficient at 80% power to detect a difference in means of 0.91, assuming a common standard deviation of 1 using a two group t-test with a two-sided significance level of 0.05.
2.2. Collection and Processing of Unstimulated Whole Saliva (UWS)
UWS was collected at approximately the same time of day by the drooling method as described [34, 35]. Briefly, subjects were asked to refrain from eating or drinking for 2 h prior to saliva collection. At least 2 mL of UWS was collected by passively drooling into a chilled centrifuge tube for 5–10 min. The tubes were codified and transferred on ice to the laboratory for processing. Each sample was clarified by centrifuging at 3500 rpm at 4°C for 10 min and stored in Complete™ Protease Inhibitor Cocktail (Roche, Mannheim, Germany). The supernatant-clarified saliva was stored at −80°C until further analysis.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for Resistin and Visfatin
All UWS samples were depleted of amylase and immunoglobulins by incubating serially with antihuman amylase mAb (1 : 2500, cat. no. ab8944; Abcam) and protein G beads (Miltenyi Biotec Inc.) at 4°C. Total protein of the precleaned saliva samples was determined by spectrophotometry and ranged between 1.3 and 7.7 mg/mL [36]. Volume equal to 1 µg of total protein in precleaned UWS was assessed for resistin, visfatin, and ghrelin per using specific sandwich ELISA kits (item no. 10007610 81, part no. 579020-96, and part no. 10006306-96, resp.; Bertin Pharma/Cayman Chemical, Ann Arbor, MI, USA) following manufacturer's instructions. The cytokines TNF-α and IL-6 in saliva were measured using specific ELISA kits (BD Biosciences, CA, USA) following the manufacturer's instructions.
2.4. Statistical Analysis
For all biomarkers, statistical significance between the healthy and diabetes cohorts was determined by two-tailed paired t-tests; p < 0.05 was considered significant.
3. Results
3.1. Clinical Characteristics
The study cohort consisted of twenty individuals with self-reported T2DM and twenty healthy individuals (Table 1). The average age of T2DM cohort was 56.5 yrs and that of healthy group was 48 yrs. The average HbA1c value of the T2DM group was 5.4 ± 1.9%. Since the HbA1c values reported were measured within the past three months, the range is consistent with the diagnostic criteria for T2DM [37].
Table 1.
Demographic characteristics, HbA1c, and total salivary protein content.
| Healthy | T2DM | ||
|---|---|---|---|
| Number of individuals | M | 10 | 12 |
| F | 10 | 8 | |
| Age (yrs) | 48 (range: 42–55) | 56.5 (range: 45–58) | |
| % A1C | 7.85 | ||
| Range: 6–12 | |||
| Salivary protein (mg/mL) | 3.4 ± 1.6 | 5.4 ± 1.9 |
3.2. Salivary Cytokines in T2DM
We observed that the UWS concentration of IL-6 and TNF-α was not significantly different between T2DM and healthy individuals with no periodontitis (Figure 1). Similar observation of comparable salivary IL-6 levels between systemically healthy and diabetes individuals with healthy periodontium has been reported by others [38].
Figure 1.
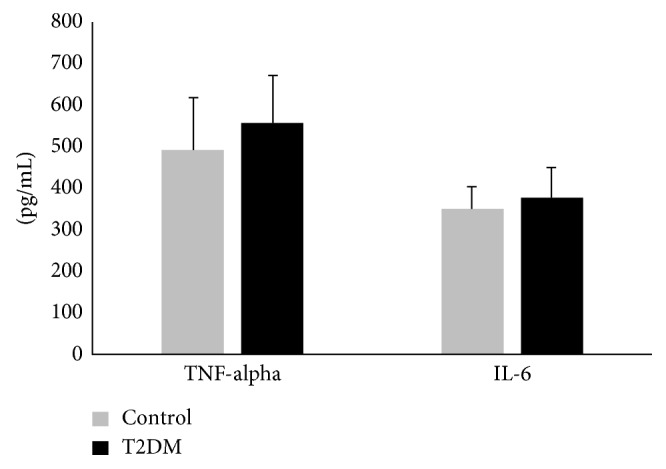
Salivary TNF-α and IL-6 in T2DM: Unstimulated whole saliva (UWS) was collected from 20 T2DM individuals and 20 healthy individuals. Each UWS sample was depleted of amylase and immunoglobulins. Precleaned UWS was assessed for TNF-α and IL-6 using specific sandwich ELISA kits.
