Abstract
Background
Standard diffusion tensor imaging measures (e.g., fractional anisotropy; FA) are difficult to interpret in brain regions with crossing white-matter (WM) fibers. Diffusion spectrum imaging (DSI) can be used to resolve fiber crossing, but has been difficult to implement in studies of patients with psychosis given long scan times.
Methods
We used four fold accelerated compressed sensing to accelerate DSI acquisition to investigate the superior longitudinal fasciculus (SLF) in 27 (20M/7F) patients with recent onset psychosis and 23 (11M/12F) healthy volunteers. Dependent measures included the number of crossing fiber directions, multi directional anisotropy (MDA), which is a measure sensitive to the anisotropy of the underlying water diffusion in regions of crossing fibers, generalized FA (GFA) computed from the orientation distribution function, FA and tract volume.
Results
Patients demonstrated a greater number of crossing WM fibers, lower MDA, GFA and FA in the left SLF compared to healthy volunteers. Patients also demonstrated a reversal in the normal (R>L) asymmetry of crossing fiber directions in the SLF and a lack of normal (L>R) asymmetry in MDA, GFA and FA compared to healthy volunteers. Lower GFA correlated significantly (p < .05) with worse overall neuropsychological functioning; posthoc tests revealed significant effects with verbal functioning and processing speed.
Conclusions
Our findings provide the first in vivo evidence for abnormal crossing fibers within the SLF among individuals with psychosis and their functional correlates. A reversal in the normal pattern of WM asymmetry of crossing fibers in patients may be consistent with an aberrant neurodevelopmental process.
Keywords: white matter, psychosis, diffusion spectrum imaging, superior longitudinal fasciculus, fractional anisotropy
Introduction
Magnetic resonance (MR) imaging studies have identified white-matter connectivity deficits in the neurobiology of psychosis, including individuals with schizophrenia (Voineskos, 2015) and bipolar disorder (Bellani et al., 2016; Mahon et al., 2009). The first generation of diffusion tensor imaging (DTI) studies used voxelwise and region of interest approaches to investigate white-matter microstructure in patients with psychosis and demonstrated that abnormalities are present early in the course of illness and prior to extensive pharmacologic intervention (Sun et al., 2015; Szeszko et al., 2005; Szeszko et al., 2008). Subsequent studies used tractography to identify abnormalities within specific white-matter bundles among individuals at risk for (Cho et al., 2016) and experiencing (Cho et al., 2016; Kikinis et al., 2015) a first episode of psychosis. Moreover, white-matter abnormalities in schizophrenia have been linked with poor treatment response (Reis Marques et al., 2014) and negative symptoms (Voineskos et al., 2013), thus making them a potentially important target for treatment intervention.
The proportion of white-matter voxels containing crossing white-matter fibers has been estimated to be approximately 63% using automatic relevance determination and 90% using constrained spherical deconvolution, thus exceeding prior estimates (Jeurissen et al., 2013). Automatic relevance determination (Behrens et al., 2007) is a selection technique that utilizes a complex model to fit the data ensuring that parameters not supported by the model contribute minimal overall effect. This stands in contrast to other model selection techniques that fit different models to the data separately and compare them through measures reflecting data fit and complexity. In spherical deconvolution (Tournier et al., 2004; Tournier et al., 2007) fiber orientation can be assessed from high angular resolution diffusion imaging without any need for assumptions regarding the number of fiber orientations that may be present within a given voxel. This signal is expressed as a convolution over a sphere to yield a response function along with a concomitant orientation distribution function (ODF), thus allowing the fiber orientation distribution to be resolved using deconvolution.
Although the commonly used DTI model can perform well in regions consisting of a single fiber direction, this approach cannot resolve fiber tracts aligned along different axes (Farquharson et al., 2013). FA, measured through DTI, does not provide an accurate representation of the underlying white-matter microstructure in regions of crossing white-matter fibers, making both interpretation of findings and tractography inaccurate. The challenge of considering fiber crossing in image processing can be overcome using several approaches. For example, Rathi et al. (2011) used unscented Kalman filter tractography with a two tensor model and reported that 20 patients with first episode schizophrenia had at least one significantly different diffusion measure in 740 among 1254 fiber bundles compared to 20 healthy controls. In addition, the field has moved toward the application of techniques that provide high angular resolution such as diffusion spectrum imaging (Wedeen et al 2012) to provide information regarding the constituent fibers and enable resolution of crossing fibers. More specifically, in contrast to DTI q-space, which provides angular coverage along a sphere, DSI samples q-space on a uniformly-spaced Cartesian grid (Figure 1 illustrates the differences in these approaches). The additional q-space samples in DSI allow for the calculation of the diffusion probability distribution function (PDF). The angular component of the PDF is the orientation distribution function (ODF). Multiple fiber directions can be resolved by identifying peaks and troughs of the ODF. Using diffusion spectrum imaging (DSI) Griffa et al. (2015) reported that connectivity strength within an affected core of brain regions, quantified using both generalized fractional anisotropy (GFA) and the apparent diffusion coefficient, was abnormal in 15 patients with schizophrenia compared to healthy volunteers. Wu and colleagues (2014) identified lower white-matter microstructural integrity involving dorsal and ventral tracts assessed using DSI and concomitant lower functional lateralization of the dorsal pathway in 18 patients with schizophrenia compared to 18 matched healthy volunteers.
Figure 1.
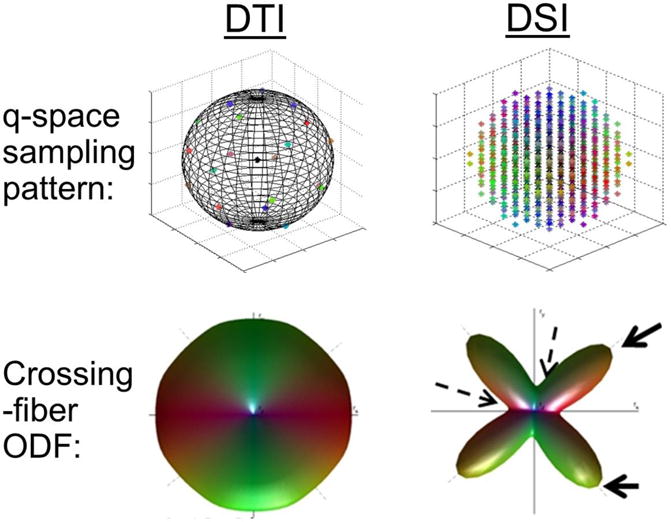
Illustration of differences between diffusion tensor imaging and diffusion spectrum imaging (DSI), regarding its diffusion-space sampling (q-space) and orientation distribution function (ODF).
