Abstract
Objectives
The goals of this study were to assess the effectiveness of WBV (whole body vibration) training through an analysis of effect sizes, identify advantages of WBV training, and suggest other effective treatment methods.
Methods
Four databases, namely, EMBASE, PubMed, EBSCO, and Web of Science, were used to collect articles on vibration. Keywords such as “vibration” and “stroke” were used in the search for published articles. Consequently, eleven studies were selected in the second screening using meta-analyses.
Results
The total effect size of patients with dementia in the studies was 0.25, which was small. The effect size of spasticity was the greatest at 1.24 (high), followed by metabolism at 0.99 (high), balance, muscle strength, gait, and circulation in the decreasing order of effect size.
Conclusions
The effect sizes for muscle strength and balance and gait function, all of which play an important role in performance of daily activities, were small. In contrast, effect sizes for bone metabolism and spasticity were moderate. This suggests that WBV training may provide a safe, alternative treatment method for improving the symptoms of stroke in patients.
1. Introduction
Stroke rehabilitation is a process through which patients with disabilities as a result of stroke manage to resume activities of daily living and reestablish their normal lifestyle through a learning process. It also aims to assist patients in gaining better understanding of their condition, adapting to difficulties they experience due to their disabilities, and preventing secondary complications [1]. Typical disabilities that follow stroke include muscle weakness, abnormal muscle stress, and dystonia. These disabilities not only limit daily activities but also affect the balancing ability and gait function [2].
To a large extent, research has been conducted with the aim of resolving these stroke-related problems. Recently, whole body vibration (WBV) has been heavily researched as a way to improve muscle function, muscle strength, and gait function in stroke patients [3, 4]. WBV training involves standing or making vigorous movements on a vibration platform placed on a static surface. In previous studies, WBV training was suggested as a potential method to improve physical functions. It was also suggested that WBV improves muscle function and balance by increasing muscle strength.
Therapies that involve WBV exercises have been on the rise recently; however, only a few studies have compared WBV therapy with other treatment modalities. Therefore, this study aims to compare the effects of WBV treatment using meta-analysis. Further, the purpose of this study is to assess the effectiveness of WBV training through an analysis of effect sizes, identify advantages of WBV training, and suggest other effective treatment methods.
2. Material and Method
2.1. Research Question
The purpose of this systemic review was determined according to PICO (patient, intervention, comparison, and outcome). In this review, the patient (P) was defined as a person having “stroke.” The intervention (I) was defined in the experimental group that underwent WBV training (static activities and vigorous exercise). The experimental group was compared (C) to the control group that did not undergo WBV training. The outcome (O) was defined as changes in motor functions and body structure. This study investigated effect sizes of WBV training on different variables and determined the ones on which WBV had the greatest effect.