3.3. Differential Expressions of Visfatin, Resistin, and Ghrelin in Diabetic Saliva
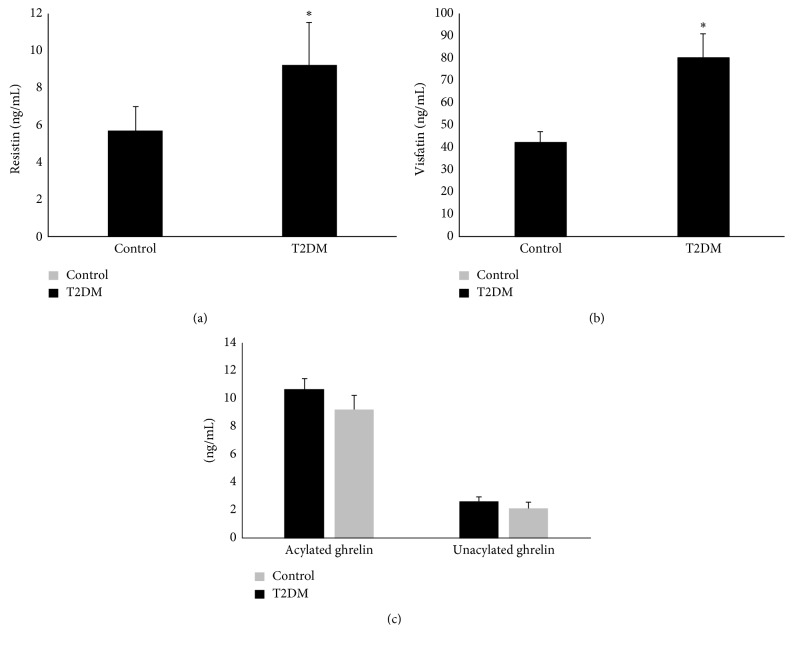
We observed that the UWS from T2DM subjects possessed significantly elevated levels of resistin (9.2 ± 2.3 ng/mL) and visfatin (80.2 ± 42.3 ng/mL) as compared to that from control subjects (5.7 ± 1.3 ng/mL and 46.0 ± 17.5 ng/mL, resp.) (Figures 2(a) and 2(b)). No significant difference was observed in the salivary concentration of either unacylated (9.2 ± 4.3 ng/mL and 10.7 ± 5.6 ng/mL, resp.) or acylated (2.7 ± 2.3 ng/mL and 2.2 ± 1.4 ng/mL, resp.) ghrelin between the T2DM and the control group (Figure 2(c)).
Figure 2.
Salivary resistin, visfatin, and ghrelin in T2DM: Unstimulated whole saliva (UWS) was collected from 20 T2DM individuals and 20 healthy individuals. Each UWS sample was depleted of amylase and immunoglobulins. Precleaned UWS was assessed for (a) resistin, (b) visfatin, and (c) acylated and unacylated ghrelin using specific assay kits. ∗p<0.05.
4. Discussion
Escalating global burden of T2DM underscores the need for multipronged screening strategies for early identification of individuals at high risk [7, 39]. Furthermore, elucidation of the molecular pathogenesis has shown that the processes that lead to T2DM are initiated very early with a long lag phase between the disease onset and the clinical diagnosis [6]. Many cross-sectional studies have evaluated multiple serum proteins as predictive biomarkers for T2DM [5, 7, 39].
Potential applications of salivary biomarkers for T2DM have gained importance with the establishment of shared characteristics of salivary and serum proteomes [26]. It has been suggested that the increased basement membrane permeability often associated with diabetes is a potential mechanism for the increased passage of proteins and metabolites from the exocrine glands as well as for the enhanced leakage of serum-derived components into whole saliva [26, 33, 40].
Clinical application of salivary components as potential biomarkers is likely to be better accepted for molecules that correlate with the pathological process of T2DM. Considerable evidence suggests that the T2DM is a multifactorial disease involving dysregulation of various biological pathways such as inflammation, adipokine signaling, and incretin signaling [6]. The prodiabetic effects of the adipokine resistin have been attributed to inhibition of insulin signaling and a pro-inflammatory mechanism that culminates in β-cell loss [9, 10, 13, 41]. The adipokine visfatin has been shown to exhibit glucose-lowering and insulin-mimicking/insulin-sensitizing effects [9, 10, 12, 13]. Circulating levels of both resistin and visfatin are upregulated in T2DM [12, 13, 18, 42]. Previously, others have shown positive correlation between the serum and salivary levels of these two adipokines [42–44]. Here, we observed that the salivary resistin and visfatin concentrations are significantly elevated in T2DM. Similar observations of elevated salivary visfatin and resistin have been reported earlier in chronic periodontitis and diabetes [42, 43, 45].