Note: In DTI, q-space is sampled on a spherical shell, whereas in DSI q-space is sampled on a Cartesian grid bounded by a spherical surface of given maximum b-value (typically much larger than usual b-values employed in DTI). In a simulation of a 90-degree, equal crossing fibers, DTI is unable to resolve the two directions, whereas the added samples in DSI allow for the computed ODF to resolve the two directions. The solid and dashed arrows point to the peaks and troughs of the ODF that are used to resolve diffusion directionalities.
The use of techniques that can resolve crossing white-matter fibers such as DSI can provide complementary measures to traditional DTI and is better suited for tracking white-matter bundles in the brain (Wedeen et al., 2005). The main drawback of DSI, however, is the long scan time making it difficult to implement in clinical practice. Accelerated acquisition of DSI may be accomplished, however, by leveraging the sparsity of diffusion data in a suitable transform domain (Khare et al., 2012; Menzel et al., 2011). This acceleration uses compressed sensing (Candès, 2006) which has become more widely adopted in MR imaging research (Lustig et al., 2007). The purpose of applying compressed sensing to DSI is to exploit the inherent sparsity of the diffusion propagator in a suitable transform domain (e.g. wavelets) by first randomly under sampling the diffusion encoding space, and then using the under sampled pattern to reconstruct missing data points.
A potentially important measure that can be derived from DSI includes the number of crossing fiber bundles within a white-matter tract by identifying the peaks and troughs of the ODF. In addition, similar to FA, GFA ranges from 0 to 1 (denoting zero to maximal anisotropy), but normalizes the angular variability within the diffusion ODF to quantify the angular dependence of diffusion mobility, thus deriving a measure that is generalized from more than three eigenvalues of the DTI model (Glenn et al 2015). More specifically, a major drawback of the tensor model is that it cannot represent non-Gaussian PDFs, which are more likely to represent water diffusion properties in the brain. Therefore, an advantage of GFA compared to FA is that it provides a more appropriate estimate of anisotropy from the computed ODF to provide a potentially more valid estimate of water diffusion properties in white matter (Tuch et al 2004). We also investigated a model independent, multi directional anisotropy (MDA) measure that is analytically and experimentally equivalent to fractional anisotropy (FA) in cases of single-direction diffusivity, but has been demonstrated empirically to be superior to FA in its sensitivity to the underlying anisotropy of multi-directional diffusivity while considering crossing white-matter fibers (Tan et al., 2015). Lastly, although not a main focus of the current study we also computed FA to enable comparisons with previously published studies.
The neurobiology of psychosis likely involves structural alterations in multiple white-matter tracts, but in the current study we focused a priori on the superior longitudinal fasciculus (SLF) in light of prior work indicating that abnormalities in this tract are particularly robust in the early stages of schizophrenia (Ruef et al., 2012) and psychotic bipolar disorder (Emsell et al., 2013; Lin et al., 2011). Abnormalities in the SLF have also been identified among individuals at risk for developing psychotic disorders (Karlsgodt et al., 2009; von Hohenberg et al., 2014), which strongly predict deficits in neuropsychological and social/role functioning (Hatton et al., 2014; Karlsgodt et al., 2009) and are predicted by childhood and adolescent risk factors for psychosis in healthy adults. Moreover, a recent study from our group (Schwehm et al., 2016) identified lower FA within the SLF using tractography in 2 independent cohorts of patients with psychosis (first episode and chronic) compared to age matched healthy volunteers, converging with prior voxelwise studies implicating lower FA in the SLF in patients with first episode (Szeszko et al., 2005) and recent onset (Szeszko et al., 2008) schizophrenia compared to healthy volunteers.
Although the overall pattern of cortical asymmetry has been identified and referred to as developmental torque (LeMay, 1976), investigation of asymmetry for the major white-matter bundles has not been well characterized, especially among individuals with psychosis. Such studies could provide novel information regarding etiopathology in psychosis given that they presumably reflect neurodevelopmental mechanisms that occur in utero (Liu et al., 2010). Findings regarding white-matter asymmetry for the SLF have been mixed with some studies reporting rightward (Park et al., 2004; Yin et al., 2013) and others leftward (Choi et al., 2010) asymmetry of FA that may be influenced by environmental factors (Oechslin et al., 2009). Asymmetry of the SLF has been linked to unique connectivity patterns (Wang et al., 2016), that have functional correlates including visuomotor processing and the control of movement (Budisavljevic et al., 2017). In addition, studies investigating the comparative neuroanatomy of the SLF implicate a unique role for this white-matter bundle in the evolution of human fronto parietal networks associated with action imitation and concomitant social learning (Hecht et al., 2015) with other evidence suggesting that self-recognition functions may relate to SLF asymmetry (Hecht et al., 2016).
In the current study we investigated fiber orientation complexity within the SLF using an accelerated DSI acquisition to reduce scan time by a factor of four in 27 patients with recent onset psychosis and 23 healthy volunteers. We tested the hypothesis that patients would have a greater number of crossing white-matter fibers and lower GFA, MDA and FA within the SLF compared to healthy volunteers and that patients would demonstrate an abnormal pattern of asymmetry in these measures. We further investigated the neuropsychological correlates of white-matter measures demonstrated to be significantly different between groups. Moreover, as a check on the validity of our approach we predicted that lower FA would be significantly correlated with a greater number of crossing white-matter fibers within the SLF.
METHODS
Subjects
Twenty seven patients with recent onset psychosis were recruited from admissions to the inpatient service at The Zucker Hillside Hospital in Glen Oaks, NY. All patients displayed acute psychotic symptoms as reflected by a rating of 4 or more on one or more of the positive symptom items from the Brief Psychiatric Rating Scale. One patient in the current study had been included in our prior investigation of FA in white-matter tracts of patients with first episode psychosis (Schwehm et al., 2016), but none of the others overlapped with any of the samples in our previously published DTI studies. Prior to the DSI exam patients may have received up to 2 years of antipsychotic treatment. Simultaneous treatment with mood stabilizers or antidepressants was not allowed at the time of the scan, although lorazepam or propranolol may have been administered for akathisia or other side effects as needed. Antipsychotic medications administered prior to the scan are provided in Table 1. Clinical raters were blind to medication status and trained using standardized clinical procedures (Robinson et al., 2015).