2.2. Selection and Collection of Articles to Be Analyzed
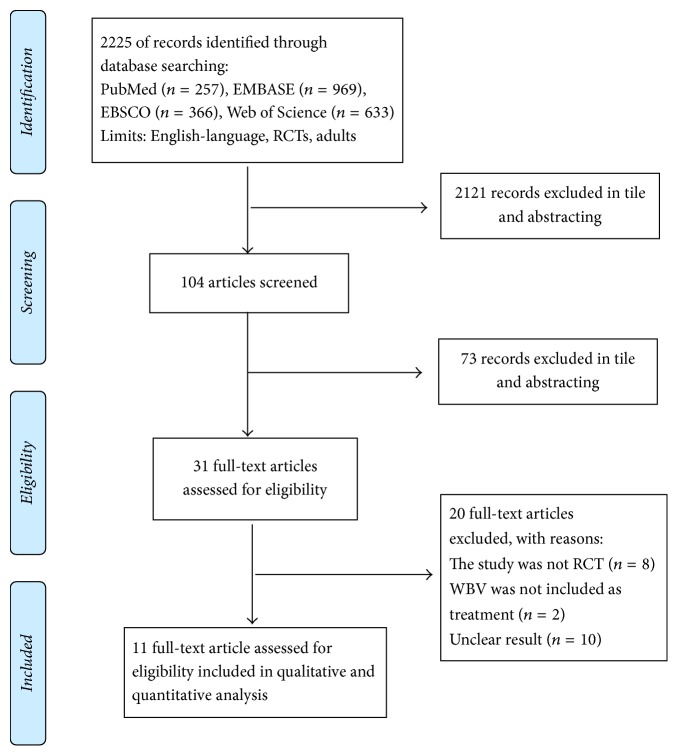
Articles related to WBV were searched for in 5 databases including EMBASE, PubMed, EBSCO, and Web of Science for inclusion in the meta-analysis. The criteria for selecting a dissertation were as follows: studies involving clinical diagnosis of stroke and its treatment in a randomized controlled clinical trial. The language was limited to English. “Vibration” and “stroke” were used as keywords in order to minimize the number of articles that would be missed when searching solely with the keyword, “WBV.” Using these keywords, a total of 2225 studies were initially selected, after which we excluded the ones that did not report sufficient statistics. The exclusion criteria were reviewed by examining the title and abstract of the papers with respect to the subject, while the main text and the theory of research were excluded from the analysis. We also excluded research that was difficult to classify after reviewing the design methods of each analyzed study. Of the 2225 initially selected articles, 2121 were excluded. Upon reviewing the titles and abstracts of the selected studies, 73 were further excluded and 31 were selected based on the research topic. Within these, 20 studies were excluded owing to the following reasons: eight studies were not randomized controlled trials, two articles did not investigate general vibration training, and 10 papers showed ambiguous results that did not provide sufficient statistical data in relation to the meta-analysis. Finally, 11 studies were selected and the characteristics of PRISMA flow chart were summarized (Figure 1, Table 1). The selected studies analyzed the treatment effects of WBV in stroke patients. The number of patients used in the final analysis was 4,413. A detailed description of the included individual studies is presented in Table 1. A review of the methodologies used in the selected articles revealed that most of them used a one-group pretest-posttest design (Table 2).
Figure 1.
Flow diagram of studies included.
Table 1.
Characteristic of included trials.
| Study | Number of participants analyzed (E/C) | Mean age (E/C) | Start REH intervention (E/C) | Duration of prog. | Time |
|---|---|---|---|---|---|
| Brogardh et al. 2012 [5] | 16/15 | 61.3 ± 8.5/63.9 ± 5.8 | 37.4/33.1 m | 2/week∗6 | 45 min |
| Chan et al. 2012 [6] | 15/15 | 56.07 ± 11.04/54.93 ± 7.45 | 30.4/38.87 m | One session | |
| Choi et al. 2014 [7] | 15/15 | 62.8 ± 9/65.1 ± 15.7 | 13/12.6 | 5/w∗4 | 15 min |
| Lau et al. 2012 [8] | 41/41 | 57.3 ± 11.3/57.4 ± 11.1 | 4.6/5.3 y | 3/w∗8 | |
| Liao et al. 2016 [9] | 28/28 | 59.8 ± 9.1/60.8 ± 8.3 | 8.5/9.0 y | 3/w∗10 | 12 min |
| Marin et al. 2013 [10] | 11/9 | 62.3 ± 10.6/64.4 ± 7.6 | 4.3/4.3 y | 12 w (17 sessions) | |
| Pang et al. 2013 [11] | 38/38 | 57.3 ± 11.3/57.4 ± 11.1 | 4.6/5.3 y | 3/w∗8 | |
| Tankisheva et al. 2014 [12] | 6/7 | 57.4 ± 13/65.3 ± 3.7 | 7.71/5.28 y | 3/w∗6 | 30 min |
| Tihanyi et al. 2007 [13] | 8/8 | 58.2 ± 9.4 | 27.2 ± 10.4 d | One session | |
| van Nes et al. 2006 [14] | 27/26 | 59.7 ± 12.3/62.6 ± 7.6 | 38.9/34.2 d | 5/w∗6 | |
| Yule et al. 2016 [15] | 4/2 | 50.5 ± 14.5/39 ± 2 | 6 m–5 y | 3/w∗4 | 15 min |
Table 2.
Review of the studies.