Ghrelin, the orexigenic peptide hormone also affects glucose metabolism. Circulating levels of ghrelin rise before and fall after a meal, thereby contributing to appetite and weight gain [15]. Plasma concentration of ghrelin has been negatively correlated with insulin resistance [14, 46]. We observed that the salivary concentration of both acylated and unacylated ghrelin was lower in T2DM saliva than that in healthy saliva although the difference did not reach statistical significance. Others have reported significant reductions in acylated ghrelin in diabetic saliva [47]. The difference may be attributed to the time and method of sample collection and preparation and the method of ghrelin assessment.
5. Conclusions
Population-based long-term studies suggest that the biomarker trajectories along the course of T2DM development diverge over time [48, 49]. This suggests that repeated measures of mechanism-based biomarkers will increase the predictive value of diabetes risk scores. The noninvasive nature and the feasibility of frequent sampling for real-time monitoring are significant advantages of saliva over peripheral blood as specimen for diagnostic/prognostic applications. In addition to the practical benefits of eliminating the need for a phlebotomist, reduced transmission of infectious disease by eliminating needle sticks and greater ease of testing of special populations of patients (e.g., institutional and children) make the assessment of biomarkers in human saliva an attractive economic strategy [24, 50]. Elevated salivary resistin and visfatin in saliva that have also been shown to correlate with serum levels suggest that the two adipokines could represent potential noninvasive T2DM biomarkers [41, 43, 44]. However, caution must be exercised since the type of sample (stimulated/unstimulated; whole/glandular), circadian variations, and susceptibility to preprocessing as well as oral health/disease are some of the confounding parameters that should be addressed in the biomarker interpretations and implementation [51, 52].
Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
References
- 1.World Health Organization (WHO) Global Report on Diabetes. Geneva, Switzerland: WHO Press; 2016. [Google Scholar]
- 2.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Rodger W. Non-insulin-dependent (type II) diabetes mellitus. Canadian Medical Association Journal. 1991;145(12):1571–1581. [PMC free article] [PubMed] [Google Scholar]
- 4.Færch K., Borch-Johnsen K., Holst J. J., Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52(9):1714–1723. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]
- 5.Sattar N. Biomarkers for diabetes prediction, pathogenesis or pharmacotherapy guidance? Past, present and future possibilities. Diabetic Medicine. 2012;29(1):5–13. doi: 10.1111/j.1464-5491.2011.03480.x. [DOI] [PubMed] [Google Scholar]
- 6.Surampudi P. N., John-Kalarickal J., Fonseca V. A. Emerging concepts in the pathophysiology of type 2 diabetes mellitus. Mount Sinai Journal of Medicine. 2009;76(3):216–226. doi: 10.1002/msj.20113. [DOI] [PubMed] [Google Scholar]
- 7.Kolberg J. A., Jorgensen T., Gerwien R. W., et al. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care. 2009;32(7):1207–1212. doi: 10.2337/dc08-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H., Yu Z., Qi Q., Li H., Sun Q., Lin X. Joint analysis of multiple biomarkers for identifying type 2 diabetes in middle-aged and older Chinese: a cross-sectional study. BMJ Open. 2011;1(1):p. e000191. doi: 10.1136/bmjopen-2011-000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabe K., Lehrke M., Parhofer K. G., Broedl U. C. Adipokines and insulin resistance. Molecular Medicine. 2008;14(11-12):741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunmore S. J., Brown J. E. The role of adipokines in beta-cell failure of type 2 diabetes. Journal of Endocrinology. 2013;216(1):T37–T45. doi: 10.1530/joe-12-0278. [DOI] [PubMed] [Google Scholar]
- 11.Kim S. J., Nian C., McIntosh C. H. Resistin is a key mediator of glucose-dependent insulinotropic polypeptide (GIP) stimulation of lipoprotein lipase (LPL) activity in adipocytes. Journal of Biological Chemistry. 2007;282(47):34139–34134. doi: 10.1074/jbc.m704896200. [DOI] [PubMed] [Google Scholar]
- 12.Haider D. G., Schaller G., Kapiotis S., Maier C., Luger A., Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49(8):1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 13.Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocrine Regulations. 2010;44(1):25–36. doi: 10.4149/endo_2010_01_25. [DOI] [PubMed] [Google Scholar]