Table 1.
Sample Demographics
| Patients (N=27) |
Healthy Volunteers (N=23) |
Statistical Test | df | p value | |
|---|---|---|---|---|---|
| Age (SD), years | 23.8 (5.4) | 26.3 (7.1) | t = 1.45 | 48 | NS |
| Sex (Male/Female) | 20M/7F | 11M/12F | χ2 = 3.63 | 1 | NS |
| Handedness (R, L)1 | 8/17 | 11/12 | χ2 = 1.26 | 1 | NS |
| Education (years)2 | 13.0 (1.8) | 14.9 (2.4) | t = 3.16 | 46 | .003 |
| Brief Psychiatric Rating Scale | 43.5 (6.3) | ||||
| MATRICS Consensus Cognitive Battery T Score3 | |||||
| Attention | 34.6 (10.7) | ||||
| Reasoning/Problem Solving | 44.8 (9.1) | ||||
| Social Cognition | 38.0 (10.2) | ||||
| Speed of Processing | 34.6 (8.4) | ||||
| Verbal Memory | 34.3 (6.1) | ||||
| Visual Processing | 40.1 (11.6) | ||||
| Working Memory | 40.1 (12.0) | ||||
| Overall Functioning | 31.8 (5.1) | ||||
| Antipsychotics received prior to MRI4 | |||||
| Risperidone | 22 | ||||
| Latuda | 1 | ||||
| Olanzapine | 2 | ||||
| Haloperidol | 1 | ||||
| Valproic Acid | 1 | ||||
| Quetiapine | 1 | ||||
| Ziprasidone | 1 |
Note: Means are provided with standard deviations in parentheses.
Data were unavailable for 2 patients.
Data were unavailable for 1 patient and 1 healthy volunteer.
Data were unavailable for 3 patients on the reasoning/problem solving, speed of processing, verbal functioning, visual functioning, and working memory domains. Five individuals were missing attention scores and 7 individuals (including 1 outlier > 3 SD from the mean) were missing social cognition scores.
Data were unavailable for 3 patients
All patients received a physical exam and laboratory screening to rule out medical causes and a substance induced psychotic disorder for this illness episode. Mean age at first psychotic symptoms was 21.5 years (SD=5.6). All patient diagnoses were based on the SCID for Axis I DSM IV Disorders supplemented by information from clinicians and, when available, family members. Diagnoses for patients included schizophrenia (n=19), schizoaffective disorder (n=1), schizophreniform disorder (n=6) or Bipolar I with psychosis (n=1). Twenty three healthy volunteers were recruited from advertisements posted on websites and by word of mouth. Exclusion criteria for healthy volunteers included the denial of any lifetime history of a major mood or psychotic disorder as determined by clinical interview using the SCID NP. Exclusion criteria for all participants included: (a) MR imaging contraindications; (b) any medical illness known to affect the brain (e.g., Huntington’s Disease, Parkinson’s disease, etc.); (c) prior psychosurgery; (d) DSM IV mental retardation; (e) stroke and (f) pregnancy. The study was approved by the Northwell Health Institutional Review Board. Written informed consent was obtained from all individuals, and from a parent or legal guardian in the case of minors. Written assent was obtained from all minors.
Handedness
Laterality scores were based on a modified version of the Edinburgh Inventory (Oldfield, 1971). The total number of right and left hand items was scored and the laterality quotient was computed: (Total R − Total L)/(Total R + Total L) yielding a range from +1.00 (totally dextral) to −1.00 (totally nondextral). Individuals with a laterality score greater than .70 were categorized as dextral while individuals with scores ≤ .70 were categorized as nondextral consistent with our prior definition (Schwehm et al., 2016). Data were missing for two subjects and handedness for one individual was based on hand preference alone.
Clinical and Neuropsychological Assessments
Patients completed the 18 item Brief Psychiatric Rating Scale (Overall and Gorham, 1962) and the total score was derived by summing all of the items. We also administered the MATRICS Consensus Cognitive Battery (Green and Nuechterlein 2004) to patients, which included: (1) speed of processing; (2) attention/vigilance; (3) working memory; (4) verbal learning; (5) visual learning; (6) reasoning and problem solving; and (7) social cognition. A measure of overall functioning was computed by standardizing the sum of these respective domain T-scores based on a community sample. Due to missing data on some domains overall neuropsychological indices could not be computed for 8 patients.
Magnetic Resonance Imaging Methods
Magnetic resonance imaging exams were conducted at the North Shore University Medical Center on a 3T whole body MRI system (GE Healthcare, Waukesha, WI USA). Each study began with a sagittal localizer, followed by a 26 minute compressed sensing DSI sequence (T2 weighted image + 127 diffusion directions, bmax = 6,000 sec/mm2, FOV = 24 cm, 128×128 matrix, slice thickness = 3 mm, TR/TE = 12 sec/125–134 msec, 27–31 slices). To accelerate DSI acquisition (from 105 minutes to 26 minutes), four-fold accelerated compressed sensing was applied (reducing 514 diffusion samples based on an 11×11-cube Cartesian q-space down to 127 randomly-distributed diffusion samples). Unlike multi-shell diffusion sampling schemes, DSI q-space samples are regularly distributed on a Cartesian grid. Based on an 11×11×11 q-space cube, 5 individual b-values along a Cartesian axis in addition to the non-diffusion encoded (T2-weighted, b=0) acquisition are sampled. For anatomical overlay and image registration, a high-resolution T1-weighted SPGR 3D spoiled gradient echo sequence with 1 mm slices was also acquired (TR/TE = 7.5/3 msec, matrix = 256×256, FOV = 240 mm).
All DSI data were visually inspected for the presence of artifacts or signal drop-out that could potentially create motion artifacts across the time series and none of the individuals demonstrated any such artifacts that would preclude study participation. We further assessed motion across the 127 volumes collected in this study by computing: (1) absolute translation from the reference volume; (2) translation from the previous volume (relative translation); (3) rotation angle from the reference volume; and (4) rotation angle from the previous volume (relative rotation). These variables were subsequently included as covariates in statistical analyses.
Diffusion Image Processing and Analysis
The undersampled DSI data were reconstructed through a compressed sensing algorithm that uses total variation and wavelets as sparsifying transforms (Khare et al., 2012; Menzel et al., 2011). Compared to DTI, which permits single directional tractography per voxel, compressed-sensing accelerated DSI provides the orientation distribution function, which allows computation of multi-directional tractography per voxel. Deterministic fiber tractography with the ODF computed from compressed sensing DSI was performed using a small angle threshold of 38°, which significantly reduced false positives (compared to 55–70° typically used in DTI).