| Study | Outcome measures | Type of intervention | control | frequency | amplitude |
|---|---|---|---|---|---|
| Brogardh et al. 2012 [5] | Muscle tone: MAS Balance: BBS Muscle strength measurements Gait performance: TUG, 10 MGS, 6 MWT Participation: SIS |
Standing barefoot on the platforms in a static position with the knee flexed 45° 60° | vibrating platform with an amplitude of 0.2 mm |
25 | 3.75 |
|
| |||||
| Chan et al. 2012 [6] | Ankle spasticity: MAS, deep tendon reflex, VAS Gait performance: TUG, 10 MWT, cadence Foot pressure |
Positioned on the platform in a semisquatting position with buttock support and were kept in an upright position with even weight distribution on both feet | same procedure, No WBV | 12 | 4 |
|
| |||||
| Choi et al. 2014 [7] | Static sitting balance: COP Dynamic sitting balance: MFRT |
Task oriented training + WBV (1) Sitting alone at a table and correcting body alignment (2) Reaching in different directions for objects located beyond arm's length using the nonparetic side (3) Reaching in different directions for objects located beyond arm's length using the paretic side (4) A bilateral reaching task |
task oriented training | 15–22 | 0–5.8 |
|
| |||||
| Lau et al. 2012 [8] | Balance: BBS Dynamic postural control: LOS Muscle strength measurements (70°) Gait performance: 10 MWT, 6 MWT Fall-related self-efficacy ABC |
Side-to-side weight shift, semisquat, forward and backward weight shift, forward lunge, standing on one leg, deep squat | same platform, No WBV | 20–30 | 0.44–0.6 |
|
| |||||
| Liao et al. 2016 [9] | Muscle tone: MAS Balance: Mini BESTest Gait performance: TUG, 6 MWT Fall-related self-efficacy ABC |
Dynamic weight shift side to side, dynamic deep squat, dynamic forward and backward weight shift, static semisquat |
same platform, No WBV | 20 | 1 |
|
| |||||
| Marin et al. 2013 [10] | Balance: BBS Muscle strength: thickness of RF, VL, MG in both legs Maximum isometric knee extension strength |
Standing on a vibration platform with knee flexion of 30° | same position, No WBV | 5–21 | 4–6 |
|
| |||||
| Pang et al. 2013 [11] | Bone turnover markers Spasticity: MAS, VAS Muscle strength: knee peak power |
Side-to-side weight shift, semisquat, forward and backward, weight shift, forward lunge, standing on one leg, deep squat | same platform, No WBV | 20–30 | 0.44–0.6 |
|
| |||||
| Tankisheva et al. 2014 [12] | Muscle tone: MAS Muscle strength measurements: isokinetic knee extension in both legs (60°/s) Isokinetic knee flexion in both legs (60°/s) Isometric knee extension in both legs Isometric knee flexion in both legs Isokinetic knee extension in nonparetic leg (240°/s) Isokinetic knee flexion in nonparetic leg (240°/s) SOT Equilibrium scores |
Standing on their toes, knee flexion of 50–60, knee flexion of 90°, wide-stance squat, one-legged squat | No | 35, 40 | 1.7, 2, 5 |
|
| |||||
| Tihanyi et al. 2007 [13] | EMG: Maximum isometric knee extension torque Maximum eccentric knee extension torque Rate of torque development Maximal voluntary eccentric torque at 60° of knee flexion Coactivation quotient of BF during Isometric knee extension Coactivation quotient of BF during eccentric knee extension |
Standing the platform with knees slightly flexed at 40° and shifting body mass to the paretic leg | same platform, No WBV | 20 | 5 |
|
| |||||
| van Nes et al. 2006 [14] | Balance: BBS BI Rivermead Mobility Index Trunk Control Test FAC Motricity Index Somatosensory threshold of affected leg |
Standing on the platform with knees slightly flexed | Exercise therapy on music | 30 | |
|
| |||||
| Yule et al. 2016 [15] | Pulse wave velocity Carotid to radial PTT Arterial stiffness Heart rate Blood pressure Augmentation index |
Static squat stance with 70° knee flexion | No | 22–26 | |
MAS: Modified Ashworth Scale, BBS: Berg Balance Scale, TUG: Timed Up & Go, SIS: Stroke Impact Scale, 10 MGS: 10 miters' gait speed, 6 MWT: six-minute walk test, COP: Center of Pressure; MFRT: Modified Functional Reach Test, LOS: Limit of Stability, 10 MWT: 10 miters' walk test, ABC: activities-specific balance confidence scale.
2.3. Data Processing
With the agreement of all members of the research team, the author names, published year, publication type, research model, study participants, assessment tools, program type, and program effectiveness were recorded for data coding. A physical therapist and a meta-analysis specialist performed the coding. Conflicts in opinions were resolved through negotiation and opinions of a physiotherapy professor. The credibility and consistency of people involved in coding were not calculated.
2.4. Data Extraction
A CMA software specialized in meta-analysis was used for data analysis. In order to interpret the effect sizes obtained from the meta-analysis, Cohen and Wolf's standard was used. According to Cohen [16], an effect is small if it is less than 0.2, moderate if it is 0.5, and large if it is greater than 0.8.