- 14.Collden G., Tschop M. H., Muller T. D. Therapeutic potential of targeting the ghrelin pathway. International Journal of Molecular Sciences. 2017;18(4):p. 798. doi: 10.3390/ijms18040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dezaki K., Sone H., Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacology and Therapeutics. 2008;118(2):239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa G., Ohta M., Ichida Y., et al. Increased serum resistin levels in patients with type 2 diabetes are not linked with markers of insulin resistance and adiposity. Acta Diabetologica. 2005;42(2):104–109. doi: 10.1007/s00592-005-0187-x. [DOI] [PubMed] [Google Scholar]
- 17.Osawa H., Ochi M., Tabara Y., et al. Serum resistin is positively correlated with the accumulation of metabolic syndrome factors in type 2 diabetes. Clinical Endocrinology. 2008;69(1):74–80. doi: 10.1111/j.1365-2265.2007.03154.x. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Yang G., Li Q., et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Experimental and Clinical Endocrinology and Diabetes. 2006;114(10):544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 19.Katsuki A., Urakawa H., Gabazza E. C., et al. Circulating levels of active ghrelin is associated with abdominal adiposity, hyperinsulinemia and insulin resistance in patients with type 2 diabetes mellitus. European Journal of Endocrinology. 2004;151(5):573–577. doi: 10.1530/eje.0.1510573. [DOI] [PubMed] [Google Scholar]
- 20.Sharifi F., Yamini M., Esmaeilzadeh A., Mousavinasab N., Shajari Z. Acylated ghrelin and leptin concentrations in patients with type 2 diabetes mellitus, people with prediabetes and first degree relatives of patients with diabetes, a comparative study. Journal of Diabetes and Metabolic Disorders. 2013;12(1):p. 51. doi: 10.1186/2251-6581-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueno H., Shiiya T., Mizuta M., Mondal S. M., Nakazato M. Plasma ghrelin concentrations in different clinical stages of diabetic complications and glycemic control in Japanese diabetics. Endocrine Journal. 2007;54(6):895–902. doi: 10.1507/endocrj.k07-007. [DOI] [PubMed] [Google Scholar]
- 22.Akin F., Bastemir M., Alkis E., Kaptanoglu B. SHBG levels correlate with insulin resistance in postmenopausal women. European Journal of Internal Medicine. 2009;20(2):162–167. doi: 10.1016/j.ejim.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Price A. H., Weir C. J., Welsh P., et al. Comparison of non-traditional biomarkers, and combinations of biomarkers, for vascular risk prediction in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Atherosclerosis. 2017;264:67–73. doi: 10.1016/j.atherosclerosis.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao P. V., Reddy A. P., Lu X., et al. Proteomic identification of salivary biomarkers of type-2 diabetes. Journal of Proteome Research. 2009;8(1):239–245. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 25.Amado F., Lobo M. J., Domingues P., Duarte J. A., Vitorino R. Salivary peptidomics. Expert Review of Proteomics. 2010;7(5):709–721. doi: 10.1586/epr.10.48. [DOI] [PubMed] [Google Scholar]
- 26.Loo J. A., Yan W., Ramachandran P., Wong D. T. Comparative human salivary and plasma proteomes. Journal of Dental Research. 2010;89(10):1016–1023. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conner S., Iranpour B., Mills J. Alteration in parotid salivary flow in diabetes mellitus. Oral Surgery, Oral Medicine, Oral Pathology. 1970;30(1):55–59. doi: 10.1016/0030-4220(70)90011-3. [DOI] [PubMed] [Google Scholar]
- 28.Jurysta C., Bulur N., Oguzhan B., et al. Salivary glucose concentration and excretion in normal and diabetic subjects. Journal of Biomedicine and Biotechnology. 2009;2009:6. doi: 10.1155/2009/430426.430426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S., Sandhu S. V., Bansal H., Sharma D. Comparison of salivary and serum glucose levels in diabetic patients. Journal of Diabetes Science and Technology. 2015;9(1):91–96. doi: 10.1177/1932296814552673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchetti P., Benzi L., Masoni A., et al. Salivary insulin concentrations in type 2 (non-insulin-dependent) diabetic patients and obese non-diabetic subjects: relationship to changes in plasma insulin levels after an oral glucose load. Diabetologia. 1986;29(10):695–698. doi: 10.1007/bf00870278. [DOI] [PubMed] [Google Scholar]
- 31.Vasconcelos A. C., Soares M. S., Almeida P. C., Soares T. C. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. Journal of Oral Science. 2010;52(2):293–298. doi: 10.2334/josnusd.52.293. [DOI] [PubMed] [Google Scholar]