Dependent measures in this study included: (1) number of crossing fiber directions, which was defined as the total number of peaks located on the orientation distribution function that was generated for 181 discretized directions on a hemisphere, equivalent to an approximate 10° tessellation angle; (2) multi dimensional anisotropy, which has been demonstrated empirically to be superior to FA in its sensitivity to the underlying anisotropy of multi directional diffusivity while considering crossing white-matter fibers (Tan et al., 2015); (3) GFA, which is a more comprehensive measure of anisotropy compared to FA that normalizes the angular variability within the diffusion ODF to quantify the angular dependence of diffusion mobility, thus deriving a measure that more accurately reflects the underlying fiber bundle anisotropy than what can be derived from the three eigenvalues of a tensor model; (4) FA; and (5) volume (based on traversed locations for tract). No thresholding or denoising was used to generate the number of crossing fibers as the ODFs were derived from DSI data and did not undergo sharpening filters, as compared to fiber orientation distribution functions obtained with spherical deconvolution that effectively sharpen the ODFs to improve peak detection. A linear interpolation kernel was used to create a smooth mask prior to creation of binary mask, to reduce pixellation effects that were then used to measure FA and MDA from images.
We identified the right and left SLF by using seed regions of interest from a template (Tan et al., 2014). Each region of interest was a sphere characterized by its position and radius, whereby each tract was defined by one or more pairs of logical conditions such as “AND” and “NOT.” Each ROI was manually optimized for visualization of each tract per subject using the Trackvis software (Wang, Wedeen, MA, USA) similar to a previous DTI study (Gruner et al., 2012) by an operator blind to group membership. Two spherical ROIs were placed along the arcuate fasciculus in regions similar to Kamali et al., (2014), and an additional spherical ROI was placed in the middle of these two ROIs. The ROIs (beginning with inferior-posterior to superior-anterior) were 1.4cm, 1.1cm, and 1.1cm in diameter and the radii were scaled to account for the size of the brain mask obtained from skull stripping (Iglesias et al., 2011). Region of interest placements are illustrated for the right and left SLF in Figure 2.
Figure 2.
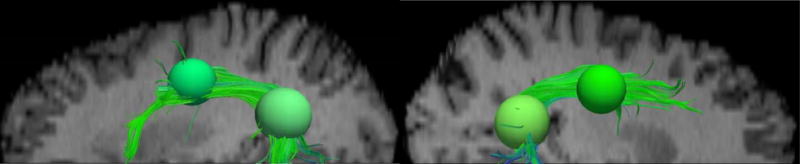
Tractography renderings in the left (left panel) and right (right panel), illustrating the superior longitudinal fasciculus (SLF) obtained using CS-DSI superimposed on the T2 (b=0) image, and the two primary spherical ROIs (superior-anterior: 1.1 cm and inferior-posterior: 1.4 cm in diameter, 5.0 cm apart) used to define the SLF. An additional superior-inferior ROI (1.1 cm diameter, not shown) in the approximate mid-section (approximately 2.7 cm superior-medial of the inferior-posterior ROI) is also used with the first ROI to capture any longitudinal fibers missed by the primary ROIs.
Statistical Analysis
We used repeated measures analysis of covariance to test the hypothesis that patients differed from healthy volunteers in the number of crossing fiber directions, GFA, MDA, FA and tract volume. In each analysis group served as the between-subjects factor and hemisphere as the within-subjects factor. Age and sex were included as statistical covariates in these models given their potential to influence white-matter microstructure and asymmetry (Kitamura et al., 2011; Powell et al., 2012). We also repeated analyses while controlling for Edinburgh laterality score and the 4 motion indices in subsequent models. We additionally computed asymmetry indices ([(right − left) / (right + left)] * 100) for all measures that demonstrated significant group × hemisphere interactions and compared them between groups using independent t-tests (alpha =.05; two-tailed). Scatterplots were visually inspected and Pearson product moment or Spearman rank order correlations were used to investigate the relationship between MR imaging measures and neuropsychological measures (alpha =.05; two-tailed). To limit Type I error in these analyses we only investigated neuropsychological correlates of MR imaging measures that differed significantly between groups; these correlations were first conducted in relationship to the overall neuropsychological domain with subsequent posthoc analyses investigating individual domains.
Results
Patients did not differ significantly (p>.05) from healthy volunteers in distributions of age, sex, or handedness, but as expected healthy volunteers had significantly (p<.05) more education compared to patients (Table 1). Mean total BPRS scores for patients at the time of the scan was 43.5 (SD=6.3). Mean (SD) values for the dependent measures derived for the SLF are provided in Table 2 for descriptive purposes only.
Table 2.
Mean and Standard Deviation (in parentheses) for Right and Left Superior Longitudinal Fasciculus Metrics and Asymmetry Indices.
| Metric | Right Superior Longitudinal Fasciculus | Left Superior Longitudinal Fasciculus | Asymmetry Index | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (N=23) |
Patients (N=27) |
p-value | Controls (N=23) |
Patients (N=27) |
p-value | Controls (N=23) |
Patients (N=27) |
p-value | |
| Number of Crossing Fibers | 1.87 (0.09) | 1.87 (0.15) | NS | 1.82 (0.10) | 1.90 (0.14) | .04 | 1.19 (2.36) | −.64 (2.30) | .008 |
| MDA | .476 (0.02) | .475 (0.01) | NS | .490 (0.02) | .479 (0.01) | 01 | −1.43 (1.64) | −.34 (1.54) | .019 |
| GFA | .179 (.007) | .180 (.006) | NS | .185 (.008) | .179 (.006) | .006 | −1.51 (1.84) | .08 (1.94) | .005 |
| FA | .463 (0.03) | .462 (0.02) | NS | .472 (0.03) | .453 (0.02) | .004 | −1.05 (2.15) | .96 (2.17) | .002 |
| Volume (cc) | 12.7 (4.5) | 11.4 (3.0) | NS | 12.3 (3.9) | 11.9 (2.9) | NS | 1.20 (10.2) | −2.28 (11.1) | NS |
Notes. Number of crossing fibers = number of ODF peaks found for each voxel; MDA (multi directional anisotropy) = anisotropy calculated from ODFs by using ratio of peaks to troughs in the ODFs and identical to FA for single-directional Gaussian diffusion with values ranging from 0 to 1; GFA (generalized fractional anisotropy) defined according to Tuch (2004); and FA (fractional anisotropy) = anisotropy calculated based on a DTI model with values ranging from 0 to 1; Volume = volume based on traversed locations for tract. The asymmetry index was computed using the following formula = [(R−L)/(R+L)] * 100.