2.5. Quality Assessment
Using the PEDro database's method of analysis, a quality assessment of randomized controlled trial articles was performed. The PEDro scale determines the scientific validity of clinical trials (9-10 = excellent, 6–8 = good, 4–6 = fair, and <4 = poor). Studies of excellent or good qualities with a sample size greater than or equal to 50 were considered as level 1 evidence [17] (Table 3).
Table 3.
Quality assessment.
| Brogardh et al. (2012) | Chan et al. (2012) | Choi et al. (2014) | Lau et al. (2012) | Liao et al. (2016) | Marin et al. (2013) | Pang et al. (2013) | Tankisheva et al. (2014) | Tihanyi et al. (2007) | van Nes et al. (2006) | Yule et al. (2016) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligibility criteria | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Random allocation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Concealed allocation | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Baseline comparability | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Blinded subjects | Yes | Yes | No | No | No | No | No | No | No | No | Not Yet |
| Blinded therapists | Yes | No | No | No | No | No | No | No | No | No | Not Yet |
| Blinded assessors | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Not Yet |
| Adequate follow-up | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Intention-to-treat analysis | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Not Yet |
| Between-group comparisons | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Point estimators and variability | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not Yet |
| Total PEDro score | 9 | 8 | 6 | 8 | 8 | 8 | 8 | 7 | 6 | 8 | Not Yet |
| Sample size ≥ 50 | No | No | No | Yes | No | No | Yes | No | Yes | Yes | No |
| Level of evidence | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 |
3. Results
3.1. Homogeneity Test and Total Effect Size
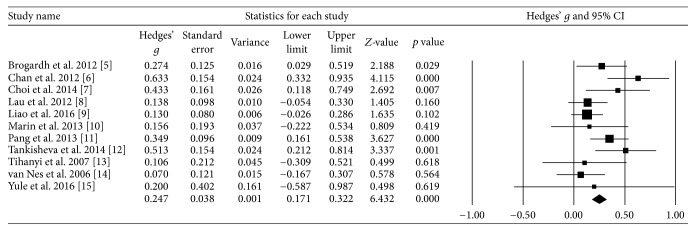
Assuming that results of each study were based on one homogeneous population, a homogeneity test with a fixed-effects model was performed. The Q value was 18.02, verifying that the studies were performed on homogeneous population. Considering each subject's result as one unit and using a random effects model, a “standardized mean difference” effect size (d) was calculated. The obtained total effect size of WBV was 0.25, and the 95% confidence interval was 0.17~0.32 (Table 4, Figure 2). Since the effect size of WBV on stroke was close to 0.2, it was interpreted that WBV has a “small effect size.”
Table 4.
Homogeneity test and the total effect size.
| N | Q-value | p | I 2 | Point estimate | 95% CI | Standard error |
|---|---|---|---|---|---|---|
| 11 | 18.02 | 0.00 | 44.5 | 0.25 | 0.17–0.32 | 0.04 |
Figure 2.
Homogeneity test.
3.2. Publication Bias Assessment
Publication biases were assessed to validate the results of meta-analysis using three different methods. A type of sensitivity analysis was performed using Duval and Tweedie's [18] trim-and-fill method. Since the correction values of articles and the observed values were identical, it was difficult to conclude if publication bias was present (Table 5).
Table 5.
Trim-and-fill publication bias assessment.
| Studies trimmed | Point estimate | 95% CI | Q-value | ||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Observed values | - | 0.25 | 0.17 | 0.32 | 18.02 |
| Adjusted values | 0 | 0.25 | 0.1 | 0.32 | 18.02 |
3.3. Effect Size according to Treatment Effectiveness
As presented in Table 5, the effect size of spasticity was the largest at 1.24, followed by bone metabolism at 0.99, balance, muscle strength, gait, and cardiac function, in decreasing order of effect size (Table 6).
Table 6.
Effect size according to treatment effectiveness.