- 32.Wang B., Du J., Zhu Z., Ma Z., Wang S., Shan Z. Evaluation of parotid salivary glucose level for clinical diagnosis and monitoring type 2 diabetes mellitus patients. BioMed Research International. 2017;2017:5. doi: 10.1155/2017/2569707.2569707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L., Xiao H., Karlan S., et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015573.e15573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan M., Kodumudi K. N., Zunt S. L. Soluble CD14 and toll-like receptor-2 are potential salivary biomarkers for oral lichen planus and burning mouth syndrome. Clinical Immunology. 2008;126(1):31–37. doi: 10.1016/j.clim.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Janardhanam B., Zunt S., Srinivasan M. Quality assessment of saliva bank samples. Biopreservation and Biobanking. 2012;10(3):282–287. doi: 10.1089/bio.2011.0039. [DOI] [PubMed] [Google Scholar]
- 36.Prakasam S., Srinivasan M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Diseases. 2014;20(2):171–177. doi: 10.1111/odi.12085. [DOI] [PubMed] [Google Scholar]
- 37.Lipska K. J., Krumholz H. M. Is hemoglobin A1c the right outcome for studies of diabetes? JAMA. 2017;317(10):1017–1018. doi: 10.1001/jama.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa P. P., Trevisan G. L., Macedo G. O., et al. Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. Journal of Periodontology. 2010;81(3):384–391. doi: 10.1902/jop.2009.090510. [DOI] [PubMed] [Google Scholar]
- 39.Meigs J. B. Multiple biomarker prediction of type 2 diabetes. Diabetes Care. 2009;32(7):1346–1348. doi: 10.2337/dc09-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson S., Munro C., Pickler R., Grap M. J., Elswick R. K., Jr. Comparison of biomarkers in blood and saliva in healthy adults. Nursing Research and Practice. 2012;2012:4. doi: 10.1155/2012/246178.246178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusminski C. M., McTernan P. G., Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clinical Science. 2005;109(3):243–256. doi: 10.1042/CS20050078. [DOI] [PubMed] [Google Scholar]
- 42.Pradeep A. R., Raghavendra N. M., Sharma A., et al. Association of serum and crevicular visfatin levels in periodontal health and disease with type 2 diabetes mellitus. Journal of Periodontology. 2011;83(5):629–634. doi: 10.1902/jop.2011.110272. [DOI] [PubMed] [Google Scholar]
- 43.Desai G. S., Mathews S. T. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World Journal of Diabetes. 2014;5(6):730–738. doi: 10.4239/wjd.v5.i6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mamali I., Roupas N. D., Armeni A. K., Theodoropoulou A., Markou K. B., Georgopoulos N. A. Measurement of salivary resistin, visfatin and adiponectin levels. Peptides. 2012;33(1):120–124. doi: 10.1016/j.peptides.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Yin J., Gao H., Yang J., Xu L., Li M. Measurement of salivary resistin level in patients with type 2 diabetes. International Journal of Endocrinology. 2012;2012:5. doi: 10.1155/2012/359724.359724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudovich N. N., Nikiforova V. J., Otto B., et al. Metabolomic linkage reveals functional interaction between glucose-dependent insulinotropic polypeptide and ghrelin in humans. American Journal of Physiology: Endocrinology and Metabolism. 2011;301(4):E608–E617. doi: 10.1152/ajpendo.00154.2011. [DOI] [PubMed] [Google Scholar]
- 47.Aydin S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. Journal of Biochemistry and Molecular Biology. 2007;40(1):29–35. doi: 10.5483/bmbrep.2007.40.1.029. [DOI] [PubMed] [Google Scholar]
- 48.Hulman A., Simmons R. K., Brunner E. J., et al. Trajectories of glycaemia, insulin sensitivity and insulin secretion in South Asian and white individuals before diagnosis of type 2 diabetes: a longitudinal analysis from the Whitehall II cohort study. Diabetologia. 2017;60(7):1252–1260. doi: 10.1007/s00125-017-4275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabak A. G., Jokela M., Akbaraly T. N., Brunner E. J., Kivimaki M., Witte D. R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. The Lancet. 2009;373(9682):2215–2221. doi: 10.1016/s0140-6736(09)60619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spielmann N., Wong D. Saliva: diagnostics and therapeutic perspectives. Oral Diseases. 2010;17(4):345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rathnayake N., Akerman S., Klinge B., et al. Salivary biomarkers for detection of systemic diseases. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061356.e61356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan M., Blackburn C., Mohamed M., Sivagami A. V., Blum J. Literature-based discovery of salivary biomarkers for type 2 diabetes mellitus. Biomarker Insights. 2015;10:39–45. doi: 10.4137/BMI.S22177. [DOI] [PMC free article] [PubMed] [Google Scholar]