There were no significant main effects of group or hemisphere for any of the dependent measures. There were significant group × hemisphere interactions for the number of crossing fibers (F = 5.83, df = 46, p = .020), MDA (F = 5.13, df = 46, p = .028), GFA (F = 7.21, df = 46, p = .01) and FA (F = 7.91, df = 46, p = .007), but no significant group × hemisphere interaction for volume. Investigation of asymmetry indices revealed significant group differences in the number of crossing fibers, MDA, GFA and FA within the SLF (Table 2). Findings remained statistically significant while controlling for handedness and movement indices. Post-hoc analyses indicated that patients demonstrated a significantly greater number of crossing fibers, lower MDA, GFA and FA in the left hemisphere compared to healthy volunteers in the absence of group differences in the right hemisphere (Table 2).
Among patients, lower GFA and FA in the left SLF correlated significantly with worse overall neuropsychological functioning (r = .46, df = 19, p = .047 and r = .61, df = 19, p = .006, respectively). Posthoc analyses revealed significant effects for GFA and FA with processing speed (r = .54, df = 24, p = .007 and r = .55, df = 24, p = .005, respectively) and verbal functioning (r = .47, df = 24, p = .019 and r = .51, df = 24, p = .012, respectively). MDA, number of crossing white-matter fibers or the asymmetry measures did not correlate significantly with overall neuropsychological functioning.
The correlation between the number of crossing fibers and FA was statistically significant for both the right (r = −.43, df = 50, p =.002) and left (r = −.42, df = 50, p = .002) SLF across both groups. When separated by group, however, the correlation between the number of crossing fibers and FA was statistically significant in healthy volunteers for the right (r = −.71, df = 23, p < .001) and left (r = −.49, df = 23, p = .018) hemispheres (Figure 3). Although these correlations were not statistically significant in patients (one case was excluded for being an outlier on the scatterplot), approximately 9% of the variance in FA was still accounted for by number of crossing fibers in both the right and left hemispheres. As expected the number of fiber directions did not correlate significantly with MDA or GFA in either the right or left hemispheres among patients or healthy volunteers. None of these findings were significantly changed when handedness score was included as a covariate in analyses.
Figure 3.
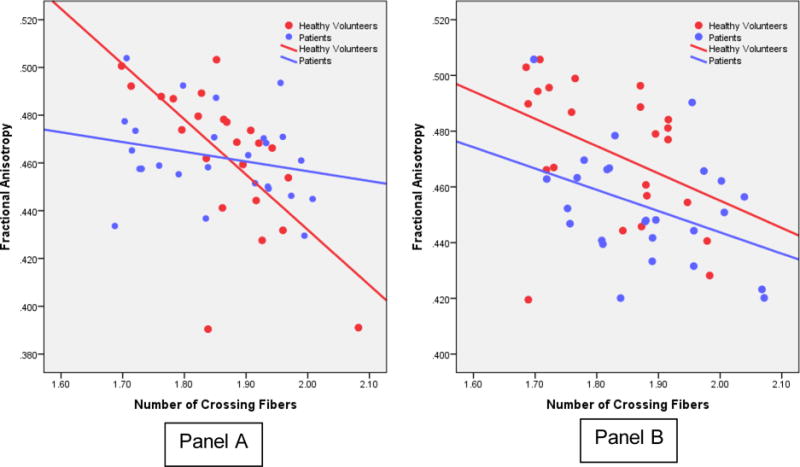
Scatterplots of Fractional Anisotropy and Number of Fiber Directions in the Right (Panel A) and Left (Panel B) Superior Longitudinal Fasciculus in Healthy Volunteers and Patients.
Discussion
The use of DSI could potentially clarify the role of crossing fibers in the neurobiology of psychosis and move beyond the standard DTI framework; however, the main drawback associated with this technique is the long scan time. In the current study we used compressed sensing DSI to reduce scan time by a factor of four. Using this approach, our primary finding is that patients with psychosis have a greater number of crossing white-matter fibers within the left SLF and demonstrate abnormal asymmetry in these crossing fibers compared to healthy volunteers. In addition, we demonstrate group differences in MDA, which can accommodate multiple crossing fiber directions and GFA, a comprehensive measure of anisotropy computed over the diffusion ODF. The validity of our approach is supported by the findings that FA differed between groups and that the number of crossing fibers within the SLF correlated significantly with FA, but not MDA.
The “normal” pattern of asymmetry in crossing fiber directions, MDA, GFA and FA was reversed in patients compared to healthy volunteers. The presence of hemispheric asymmetry in the human brain is evident at both the microscopic and macroscopic level (Chance, 2014). An abnormality in asymmetry involving the left SLF among patients is consistent with long standing hypotheses regarding the failure of left hemisphere lateralization in the pathophysiology of schizophrenia (Crow, 1999; Ribolsi et al., 2009). Empirical studies reported both functional and structural abnormalities in brain asymmetry in patients with psychosis compared to healthy volunteers (Bilder et al., 1994; Ratnanather et al., 2013; Royer et al., 2015; Szeszko et al., 2003) and greater gray matter volume loss over time in left (compared to right) hemisphere regions assessed using meta-analysis and meta-regression (Vita et al., 2012). Although it is possible that postnatal effects may play a role in this process, we are unaware of any data indicating that asymmetry of crossing white-matter fibers is influenced by neurodegenerative or pharmacologic mechanisms and it may be noteworthy that prior work has demonstrated that identification of asymmetry may be improved at higher field strengths (Okada et al., 2006).
An aberrant neurodevelopmental process involving the white matter could reflect a defect in the ability to appropriately prune connections throughout adolescence and contribute to inefficient signal transduction and concomitant functional deficits in patients (Clark et al., 2012). In this regard we found that lower left hemisphere GFA was associated with worse processing speed and lower verbal functioning in patients. Our findings thus converge with prior studies identifying abnormal functional correlates involving FA in this white-matter tract (Hatton et al., 2014; Karlsgodt et al., 2008), but now extend this to more comprehensive measures such as GFA. It may be noteworthy that brain abnormalities were associated with verbal memory deficits given the considerable evidence that individuals at genetic high risk for schizophrenia demonstrate both structural and functional abnormalities in brain regions associated with language functions mediated by left hemisphere brain regions (Li et al., 2009).