| Group | Number of studies | Point estimate | Standard error | 95% CI |
|---|---|---|---|---|
| Balance | 19 | 0.28 | 0.08 | 0.12–0.43 |
| Muscle strength | 40 | 0.16 | 0.05 | 0.07–0.25 |
| Gait function | 15 | 0.09 | 0.07 | −0.06–0.24 |
| Spasticity | 3 | 1.24 | 0.23 | 0.76–1.7 |
| Bone metabolism | 2 | 0.99 | 0.18 | 0.65–1.35 |
| Cardiac function | 3 | 0.2 | 0.4 | −0.59–0.99 |
|
| ||||
| Total | 82 | 0.22 | 0.04 | 0.16–0.29 |
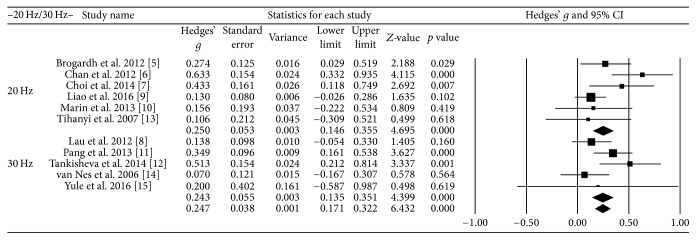
3.4. Effect Size at Different Vibration Frequencies
Vibration frequencies below 20 Hz were considered low frequencies and those over 30 Hz were considered high frequencies. The effect size was 0.25 at high frequency and 0.24 at low frequency; therefore, there was no significant difference in the effect sizes between high and low frequency (Figure 3).
Figure 3.
Effect size at different vibration frequencies.
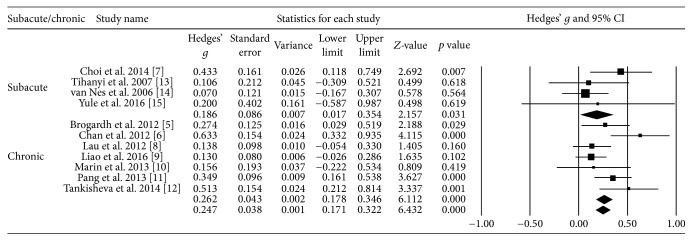
3.5. Effect Size according to the Time Lapse after the Onset of Stroke
The effect size was 0.26 when time lapse after the onset of stroke was over a year (chronic) and 0.19 when the time lapse was under one year (acute/subacute). The effect size of acute/subacute stroke was close to 0.2, which signifies a small effect size. In contrast, the effect size was relatively large for chronic stroke (Figure 4).
Figure 4.
Effect size according to the time lapse after the onset of stroke.
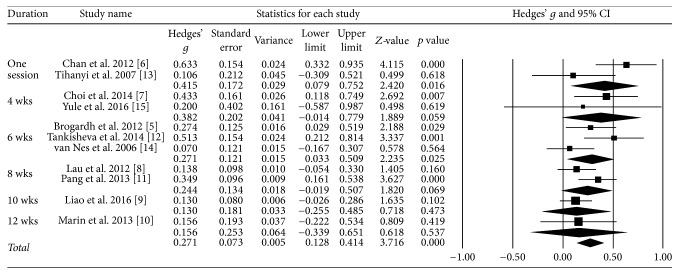
3.6. Effect Size according to the Treatment Period
The effect size was 0.42 for one session and 0.4 for four weeks of therapy; both effect sizes were moderate (Figure 5).
Figure 5.
Effect size according to the treatment period.
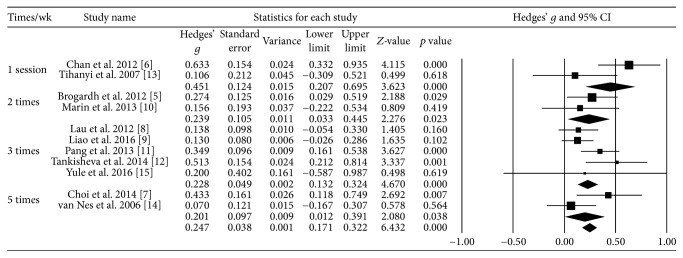
3.7. Effect Size according to the Number of Treatments per Week
The effect size was 0.45 for one session and the effect size for the other times was 0.2 (Figure 6).
Figure 6.
Effect size according to the number of treatments per week.
3.8. Changes per Published Year
Research on WBV for stroke patients started recently, and the number of studies is gradually increasing every year (Figure 7).
Figure 7.
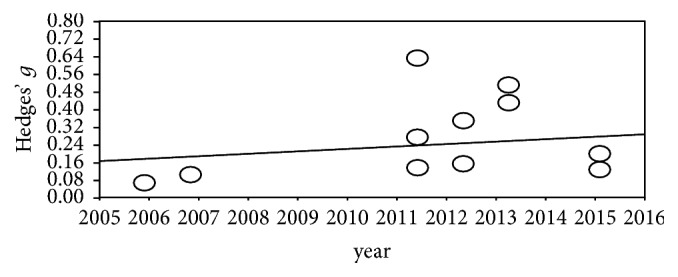
Changes per published year.