Although not a primary focus of this study, we report lower FA in the left SLF of patients compared to healthy volunteers replicating prior findings by our group (Szeszko et al., 2005; Szeszko et al., 2008) and others (Hatton et al., 2014) using independent cohorts of patients with psychosis. Although we did not find evidence that MDA within the SLF was superior to FA in distinguishing the groups, our results nevertheless implicate water diffusion abnormalities in patients with psychosis compared to healthy volunteers while considering fiber crossing. Moreover, DSI may provide complementary information regarding crossing fibers that is directly related to FA. For example, the observation of lower MDA in patients compared to healthy volunteers, suggests that additional factors other than number of fiber directions (e.g., fiber structural integrity) may be influencing findings of abnormal water diffusion among patients. Specifically, the lower correlations between number of fiber directions and MDA compared to FA (at least in healthy volunteers) provide direct in-vivo evidence that this measure is less affected by multiple fiber directions.
The neurobiological mechanism(s) potentially leading to more crossing fibers in patients compared to healthy volunteers is largely unknown, but may be neurodevelopmental in origin. Nonhuman primate studies indicate that brain fiber pathways are organized in a curved 3-dimensional grid characterized by 3 primordial gradients (Wedeen et al 2012), likely established during early embryogenesis. Thus, it is conceivable that abnormal fiber crossings occur in-utero and requires an environmental “trigger” for psychosis onset. In addition, several reviews indicate that patients with psychosis have a defect in the ability to prune white-matter connections over the course of illness (Peters and Karlsgodt, 2015; Kochunov and Hong, 2014), which may, in part, be genetic in origin (Voineskos, 2015; Duff et al 2013). A possible interpretation of these findings is that a greater number of crossing fibers may, in part, be influencing FA calculations in patients, consistent with the results of this study and the large literature demonstrating lower FA in patients compared to healthy volunteers.
An important advantage of using DSI compared with other advanced diffusion acquisition approaches such as high angular resolution diffusion-weighted imaging is that diffusion encoding space (or q space) is sampled on a Cartesian grid, allowing for the diffusion propagator to be reconstructed using standard Fourier transforms. Therefore, DSI relies on less stringent assumptions and does not depend on model based reconstruction (such as q-ball, spherical transforms, and spherical deconvolution) that is typically required with high angular resolution diffusion-weighted imaging (Tuch et al., 2002). Similar to prior work that used spherical deconvolution to control for crossing fiber pathways (Reijmer et al., 2012) our approach yielded larger and longer pathways of the SLF compared to our previously reported DTI based methods. An additional advantage of using compressed sensing on a full sphere is that all directions are uniformly sampled. In that regard one disadvantage of most half sphere approaches is that a plane for separating the half sphere must be chosen that could potentially lead to bias in directionality. In addition, half-sphere sampling speeds up the process only by a factor of 2.
There were a number of study limitations that should be acknowledged. We did not localize abnormal crossing fibers to specific parts of the SLF and it is known that this tract is heterogeneous and comprised of different subcomponents (e.g., SLF I, II and III) and arcuate fasciculus (Martino et al., 2013; Thiebaut de Schotten et al., 2011). It should be acknowledged that patients were receiving antipsychotic medications at the time of the scan and it is conceivable that they could influence measures of water diffusion such as FA, although the relationship between antipsychotic medications and crossing white-matter fibers remains unknown. An additional study caveat is that we were unable to determine whether the observed abnormalities in crossing white-matter fibers are specific to the white matter within the SLF and/or are associated with greater crossing of other white-matter tracts traversing the SLF. Thus, the underlying mechanisms contributing to abnormal crossing white-matter fibers could not be determined in the current study.
In sum, using ODF-based metrics our DSI findings implicate a greater number of crossing white-matter fibers within the SLF and concomitant lower GFA and MDA in patients with recent onset psychosis compared to healthy volunteers. We also provide direct in-vivo evidence for a relationship between fiber crossing and FA among healthy humans.
Highlights.
Diffusion tensor imaging cannot resolve fiber tracts aligned along different axes in the same voxel.
Diffusion spectrum imaging (DSI) can quantify crossing white-matter fibers.
The main drawback associated with DSI is the long scan time.
Compressed sensing was used to accelerate DSI acquisition.
Patients with psychosis demonstrated abnormalities in crossing white-matter fibers.
ETHICAL STATEMENT.
The current work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Written informed consent was obtained from all individuals, and from a parent or legal guardian in the case of minors. Written assent was obtained from all minors. This statement is provided in the manuscript.
Acknowledgments
Drs. Szeszko and Tan had full access to all the data in the study and take responsibility for the integrity of the data. Dr. Szeszko conducted and is responsible for the data analysis.
The authors would like to thank Jonathan Sperl, Marion Menzel, and Tim Sprenger for their useful comments.
FUNDING
This work was supported in part by grants from NARSAD (PRS) and the National Institute of Mental Health (R01MH076995; R21MH101746; R21MH107855), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Dr. Malhotra serves on the advisory boards of Genomind, LLC, FORUM Pharmaceuticals and Takeda Pharmaceuticals and a consultant to Vanda Pharmaceuticals. Dr. Robinson has served as a consultant to Janssen, Otsuka, Costello, Neurocrine and Innovative Science Solutions. Drs. Tan and Marinelli are employed by the General Electric company. Drs. Szeszko, Gallego, Kingsley, Uluğ, and Rhindress report no potential conflicts of interest.
References
- Behrens TEJ, Johansen-Berg H, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani M, Boschello F, Delvecchio G, Dusi N, Altamura CA, Ruggeri M, Brambilla P. DTI and Myelin Plasticity in Bipolar Disorder: Integrating Neuroimaging and Neuropathological Findings. Front Psychiatry. 2016;7:21. doi: 10.3389/fpsyt.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, Snyder PJ, Lieberman JA. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151(10):1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Dell’Acqua F, Zanatto D, Begliomini C, Miotto D, Motta R, Castiello U. Asymmetry and Structure of the Fronto-Parietal Networks Underlie Visuomotor Processing in Humans. Cereb Cortex. 2017;27(2):1532–1544. doi: 10.1093/cercor/bhv348. [DOI] [PubMed] [Google Scholar]
- Candès EJ. Modern statistical estimation via oracle inequalities. Acta Numerica. 2006;15:257. [Google Scholar]
- Chance SA. The cortical microstructural basis of lateralized cognition: a review. Front Psychol. 2014;5:820. doi: 10.3389/fpsyg.2014.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KI, Shenton ME, Kubicki M, Jung WH, Lee TY, Yun JY, Kim SN, Kwon JS. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr Bull. 2016;42(3):723–731. doi: 10.1093/schbul/sbv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Lee JM, Koo BB, Park JS, Kim DS, Kwon JS, Kim IY. Sex differences in the temporal lobe white matter and the corpus callosum: a diffusion tensor tractography study. Neuroreport. 2010;21(1):73–77. doi: 10.1097/WNR.0b013e3283345eb0. [DOI] [PubMed] [Google Scholar]
- Clark K, Narr KL, O’Neill J, Levitt J, Siddarth P, Phillips O, Toga A, Caplan R. White matter integrity, language, and childhood onset schizophrenia. Schizophr Res. 2012;138(2–3):150–156. doi: 10.1016/j.schres.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ. Twin studies of psychosis and the genetics of cerebral asymmetry. Br J Psychiatry. 1999;175:399–401. doi: 10.1192/bjp.175.5.399. [DOI] [PubMed] [Google Scholar]
- Duff BJ, Macritchie KAN, Moorhead TWJ, Lawrie SM, Blackwood DHR. Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr Res. 2013;147(1):1–13. doi: 10.1016/j.schres.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Emsell L, Leemans A, Langan C, Van Hecke W, Barker GJ, McCarthy P, Jeurissen B, Sijbers J, Sunaert S, Cannon DM, McDonald C. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry. 2013;73(2):194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Farquharson S, Tournier JD, Calamante F, Fabinyi G, Schneider-Kolsky M, Jackson GD, Connelly A. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg. 2013;118(6):1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- Glenn GR, Helpern JA, Tabesh A, Jensen JH. Quantitative assessment of diffusional kurtosis anisotropy. NMR Biomed. 2015;28(4):448–459. doi: 10.1002/nbm.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Griffa A, Baumann PS, Ferrari C, Do KQ, Conus P, Thiran JP, Hagmann P. Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp. 2015;36(1):354–366. doi: 10.1002/hbm.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner P, Vo A, Ikuta T, Mahon K, Peters BD, Malhotra AK, Ulug AM, Szeszko PR. White matter abnormalities in pediatric obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37(12):2730–2739. doi: 10.1038/npp.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton SN, Lagopoulos J, Hermens DF, Hickie IB, Scott E, Bennett MR. White matter tractography in early psychosis: clinical and neurocognitive associations. J Psychiatry Neurosci. 2014;39(6):417–427. doi: 10.1503/jpn.130280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EE, Gutman DA, Bradley BA, Preuss TM, Stout D. Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage. 2015;108:124–137. doi: 10.1016/j.neuroimage.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EE, Mahovetz LM, Preuss TM, Hopkins WD. A neuroanatomical predictor of mirror self-recognition in chimpanzees. Soc Cogn Affect Neurosci. 2016;12(1):37–48. doi: 10.1093/scan/nsw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Liu CY, Thompson PM, Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans Med Imaging. 2011;30(9):1617–1634. doi: 10.1109/TMI.2011.2138152. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34(11):2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct. 2014;219(1):269–281. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63(5):512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Khare K, Hardy CJ, King KF, Turski PA, Marinelli L. Accelerated MR imaging using compressive sensing with no free parameters. Magn Reson Med Sci. 2012;68(5):1450–1457. doi: 10.1002/mrm.24143. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Fitzsimmons J, Dunn C, Vu MA, Makris N, Bouix S, Goldstein JM, Mesholam-Gately RI, Petryshen T, Del Re EC, Wojcik J, Seidman LJ, Kubicki M. Anterior commissural white matter fiber abnormalities in first-episode psychosis: a tractography study. Schizophr Res. 2015;162(1–3):29–34. doi: 10.1016/j.schres.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Morikawa M, Kiuchi K, Taoka T, Fukusumi M, Kichikawa K, Kishimoto T. Asymmetry, sex differences and age-related changes in the white matter in the healthy elderly: a tract-based study. BMC Res Notes. 2011;4:378. doi: 10.1186/1756-0500-4-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40(4):721–8. doi: 10.1093/schbul/sbu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann N Y Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- Li X, Branch CA, DeLisi LE. Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Curr Opin Psychiatry. 2009;22(2):131–139. doi: 10.1097/YCO.0b013e328324bc43. [DOI] [PubMed] [Google Scholar]
- Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. J Affect Disord. 2011;131(1–3):299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Liu F, Garland M, Duan Y, Stark RI, Xu D, Bansal R, Dong Z, Peterson BS, Kangarlu A. Techniques for in utero, longitudinal MRI of fetal brain development in baboons at 3T. Methods. 2010;50(3):147–156. doi: 10.1016/j.ymeth.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med Sci. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- Mahon K, Wu J, Malhotra AK, Burdick KE, DeRosse P, Ardekani BA, Szeszko PR. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34(6):1590–1600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218(1):105–121. doi: 10.1007/s00429-012-0386-5. [DOI] [PubMed] [Google Scholar]
- Menzel MI, Tan ET, Khare K, Sperl JI, King KF, Tao X, Hardy CJ, Marinelli L. Accelerated diffusion spectrum imaging in the human brain using compressed sensing. Magn Reson Med Sci. 2011;66(5):1226–1233. doi: 10.1002/mrm.23064. [DOI] [PubMed] [Google Scholar]