4. Discussion
This study was conducted to investigate the effectiveness of WBV through a meta-analysis of numerous studies on WBV therapy that were published recently. According to Lee et al. [2], muscle dystrophy, muscle tone, and loss of sensation in the aftermath of stroke affect the ability to function and walk. In this study, we analyzed the magnitude of these effects on muscle strength, locomotion, muscular dystrophy, and balance and analyzed the effects of bone density and circulation in order to prevent secondary complications. Previous meta-analyses have investigated the effects of WBV on balance, gait function, and limb movement [19], activity and participation after stroke [20], muscle strength, proprioceptive sense, and quality of life [21], and muscle stress [22]. However, in a meta-analysis by Lu et al. [23], WBV did not have significant effects on muscle strength, balance, and gait function. Further, this suggests a need for investigations based on the therapeutic efficacy of WBV in stroke patients.
In our meta-analysis, the total effect size was 0.25, which signifies a small effect size. However, when we evaluated the therapeutic efficacy of WBV for stroke symptoms, the effect size for spasticity was the largest at 1.24, followed by bone turn over test at 0.99, balance, muscle strength, gait function, and circulation in the decreasing order of effect size. Evaluation of spasticity comprised MAS, ATR, and so forth; muscle strength was isometric and isometric exercise was also evaluated. The evaluation comprised TUG, 10 MWT, and so forth, and the balance was between BBS, MFRT, and so forth. The goal was to measure bone metabolism using CTx and BAP, while cardiac function was evaluated by measuring HR and BP. According to the results of this study, WBV was more effective for spasticity that affects gait function than for gait function itself and muscle strength. This is consistent with the results of a study by Chan et al. [6], who reported that WBV reduced ankle plantarflexion spasticity in chronic stroke patients and therefore would be useful in gait function improvement. Moreover, they reported that WBV did improve gait function and would improve movements and movement speed. Another previous study also reported that a reduction in ankle plantarflexion spasticity affects gait function, limb movement, and movement speed [24, 25].
Even if bone metabolism does not affect gait function and risk of falls, it may prevent secondary physical problems that occur upon falling among stroke patients. Pang et al. [11] used a bone turnover test to evaluate the effects on bone mineral density. While no significant differences in the effects between the experimental and control groups were noted, it was suggested that the treatment period be extended or the therapeutic intensity be increased. Garnero et al. [26] also reported that the level of bone turnover could be useful for osteoporosis risk assessment. Considering that the majority of stroke patients are at an advanced age and have a high risk of falls due to the reduced control of their bodies, it is expected that WBV may be an effective treatment for bone weakening.
While Lau et al. [8] reported that WBV had no effect on risk of falls or motor functions, their study focused on self-efficacy of falls, which is related to balance, postural control, mobility, and muscle strength and balance. Although it cannot be definitively concluded that WBV directly improves motor functions and thereby prevents falls, WBV may certainly prevent secondary problems that occur in patients who had strokes due to an accidental fall. In this study, the effect size was small for balance, gait function, and muscle strength.
With regard to balance and muscle strength, Tihanyi et al. [13] reported that WBV was effective in increasing voluntary muscle strength, which further helped balancing and gait function. Lau et al. [8] reported that WBV eliminated risk of falls and enhanced motor functions in stroke patients during leg exercises. Regarding balance, van Nes et al. [14] reported improvements in performance of activities of daily life and balance in the WBV group compared to controls after a 12-week program. As reported by Choi et al. [7], WBV improved sitting balance and was suggested as an effective training method to improve balance. It was also reported that WBV was helpful for stimulation of the vestibular system, posture improvement, and posture correction [27] as well as postural sway enhancement [28].
However, WBV in our analysis had a small effect compared to these individual studies. The results of our study are consistent with those of Brogardh et al. [5], in which WBV had a small effect on balance and gait function improvement. They are also consistent with the results obtained by Marin et al. [10], in which there was no significant difference in muscle strength and balance between the WBV and control groups.