- Oechslin MS, Imfeld A, Loenneker T, Meyer M, Jancke L. The plasticity of the superior longitudinal fasciculus as a function of musical expertise: a diffusion tensor imaging study. Front Hum Neurosci. 2009;3:76. doi: 10.3389/neuro.09.076.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Miki Y, Fushimi Y, Hanakawa T, Kanagaki M, Yamamoto A, Urayama S, Fukuyama H, Hiraoka M, Togashi K. Diffusion-tensor fiber tractography: intraindividual comparison of 3.0-T and 1.5-T MR imaging. Radiology. 2006;238(2):668–678. doi: 10.1148/radiol.2382042192. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale (BPRS) Psychol Rep. 1962;10:799–812. [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23(1):213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Karlsgodt KH. White matter development in the early stages of psychosis. Schizophr Res. 2015;161(1):61–9. doi: 10.1016/j.schres.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JL, Parkes L, Kemp GJ, Sluming V, Barrick TR, Garcia-Finana M. The effect of sex and handedness on white matter anisotropy: a diffusion tensor magnetic resonance imaging study. Neuroscience. 2012;207:227–242. doi: 10.1016/j.neuroscience.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Rathi Y, Kubicki M, Bouix S, Westin CF, Goldstein J, Seidman L, Mesholam-Gately R, McCarley RW, Shenton ME. Statistical analysis of fiber bundles using multi-tensor tractography: application to first-episode schizophrenia. Magn Reson Imaging. 2011;29(4):507–515. doi: 10.1016/j.mri.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnanather JT, Poynton CB, Pisano DV, Crocker B, Postell E, Cebron S, Ceyhan E, Honeycutt NA, Mahon PB, Barta PE. Morphometry of superior temporal gyrus and planum temporale in schizophrenia and psychotic bipolar disorder. Schizophr Res. 2013;150(2–3):476–483. doi: 10.1016/j.schres.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer YD, Leemans A, Heringa SM, Wielaard I, Jeurissen B, Koek HL, Biessels GJ, Vascular Cognitive Impairment Study, g Improved sensitivity to cerebral white matter abnormalities in Alzheimer’s disease with spherical deconvolution based tractography. PLoS One. 2012;7(8):e44074. doi: 10.1371/journal.pone.0044074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis Marques T, Taylor H, Chaddock C, Dell’acqua F, Handley R, Reinders AA, Mondelli V, Bonaccorso S, Diforti M, Simmons A, David AS, Murray RM, Pariante CM, Kapur S, Dazzan P. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 2014;137(Pt 1):172–182. doi: 10.1093/brain/awt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribolsi M, Koch G, Magni V, Di Lorenzo G, Rubino IA, Siracusano A, Centonze D. Abnormal brain lateralization and connectivity in schizophrenia. Rev Neurosci. 2009;20(1):61–70. doi: 10.1515/revneuro.2009.20.1.61. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Gallego JA, John M, Petrides G, Hassoun Y, Zhang JP, Lopez L, Braga RJ, Sevy SM, Addington J, Kellner CH, Tohen M, Naraine M, Bennett N, Greenberg J, Lencz T, Correll CU, Kane JM, Malhotra AK. A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophr Bull. 2015;41(6):1227–1236. doi: 10.1093/schbul/sbv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer C, Delcroix N, Leroux E, Alary M, Razafimandimby A, Brazo P, Delamillieure P, Dollfus S. Functional and structural brain asymmetries in patients with schizophrenia and bipolar disorders. Schizophr Res. 2015;161(2–3):210–214. doi: 10.1016/j.schres.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Ruef A, Curtis L, Moy G, Bessero S, Badan Ba M, Lazeyras F, Lovblad KO, Haller S, Malafosse A, Giannakopoulos P, Merlo M. Magnetic resonance imaging correlates of first-episode psychosis in young adult male patients: combined analysis of grey and white matter. J Psychiatry Neurosci. 2012;37(5):305–312. doi: 10.1503/jpn.110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwehm A, Robinson DG, Gallego JA, Karlsgodt KH, Ikuta T, Peters BD, Malhotra AK, Szeszko PR. Age and sex effects on white matter tracts in psychosis from adolescence through middle adulthood. Neuropsychopharmacology. 2016;41(10):2473–2480. doi: 10.1038/npp.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YW, Hu H, Wang Y, Ding WN, Chen X, Wan JQ, Zhou Y, Wang Z, Xu JR. Inter-hemispheric functional and anatomical connectivity abnormalities in traffic accident-induced PTSD: a study combining fMRI and DTI. J Affect Disord. 2015;188:80–88. doi: 10.1016/j.jad.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62(7):782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Goldman RS, Bates J, Ashtari M, Snyder PJ, Lieberman JA, Bilder RM. Lack of normal association between cerebellar volume and neuropsychological functions in first-episode schizophrenia. Am J Psychiatry. 2003;160(10):1884–1887. doi: 10.1176/appi.ajp.160.10.1884. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Ardekani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33(5):976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Tan ET, Liu X, Ulug AM, Kingsley PB, Malhotra AK, Robinson DG, Szeszko PR, Marinelli L. Robust automated tractography of the brain using diffusion spectrum imaging abstract presented at the International Society for Magnetic Resonance in Medicine. Twenty-Second Annual Meeting; Milan, Italy. May 10–16, 2014. [Google Scholar]
- Tan ET, Marinelli L, Sperl JI, Menzel MI, Hardy CJ. Multi-directional anisotropy from diffusion orientation distribution functions. J Magn Reson Imaging. 2015;41(3):841–850. doi: 10.1002/jmri.24589. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23(3):1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35(4):1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tuch DS. Q-ball imaging. Magn Reson Med. 2004;52(6):1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN. Genetic underpinnings of white matter ‘connectivity’: heritability, risk, and heterogeneity in schizophrenia. Schizophr Res. 2015;161(1):50–60. doi: 10.1016/j.schres.2014.03.034. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70(5):472–480. doi: 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- von Hohenberg CC, Pasternak O, Kubicki M, Ballinger T, Vu MA, Swisher T, Green K, Giwerc M, Dahlben B, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, Woodberry KA, Thermenos HW, Mulert C, McCarley RW, Seidman LJ, Shenton ME. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr Bull. 2014;40(4):895–903. doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pathak S, Stefaneanu L, Yeh FC, Li S, Fernandez-Miranda JC. Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct. 2016;221(4):2075–2092. doi: 10.1007/s00429-015-1028-5. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Res Med. 2005;54(6):1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Rosene DL, Wang R, Dai G, Mortazavi F, Hagmann P, Kaas JH, Tseng WI. The geometric structure of the brain fiber pathways. Science. 2012;335(6076):1628–1634. doi: 10.1126/science.1215280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Hwang TJ, Chen PJ, Chou TL, Hsu YC, Liu CM, Wang HL, Chen CM, Hua MS, Hwu HG, Tseng WY. Reduced structural integrity and functional lateralization of the dorsal language pathway correlate with hallucinations in schizophrenia: a combined diffusion spectrum imaging and functional magnetic resonance imaging study. Psychiatry Res. 2014;224(3):303–310. doi: 10.1016/j.pscychresns.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Yin X, Han Y, Ge H, Xu W, Huang R, Zhang D, Xu J, Fan L, Pang Z, Liu S. Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp. 2013;34(4):796–813. doi: 10.1002/hbm.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]