Similarly, Yule et al. [15] concluded that WBV does not effectively improve physical functions related to muscle strength and balance that are related to walking and activities of daily life; however, they suggested that WBV is a safe method to improve spasticity, which is related to safety and sitting balance. Likewise, Liao et al. [9] and Tankisheva et al. [12] reported that WBV is a safe therapy that can be used to improve physical functions, structure, activity, and muscle gain. Since WBV safely reduces spasticity and has an effect on bone mineral density, it may be expected to prevent secondary problems caused by accidental falls. Although these analyses compared the effects of vibration frequencies, there was no difference in the magnitude of effects according to frequency. Further, there were no significant differences in the amount of effects caused by the period of stroke; however, it appeared somewhat higher in the chronic period. This implies that WBV is therapeutic for stroke when considered in terms of spontaneous recovery. There was no difference in the effectiveness on the basis of number of sessions and weeks of treatment. Because our meta-analysis studies lacked a sufficiently large number of studies, we need to further evaluate studies on WBV for stroke. A small sample size was used in this study to establish indisputable evidence. In future, a higher number of studies on effectiveness according to the timing of stroke are required.
5. Conclusion
This study investigated effect sizes of WBV training therapy for stroke patients through a meta-analysis. The number of analyzed articles was perhaps too small because studies that included subjects without a diagnosis of stroke were excluded, several studies investigated the effects of intervention qualitatively, and several others were nonrandomized controlled studies or did not have control groups.
The purpose of our study was to verify the efficacy of WBV training as a novel approach to stroke treatment and suggest more effective treatment methods. Effect sizes from WBV studies with a pretest-posttest design and a control group were obtained, and the total effect size was small. The effect sizes for muscle strength and balance and gait function, all of which play an important role in performance of daily activities were small. In contrast, effect sizes for bone metabolism and spasticity were moderate. WBV training is a safe therapeutic method for improving symptoms in stroke patients.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Sacco R. L., Kasner S. E., Broderick J. P., et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee G., Song C., Lee Y., Cho H., Lee S. Effects of motor imagery training on gait ability of patients with chronic stroke. Journal of Physical Therapy Science. 2011;23(2):197–200. doi: 10.1589/jpts.23.197. [DOI] [Google Scholar]
- 3.Cochrane D. J. Vibration exercise: the potential benefits. International Journal of Sports Medicine. 2011;32(2):75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 4.Lee G. C. Does whole-body vibration training in the horizontal direction have effects on motor function and balance of chronic stroke survivors? A preliminary study. Journal of Physical Therapy Science. 2015;27(4):1133–1136. doi: 10.1589/jpts.27.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogardh C., Flansbjer U. B., Lexell J. No specific effect of whole-body vibration training in chronic stroke: a double-blind randomized controlled study. Archives of Physical Medicine and Rehabilitation. 2012;93(2):253–258. doi: 10.1016/j.apmr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Chan K.-S., Liu C.-W., Chen T.-W., Weng M.-C., Huang M.-H., Chen C.-H. Effects of a single session of whole body vibration on ankle plantarflexion spasticity and gait performance in patients with chronic stroke: a randomized controlled trial. Clinical Rehabilitation. 2012;26(12):1087–1095. doi: 10.1177/0269215512446314. [DOI] [PubMed] [Google Scholar]
- 7.Choi S.-J., Shin W.-S., Oh B.-K., Shim J.-K., Bang D.-H. Effect of training with whole body vibration on the sitting balance of stroke patients. Journal of Physical Therapy Science. 2014;26(9):1411–1414. doi: 10.1589/jpts.26.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau R. W. K., Yip S. P., Pang M. Y. C. Whole-body vibration has no effect on neuromotor function and falls in chronic stroke. Medicine & Science in Sports & Exercise. 2012;44(8):1409–1418. doi: 10.1249/MSS.0b013e31824e4f8c. [DOI] [PubMed] [Google Scholar]
- 9.Liao L.-R., Ng G. Y. F., Jones A. Y. M., Huang M.-Z., Pang M. Y. C. Whole-Body Vibration Intensities in Chronic Stroke: A Randomized Controlled Trial. Medicine & Science in Sports & Exercise. 2016;48(7):1227–1238. doi: 10.1249/MSS.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 10.Marin P. J., Ferrero C. M., Menéndez H., Martín J., Herrero A. J. Effects of whole-body vibration on muscle architecture, muscle strength, and balance in stroke patients: a randomized controlled trial. American Journal of Physical Medicine & Rehabilitation. 2013;92(10):881–888. doi: 10.1097/phm.0b013e318292336c. [DOI] [PubMed] [Google Scholar]
- 11.Pang M. Y., Lau R. W., Yip S. P. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine. 2013;49(4):439–450. [PubMed] [Google Scholar]
- 12.Tankisheva E., Bogaerts A., Boonen S., Feys H., Verschueren S. Effects of intensive whole-body vibration training on muscle strength and balance in adults with chronic stroke: a randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation. 2014;95(3):439–446. doi: 10.1016/j.apmr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Tihanyi T. K., Horváth M., Fazekas G., Hortobágyi T., Tihanyi J. One session of whole body vibration increases voluntary muscle strength transiently in patients with stroke. Clinical Rehabilitation. 2007;21(9):782–793. doi: 10.1177/0269215507077814. [DOI] [PubMed] [Google Scholar]
- 14.van Nes I. J. W., Latour H., Schils F., Meijer R., Van Kuijk A., Geurts A. C. H. Long-term effects of 6-week whole-body vibration on balance recovery and activities of daily living in the postacute phase of stroke: a randomized, controlled trial. Stroke. 2006;37(9):2331–2335. doi: 10.1161/01.STR.0000236494.62957.f3. [DOI] [PubMed] [Google Scholar]
- 15.Yule C. E., Stoner L., Hodges L. D., Cochrane D. J. Does short-term whole-body vibration training affect arterial stiffness in chronic stroke? A preliminary study. Journal of Physical Therapy Science. 2016;28(3):996–1002. doi: 10.1589/jpts.28.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, Mich, USA: Routledge; 1988. (Lawrence Erlbaum Associate). [DOI] [Google Scholar]
- 17.Bhogal S. K., Teasell R. W., Foley N. C., Speechley M. R. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. Journal of Clinical Epidemiology. 2005;58(7):668–673. doi: 10.1016/j.jclinepi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 19.Yang X., Wang P., Liu C., He C., Reinhardt J. D. The effect of whole body vibration on balance, gait performance and mobility in people with stroke: A systematic review and meta-analysis. Clinical Rehabilitation. 2015;29(7):627–638. doi: 10.1177/0269215514552829. [DOI] [PubMed] [Google Scholar]
- 20.Liao L.-R., Huang M., Lam F. M. H., Pang M. Y. C. Effects of whole-body vibration therapy on body functions and structures, activity, and participation poststroke: A systematic review. Physical Therapy in Sport. 2014;94(9):1232–1251. doi: 10.2522/ptj.20130366. [DOI] [PubMed] [Google Scholar]
- 21.Pozo-Cruz B. D., Adsuar J. C., Parraca J. A., Pozo-Cruz J. D., Olivares P. R., Gusi N. Using whole-body vibration training in patients affected with common neurological diseases: a systematic literature review. The Journal of Alternative and Complementary Medicine. 2012;18(1):29–41. doi: 10.1089/acm.2010.0691. [DOI] [PubMed] [Google Scholar]
- 22.Huang M., Liao L.-R., Pang M. Y. C. Effects of whole body vibration on muscle spasticity for people with central nervous system disorders: A systematic review. Clinical Rehabilitation. 2017;31(1):23–33. doi: 10.1177/0269215515621117. [DOI] [PubMed] [Google Scholar]
- 23.Lu J., Xu G., Wang Y. Effects of whole body vibration training on people with chronic stroke: A systematic review and meta-analysis. Topics in Stroke Rehabilitation. 2015;22(3):161–168. doi: 10.1179/1074935714Z.0000000005. [DOI] [PubMed] [Google Scholar]
- 24.Goldie P. A., Matyas T. A., Evans O. M. Gait after stroke: initial deficit and changes in temporal patterns for each gait phase. Archives of Physical Medicine and Rehabilitation. 2001;82(8):1057–1065. doi: 10.1053/apmr.2001.25085. [DOI] [PubMed] [Google Scholar]
- 25.Lamontagne A., Malouin F., Richards C. L., Dumas F. Mechanisms of disturbed motor control in ankle weakness during gait after stroke. Gait & Posture. 2002;15(3):244–255. doi: 10.1016/s0966-6362(01)00190-4. [DOI] [PubMed] [Google Scholar]
- 26.Garnero P., Sornay-Rendu E., Chapuy M. C., Delmas P. D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. Journal of Bone and Mineral Research. 1996;11(3):337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 27.Priplata A. A., Niemi J. B., Harry J. D., Lipsitz L. A., Collins J. J. Vibrating insoles and balance control in elderly people. The Lancet. 2003;362(9390):1123–1124. doi: 10.1016/s0140-6736(03)14470-4. [DOI] [PubMed] [Google Scholar]
- 28.Haas C. T., Turbanski S., Kessler K., Schmidtbleicher D. The effects of random whole-body-vibration on motor symptoms in Parkinson's disease. NeuroRehabilitation. 2006;21(1):29–36. doi: 10.1007/3-540-36741-1_8. [DOI] [PubMed] [Google Scholar]